Abstract
The present experiment compared the effects of a food-based conditioned inhibitor on food seeking vs. cocaine seeking behavior. In two groups of rats, the A+/AB− Pavlovian conditioned inhibition procedure was used to create a conditioned inhibitor for food. Then, for one group of rats (Food-Food Group), a click stimulus was established as an operant discriminative stimulus (SD) for food-reinforced lever pressing. In the other group (Food-Cocaine Group), the click was established as an SD for cocaine self-administration. In testing, the putative inhibitor for food was simultaneously presented with the click for the first time in both groups. In the Food-Food Group, the food-based inhibitor suppressed responding occasioned by the click significantly more than did a neutral control stimulus. In contrast, in the Food-Cocaine Group, there was no difference in the amount of suppression produced by the food-based inhibitor and the control stimulus. These results suggest that the effects of food-based Pavlovian conditioned inhibitors are specific for food-motivated behavior and do not easily transfer to cocaine-motivated behavior.
There have been a number of theorists that have postulated the existence of two different motivational systems: an appetitive system and an aversive system (e.g. Gray, 1975; Konorski, 1967; Mowrer, 1960). Stimuli capable of activating either of these systems are assumed to be able to control organisms’ behavior and to determine the type of activity the organisms will display towards these stimuli. Appetitive stimuli usually elicit approaching reactions, while aversive stimuli tend to provoke withdrawal reactions. An influential general theory based on these assumptions is Konorski’s (1967) appetitive-aversive interaction theory that has been amplified by Dickinson and colleagues (Dickinson & Dearing, 1979; Dickinson & Pearce, 1977). A central tenet of this theory is that reinforcers within the same incentive class, appetitive or aversive, have functionally comparable motivational properties. According to this formulation, a conditioned inhibitor based on a reinforcer in one incentive class should be capable of suppressing responding motivated by another reinforcer within that same incentive class (Dickinson & Pearce, 1977).
There has been conflicting evidence related to this prediction of appetitive-aversive interaction theory. Evidence in favor of this theory using appetitive class reinforcers was provided by Holland (1989). He reported that a food-based conditioned inhibitor suppressed sucrose-motivated behavior and, symmetrically, a sucrose-based inhibitor suppressed food-motivated responding. Colwill (1991) also found that inhibitors for food suppressed responding for sucrose, and vice versa, although this transfer was not complete (i.e., responding for one reinforcer was suppressed to a greater degree by an inhibitor based on that reinforcer than by an inhibitor based on the other reinforcer). In opposition to these two studies that support, to some extent, appetitive-aversive interaction theory, Kruse, Overmier, Konz, and Rokke (1983) found no transfer of the effects of food- or sucrose-based inhibitors to responding for sucrose or food, respectively. Similarly, Delamater, LoLordo and Sosa (2003) reported that the effects of food- and sucrose-based inhibitors were specific to responding motivated by the same reinforcers on which the inhibitors were based.
One of the main differences between the studies that found cross-appetitive-reinforcer transfer of a conditioned inhibitor (i.e., Colwill, 1991; Holland, 1989) and those that did not (i.e., Delamater et al., 2003; Kruse et al., 1983) is the procedure employed to create the conditioned inhibitor. The studies that found transfer used the A+/AB− procedure (i.e., Colwill, 1991; Holland, 1989), whereas the studies that failed to observe transfer used either differential conditioning (A+/B−; Kruse et al., 1983) or backward conditioning (Delamater et al., 2003). With the A+/AB− procedure, the inhibitor signals the absence of the reinforcer against an excitatory background provided by stimulus A. This situation should create a potent conditioned inhibitor (Wagner & Rescorla, 1972). Differential and backward conditioning should produce a weaker conditioned inhibitor since in both of these procedures the inhibitor signals the absence of the reinforcer during times in which expectation for the reinforcer is low (i.e., during CS+ intertrial intervals). Therefore, the apparent discrepant outcomes of the cross-reinforcer inhibitory transfer experiments described above may be produced by differences in the strength of the inhibitor. That is, transfer is observed when the inhibitor is strong (as it would be when created with the A+/AB− procedure), but not when it is weak (e.g., one created by differential or backward conditioning).
It is noteworthy that the studies that have found cross-appetitive-reinforcer transfer of conditioned inhibition still only provide weak support for appetitive-aversive interaction theory’s prediction, since the two reinforcers used were food and liquid sucrose – both ingestion-related reinforcers that the rat orally consumed. Therefore, it is plausible that the cross-appetitive-reinforcer inhibitory transfer might be the result of the high degree of physical similarity between the reinforcers. A more stringent test of the hypothesis might examine the transfer of the effects of an inhibitor based on a feeding-related reinforcer (e.g., food) to behavior motivated by a non-feeding-related reinforcer (e.g., cocaine).
Recently, Weiss et al., (2007) have reported that the effects of a food-based inhibitor transferred to operant lever pressing previously reinforced by cocaine. In that experiment, rats were trained to press a lever for food whenever a click discriminative stimulus (SD) was present and for cocaine whenever a tone SD was present. Occasionally, a light stimulus was presented simultaneously with the click and food reinforcement was discontinued. This A+/AB− training was intended to establish the light as an operant discriminative inhibitor for food. In testing, the light was compounded with the tone for the first time and suppressed lever pressing occasioned by the tone by approximately 90%.
Although the results of Weiss et al. (2007) are consistent with appetitive-aversive interaction theory’s prediction that a food-based conditioned inhibitor should transfer to behavior motivated by cocaine, there is an alternative explanation of these results that does not involve transfer of conditioned inhibition. The light in that study was a discriminative inhibitor. Such stimulus is different from a Pavlovian conditioned inhibitor. A discriminative inhibitor not only signals a negative stimulus–reinforcer relation, because it is associated with the absence of the reinforcing stimulus, but it also signals a negative stimulus–response relation, because it is also associated with a decrease in response rate. Thus, as noted by Weiss et al. (2007), a discriminative inhibitor like the light they used can potentially suppress responding through two different processes: 1) Pavlovian conditioned inhibition that is a function of the negative stimulus-reinforcer association that it signals, and 2) an operant discriminative process that is a function of the stimulus-response association that it signals. The objective of Weiss et al. (2007) was to develop a highly effective procedure that could be used to reduce drug seeking and, therefore, that study did not attempt to separate the relative influences of these two processes.
The goal of the present experiment was to determine whether a purely Pavlovian conditioned inhibitor for food – an inhibitor not specifically trained to produce response cessation – would suppress cocaine seeking. The employment of a Pavlovian inhibitor in our study, instead of a discriminative inhibitor, allows measuring exclusively the effect of motivational properties of the stimulus on drug seeking, since during training with the Pavlovian inhibitor, operant responding is not involved. For comparison, this experiment also investigated whether a Pavlovian food-based inhibitor would suppress operant food seeking. A design schematic of the experiment is presented in Table 1.
Table 1. Design of the experiment.
T, C and R represent the Tone, the Click and lever pressing, respectively. I and N represent the putative conditioned inhibitor and the neutral stimulus, respectively. Stimuli I and N consisted of a light or a wind, which were counterbalanced. In the first phase, all animals received a Tone/no-Tone discrimination wherein food was presented response-independently in the presence of the tone. Later, they received Conditioned Inhibition Training. Stimulus I was differentially reinforced, while stimulus N was non-differentially reinforced. In the next phase, animals were trained on a Click/no-Click discrimination in which lever pressing was reinforced in the presence of the Click. The reinforcement used in the Food-Food Group was food, while in the Food-Cocaine it was cocaine. Finally, animals received a test consisting of Click, Click+Conditioned Inhibitor (C+I) compound, and Click+Neutral Stimulus compound (C+N) components.
| Group | Tone/no-Tone | Inhibition Training | Click/no-Click | Test |
|---|---|---|---|---|
| Food-Food |
T: Food | T: Food | C: R→ Food |
C, C+I, C+N |
| T+I: No Food | ||||
| Food-Cocaine | T+N: Food | C: R→ Cocaine | ||
| N: No Food | ||||
According to appetitive-aversive interaction theory, the food-based Pavlovian conditioned inhibitor should suppress both cocaine and food seeking. Such results would also suggest that the suppression of responding reported by Weiss et al. (2007) were in fact due, at least in part, to transfer of conditioned inhibition. On the other hand, if a food based conditioned inhibitor suppresses food seeking but not cocaine seeking, this would 1) contradict a prediction of appetitive-aversive interaction theory, and 2) suggest that the suppression of cocaine seeking observed in the Weiss et al. (2007) study was due primarily to the response discriminative process, rather than to inhibitory associative or incentive-motivational factors.
METHOD
Subjects
Thirteen naïve adult male Long-Evans rats, initially weighing 390 g on average (range: 370–440 g), were used as subjects. Rats were housed in individual metal cages in a colony room with a 12-h light cycle (lights on at 08:00 a.m.). Training sessions were conducted during the light-on cycle. Training commenced after rats had been deprived to approximately 80 % of their ad libitum weight. Weights were maintained at this level by feeding them 12–15 g of laboratory rat chow following their training sessions, which were conducted 5 days per week. Water was available continuously, except during the experimental sessions.
Surgery
Rats were surgically prepared with chronic indwelling jugular vein catheters and headmounts using a modification of Weeks (1962) procedure described by Panlilio, Weiss and Schindler (1996). In brief, under ketamine (60 mg/kg) and xylazine (10 mg/kg) anesthesia, approximately 3 cm of Silastic® tubing (0.044 mm ID, 0.814 mm OD) was inserted into the right jugular vein. This Silastic® tubing was connected to 5 cm of vinyl tubing (dural plastic: 0.5 mm ID, 1.0 mm OD) that was passed under the skin around the shoulder and exited at the back. The vinyl tubing was threaded through a 10-mm2 section of Tygon tubing that served as a subcutaneous anchor. Four stainless steel jeweler’s screws were implanted in the skull to which a 20-mm plastic screw was cemented with dental acrylic. After surgery, catheters were flushed daily with 0.1 ml of a saline solution containing 1.25 U/ml heparin and 0.08 mg/ml gentamycin.
Apparatus
Training sessions were conducted in six operant chambers (Weiss & Schindler, 1989) that were enclosed in sound-attenuation chests (Weiss, 1970). Each chamber measured 20 cm high, 23 cm long, and 18 cm wide, and was dimly lighted at all times by a shielded 7.5-W houselight operated at 3 W. The level of illumination created by this houselight was enough to make the rat barely discernible. Each chamber contained a lever operandum and food trough on the front wall. A response on the lever closed a Gerbrands microswitch, requiring a force of 0.14 to 0.18 N (15 to 20 g). Ambient noise with the exhaust fan running was measured at 70 dB (Realistic SPL meter). An approximately 2000-Hz, 85-dB tone was generated by a BRS AO-201 audio oscillator, amplified by a BRS AA-201 amplifier, and presented through an 8-Ohm, 20-cm speaker mounted in an enclosure 21.5 cm above the training chamber. There were two 15-cm, 25-W, 120-V tubular light bulbs 10 cm behind the two translucent sidewalls, which provided the visual stimulus. These lights were operated at 60 V. A ventilation fan was mounted on the exterior of the rear wall of each chamber. These fans operated at 28 V. The creation of small holes in the rear wall permitted the circulation of airflow into the boxes. This “wind” and associated sound generated by the fan was used as a stimulus.
Cocaine (National Institute on Drug Abuse, Bethesda, MD) in saline solution at a concentration of 2.56 mg/ml was infused at a rate of 3.19 ml/min by 10-ml syringes driven by Harvard Apparatus (South Natick, MA) or MED Associates (East Fairfield, VT) syringe pumps located outside of the sound attenuation chests. Tygon tubing (Saint Gobain Performance Plastics, Akron, OH) extended from the 10-ml syringes to a 22-gauge rodent single-channel fluid swivel and tether apparatus (Alice King Chatham Medical Arts, Hawthorne, CA) that descended through the ceiling of the training chamber. Cocaine was delivered to the subject through Tygon tubing that passed through the metal spring of the tether apparatus. This metal spring was attached to the plastic screw that was cemented to the rat’s head to reduce tension on the catheter.
Experimental events were controlled by a MED Associates (St. Albans, VT) computer system located in a room adjacent to the one where the training chambers were located. Cumulative recorders used to monitor rats´ ongoing behavior were also located in this room.
Procedure
For a summary of the procedure see Table 2.
Table 2. Summary of the procedure of the experiment.
VT and FR refer to variable time and fixed ratio schedules, respectively. EXT indicates that responding was not reinforced. RC refers to response correction schedule. G.i. is an abbreviation for “gradually increased”. Stimuli I and N represent the putative conditioned inhibitor and the neutral stimulus, respectively.
| Tone/no-Tone Discrimination |
| Tone: VT 45-s |
| Tone-off: EXT |
| Conditioned Inhibition Training |
| Both groups treated the same in this phase |
| Food Components (50% of Total Components) |
| Tone: VT 45-s (80% of the Food Components) |
| Tone+N: VT 45-s (20% of the Food Components) |
| Extinction Components (50% of Total Components) |
| Tone+I: EXT (40% of the Extinction Components) |
| N: EXT (20% of the Extinction Components) |
| All stimuli-off: EXT (40% of the Extinction Components) |
| Click/no-Click Discrimination Training |
| Reinforcer in Click = Cocaine for Food-Cocaine Group |
| Reinforcer in Click = Food for Food-Food Group |
|
| Stimulus Compounding Tests |
| Warm-up |
| Click: VT 45-s |
| Click-off: EXT + RC 60-s |
| Stimulus Compounding Test |
| Click: EXT |
| Click+I: EXT |
| Click+N: EXT |
| All stimuli-off: EXT |
Tone/no-Tone Discrimination
In this phase all rats received tone-on components that alternated with tone-off components. All components lasted 60 sec on average (range: 30–120). During tone components, food pellets (45 mg, P.J. Noyes Co.) were presented on a variable-time (VT) 45-sec schedule, while during tone-off components, no food was delivered (extinction [EXT]). Sessions lasted 90 min. All animals received 7 sessions of training on this multiple (mult) VT EXT discrimination.
Conditioned Inhibition Training
In this phase, as previously, VT 45-s components, where food was presented non-contingently, alternated with EXT components, where no food was delivered and both types of components lasted 60 s on average. However, now there was a change in the stimuli that signaled these components. For six subjects, 80% of the VT components were signaled by tone alone (as previously), but the remaining 20% of the VT components were signaled by tone+wind. For these subjects, 40% of the EXT components were signaled by tone+light, 20% by wind alone, and the remaining 40% of the components were signaled by the absence of all stimuli. Thus, for these subjects the light would be the putative inhibitor and the wind would be the control stimulus. For the remaining seven subjects, everything was as described above except the roles of the light and the wind were reversed.
According to the arrangement described above, components containing the putative inhibitor and components containing the control stimulus occurred at the same frequency (i.e., 40% of total components). However, the putative inhibitor always signaled the absence of food, while half the control stimulus components signaled food and half signaled no food. Furthermore, the putative inhibitor always signaled the absence of food against the excitatory background provided by the tone, which, when presented without the putative inhibitor, always signaled food. All animals received 14 sessions (lasting 2 h each) during this phase.
Click/no-Click Discrimination Training
Subjects were randomly assigned to the Food-Food Group (n=6) or the Food- Cocaine Group (n=7). In both groups, there were three subjects that were previously trained with the light as the putative conditioned inhibitor and with the wind as the neutral stimulus. There were three and four rats from the opposite counterbalanced conditions in the Food-Food and Food-Cocaine Groups, respectively. At this point, rats belonging to Food-Cocaine Group were implanted with a catheter, as described in the Surgery section. After surgery, rats were given at least 5 days to recover in their home cages.
Food-Food Group
Rats were then trained to press the lever to obtain food pellets on a fixed-ratio (FR-1) schedule with the click stimulus on continuously. Simultaneously, food pellets were presented on a variable-time (VT) 120-sec schedule. This VT schedule was in effect until a subject pressed the lever eight times within a session. As rats learned to lever-press, the reinforcement schedule was gradually changed from FR-1 to FR-10 over sessions. Once animals were responding on the FR-10 schedule at a moderate to high rate, a variable-interval (VI) 30-s schedule was implemented. The values for the VI schedule came from a custom-generated list of 58 values that ranged from 1 to 79. In this list, there were 24 values that were less than 20, 13 values from 20 to 39, 7 values from 40 to 59, and 13 values from 60 to 79. The VI schedule was soon changed from VI 30-s to VI 45-s by multiplying each value from the list by 1.5.
Once rats were responding on a regular basis on the VI 45-sec schedule, click/no-click discrimination training began. Now, click components lasting 60 sec on average (range: 30–120) alternated with click-off periods, which also lasted 60 sec on average (range: 40–90). During click components, the VI 45-sec schedule remained in effect, while during click-off components, lever presses were not followed by food (extinction [EXT]), creating a mult VI 45-sec EXT schedule. In addition, a 10-sec response correction contingency was added to the end of click-off components in order to promote response cessation in EXT components. A lever press during the response correction period delayed the presentation of the next click component by 10 sec. The response correction was increased to 30 sec and finally to 60 sec over sessions. Training on this schedule continued until the response rate in click components was stable and at least 5 times greater than in click-off components for two consecutive sessions. Sessions in this phase lasted until rats received approximately 150 pellets or until 2 hours elapsed.
Food-Cocaine Group
Rats of this group were trained the same way as in the previous group, except that cocaine infusions served as the reinforcer instead of a food pellet. The dose was set to 1.0 mg/kg/infusion during initial FR-1 training. As rats came to regularly respond, the dose per infusion was gradually reduced to 0.25 mg/kg, where it remained for the rest of the experiment.
Stimulus Compounding (Summation) Tests
Click+Conditioned Inhibitor and Click+Neutral Stimulus Test. All animals received three consecutive test sessions. Each test session was preceded by a 45-min warm-up period, wherein the click/no-click training baseline was effective. Each test session consisted of 6 blocks of three components. In each block a 60-sec period of click, click+light and click+wind components were presented. No reinforcement was delivered in any of these components. Each type of component was presented only once per block, with the order of stimulus presentation randomized within each block. These components were separated by 60-sec periods where all stimuli were off.
RESULTS
A significance level of p < .05 was adopted for statistical tests. Training during the tone/no-tone discrimination and during the conditioned inhibition phases proceeded uneventfully. Prior to training in the click/no-click discrimination rats were trained on a fixed-ratio schedule an average of 1 and 6 sessions for the Food-Food and Food-Cocaine Groups, respectively. The mean number of sessions necessary for the rats to reach the criterion during the click/no-click discrimination training was 5 for the Food-Food and 15 for the Food-Cocaine Groups. The mean response rates over the two criterion sessions in click were 31.5 and 8.0 responses/min for the Food-Food and Food-Cocaine Groups, respectively. In click-off, response rates were 4.5 and 1.2 responses/min, respectively, for these groups.
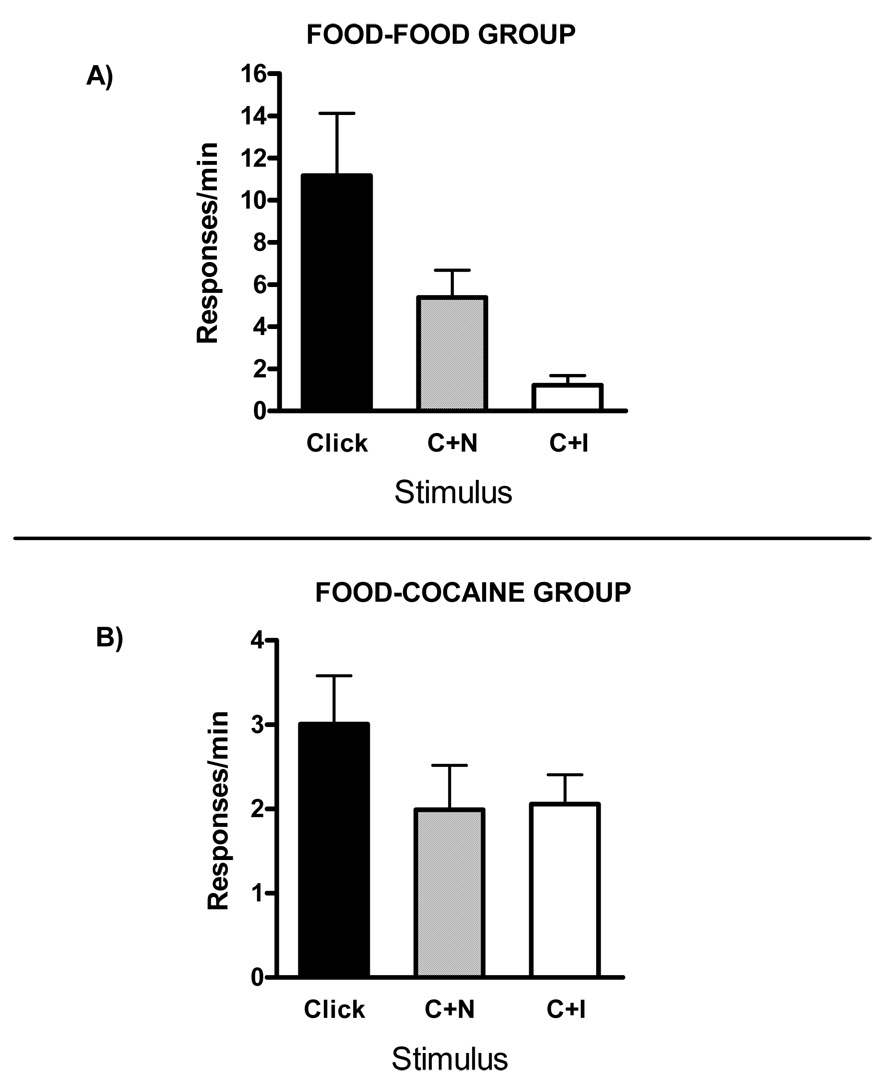
Figure 1 illustrates mean (± SEM) response rates (responses per min) pooled across the three compounding test sessions in click, in the click+neutral stimulus compound (C+N) and in the click+conditioned inhibitor compound (C+I) for the Food- Food Group (panel A) and the Food-Cocaine Group (panel B). Note that the scales of the Y-axes differ across panels. This figure illustrates that in both groups the response rate in the C+N and C+I compounds were lower than in click alone. However, in the Food-Food Group, the inhibitor produced substantially more suppression of click responding than the neutral stimulus. In contrast, in the Food-Cocaine Group, there was no difference in the amount of suppression produced by the neutral stimulus and the inhibitor.
Figure 1.
Mean (± SEM) response rates (responses per min) pooled across the three compounding test sessions in click, in the click+neutral stimulus compound (C+N) and in the click+conditioned inhibitor compound (C+I) for Food-Food Group (panel A) and Food-Cocaine Group (panel B).
These impressions were confirmed by statistical analyses. A repeated measures analysis of variance (ANOVA) conducted on these response rates, with group (Food-Food or Food-Cocaine) and stimulus (Click, C+N or C+I) as variables, revealed that there was a significant effect of group, F(1,11) = 7.56, and of stimulus, F(2,2) = 12.77. The interaction between these two factors was also significant, F(2,2) = 8.54. In order to investigate this interaction, further independent statistical analyses were performed. Repeated measures ANOVAs conducted on each group revealed that there was a significant effect of stimulus in the Food-Food Group, F(2,10) = 9.22, as well as in the Food-Cocaine Group, F(2,12) = 4.26. Paired t-tests revealed that in the Food-Food Group, the response rate in click was significantly greater than the response rate in C+N, t(5) = 3.14, and in C+I as well, t(5) = 3.05. More importantly, another paired t-test revealed that the response rate in the C+I compound was statistically lower than the response rate in the C+N compound, t(5) = −2.76. The same analyses were conducted on the Food-Cocaine Group, showing that, like in the Food-Food Group, the response rate in click was significantly different from the response rate in C+N, t(6) = 2.76, and also from the response rate in C+I, t(6) = 2.68. However, in contrast to the Food-Food Group, the response rate in C+I compound was not statistically different from the response rate in C+N compound, t(6) = .14.
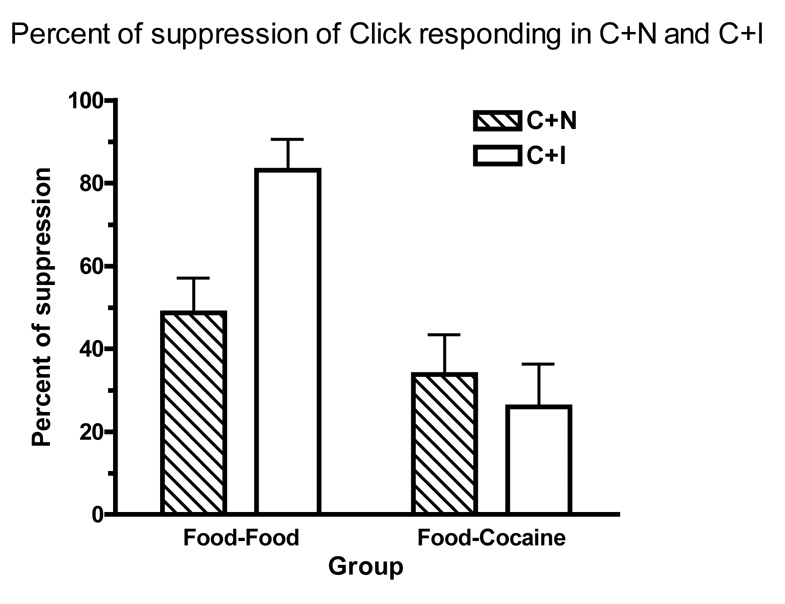
Figure 2 presents test results in terms of mean percent suppression of click responding (e.g., [([Click rate – C+I rate]/Click rate) × 100]). A percentage of 0 indicates no suppression, whereas a percentage of 100 means that the suppression was complete. This measure allows the relative difference between response rates in click and the C+I or C+N compounds observed in each subject to be weighted equally regardless of absolute response rates in these stimuli. In the Food-Food Group, the putative inhibitor suppressed click responding by approximately 85%, while the neutral stimulus only suppressed click responding by approximately 50%. Hence, in this group, the percent suppression of click rates was substantially greater in the C+I compound as compared to the C+N compound. On the other hand, in the Food-Cocaine Group, the percent suppression of click rates observed in the C+I and C+N conditions were 25% and 35%, respectively. Therefore, in this group, and in contrast to the previous one, the suppression observed in the C+I compound was slightly smaller than in the C+N compound. A repeated measures ANOVA conducted on these percentages of suppression, with group (Food-Food or Food-Cocaine) and stimulus (C+N or C+I) as variables, revealed that there was a significant effect of group, F(1,11) = 12.36, but the effect of stimulus did not reach statistical reliability, F(1,11) = 2.69. Additionally, the interaction between these two factors was also significant, F(1,11) = 6.75. This interaction was explored performing t-test analyses. Paired t-tests revealed that in the Food-Food Group, the percent suppression in C+I was significantly greater than in C+N, t(5) = 6.52. In contrast, in the Food-Cocaine Group this difference was not statistically reliable, t(6) = −.54. Finally, unpaired t-tests revealed that C+I produced significantly greater suppression in the Food-Food Group than in Food-Cocaine Group, t(11) = 4.34, whereas the percent of suppression observed in C+N did not differ significantly between groups, t(11) = 1.14.
Figure 2.
Mean (± SEM) percent suppression of click responding in the click+inhibitor (C+I) and click+neutral stimulus (C+N) compounds during the summation tests for the Food-Food Group and the Food-Cocaine Group.
DISCUSSION
The present experiment demonstrated that a Pavlovian conditioned inhibitor, which was associated with the omission of food, suppressed responding occasioned by a discriminative stimulus (SD) for food to a greater extent than did a neutral stimulus which was not differentially associated with food. This result replicates that of previous studies that used a similar design (Gutman & Maier, 1978; Lombas, Kearns, & Weiss, 2008; though see also Bonardi, 1988). However, a food-based Pavlovian conditioned inhibitor did not suppress responding occasioned by an SD for cocaine-reinforced responding any more than a control stimulus did.
The finding that the effects of a food-based conditioned inhibitor did not transfer to behavior motivated by cocaine stands in apparent contrast to results recently reported by Weiss et al. (2007). However, as acknowledged in the discussion of Weiss et al. (2007), the reported suppression of cocaine seeking produced by a discriminative inhibitor for food, created within an operant A+/AB− paradigm in that study, could have been due to 1) a cross-appetitive-reinforcer transfer of conditioned inhibition, 2) the fact that the inhibitor in that study was discriminative for response cessation or 3) some combination of the these two factors. The failure to find any transfer of conditioned inhibition in the present experiment suggests that the results of the Weiss et al. (2007) study could have primarily been due to the second alternative listed above. That is, it is likely that the suppression of cocaine seeking observed in that study was due mainly to the fact rats had learned to stop lever pressing in the presence of the food-based discriminative inhibitor.
One unexpected finding in the present study was the degree to which the neutral stimulus suppressed responding occasioned by the click during testing in both groups. The neutral stimulus suppressed click responding by approximately 50% and 35% in the Food-Food and Food-Cocaine Groups, respectively. Because the neutral stimulus was uncorrelated with the presence or absence of food, it should not have acquired inhibitory or excitatory properties. Therefore, any suppression produced by the neutral stimulus likely was due to external inhibition or generalization decrement that might be related to the novelty of having the click and neutral stimulus presented simultaneously.
Support for this hypothesis is provided by a previous study performed by Bonardi (1988). Using a within-subject control procedure similar to the Food-Food Group in the present work, she also found suppression of responding when a novel visual stimulus was compounded with an auditory SD for food-reinforced lever pressing (Group VR). However, it should be noted that the magnitude of this suppression was smaller than that produced by the neutral stimulus in the present study. This difference may have been due to the fact that rats were occasionally reinforced for lever pressing during novel stimulus presentations on the summation test in the Bonardi study, while in the present study no reinforcement was delivered during the tests.
Despite the suppression produced by the control stimulus in both groups of the present study, the signal for the absence of food suppressed click responding to a significantly greater degree than the neutral stimulus in the Food-Food Group, indicating that this stimulus did in fact acquire inhibitory properties that acted to suppress responding for food. In the Food-Food Group, this food-based inhibitor suppressed responding by approximately 85% as compared to approximately 50% suppression produced by the neutral stimulus. This indicates that approximately 35% of the suppression produced by the inhibitor was due to inhibitory conditioning rather than nonassociative influences (e.g., external inhibition or generalization decrement). On the other hand, in the Food-Cocaine Group the amount of suppression produced by the food-inhibitor (25%) was approximately the same as that produced by a neutral stimulus (33%). This means that in the Food-Cocaine Group all of the response suppression produced by the food-based inhibitor was due to non-associative factors and that the food-related inhibitory properties conditioned to this stimulus did not affect responding for cocaine.
Another unanticipated result in the present experiment was the difference between groups in click response rates. The Food-Food Group made approximately four times as many responses per minute in the click as the Food-Cocaine Group during training (31.5 vs. 8.0 responses/min) and testing (11.2 vs. 3.0 responses/min). However, there are several reasons why this difference between groups in click response rates is unlikely to account for differential effects of the food-based inhibitor between groups reported here. First, the mean cocaine response rates during training and testing in the present experiment were actually slightly higher than those in a previous study (Kearns, Weiss, Schindler & Panlilio., 2005) that found that a cocaine-based inhibitor in fact did suppress cocaine seeking by over 90%. That previous study used the same dose of cocaine (0.25 mg/kg) and the same variable-interval schedule value (VI 45-s) that was employed here. Second, if anything, the lower response rate to the click in the Food-Cocaine Group might have biased outcomes toward finding a greater percent suppression in this group. If the inhibitor had reduced response rate in the same amount in both groups, suppression would have been more profound in the group with the lower response rate (Food-Cocaine Group) than in the group with a greater response rate (Food-Cocaine Group). Instead, the opposite result was found. Finally, there was no significant difference between the groups in the percentage by which the neutral stimulus suppressed click responding. This percentage measure factors out differences in absolute response rates. This suggests that in the two groups the click response rates were equally sensitive to the non-associative suppressive effects produced by the simultaneous presentation of the neutral stimulus. It is unlikely that the different click rates of the two groups would be equally sensitive to one source of suppression (i.e., non-associative factors such as external inhibition or generalization decrement), but at the same time differentially sensitive to another source of potential suppression (i.e., food-based conditioned inhibition).
It has been found that cocaine produces interoceptive cues that can produce statedependent learning (e.g., D’Mello and Stolerman, 1977). In the Food-Cocaine Group the food-based inhibitor was created before cocaine self-administration began, but testing occurred after having self-administered cocaine during the pre-test warm-up. Therefore, it might be thought that this difference in drug context over training and testing conditions could have interfered with the transfer of inhibition in this group. However, this seems unlikely as multiple studies have shown that the effects of conditioned inhibitors transfer very well across contexts provided by exteroceptive cues (Bouton & Nelson, 1994; Nelson, 2002; Nelson & Bouton, 1997).
It might also be thought that general stimulus processing capabilities might be impaired under the influence of cocaine and that this may have prevented the food-inhibitor from suppressing responding in the Food-Cocaine Group. However, this possibility seems unlikely because rats in the Kearns et al. (2005) and Weiss et al. (2007) studies were also tested under the influence of cocaine, but nevertheless displayed 90% suppression of cocaine seeking when a discriminative inhibitor was presented. Furthermore, the training data from the click/no-click phase from the present experiment indicate that, although response rates were lower for the group responding for cocaine, click/click-off discrimination ratios were essentially the same in both groups (approximately 4:1).
There are studies that have provided evidence that, in addition to its positive properties, cocaine administration is responsible for producing a negative affective state in the subject (e.g., Ettenberg, Raven, Danluck & Necessary, 1999; for different results see Lombas, Freeman, Roma & Riley, 2007). There has been a disagreement about which of these properties reinforce drug addiction behaviour. Theories that concentrate on the drug’s positive properties state that drug taking is motivated by the hedonic effects of drugs of abuse (positive reinforcement). In contrast, theories that have focused on the negative properties consider that drug consumption behaviour is maintained because it avoids negative affective consequences of drug withdrawal (negative reinforcement). Hence, in our study rats might respond for cocaine because cocaine has positive effects or in order to escape or avoid aversive withdrawal symptoms. However, studies that have induced cocaine withdrawal symptoms employed larger amounts of cocaine given over a longer timer periods (e.g., Markou & Koob, 1991) than were used in the present experiment. Further, the negative reinforcement account could be applied just as easily to food. That is, animals could be thought to be responding for food to escape or avoid aversive food-deprivation symptoms. It is clear that the present experiment cannot determine whether rats were responding for cocaine or for food because of their positively- or negatively-reinforcing qualities. Nevertheless, this is not important because the essential thing for appetitive-aversive interaction theory is that rats were responding for these stimuli. That they did so means that both food and cocaine were acting as appetitive stimuli as defined by the theory.
To conclude, the results of the present experiment suggest that the motivational properties acquired by an inhibitory CS for food have suppressive effects on responding when it is maintained by food, but not when it is maintained by cocaine. These findings are inconsistent with the prediction of appetitive-aversive interaction theory that an inhibitor for one appetitive stimulus should suppress responding maintained by a different reinforcer but with hedonically similar properties (Dickinson & Dearing, 1977; Dickinson & Pearce, 1979; Weiss et al., 1996). Rather, they suggest that Pavlovian conditioned inhibitors are reinforcer-specific (in agreement with Delamater et al., 2003; and Kruse et al., 1983).
Acknowledgements
This research was supported by a post-doctoral fellowship from the Basque Government (Programa de Formación de Investigadores del Departamento de Educación, Universidades e Investigación) awarded to Andrés Sebastián Lombas, and by a grant from National Institute on Drug Abuse Grant Awarded by Stanley J. Weiss. The principles of laboratory animal care as described in the Guide for the Care and Use of Laboratory Animals (National academy of Sciences, 1996) were followed during this research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bonardi C. Mechanisms of inhibitory discriminative control. Animal Learning & Behavior. 1988;16:445–450. [Google Scholar]
- Bouton ME, Nelson JB. Context specificity of target versus feature inhibition in a feature-negative discrimination. Journal of Experimental Psychology: Animal Behavior Processes. 1994;20:51–65. [PubMed] [Google Scholar]
- Colwill RM. Negative discriminative stimuli provide information about the identity of omitted response-contingent outcomes. Animal Learning & Behavior. 1991;19:326–336. [Google Scholar]
- Delamater AR, LoLordo VM, Sosa W. Outcome-specific conditioned inhibition in Pavlovian backward conditioning. Learning & Behavior. 2003;31:393–402. doi: 10.3758/bf03196000. [DOI] [PubMed] [Google Scholar]
- Dickinson A, Dearing MF. Appetitive-aversive interactions and inhibitory processes. In: Dickinson A, Boakes RA, editors. Mechanisms of animal learning and motivation: A memorial volume to Jerzy Konorski. Hillsdale, NJ: Lawrence Erlbaum Associates, Inc; 1979. pp. 203–232. [Google Scholar]
- Dickinson A, Pearce JM. Inhibitory interaction between appetitive and aversive stimuli. Psychological Bulletin. 1977;84:690–711. [Google Scholar]
- D’Mello GD, Stolerman IP. Comparison of the discriminative stimulus properties of cocaine and amphetamine in rats. British Journal of Pharmacology. 1977;61:415–422. doi: 10.1111/j.1476-5381.1977.tb08434.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ettenberg A, Raven MA, Danluck DA, Necessary BD. Evidence for opponent-process actions of intravenous cocaine. Pharmacology, Biochemistry and Behavior. 1999;64:507–512. doi: 10.1016/s0091-3057(99)00109-4. [DOI] [PubMed] [Google Scholar]
- Gray JA. Elements of two-process learning theory. London: Academic Press; 1975. [Google Scholar]
- Gutman A, Maier SF. Operant and Pavlovian factors in cross-response transfer of inhibitory stimulus control. Learning & Motivation. 1978;9:231–254. [Google Scholar]
- Holland PC. Transfer of negative occasion setting and conditioned inhibition across conditioned and unconditioned stimuli. Journal of the Experimental Psychology: Animal Behavior Processes. 1989;15:311–328. [PubMed] [Google Scholar]
- Kearns DN, Weiss SJ, Schindler CW, Panlilio LV. Conditioned inhibition of cocaine seeking in rats. Journal of the Experimental Psychology: Animal Behavior Processes. 2005;31:247–253. doi: 10.1037/0097-7403.31.2.247. [DOI] [PubMed] [Google Scholar]
- Konorski J. Integrative activity of the brain: An interdisciplinary approach. Chicago, IL: University of Chicago Press; 1967. [Google Scholar]
- Kruse JM, Overmier JB, Konz WA, Rokke E. Pavlovian conditioned stimulus effects upon instrumental choice behavior are reinforcer specific. Learning & Motivation. 1983;14:165–181. [Google Scholar]
- Lombas AS, Freeman KB, Roma PG, Riley AL. Intravenous cocaine priming reinstates cocaine-induced conditioned place preference. Psicológica. 2007;28:55–62. [Google Scholar]
- Lombas AS, Kearns DN, Weiss SJ. A comparison of the effects of discriminative and Pavlovian inhibitors and excitors on instrumental responding. Behavioural Processes. 2008;78:53–63. doi: 10.1016/j.beproc.2008.01.003. [DOI] [PubMed] [Google Scholar]
- Markou A, Koob GF. Postcocaine anhedonia. An animal model of cocaine withdrawal. Neuropsychopharmacology. 1991;4:17–26. [PubMed] [Google Scholar]
- Mowrer OH. Learning theory and behavior. New York: Wiley; 1960. [Google Scholar]
- Nelson JB. Context Specificity of Excitation and Inhibition in Ambiguous Stimuli. Learning and Motivation. 2002;33:284–310. [Google Scholar]
- Nelson JB, Bouton ME. The effects of a context switch following serial and simultaneous feature-negative discriminations. Learning and Motivation. 1997;28:56–84. [Google Scholar]
- Panlilio L, Weiss SJ, Schindler CW. Cocaine self-administration increased by compounding discriminative stimuli. Psychopharmacology. 1996;125:202–208. doi: 10.1007/BF02247329. [DOI] [PubMed] [Google Scholar]
- Wagner AR, Rescorla RA. Inhibition in Pavlovian conditioning: Application of a theory. In: Boaks RA, Halliday MS, editors. Inhibition and learning. London: Academic Press; 1972. pp. 301–336. [Google Scholar]
- Weeks JR. Experimental morphine addiction: method for automatic intravenous injections in unrestrained rats. Science. 1962;138:143–144. doi: 10.1126/science.138.3537.143. [DOI] [PubMed] [Google Scholar]
- Weiss SJ. An effective and economical sound-attenuation chamber. Journal of the Experimental Analysis of Behavior. 1970;13:37–39. doi: 10.1901/jeab.1970.13-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss SJ, Kearns DN, Christensen CJ, Huntsberry ME, Schindler CW, Panlilio LV. Reduction of cocaine-seeking by a food-based inhibitor in rats. Experimental & Clinical Psychopharmacology. 2007;15:359–367. doi: 10.1037/1064-1297.15.4.359. [DOI] [PubMed] [Google Scholar]
- Weiss SJ, Schindler CW. Integrating control generated by positive and negative reinforcement on an operant baseline: Appetitive-aversive interactions. Animal Learning & Behavior. 1989;17:433–446. [Google Scholar]
- Weiss SJ, Thomas DA, Weissman RD. Combining operant-baseline-derived conditioned excitors and inhibitors from the same and different incentive classes: an investigation of appetitive-aversive interactions. The Quarterly Journal of Experimental Psychology. 1996;49:357–381. doi: 10.1080/713932635. [DOI] [PubMed] [Google Scholar]