Abstract
Background
Prostate cancer (PCa) incidences vary with genetic, geographical and ethnic dietary background of patients while angiogenesis is modulated through exquisite interplay of tumor-stromal interactions of biological macromolecules. We hypothesized that comprehensive analysis of four biomarkers modulating angiogenesis in PCa progression in two diverse populations might explain the variance in the incidence rates.
Results
Immunohistochemical analysis of 42 PCa biopsies reveals that though Anx-II expression is lost in both the Indian and American population with Gleason scores (GS) ranging between 6 and 10, up to 25 % of cells in the entire high grade (GS > 8) PD PCa samples from US show intense focal membrane staining for Anx-II unlike similarly graded specimens from India. Consistent with this observation, the prostate cancer cell lines PC-3, DU-145 and MDA PCa 2A, but not LNCaP-R, LNCAP-UR or MDA PCa 2B cell lines, express Anx-II. Transcriptional reactivation of Anx-II gene with Aza-dC could not entirely account for loss of Anx-II protein in primary PCa. Cyclooxygenase-2 (COX-2) was moderately expressed in most of high grade PIN and some MD PCa and surrounding stroma. COX-2 was not expressed in PD PCa (GS ~7–10), while adjacent smooth muscles cells stained weakly positive. Decorin expression was observed only in high grade PIN but not in any of the prostate cancers, atrophy or BPH while stromal areas of BPH stained intensively for DCN and decreased with advancing stages of PCa. Versican expression was weak in most of the MD PCa, moderate in all of BPH, moderately focal in PD PC, weak and focal in PIN, atrophy and adjacent stroma.
Conclusions
Expression of pro- and anti-angiogenic modulators changes with stage of PCa but correlates with angiogenic status. Focal membrane staining of Anx-II reappears in high grade PCa specimens only from US indicating differential expression of Anx-II. COX-2 stained stronger in American specimens compared to Indian specimens. The sequential expression of DCN and VCN in progressive stages was similar in specimens from India and USA indicating no population-based differences. The mechanistic and regulatory role of Anx-II in PCa progression warrants further investigation.
Background
Prostate cancer is the most common form of cancer in males and the second leading cause of cancer related death. The latest estimates of global incidences of PCa show that it is more common in countries with higher proportions of elderly men in their population and PCa accounts for about 15% of all cancers in developed countries as compared to 4% in developing countries [1]. The incidence of PCa increased in the American population in the late 1980s and early 1990s, but increased incidence rates have also occurred in low risk countries like India. While the incidence rates may be low in India, one study found that 84% of patients in India present with advanced stages of PCa [2]. Another study encompassing 110 Indian PCa specimens, found that high grade PIN was present in 85% of the samples [3]. In India, early detection of PCa is difficult because of the high-cost of PSA screening resulting in limited PSA screening, and the fact that there are rarely any symptoms in the early stages of the disease. Thus, it is important to understand the molecular changes associated with early stages of PCa as well as progression of the disease. Numerous biomarkers have been identified; however, our particular study focuses on specific biomarkers that are related to interaction of tumor cell and stromal microenvironment and its cooperative effect in prostate cancer progression through promotion of angiogenesis. We studied archival prostate tissues from India and USA patient populations to investigate ethnic differences in the expression pattern of biomarkers that could point to genetic and lifestyle-related contribution to prostate cancer progression.
Angiogenesis is the formation of new blood vessels from pre-existing ones. It is well known that any increase in tumor mass must be preceded by an increase in vascular supply that helps support growth and dissemination of tumor cells [4]. The process of angiogenesis is a result of changes in the equilibrium between positive and negative angiogenic factors [5]. Some important mediators that have been studied include acidic and basic fibroblast growth factors (FGFs), vascular endothelial growth factors (VEGFs), transforming growth factors (TGF-α and TGF-β), platelet-derived growth factor (PDGF), angiogenins (ANG), interleukin (IL)-8, and tumor necrosis factor-alpha (TNFα) [6-8]. Interestingly, both the development of a vascular supply and stromal support are essential for tumor growth [9,10]. To test this hypothesis, we investigated biomarkers that are known as direct or indirect modulators of angiogenesis through interaction in the extracellular matrix milieu with other biomolecules involved in tumor progression. To our knowledge this is the first study with a comprehensive approach to study prostate cancer progression involving probable crosstalk of angiogenic modulators.
Anx-II belongs to a family of Ca2+-dependent phospholipid and membrane binding proteins called annexins. Anx-II is implicated in the tissue plasminogen activator (tPA)-plasmin pathway, and facilitates formation of a complex that permits plasmin formation known to stimulate matrix remodeling and activation of matrix metalloproteinases (MMPs) required for angiogenesis [11,12]. Anx-II has been frequently found to be lost in primary prostate adenocarcinoma [13] but is known to be over-expressed in other type of cancers [14-16].
Cyclooxygenase-2 (COX-2) is the inducible form of cyclooxygenase that converts arachidonic acid to prostaglandins. Its expression is undetectable in normal tissue, but COX-2 is expressed in higher amounts in inflamed and malignant tissues. Prostaglandins are known pro-angiogenic factors and cells that overexpress COX-2 are able to stimulate endothelial migration and tube formation [17]. COX-2 is also said to contribute to tumor progression by increasing adhesion of cells to the extracellular matrix (ECM) and making them resistant to apoptosis [18]. Studies have also shown that COX-2 inhibitors are anti-angiogenic [19,20].
Decorin (DCN) is small leucine-rich proteoglycan found in many connective tissues. It converges with EGF functionally to regulate the cell cycle through a pathway that leads to growth suppression. Several studies have shown that wild-type decorin has the ability to reduce VEGF levels in tumors [21]. The authors of this study hypothesized that if native decorin could be released and spread diffusely through the stroma, it could stop growing tumor cells by suppressing the production of a powerful angiogenic stimulus. However, there is limited information on decorin expression levels and molecular studies of decorin in prostate cancer [22].
Versican (VCN) is a large chondroitin sulphate proteoglycan secreted by fibroblasts and malignant epithelial cells have the ability to regulate versican secretion. TGF-β1, a known proangiogenic factor, enhances the level of versican accumulation in prostate cancer [23]. It is believed that it plays a role in tumor progression by destabilizing cell adhesion, hence promoting cell proliferation and motility especially of endothelial cells [24].
We hypothesize that tumor angiogenesis and subsequent progression is modulated by complex interplay between tumor cells and stromal components through expression of various biological macromolecules. Therefore, analysis of comprehensive expression status of these molecules may help design better intervention strategies for anti-angiogenic therapy in prostate cancer.
Results and Discussion
We have examined a total of 42 prostate archival tissue specimens from Indian and American populations that are geographically different with varying lifestyles. Ethnicity, diet and treatment regimens have been reported to cause variance in prostate cancer incidence and progression rates [25-28]. We specifically looked for differences in angiogenic status and respective modulators between PCa specimens from India and USA. The specimens were classified accordingly into areas of BPH, atrophy, moderately differentiated PCa, poorly differentiated PCa or stroma. Though the classification was done as per the major area represented in the specimens, individual islands of BPH, high grade PIN, atrophy or PCa were present within the same specimen, thereby providing us an opportunity to observe differences in staining intensity. As mentioned in the methods section, the Gleason grades of PCa tissues ranged from 6–10, thus no well-differentiated PCa (Gleason grade 2–4) were available for our study. The representative progression stages for H & E stained prostate tissue specimens of various stages are presented in Fig. 1A,1B,1C,1D. Figures for atrophy were not included as it a common veritable feature in either BPH or PCa in elderly men. The results of our comprehensive immunohistochemical profiling studies and scoring data of respective stage specific biomarkers are shown in Table I. The significant correlation statistics had p ≤ 0.05.
Figure 1.
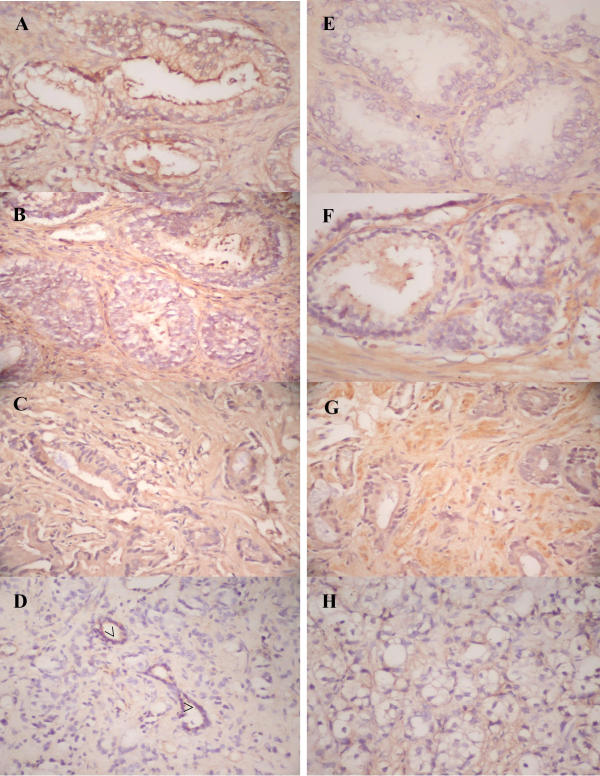
The photomicrograph of representative prostate archival specimens stained with H&E. (A-D) shows areas of BPH, high grade PIN, moderately-differentiated and poorly-differentiated prostate adenocarcinoma (PCa) and residual normal glands (indicated as open arrows). Immunostaining for endothelial cell specific marker (anti-CD34, E-H) at magnification × 200 shows the extent of anti-CD34 stained organizing endothelial cell in areas of PIN as well as intraductal venule cross-sections in PCa (indicated as stealth arrows). No CD34 staining was seen in BPH area.
Table 1.
Angiogenesis modulating biomarker status in various prostate archival specimens. (n= 42)
| Antigen | BPH | Atrophy | PIN | MD PCa | PD PCa | Stroma |
| Annexin II | +(3) | ++(4)* | ++(2)* | -(4) | -(3) | +(4) |
| Cyclooxygenase-2 | +(4) | +(4) | ++(3)* | +(2) | +(1) | ++(2)* |
| Decorin | -(4) | -(4) | ++(2)* | -(4) | -(4) | +++(4)* |
| Versican | ++(4) | + (4) | +(2) | + (3) | ++(1) | +(3) |
| CD34 | -(4) | +(4) | ++(3)* | +(1) | ++(3)* | ND |
$ Benign Prostatic Hyperplasia (BPH), high-grade Prostatic Intraepithelial Neoplasia (PIN), moderately-differentiated prostate adenocarcinoma (MD PCa), poorly-differentiated prostate adenocarcinoma (PD PCa), not determined (ND). Staining Intensity was scored as negative(-), weak (+), moderate (++) or strong (+++). Average percentage of cells were estimated and placed empirically into four categories 1–25%, 26–50%, 51–75%, or 75–100% and denoted in parenthesis as (1), (2), (3) or (4) respectively. The combination factor (product) of staining intensity and percentage of cells was used for statistical significance analysis of the data. Significant correlation is denoted as '*' for p ≤ 0.05. The 95 % confidence limits for correlation coefficient were negative for Anx-II (0.015) and positive for COX-2 (0.019) expression in PCa.
CD34 is a myeloid progenitor cell antigen that is found in endothelial cells. This biomarker can be found in all types of endothelial cells and has been used previously to correlate angiogenesis in different stages of cancer [29]. We have used this endothelial marker to correlate overall angiogenesis status in prostate cancer samples used in our studies (Fig. 1E,1F,1G,1H). Strong staining intensity for CD34 was observed in endothelial cells associated with high grade PIN (MVD~20.0), where as no CD34 staining was seen in BPH. We observed intra- and inter-nodular staining of microvessels and organizing endothelial cells within the stromal compartment in MD PCa (MVD~9.0) or PD PCa (MVD~36.0) specimens. As mentioned earlier, the staining pattern of the endothelial cell specific marker CD34 in these prostate tissues was carried out to correlate the stage specific extent of angiogenesis as a combined effect of all the markers studied. We found that areas around PIN stain heavily for CD34. The interstitial tissues of prostate carcinoma also stained positive implying that angiogenesis is a universal phenomenon in prostate cancer progression from premalignancy to malignant stages but the players regulating the process may be different and sequential at each stage.
The immunostained sections of prostate archival specimens showed that Anx-II expression was lost with malignant transformation in prostate cancer (Fig. 2A,2B,2C,2D). None of the MDPCa or PD PCa specimens (0%) from India expressed detectable Anx-II as compared to BPH or atrophy where 75–100% stained positive. The 95 % confidence limit for loss of ANX-II had a correlation coefficient of 0.015. Anx-II staining intensity was stronger in areas of atrophy than in BPH samples. In BPH the staining was mainly on luminal edge of glandular epithelial cells while in atrophy it was more cytoplasmic and membrane bound. Because it is still expressed heterogeneously in low grade PIN with diminishing intensity levels, but not in high grade PIN or prostate adenocarcinoma, loss of Anx-II may be a possible biomarker for impending malignancy of PIN into advanced prostate cancer. The individual acini of PIN at the same time correlates with intense staining for endothelial marker CD34 and therefore could well be a stage specific to "angiogenic switch". A threshold intensity level and extent of CD34 staining needs to be defined further using quantitative criteria for pathological implications of angiogenesis in PCa progression. Most of the Anx-II was observed as membrane staining in the prostate epithelial cells in contrast to cytoplasmic staining in infiltrating immune cells (lymphocytes and mast cells). The stromal fibroblasts expressed weaker intensity as compared to any prostate epithelial cells. We also observed membrane staining of nerves in few samples.
Figure 2.
The photomicrograph of representative prostate archival specimens immunostained with anti-Anx-II (A-D) and anti-COX-2 (E-H) antibodies at magnification × 200 shows positively stained Anx-II regions of normal epithelia (open arrows) and BPH as opposed to loss of Anx-II in PCa. High grade PIN is negative. The COX-2 immunostaining staining is present in high grade PIN but decreases in intensity from moderately-differentiated carcinoma to poorly-differentiated carcinoma. BPH and normal epithelia are negative. The stromal cells in the areas of poorly differentiated PCa however stain strongly.
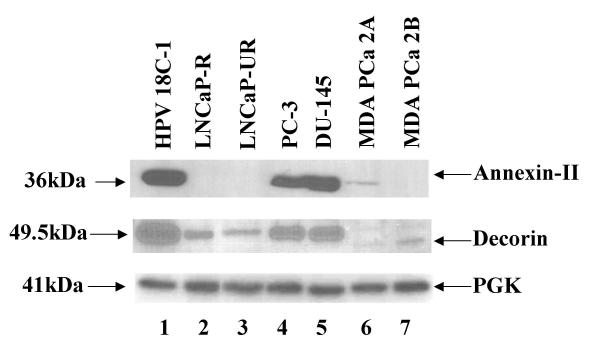
On examination of poorly-differentiated PCa (Gleason grade greater than 8) from American specimens, we found intense focal membrane staining in about 0–25 % cells (Fig. 3), and this was not observed in corresponding Indian specimens. These observations are surprising but consistent with absence or greatly reduced expression of Anx-II in androgen-responsive LNCaP-R and androgen-unrepsonsive LNCaP-UR, MDA PCa 2A and 2B cell lines, but expression of the protein in the metastatic androgen-unresponsive PC-3 and DU-145 cell lines (Fig. 4).
Figure 3.
Representative magnified images of high grade PCa specimens from American (panel A) and Indian (panel B) shows distinct pattern of Anx-II immunostaining. Intense focal staining of cell membranes in American specimens contrasts to very weak staining in Indian specimens.
Figure 4.
Western Blot profile of Anx-II and DCN expressed in prostate cancer cell lines. Each lane was loaded with 20 μg of total protein from lysates of indicated cell lines and detected with appropriate antibodies as mentioned in methods section. The samples are HPV-18C-1 (lane 1), LNCaP-R (lane 2), LNCaP-UR (lane 3), PC-3 (lane 4), DU-145 (lane 5), MDA PCa 2A (lane 6) and MDA PCa 2B (lane 7). PGK was included as internal control.
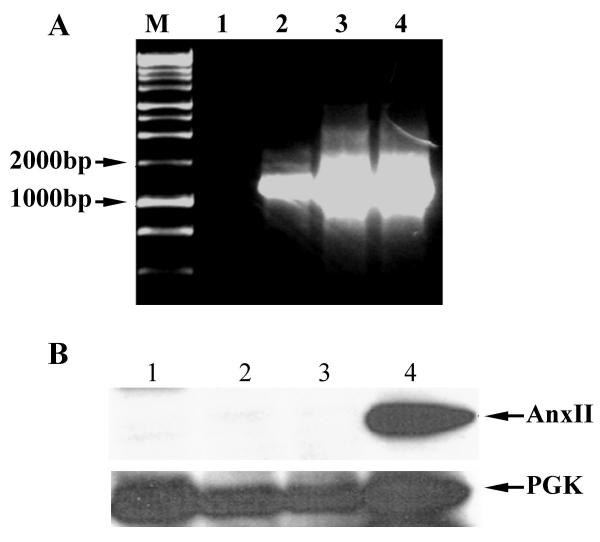
Previous studies by Chetcuti et al., [30] showed that treatment of LNCaP cells with the methylation inhibitor 5-aza-2'-deoxycytidine (Aza-dC) resulted in reexpression of annexin II, leading the authors to conclude that annexin II is silenced in LNCaP cells by methylation. Treatment with Aza-dC appears to reactivate many genes at the transcriptional level, without a corresponding reactivation at the protein level. We examined if annexin II protein appears in Aza-dC treated LNCaP cells (Fig. 5). Robust mRNA expression of annexin II is observed in LNCaP cells after Aza-dC treatment (Fig. 5 panel A). However, when protein levels are examined by immunoblot analysis, we find that Aza-dC-treated LNCaP cells do not express annexin II protein (Fig. 5 panel B). Our data lead us to conclude that methylation status does not account solely for silencing of annexin II in LNCaP cells.
Figure 5.
Anx-II mRNA expression, but not protein, is induced by 5-aza-2'-deoxycytidine, A: LNCaP and DU-145 cells were treated with or without 3 μM 5-aza-2'-deoxycytidine for 48 hours, and the annexin II expression was measured by RT-PCR. M: marker; lane 1: untreated LNCaP; lane 2: Aza-dC treated LNCaP; lane 3 untreated DU-145; lane 4 Aza-dC treated DU-145. B: LNCaP cells were treated with or without 3 μM 5-aza-2'-deoxycytidine for 48 hours. 10 μg of total protein was subjected to Western blot as described in Materials and Methods. 10 μg of whole cell extract of K562 cells was used as a positive control. Lane 1: treated LNCaP; Lane 2: untreated LNCaP; Lane 3: mock-treated LNCaP (with Lipofectamine); Lane 4: K562 cell extract. The bottom panel shows expression of an internal control in the same samples. PGK was used as an internal control.
Our results indicate that COX-2 is expressed at moderate amounts in high grade PIN (50–75%) indicating angiogenic switch in contrast to BPH and atrophy, and is a causal event for prostate cancer progression through promotion of angiogenesis. COX-2 expression was also observed in 26–50 % of moderately-differentiated PCa samples but weak focal expression (0–25 % cells) was seen in poorly differentiated PCa (Fig. 2E,2F,2G,2H). However, the smooth muscle cells of stroma and blood vessels in the vicinity of high grade PCa of Gleason grade of more than 7 were intensively stained for COX-2, indicating possible contribution of extracellular matrix modulation in prostate cancer progression. The 95 % confidence limit for COX-2 expression had correlation coefficient of 0.019. Multiple activities of COX-2 have been shown to regulate angiogenesis, increase cellular adhesion to the extracellular matrix, and make cells resistant to apoptosis. Therefore, biology of prostate cancer and progression to metastasis needs to be looked at total tumor environment milieu and not just at prostate epithelial cell level. All of the BPH samples were weakly positive for this biomarker. All other prostate specimens including BPH exhibited weak to moderate staining of the stromal fibroblast or smooth muscle cells depending on differentiation stage of nearby prostate epithelial population. We also observed staining of seminal vesicles cells adjacent to the prostate tissue. We observed that COX-2 staining in American population was generally more intense than corresponding specimens from Indian population.
Two extracellular proteoglycans implicated in angiogenesis, invasion and metastasis viz. DCN and VCN were also studied. We find that DCN expression is negative in prostate epithelial cells from all lesions with exception of PIN (Fig. 6A,6B,6C,6D). However, decorin was expressed in only 26–50% of PIN samples and was heterogeneous in distribution. Decorin is believed to have anti-angiogenic and tumor suppressor activities [31]. Since carcinoma cells do not express decorin, both of the properties mentioned previously are presumably lost. This biomarker was strongly expressed in stromal cells in BPH with 100% of specimens staining positive which diminishes in intensity with sequential progressive stages. Stromal expression of decorin is expected since it also functions in collagen fibrillogenesis and extracellular matrix organization [32]. In contrast to the lack of DCN expression in tissue specimens, we find that prostate cancer cell lines express DCN (Figure 4). Highest DCN expression was observed in the androgen-unresponsive PC-3 and DU-145 cell lines. The androgen-sensitive LNCaP cells express DCN, while the other androgen-sensitive MDA PCa 2A and 2B cell lines appear to express a truncated form of DCN.
Figure 6.
The photomicrograph of representative prostate archival specimens stained with anti-DCN (A-D) and anti-VCN (E-H) at magnification × 200 shows only PIN regions as DCN positive while BPH, moderately-differentiated and poorly-differentiated carcinomas are negative. VCN staining is strongly positive in BPH and moderately-differentiated carcinoma while poorly-differentiated carcinoma shows moderate staining. PIN is negative for VCN expression.
VCN was expressed in both moderately-differentiated (50–75 %) and poorly-differentiated (1–25 %) PCa and were mostly restricted to a focal group of cells (Fig. 6E,6F,6G,6H). VCN is known to promote cell proliferation and endothelial migration by destabilizing cell adhesion and promoting invasion into vascular stroma. Again our studies show that carcinoma cells strongly express this biomarker, hence the spread of malignant cells is promoted in conjunction with organizing endothelial cells. Our study also shows that 26–50 % of the cells in PIN are negative for VCN, but malignant cells over express, with polarity of staining towards the migratory apex of invasive epithelial cells. It needs to be mentioned here that in high-grade cribiform invasive PCa specimens the intensity of VCN staining was quite intense in almost all of epithelial cells. Therefore proteoglycan expression switch (DCN to VCN) occurs simultaneous to decorin loss and represents sequential expression pattern that possibly controls overall angiogenesis in progression stages. However, VCN was also expressed strongly in most of the BPH and atrophy samples but its role in these pathological conditions remains unclear, as we have not seen any correlating angiogenesis. VCN is expressed weakly in the stromal compartment as well (100%).
Conclusions
It is believed that Anx-II plays a role in the pro- and anti-angiogenic switch mechanism and that it may contribute to invasive potential through extracellular matrix degradation through increased plasminolysis at the cell surface [33]. Through plasmin reductase activity of its own or in association of phosphoglycerate kinase (PGK) it may give rise to an anti-angiogenic factor – angiostatin [34] as well as affect DNA replication and cell proliferation [35]. The mechanistic role of Anx-II in control of the "angiogenic switch" remains an area of active investigation by our group as well as others. Based on our results we suggest that COX-2 probably cooperates in modulation of angiogenesis and VCN modulates adhesion [36] of invasive tumor cells to organizing endothelial cells while loss of Anx-II and DCN is unable to restrict the cell proliferation and angiogenesis respectively in prostate cancer. On the other hand, intense membrane staining may aid in angiogenesis and subsequent metastasis in high grades of prostate cancer due to neovascular stroma generation. The tissue remodeling events associated with dissemination of tumor cells into other organs as well as failure of immunosurveilance mechanisms with enhanced angiogenesis may thus play together in tumor progression. Aptly, major angiogenic factors such as VEGFs have also been shown to down-regulate natural immune response as well [37]. Further studies are necessary to understand the intricate cooperative mechanisms of these biomarkers that affect tumor progression through modulation of angiogenesis and innate immunity.
Additionally, since Anx-II is expressed in BPH and atrophy but not expressed in high-grade PIN or malignant cells, it could be a specific marker for progression of prostate cancer. Nuclear accumulation of Anx-II is a negative regulator of prostate cell proliferation [38]. However, the mechanisms of cellular proliferation control may be different due to localization of Anx-II, and has been demonstrated in breast, colorectal, hepatic or hematological malignancies [15,39-41]. The focal membrane staining of Anx-II in high grade PD PCa in American specimens correlates with Anx-II expression in the metastatic prostate carcinoma cell lines PC-3 and DU-145. The loss of Anx-II in PCa appears to involve multiple mechanisms, and DNA methylation does not appear to be the sole process. The mechanisms leading to reappearance of Anx-II in high grade PCa and in metastatic cell lines are yet unknown and need further investigation.
The up-regulated COX-2 and VCN expression in prostate adenocarcinoma stages support the notion that different biomolecules could be factor for tumor angiogenesis, invasion, dissemination to organs contributing individually in each stage of progression and metastasis. COX-2 is known modulator of apoptosis and angiogenesis as discussed in the introduction while VCN is an anti-adhesive molecule which helps in dissemination of prostate cells. The negative modulator in such processes such as decorin is lost upon malignant transformation and unable to check TGF-β mediated constitutive signaling. This also supports our hypothesis that tumor progression is modulated by interactions between tumor cells and stromal components and anti-angiogenic strategies can be based on combinatorial approach targeting various modulators simultaneously. Since existing literature supports the view that all of the angiogenesis modulators studied by us are regulated in the TGF-β pathway dependent manner, it remains to be investigated whether TGF-β signaling antagonists will show therapeutic efficacy in preventing prostate cancer angiogenesis and progression in future.
Methods
Tissue specimens and Cell lines
The prostate tissue archival specimens were core needle biopsies, transurethral resection specimens (TURPs) or radical prostatectomy from patients with prostate adenocarcinoma and were obtained from the All India Institute of Medical Sciences (AIIMS) in New Delhi, India and University of Nebraska Medical Center (UNMC), Omaha, USA. These specimens were used according to institutional guidelines and approved protocols from UNMC Institutional Review Board. In total, 17 core needle biopsies from India and 25 US specimens comprising of radical prostatectomy (23) and TURPs (2) were examined by immunohistochemistry for profiling the angiogenesis modulator biomarkers. Two anatomic pathologists independently graded the H&E stained paraffin sections from the same block to determine the Gleason scores. The Gleason grades for these cancers varied between 6 and 10. The presence of BPH, high grade PIN and atrophy was recorded. The archival tissues samples examined were heterogeneous in distribution of different type of progression stages and consisted of distinct regions of BPH, atrophy, PIN, MD PCa or PD PCa in combination within same specimen.
HPV-18 C-1 cells were grown in Keratinocyte-SFM supplemented with bovine pituitary extract (25 μg/ml) and recombinant epidermal growth factor (0.15 ng/ml). LNCaP-R and LNCaP-UR cells [42] were maintained in RPMI 1640 supplemented with 7% FBS and 1% PS. DU-145 and PC-3 were maintained in DMEM supplemented with 5% FBS and 1% PS. MDAPCa 2A and 2B cells were maintained in BRFF media (Biological Research Faculty and Facility, Inc, Ijamsville, MD, USA) supplemented with 20% FBS and 1% PS. Cells were routinely fed with fresh media and incubated in a 37°C incubator at 5% CO2.
Immunohistochemistry
Neutral buffered formalin fixed and paraffin embedded tissue sections (5 μm) were treated with EZ-DeWax™ solution (Biogenex Inc., San Ramon, CA) for 10 minutes followed by a rinse with deionized water for 2 minutes. Tissue sections were rehydrated with phosphate buffered saline (PBS) and treated subsequently with 0.3% H2O2 in absolute methanol for 30 minutes to block endogenous peroxidase activity. The slides were washed three times with PBS and immersed in 2.5% normal horse serum (Vector Laboratories, Burlingame, CA) for 30 minutes to inhibit nonspecific binding. To reduce the amount of free avidin and biotin in the tissue, the slides were incubated in avidin and biotin blocking solutions (Vector Laboratories, Burlingame, CA) for 15 minutes each. The tissue sections were then incubated in primary antibody overnight at 4°C. Primary antibodies used are as follows: polyclonal goat anti-human COX-2 (cat # sc1745; SantaCruz Biotechnology Inc, Santa Cruz, CA, 1:250 dilution), Anx-II (α-774, 1:1000 dilution [43]), VCN (LF-99; 1:1000 dilution [44]), DCN (cat # AF-143; R & D Systems, MN, 1:100 dilution), and antibody against CD34 (gift from Dr. Rakesh Singh, University of Nebraska Medical Center, NE, 1:75 dilution). Slides were washed with PBS and incubated for 30 minutes with biotinylated anti-mouse/rabbit/goat antibody (H+L) made in horse (Vector Laboratories, Burlingame, CA). After washing in PBS, the sections were treated with Streptavidin / Peroxidase complex solution (Vector Laboratories, Burlingame, CA) for 45 minutes. Visualization of signal was achieved with incubation in a substrate solution composed of 250 μL Tris pH 7.5, 2.5 mg diaminobenzidine tetrachloride (DAB) and 1.7 μL 0.3% H2O2 in 5 mL of ddH2O for up to two minutes. Sections were counterstained with hematoxylin and mounted using Vectamount medium (Vector Laboratories, Burlingame, CA). All procedures were carried out at room temperature unless otherwise specified. Slides were examined in blinded manner by a pathologist and scored for staining intensity & percentage of stained cells. The combined score of staining intensity and percentage of stained cells (greater than 5 %) was considered positive staining for each type of samples. Statistical significance analysis was performed using correlation coefficient test as described earlier [45]. Average microvessel density (MVD) was enumerated by counting CD 34 positive sprouting tubular structures at 400× magnification of stained slides in different stages of PCa.
RT-PCR
Total RNA was extracted from LNCaP and DU-145 cell lines using RNeasy Kit (Qiagen, CA). First Strand cDNA was synthesized from 1 μg RNA with Oligo dT as primer using SuperScript RTs (Invitrogen). One quarter of each RT product was added to PCR mixtures (50 μl), which contained 2.5 U Taq DNA polymerase and buffer (Promega), 200 μM dNTPs and 0.5 μM of annexin II-specific primers ANX-II-F (5'-AACACATTGGCCTCAGGAAG-3') and ANX-II-R (5'-CAGGAATGCTTAGGCAACT-3'). These primers span part of the 3'and 5'-untranslated region. The PCR temperature program was 94°C for 30 seconds, 60°C for 1 minute, and 72°C for 1 minute for 30 cycles with a final 10 minutes extension at 72°C. 15 μl of each PCR product was taken for electrophoresis in 0.8% agarose gel and the gel was stained with ethidium bromide. The gel was visualized with UV light and photographed.
Western Blot analysis
Protein extracts were prepared from prostate cancer cell lines using a protein lysis buffer containing 50 mM Tris-HCl, pH 7.5, 2.0 mM phenylmethylsulfonyl fluoride (PMSF), 5.0 mM iodoacetamide, 5.0 mM ethylene diamine tetraacetic acid (EDTA), 150 mM NaCl, 0.5% nonylphenoxy polyethoxy ethanol (NP-40), and 0.5% nonanoyl-N-methylglucamide (Mega-9). Protease inhibitors leupeptin (2 μg/ml) and pepstatin (1 μg/ml) (Roche, Mannheim, Germany) were added just prior to the addition of lysis buffer to the cells. Protein concentrations in the extracts were quantitated using bicinchoninic acid (BCA) protein assay (Pierce, Rockford, IL). From each extract, 20 μg of total protein were separated on a 12% SDS-PAGE with a 5% stacking gel. After electrophoresis, proteins were transferred to 0.2 μm PVDF membranes (Millipore, Bedford, MA) using transfer buffer that contained 25 mM Tris-HCl and 700 mM glycine. Membranes were blocked in 7% powdered milk dissolved in 1X TTBS buffer (14 mM Tris, 154 mM NaCl, and 0.5 % Tween-20, pH adjusted to 7.5 with HCl) overnight at 4°C. Primary antibodies were incubated for 2 hours at room temperature in 5% powdered milk dissolved in 1X TTBS. Primary antibodies used were monoclonal mouse anti-human Annexin II (BD Biosciences) at 1:5000 dilution and polyclonal rabbit antiserum against human Decorin (LF136, obtained from Dr. Larry Fisher, NIH) at 1:4000 dilution. The membranes were washed in 1X TTBS for three times, 15 minutes each at room temperature (RT). Appropriate secondary antibodies conjugated to horseradish peroxidase (Promega, Madison, WI) were incubated with respective membranes for 1 hour at RT. Follwing Five intermittent washes with 1X TTBS membranes were developed using ECL+ (Amersham Pharmacia Biotech, Arlington Heights, IL) and films exposed for appropriate times to detect signal. Immunoblot for 3-phosphoglycerate kinase (PGK) was used as an internal control for gel loading.
Authors' Contributions
AGB performed the standardization of Immunostaining protocols, immunohistochemistry for decorin and versican and prepared the manuscript. JL performed the demethylation experiment. YY performed the immunohistochemistry for annexin II. VKG maintained prostate cancer cell lines and performed immunoblot analyses. SLJ examined the tissue sections, graded them for Gleason score and interpreted the immunohistochemical data. AKD examined the tissue sections, graded them for Gleason score and interpreted the immunohistochemical data. NPG assisted in specimen collection and interpretation of data. LT performed immunohistochemical staining under the supervision of AGB and YY. JKV coordinated the research project, analyzed the data and prepared the final manuscript. All authors read and approved the final manuscript.
Acknowledgments
Acknowledgements
The authors thankfully acknowledge the generous gifts of MDA PCa 2A and 2B cell lines from Dr. Nora Navone (MD Anderson Cancer Center, Houston, TX), HPV-18-C1 cell lines from Dr. Johng Rhim (Uniformed Services University of the Health Sciences, Bethesda, MD), antibodies to decorin and versican from Dr. Larry Fischer (Dept of Intramural Research, NIDCR, NIH, MD), and anti-CD34 from Dr. Rakesh Singh (Dept. of Pathology and Microbiology, UNMC, Omaha, NE).
This work was supported by grants from Nebraska Cancer and Smoking Disease Research Program (2003–30), the Philip Morris Incorporated and Pfeiffer (Gustavus and Louise) Foundation. YY is a Visiting Scientist from the Faculty Exchange Program between the Shanghai Joint Cancer Institute and the UNMC Eppley Cancer Center. LT was as an undergraduate summer intern from St. Mary's University, San Antonio, TX
Contributor Information
Abhijit G Banerjee, Email: abanerjee@unmc.edu.
Jie Liu, Email: jieliu@unmc.edu.
Yawei Yuan, Email: ywyuan66@yahoo.com.
Velliyur K Gopalakrishnan, Email: vkgopal@unmc.edu.
Sonny L Johansson, Email: sjohanss@unmc.edu.
Amit K Dinda, Email: amit_dinda@yahoo.com.
Narmada P Gupta, Email: narmadagupta@hotmail.com.
Lindsey Trevino, Email: ltre8@aol.com.
Jamboor K Vishwanatha, Email: jvishwan@unmc.edu.
References
- Quinn M, Babb P. Patterns and trends in prostate cancer incidence, survival, prevalence and mortality. Part I: international comparisons. BJU Int. 2002;90:162–173. doi: 10.1046/j.1464-410X.2002.2822.x. [DOI] [PubMed] [Google Scholar]
- Srinivas V, Mehta H, Amin A, Choudary R, Gadgil N, Ravishanker D, Phadke AG. Carcinoma of the prostate--state at initial presentation. Int Urol Nephrol. 1995;27:419–422. doi: 10.1007/BF02550077. [DOI] [PubMed] [Google Scholar]
- Desai SB, Borges AM. The prevalence of high-grade prostatic intraepithelial neoplasia in surgical resection specimens: an Indian experience. Cancer. 2002;94:2350–2352. doi: 10.1002/cncr.10495.abs. [DOI] [PubMed] [Google Scholar]
- Weidner N, Carroll PR, Flax J, Blumenfeld W, Folkman J. Tumor angiogenesis correlates with metastasis in invasive prostate carcinoma. Am J Pathol. 1993;143:401–409. [PMC free article] [PubMed] [Google Scholar]
- Shih SC, Robinson GS, Perruzzi CA, Calvo A, Desai K, Green JE, Ali IU, Smith LE, Senger DR. Molecular profiling of angiogenesis markers. Am J Pathol. 2002;161:35–41. doi: 10.1016/S0002-9440(10)64154-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doll JA, Reiher FK, Crawford SE, Pins MR, Campbell SC, Bouck NP. Thrombospondin-1, vascular endothelial growth factor and fibroblast growth factor-2 are key functional regulators of angiogenesis in the prostate. Prostate. 2001;49:293–305. doi: 10.1002/pros.10025. [DOI] [PubMed] [Google Scholar]
- Ferrer FA, Miller LJ, Andrawis RI, Kurtzman SH, Albertsen PC, Laudone VP, Kreutzer DL. Angiogenesis and prostate cancer: in vivo and in vitro expression of angiogenesis factors by prostate cancer cells. Urology. 1998;51:161–167. doi: 10.1016/S0090-4295(97)00491-3. [DOI] [PubMed] [Google Scholar]
- Lutsenko SV, Kiselev SM, Severin SE. Molecular mechanisms of tumor angiogenesis. Biochemistry (Mosc ) 2003;68:286–300. doi: 10.1023/A:1023002216413. [DOI] [PubMed] [Google Scholar]
- Liao Z, Boileau TW, Erdman JW, Clinton SK. Interrelationships among angiogenesis, proliferation, and apoptosis in the tumor microenvironment during N-methyl-N-nitrosourea androgen-induced prostate carcinogenesis in rats. Carcinogenesis. 2002;23:1701–1711. doi: 10.1093/carcin/23.10.1701. [DOI] [PubMed] [Google Scholar]
- Tuxhorn JA, Ayala GE, Rowley DR. Reactive stroma in prostate cancer progression. J Urol. 2001;166:2472–2483. doi: 10.1097/00005392-200112000-00126. [DOI] [PubMed] [Google Scholar]
- Cesarman GM, Guevara CA, Hajjar KA. An endothelial cell receptor for plasminogen/tissue plasminogen activator (t-PA). II. Annexin II-mediated enhancement of t-PA-dependent plasminogen activation. J Biol Chem. 1994;269:21198–21203. [PubMed] [Google Scholar]
- Hajjar KA, Acharya SS. Annexin II and regulation of cell surface fibrinolysis. Ann N Y Acad Sci. 2000;902:265–271. doi: 10.1111/j.1749-6632.2000.tb06321.x. [DOI] [PubMed] [Google Scholar]
- Liu JW, Shen JJ, Tanzillo-Swarts A, Bhatia B, Maldonado CM, Person MD, Lau SS, Tang DG. Annexin II expression is reduced or lost in prostate cancer cells and its re-expression inhibits prostate cancer cell migration. Oncogene. 2003;22:1475–1485. doi: 10.1038/sj.onc.1206196. [DOI] [PubMed] [Google Scholar]
- Vishwanatha JK, Chiang Y, Kumble KD, Hollingsworth MA, Pour PM. Enhanced expression of annexin II in human pancreatic carcinoma cells and primary pancreatic cancers. Carcinogenesis. 1993;14:2575–2579. doi: 10.1093/carcin/14.12.2575. [DOI] [PubMed] [Google Scholar]
- Mai J, Waisman DM, Sloane BF. Cell surface complex of cathepsin B/annexin II tetramer in malignant progression. Biochim Biophys Acta. 2000;1477:215–230. doi: 10.1016/S0167-4838(99)00274-5. [DOI] [PubMed] [Google Scholar]
- Wu W, Tang X, Hu W, Lotan R, Hong WK, Mao L. Identification and validation of metastasis-associated proteins in head and neck cancer cell lines by two-dimensional electrophoresis and mass spectrometry. Clin Exp Metastasis. 2002;19:319–326. doi: 10.1023/A:1015515119300. [DOI] [PubMed] [Google Scholar]
- Tsujii M, Kawano S, Tsuji S, Sawaoka H, Hori M, DuBois RN. Cyclooxygenase regulates angiogenesis induced by colon cancer cells. Cell. 1998;93:705–716. doi: 10.1016/s0092-8674(00)81433-6. [DOI] [PubMed] [Google Scholar]
- Singh B, Lucci A. Role of cyclooxygenase-2 in breast cancer. J Surg Res. 2002;108:173–179. doi: 10.1006/jsre.2002.6532. [DOI] [PubMed] [Google Scholar]
- Liu XH, Kirschenbaum A, Yao S, Lee R, Holland JF, Levine AC. Inhibition of cyclooxygenase-2 suppresses angiogenesis and the growth of prostate cancer in vivo. J Urol. 2000;164:820–825. doi: 10.1097/00005392-200009010-00056. [DOI] [PubMed] [Google Scholar]
- Baines A, Taylor-Parker M, Goulet AC, Renaud C, Gerner EW, Nelson MA. Selenomethionine Inhibits Growth and Suppresses Cyclooxygenase-2 (COX-2) Protein Expression in Human Colon Cancer Cell Lines. Cancer Biol Ther. 2002;1:370–374. [PubMed] [Google Scholar]
- Grant DS, Yenisey C, Rose RW, Tootell M, Santra M, Iozzo RV. Decorin suppresses tumor cell-mediated angiogenesis. Oncogene. 2002;21:4765–4777. doi: 10.1038/sj.onc.1205595. [DOI] [PubMed] [Google Scholar]
- Ricciardelli C, Mayne K, Sykes PJ, Raymond WA, McCaul K, Marshall VR, Horsfall DJ. Elevated levels of versican but not decorin predict disease progression in early-stage prostate cancer. Clin Cancer Res. 1998;4:963–971. [PubMed] [Google Scholar]
- Sakko AJ, Ricciardelli C, Mayne K, Tilley WD, Lebaron RG, Horsfall DJ. Versican accumulation in human prostatic fibroblast cultures is enhanced by prostate cancer cell-derived transforming growth factor beta1. Cancer Res. 2001;61:926–930. [PubMed] [Google Scholar]
- Kahari VM, Larjava H, Uitto J. Differential regulation of extracellular matrix proteoglycan (PG) gene expression. Transforming growth factor-beta 1 up-regulates biglycan (PGI), and versican (large fibroblast PG) but down-regulates decorin (PGII) mRNA levels in human fibroblasts in culture. J Biol Chem. 1991;266:10608–10615. [PubMed] [Google Scholar]
- Gronberg H. Prostate cancer epidemiology. Lancet. 2003;361:859–864. doi: 10.1016/S0140-6736(03)12713-4. [DOI] [PubMed] [Google Scholar]
- Sanchez-Chapado M, Olmedilla G, Cabeza M, Donat E, Ruiz A. Prevalence of prostate cancer and prostatic intraepithelial neoplasia in Caucasian Mediterranean males: an autopsy study. Prostate. 2003;54:238–247. doi: 10.1002/pros.10177. [DOI] [PubMed] [Google Scholar]
- Lee MM, Chang JS, Jacobs B, Wrensch MR. Complementary and alternative medicine use among men with prostate cancer in 4 ethnic populations. Am J Public Health. 2002;92:1606–1609. doi: 10.2105/ajph.92.10.1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolonel LN, Hankin JH, Whittemore AS, Wu AH, Gallagher RP, Wilkens LR, John EM, Howe GR, Dreon DM, West DW, Paffenbarger R.S.,Jr. Vegetables, fruits, legumes and prostate cancer: a multiethnic case-control study. Cancer Epidemiol Biomarkers Prev. 2000;9:795–804. [PubMed] [Google Scholar]
- Bettencourt MC, Bauer JJ, Sesterhenn IA, Connelly RR, Moul JW. CD34 immunohistochemical assessment of angiogenesis as a prognostic marker for prostate cancer recurrence after radical prostatectomy. J Urol. 1998;160:459–465. doi: 10.1097/00005392-199808000-00047. [DOI] [PubMed] [Google Scholar]
- Chetcuti A, Margan SH, Russell P, Mann S, Millar DS, Clark SJ, Rogers J, Handelsman DJ, Dong Q. Loss of annexin ii heavy and light chains in prostate cancer and its precursors. Cancer Res. 2001;61:6331–6334. [PubMed] [Google Scholar]
- Stander M, Naumann U, Wick W, Weller M. Transforming growth factor-beta and p-21: multiple molecular targets of decorin-mediated suppression of neoplastic growth. Cell Tissue Res. 1999;296:221–227. doi: 10.1007/s004410051283. [DOI] [PubMed] [Google Scholar]
- Neame PJ, Kay CJ, McQuillan DJ, Beales MP, Hassell JR. Independent modulation of collagen fibrillogenesis by decorin and lumican. Cell Mol Life Sci. 2000;57:859–863. doi: 10.1007/s000180050048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuszynski GP, Sharma MR, Rothman VL, Sharma MC. Angiostatin Binds to Tyrosine Kinase Substrate Annexin II through the Lysine-Binding Domain in Endothelial Cells. Microvasc Res. 2002;64:448–462. doi: 10.1006/mvre.2002.2444. [DOI] [PubMed] [Google Scholar]
- Kwon M, Caplan JF, Filipenko NR, Choi KS, Fitzpatrick SL, Zhang L, Waisman DM. Identification of annexin II heterotetramer as a plasmin reductase. J Biol Chem. 2002;277:10903–10911. doi: 10.1074/jbc.M111219200. [DOI] [PubMed] [Google Scholar]
- Kumble KD, Iversen PL, Vishwanatha JK. The role of primer recognition proteins in DNA replication: inhibition of cellular proliferation by antisense oligodeoxyribonucleotides. J Cell Sci. 1992;101 ( Pt 1):35–41. doi: 10.1242/jcs.101.1.35. [DOI] [PubMed] [Google Scholar]
- Sakko AJ, Ricciardelli C, Mayne K, Suwiwat S, LeBaron RG, Marshall VR, Tilley WD, Horsfall DJ. Modulation of prostate cancer cell attachment to matrix by versican. Cancer Res. 2003;63:4786–4791. [PubMed] [Google Scholar]
- Ohm JE, Carbone DP. VEGF as a mediator of tumor-associated immunodeficiency. Immunol Res. 2001;23:263–272. doi: 10.1385/IR:23:2-3:263. [DOI] [PubMed] [Google Scholar]
- Liu J, Rothermund CA, Ayala-Sanmartin J, Vishwanatha JK. Nuclear annexin II negatively regulates growth of LNCaP cells and substitution of ser 11 and 25 to glu prevents nucleo-cytoplasmic shuttling of annexin II. BMC Biochem. 2003;4:10. doi: 10.1186/1471-2091-4-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang Y, Rizzino A, Sibenaller ZA, Wold MS, Vishwanatha JK. Specific down-regulation of annexin II expression in human cells interferes with cell proliferation. Mol Cell Biochem. 1999;199:139–147. doi: 10.1023/A:1006942128672. [DOI] [PubMed] [Google Scholar]
- Zhang X, Zhou H, Xia L, Shen G, Hu Y, Wei W, Song S. Effect of antisense oligonucleotide to annexin II on the t-PA-mediated plasminogen activation in vitro. J Huazhong Univ Sci Technolog Med Sci. 2002;22:183–185. doi: 10.1007/BF02828174. [DOI] [PubMed] [Google Scholar]
- Emoto K, Yamada Y, Sawada H, Fujimoto H, Ueno M, Takayama T, Kamada K, Naito A, Hirao S, Nakajima Y. Annexin II overexpression correlates with stromal tenascin-C overexpression: a prognostic marker in colorectal carcinoma. Cancer. 2001;92:1419–1426. doi: 10.1002/1097-0142(20010915)92:6<1419::AID-CNCR1465>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Rothermund CA, Kondrikov D, Lin MF, Vishwanatha JK. Regulation of Bcl-2 during androgen-unresponsive progression of prostate cancer. Prostate Cancer Prostatic Dis. 2002;5:236–245. doi: 10.1038/sj.pcan.4500582. [DOI] [PubMed] [Google Scholar]
- Chiang Y, Davis RG, Vishwanatha JK. Altered expression of annexin II in human B-cell lymphoma cell lines. Biochim Biophys Acta. 1996;1313:295–301. doi: 10.1016/0167-4889(96)00103-6. [DOI] [PubMed] [Google Scholar]
- Bernstein EF, Fisher LW, Li K, LeBaron RG, Tan EM, Uitto J. Differential expression of the versican and decorin genes in photoaged and sun-protected skin. Comparison by immunohistochemical and northern analyses. Lab Invest. 1995;72:662–669. [PubMed] [Google Scholar]
- Rahman MA, Dhar DK, Yamaguchi E, Maruyama S, Sato T, Hayashi H, Ono T, Yamanoi A, Kohno H, Nagasue N. Coexpression of inducible nitric oxide synthase and COX-2 in hepatocellular carcinoma and surrounding liver: possible involvement of COX-2 in the angiogenesis of hepatitis C virus-positive cases. Clin Cancer Res. 2001;7:1325–1332. [PubMed] [Google Scholar]