Abstract
Engineered magnetic nanoparticles (MNPs) represent a cutting-edge tool in medicine because they can be simultaneously functionalized and guided by a magnetic field. Use of MNPs has advanced magnetic resonance imaging (MRI), guided drug and gene delivery, magnetic hyperthermia cancer therapy, tissue engineering, cell tracking and bioseparation. Integrative therapeutic and diagnostic (i.e., theragnostic) applications have emerged with MNP use, such as MRI-guided cell replacement therapy or MRI-based imaging of cancer-specific gene delivery. However, mounting evidence suggests that certain properties of nanoparticles (e.g., enhanced reactive area, ability to cross cell and tissue barriers, resistance to biodegradation) amplify their cytotoxic potential relative to molecular or bulk counterparts. Oxidative stress, a 3-tier paradigm of nanotoxicity, manifests in activation of reactive oxygen species (ROS) (tier I), followed by a pro-inflammatory response (tier II) and DNA damage leading to cellular apoptosis and mutagenesis (tier III). In vivo administered MNPs are quickly challenged by macrophages of the reticuloendothelial system (RES), resulting in not only neutralization of potential MNP toxicity but also reduced circulation time necessary for MNP efficacy. We discuss the role of MNP size, composition and surface chemistry in their intracellular uptake, biodistribution, macrophage recognition and cytotoxicity, and review current studies on MNP toxicity, caveats of nanotoxicity assessments and engineering strategies to optimize MNPs for biomedical use.
Keywords: magnetic nanoparticles, iron oxide, nanotoxicity, oxidative stress, macrophages, ROS, surface chemistry, DMSA
Introduction
Nanotechnology is at the leading edge of the rapidly developing new therapeutic and diagnostic concepts in all areas of medicine [1]. Magnetic nanoparticles (MNPs) are a class nanoparticles (i.e., engineered particulate materials of < 100 nm) that can be manipulated under the influence of an external magnetic field. MNPs are commonly composed of magnetic elements, such as iron, nickel, cobalt and their oxides.
The unique ability of MNPs to be guided by an external magnetic field has been utilized for magnetic resonance imaging (MRI), targeted drug and gene delivery, tissue engineering, cell tracking and bioseparation [2–5]. When further “functionalized” with drugs and bioactive agents, such as peptides and nucleic acids, MNPs form distinct particulate systems that penetrate cell and tissue barriers and offer organ-specific therapeutic and diagnostic modalities [4]. The ability of MNPs to be functionalized and concurrently respond to a magnetic field has made them a useful tool for theragnostics - the fusion of therapeutic and diagnostic technologies that targets to individualize medicine [6]. Through multilayered functionalization, MNPs can simultaneously act as diagnostic molecular imaging agents [7, 8] and drug carriers. MNP-based MRI imaging has been already combined with cell replacement therapy [9], organ-specific gene delivery [10], intra-operative tumor removal surgery [11, 12] and other applications discussed below. However, the resulting variety of MNP composition, shape, size, surface chemistry and state of dispersion each may influence their biodistribution and toxic potential [3].
The increased toxic potential of nano-sized materials relative to their bulk and molecular counterparts is owed to their largely enhanced reactive surface area, ability to cross cell and tissue barriers, and resistance to biodegradation [13]. Activation of oxidative stress and inflammatory signaling leading to apoptosis and genotoxicity are the key paradigm(s) of nanotoxicity [14–18]. In an in vivo setting, macrophages of the defense reticuloendothelial system (RES) quickly challenge and internalize MNPs, neutralizing their cytotoxic potential [5]. But in order to promote their circulation time, engineering strategies to modify MNP surface chemistry are used to allow for evasion of macrophages [5]. Therefore, an integrative approach to advancing MNP designs and understanding their interface with specific organ systems, with regards to their application and safety, are imperative to advancing nanomedicine [14, 16–18].
Several recent reviews have discussed engineering designs, physiochemical characteristics [19, 20] and biomedical applications of MNP [3–5]. Here, we will review current studies of MNP toxicity and issues relevant to the development of the discipline of magnetic nanotoxicology.
Formulations of MNPs for biomedical applications
Iron oxide MNPs, such as magnetite Fe3O4 or its oxidized and more stable form of maghemite γ-Fe2O3, are superior to other metal oxide nanoparticles for their biocompatibility and stability and are, by far, the most commonly employed MNPs for biomedical applications [2–4, 21, 22]. Thus, we here refer to iron oxide MNPs as “MNPs”, unless otherwise specified. Typically, magnetic nanoparticles are synthesized and dispersed into homogenous suspensions, called ferrofluids, composed of a large number of engineered composite nanoparticles. Each MNP consists of a magnetic core and a non-magnetic coating of different surface chemistry.
Thermal energy, quantum size effects and the large surface area of individual MNPs are responsible for superparamagnetic phenomena of ferrofluids [23]. Hydrodynamic particle sizes range from superparamagnetic (50–500 nm) and ultrasmall superparamagnetic (< 50 nm), and influence their magnetization values, dispersibility, stability in solution, and determine their biomedical modalities [2, 19]. Based on the biokinetics of particles, the sizes of 10–100 nm are optimal for in vivo delivery, as they escape rapid renal clearance (< 10 nm) and sequestering by the reticuloendothelial system (RES) of the spleen and liver (> 200 nm) [2]. Numerous engineering approaches aimed at achieving uniformity of MNP size, shape and composition (e.g., types of salts used, Fe2+ and Fe3+ ratio, pH and ionic strength of the media), and each may influence MNP size and magnetization properties [3, 19].
MNP shapes and structure designs range considerably from particle suspensions, sheets, tubes, shells and arrays. An emerging theme in MNP biomedical research is to influence function and magnetic properties in biological systems by control of shape. For example, increased in vivo circulation time for up to 48 h and effective tumor targeting was achieved through the use of magnetic nanoworms, representing elongated assemblies of dextran-coated iron oxide MNPs [24]. Surface chemistry is another critical determinant that regulates physiochemical characteristics of MNPs, including their size, solubility, state of dispersion and magnetization values. Because surface chemistry greatly influences MNP fate in the biological system, including the mechanisms of their cell recognition, biodistribution and immune response [3, 25] it presents a specific focus for advancing engineering strategies to minimize potential nanotoxicity.
Surface chemistry and biocompatibility
Without a coating, MNPs have hydrophobic surfaces with large surface area to volume ratios and a propensity to agglomerate [19]. A proper surface coating allows iron oxide MNPs to be dispersed into homogenous ferrofluids and improve MNP stability. Several groups of coating materials are used to modify MNP surface chemistry:
organic polymers, such as dextran, chitosan, polyethylene glycol, polysorbate, polyaniline
organic surfactants, such as sodium oleate and dodecylamine
inorganic metals, such as gold
inorganic oxides, such as silica and carbon
bioactive molecules and structures, such as liposomes, peptides and ligands/receptors
Biodegradable polymers
Clinical preparations of MNPs have traditionally relied upon organic biodegradable dextran and carbohydrate derivatives because of their common use as plasma expanders and high affinity to iron oxides [26]. Several such formulations are now available, including Ferridex, Resovist, Combidex, and AMI-288/ferumoxytol, successfully utilized as MRI contrast agents [4]. Through creating steric repulsion, polymers stabilize MNPs in suspension. But polymer-coated MNPs are unstable at high temperatures and are not suitable to protect reactive MNPs due to poor air stability and susceptibility to leaching in acidic conditions [19]. Poor functionalization and conjugation capacity of polymer-coated MNPs [4] limit their use for more complex, integrative applications. However, amination or carboxylation of dextran resulting in cross-linked iron oxide MNPs (CLIO) improves their conjugation capacity. For example, CLIO conjugated to fluorescent probes are successfully used as reporters to αvβ3 integrin, cathepsin B, E-selectin, VCAM-1, macrophage-specific and other ligands for molecular MRI imaging [4, 8, 27]. Polyethylene glycol (PEG) is a polymer widely used for its hydrophilicity and low antigenicity. In addition to steric stabilization of MNPs, PEG prevents their plasma opsonization and uptake by macrophages, increasing MNP circulation in vivo [28]. PEG-coated MNPs are efficiently internalized by cells via fluid-phase endocytosis and through amphiphilic affinity to lipid bilayers on plasma membranes [28]. These qualities, however, increase their potential to overload cells with iron and become toxic [3]. Polyvinyl alcohol (PVA) has excellent film-forming, emulsifying and adhesive properties [3]. While PVA use for intravenous (i.v.) delivery is limited due to poor persistence and agglomeration parameters in a ferrofluid [3], when cross-linked to form a magnetic gel, PVA can be used as a vitreous eye substitute [29]. Potentially, this property can be utilized in targeted drug delivery, tissue engineering and biosensor technology [3]. Chitosan provides a natural, biocompatible, cationic and hydrophilic polymer coating, suitable for affinity purification of proteins and magnetic bioseparation [3]. Interestingly, labeling of fibroblasts with chitosan-coated MNPs improved their invasive potential under magnetic force, showing promise for tissue engineering [30].
Precious metals, such as gold, have been used to protect iron oxide cores against oxidation, as they form highly stable particles of low reactivity [31, 32]. Figure 1, B-C depicts gold-coated MNPs. Nanogold is known for its superior optical properties, biocompatibility and outstanding capacity for functionalization [31, 32]. These properties make gold particularly attractive surface chemistry for MNPs. One limitation is that gold coating can weaken magnetic properties of iron oxide MNPs [20] and is considered difficult to achieve due to the dissimilar nature of the two surfaces [19, 32]. When achieved, gold-coated MNPs are stable under neutral and acidic conditions [33, 34]. Engineering a continuous gold shell over an MNP core is a challenge, but it can provide an extremely effective barrier against oxidizing agents [3].
Figure 1. Examples of MNPs of different size, composition and surface chemistry.
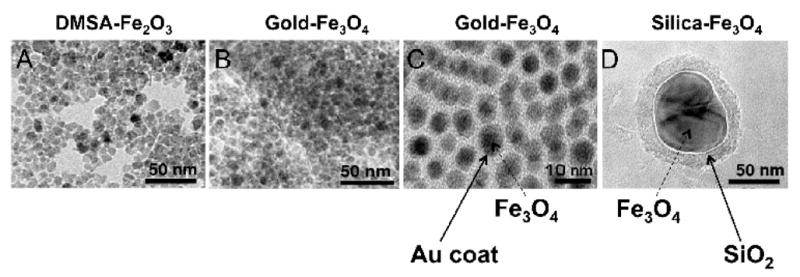
Transmission electron microscopy (TEM) of A, maghemite (Fe2O3) nanoparticles coated with anionic DMSA forming ~ 8-nm-thick round-shaped AMNPs. B, magnetite (Fe3O4) of ~8-nm-think core coated with ~2-nm-thick gold (Au), forming core-shell nanoparticles. C, magnification x 50 of B. (D). Silica coated Fe3O4 nanoparticles of ~ 100 nm, synthesized by sol gel method.
An inert silica, shell of different thicknesses is used to tune the MNP core to different extents of dipolar coupling (i.e., direct magnetic dipole-dipole interaction) and cooperative magnetic switching [19]. Based on the negative charge of the silica shell, silica-coated MNPs are redispersible and stable in aqueous conditions [19]. They provide good control of interparticle interactions both in solutions and within structures through variations of the shell thickness [19]. Depending on the protocol for their synthesis, silica-coated iron oxide MNP range in size from 1–2 nm [35] to 150 nm [36], but the development of monodispersed solutions often proves difficult. Silica-coated MNPs have longer circulation times and their hydrophilic negatively charged surface provides ideal anchorage for covalent binding to ligands presenting an excellent platform for drug delivery [3]. Figure 1, D depicts a 100 nm silica-coated iron oxide (magnetite) MNP.
Dimercaptosuccinic acid (DMSA) is a negatively charged sulfur-containing chelating agent. DMSA- conjugated iron oxide MNPs are referred to as anionic MNPs (AMNPs, see Figure 1, A). DMSA prevents MNPs from aggregation and interacts strongly with the positively charged regions of the plasma membrane due to its negative charge. AMNPs are suitable for high-efficiency cellular uptake via adsorptive endocytosis [37–40]. Because of the scattered positioning of cationic sites on the plasma membrane, AMNPs cluster at the cell surface prior to their endocytosis; this clustering phenomenon is believed to contribute to their high efficiency uptake [26, 37]. Cellular internalization of AMNPs can be reduced with dextran coating that is known to limit efficiency of intracellular MNP uptake [2, 37]. A very wide range of cells have shown rapid, uniform and efficient labeling with AMNPs [41]. However, due to the efficient intracellular uptake, considerations for functional effects of iron accumulation and concentration-dependent cytotoxicity should be given [42].
Functionalized MNPs
Surface functionalization, a process of chemical conjugation of functional groups to the surface, is now integral to MNP designs. As we already mentioned, certain coating surface chemistries have inherently superior functionalization capabilities, such as silica and gold [3]. Both coated and uncoated MNPs have been functionalized with peptides, nucleic acids, small molecules and sometimes antibodies. Antibodies are used as conjugates for magnetic beads in cell sorting and imaging, but because they are as large in size as MNP cores, they often suffer from steric hindrance, poor cell internalization and display low affinity constants [4]. Both the HIV-derived TAT peptide [12, 43–45] and transferrin, a plasma protein for iron delivery, have been effectively used as MNP delivery systems [46, 47]. MNP functionalization with the RGD (arginine-glycine-aspartic acid) peptide allowed for tumor detection of integrins and imaging of tumor vascularization [48]. Surface chemistry of MNPs has functional effect, e.g., albumin conjugation significantly promoted proliferation of primary human fibroblasts, whereas uncoated and dextran-coated MNPs inhibited their proliferation [49]. Numerous successful functionalization protocols have been developed, some of which are described under specific biomedical applications. Other types of MNP surface chemistries and protocols of their synthesis applicable to biomedical use have been reviewed elsewhere [3, 4].
MNPs for theragnostic platforms
Theragnostics (the fusion of therapeutic and diagnostic approaches) aims to personalize and advance medicine [6]. MNPs represent a particularly appropriate tool based on their ability to be simultaneously functionalized and guided by an external magnetic field. Novel designs have focused on sophisticated multilayering of MNPs with the goal to develop controlled nanodelivery systems. Already, functionalized MNPs have been used in combination theragnostic approaches for gene delivery with selective tumor imaging [10], MRI-guided therapeutic cell replacement [9] and MRI-assisted diagnostic and surgeries using a single MNP formulation [11, 12]. After removal of the magnetic field MNPs do not retain magnetism [21], providing additional versatility. We here touch upon MNP-based therapeutic applications (e.g. magnetic hyperthermia, drug/gene delivery and tissues engineering) and diagnostic imaging or biosensor platforms, depicting integrative approaches that capitalize on the MNPs’ dual magnetic and functionalization modalities.
Magnetic hyperthermia
Hyperthermia is a cancer therapy that relies on the localized heating of tumors above 43°C for about 30 minutes [50]. MNPs can generate heat under alternating magnetic fields due to energy losses in the traversing of the magnetic hysteresis loop [20]. Generation of different degrees of heat depends on the magnetization properties of specific MNP formulations and magnetic field parameters [2]. Selectivity to tumors was considerably improved through the use of silane coatings [51, 52] and through functionalization approaches. For example, MNPs conjugated with antibodies to cancer-specific antigens improved selectivity of MNP uptake by tumors during hyperthermia therapy [22]. Magnetic hyperthermia using magnetic cationic liposomes has been used in a combination approach with TNF-α gene therapy and stress-inducible gadd153 promoter, resulting in a dramatic arrest in tumor growth [53].
Magnetic resonance imaging (MRI)
MNP-enhanced MRI is based on the superior superparamagnetic qualities of iron oxide MNPs [22]. Several dextran-coated MNP formulations have been approved for clinical use as MRI contrast agents, including ferumoxides, ferumoxtran and ferucarbotran [7]. Experimental studies show that MNP-enhanced MRI is vastly superior to other noninvasive methods of identifying lymph node metastases from solid tumors and histologically positive lymph nodes outside of the usual field of resection [54]. MNP application in delineating primary tumors and detecting metastases, in imaging angiogenesis and mapping vascular supply to primary tumors has a realistic translational potential [4, 7]. Macrophage-specific MNP labeling protocols are used to image inflammatory pathologies, including atherosclerosis, multiple sclerosis and rheumatoid arthritis [4, 7, 55]. Long-term MRI-guided in vivo cell tracking of CNS regeneration became possible with the use of grafted MNP-labeled stem cells [9] and neuroprotective glia (Schwann cells and olfactory ensheathing cells) [56]. FITC-conjugated MNPs have been used by surgeons to delineate gliomas both preoperatively, based on their MRI modality, and intraoperatively, based on fluorescent tag [11, 12]. This is particularly valuable since certain tumors, such as gliomas, can shift position during surgery.
MRI has advanced to cellular and molecular sensitivities, with nanofunctionalized MRI contrast agent libraries now available [7, 8]. For example, the HIV-derived TAT peptide conjugated to ultrasmall iron oxide MNP allows efficient T cell labeling within 5 minutes [57]. A number of studies demonstrate the immense potential of MNP-based molecular MRI as a combination imaging and drug/gene-delivery strategy. For example, monocrystalline iron oxide MNPs (3 nm core) sterically protected by a layer of low-molecular weight dextran and covalently conjugated to holo-transferrin, promotes overexpression of engineered transferrin receptor (ETR) to selectively visualize tumors in vivo in real time at exceptionally high spatial resolution [10].
Bioseparation and biosensors
In vitro streptavidin-coated magnetic beads are used for phenotypic selection in different cell sorting protocols, including stem cells [58], sensory neurons [59] and others [3, 4]. Conjugated to monoclonal antibodies, MNPs could be used to decontaminate blood from infective agents [60]. The principles of MNP-based magnetic bioseparation have been applied to integrative biosensor technologies. For example, ultrasensitive bio-barcodes can detect protein analytes using specific MNP-conjugated antibodies, followed by dehybridization of the co-tagged oligonucleotides [61].
Targeted drug delivery
The development of drug delivery systems with selectivity to pathologic sites is an ambitious goal. The principles of magnetic guidance of MNP-conjugated drugs have been applied experimentally, and have reached clinical trials as a cancer therapy [5]. Following i.v. delivery of MNPs, an external magnetic field is used to concentrate MNPs at a specific target site; this procedure has been well tolerated in cancer patients [5, 50]. Nanoparticle-based drug and gene delivery systems may solve the insurmountable obstacle of treating neurological diseases: delivery across the blood-brain barrier [62, 63]. But issues of potential embolization with MNP aggregates in capillaries and the necessity for large distances between the pathological site and external magnetic field still present a challenge [3, 5]. When considering the MNP surface chemistry for drug delivery, it is favored that MNPs retain sufficient hydrophilicity and, with coating, do not exceed 100 nm in size to avoid rapid clearance by RES [64, 65]. Overall, the smaller, more neutral and more hydrophilic the nanoparticle surface, the longer its plasma half-life [5].
Magnetic transfections
When functionalized with DNA vectors, MNPs could be used as effective gene transfection systems under external magnetic field, termed magnetofections (MF) [2]. Direct comparison of state-of-the-art magnetic polycation polyethylenimine-coated MNP and corresponding standard gene vectors demonstrated a profound increase in transfection efficiency for both non-viral and viral vectors in permissive and non-permissive cells [66]. In endothelial cells, MF increased the efficiency of the luciferase reporter by 360-fold [67] and effective antisense oligonucleotide delivery in vivo and in vitro [68]. An example of magnetic nonviral gene transfer protocol includes steps of MNP synthesis, testing intact DNA binding with MNP core, preparation of magnetic lipoplexes and polyplexes, magnetofection and data processing [69].
Tissue engineering
Iron oxide MNPs have several distinct applications in tissue engineering. They have been used in stem cell replacement therapy for cell labeling, sorting, monitoring, engraftment and targeted in vivo delivery [9]. MNPs can be also used to weld the joining tissue surfaces under high temperatures, a process typically accompanied by protein denaturation followed by re-polymerization of adjacent protein chains [2, 3]. With regard to nanomagnetic welding, gold and silica coating are expected to increase MNP sensitivity and robustness to light absorption [70, 71] and allow for selection of light sources and wavelengths with minimal damage to tissues [2]. Magnetic nanoparticles have been used for construction and harvesting of multilayered keratinocyte sheet-like 3-D constructs [22]. Self-assembled magnetic nanowire arrays [72] could potentially be used in tissue engineering.
Iron detection and chelation therapy
Excess metal deposits are associated with virtually every neurodegenerative disease, including multiple sclerosis, Friedreich’s ataxia, Alzheimer’s, Parkinson’s, and Huntington’s diseases [73]. Advanced technologies of nano-sized iron detection in neuronal tissues, such as by superconducting quantum interference device (SQUID) magnetometry, have found use as a diagnostic strategy in identifying iron deposits in the brains of Alzheimer’s [74] and neuroferritinopathy patients [75]. As experimental therapeutics, MNPs may be used as iron chelators for neurodegenerative diseases [76], potentially presenting another theragnostic application.
MNP biodistribution, clearance and toxicity
MNP metabolism
Typically, upon their intracellular internalization via endocytosis, MNPs are clustered within lysosomes where, presumably, they are degraded into iron ions by an array of hydrolyzing enzymes at low pH according to endogenous iron metabolism pathways [3]. MNP size, charge, surface chemistry and route of delivery each influence their circulation time and biodistribution patterns in the body [5]. Large (> 200 nm) particles are usually sequestered by the spleen via mechanical filtration followed by phagocytosis, whereas smaller < 10 nm particles are rapidly removed through extravasation and renal clearance, with particles 10–100 nm believed to be optimal for i.v. administration [2]. The typical final biodistribution of particles is 80–90% in liver, 5–8% in spleen and 1–2% in bone marrow [5]. Surface chemistry dictates the efficiency and the mechanism of MNP internalization by cells as well as their overall biodistribution in organ systems [2, 77], metabolism and potential toxicity [13–15]. Depending on the routes of their delivery/exposure, MNP surfaces may interact with extracellular matrix components and the plasma cell membranes of macrophages, endothelial cells, skin epithelium, and respiratory or gastrointestinal tracts [14, 78–80]. A point worth noting is that MNPs accumulate in the brain, liver, spleen and lungs after their inhalation [81], demonstrating their ability to cross the blood-brain-barrier. MNPs can also penetrate the hair follicles and stratum corneum, reaching viable skin epidermis [82].
Macrophages in nanoparticle clearance: a double-edged sword
Upon their in vivo administration, within minutes nano-sized particles are challenged by macrophages of the RES [5]. The efficacy of particle clearance by the RES depends upon the size, chemical composition, cumulative projected area and surface chemistry of the MNPs and can influence mechanisms of macrophage activation and particle internalization [83, 84].
Multiple mechanisms of intracellular internalization of nanoparticles have been described, including phagocytosis (mediated by mannose, complement, Fcγ and scavenger receptors) and endocytosis (clatrin- and calveolin-mediated, fluid-phase) [25]. MNPs have been reported to be internalized via phagocytosis, scavenger receptor-mediated endocytosis, fluid-phase endocytosis and diffusion [77]. Depending on surface hydrophobicity and charge, nanoparticles can get opsonized by plasma proteins (e.g., albumin, apolipoprotein, immunoglobulins, complement, fibrinogen), which promotes their recognition and clearance by cells of RES [25, 55]. Opsonization can change the mechanisms of particle uptake by cells of RES. For example, iron oxide MNP binding to plasma fibronectin and vitronectin changed from receptor-mediated to fluid-phase endocytosis [85]. Efforts are made to engineer MNPs of size and surface chemistry that will minimize their opsonization and macrophage-mediated clearance and thus increase their circulation time [77]. For example, surface coating with amphiphilic polymeric surfactants, such as polyethylene glycol (PEG), significantly reduces MNP interactions with plasma proteins, minimizing their internalization and clearance by macrophages [5, 86–88]. But because PEG-coated MNP uptake is highly efficient [28], it is anticipated that their cytotoxic potential in non-immune cells will be increased [3]. It is likely that cytotoxic de-differentiation and cell death after exposure to anionic DMSA-coated MNPs (AMNPs) relates to the very efficiency of uptake, resulting in dose-dependent intracellular iron overload [42]. In vitro, AMNPs are internalized by macrophages [37, 89] and neuron-like PC12 cells [42] and other cells. In fact, their rapid and efficient internalization was noted to be uniform between 14 different cell types in vitro, including hepatocytes, fibroblasts, smooth muscle cells, stem and progenitor cells and immune cells [41]. MNPs internalization by macrophages is mediated via integrin MAC-1 (also known as CD11b, CD18) [90] and scavenger receptors SR-A [91].
It is not yet clear whether in vivo AMNPs are internalized uniformly between the cells, particularly immune or non-immune tissue cells. Potency of macrophages to “sense”, migrate towards and phagocytose nanoparticles depends highly on MNP surface chemistry, and occurs typically 6–12 h after their in vivo exposure [79]. In addition, inherent immunocompetence of organ and tissue systems is likely to play an important role. For example, immunocompetence of the nervous system is not homogenous. Peripheral nerve is capable of recruiting highly potent macrophages that, by clearing degenerating debris, creates a permissive environment for neurite growth [92]. This immunocompetence is believed to be promote propensity of peripheral nerve for regeneration, in great contrast to the central nervous system (CNS), where macrophages are few and ineffective [92]. We found AMNPs to stimulate macrophage recruitment into peripheral nerve 24 h after their injection, where they seem to predominantly label infiltrating macrophages (Fig. 2). Thus, MNP microinjection into peripheral nerve may present an advantageous experimental model for studying the MNP-macrophage interface within a mammalian nervous system, preventing MNP clearance issues characteristic of intravenous delivery. Understanding the molecular interactions at the macrophage-nanoparticle interface has lead to new approaches for selective MR imaging of cardiovascular diseases, multiple sclerosis, stroke and other diseases [8, 93–96].
Figure 2. Anionic DMSA-coated MNPs promote macrophage infiltration in nerve.

Plastic-embedded transverse rat sciatic nerve sections after intrafascicular injection of DMSA-coated MNPs (B) or DMSA (A). Stained with Methylene blue Azure II. A. Uniform axonal morphology typical of uninjured nerve and no evidence of infiltrating immune cells is seen after DMSA (control coating) injection. B. Increased incidence of infiltrating macrophages (yellow arrows), particularly in the perivascular spaces, is noted after DMSA-coated MNP injection. Note pixilated MNPs phagocytosed within macrophages and axonal myelin splitting (red asterisks) at the interface with MNP-filled macrophages. Objective magnification x 100 (scale bar = 25 μm). Legend: a, myelinated axon; v, vessel; red asterisks, myelin splitting; yellow arrow, infiltrating macrophages.
Macrophages are critical elements in the body’s defense system against diseases, and introduction of nanoparticles into the body may affect macrophage defensive function. It is known that nanoparticles promote activation as well as phagocytotic, cytoskeletal and cytokine-releasing functions of macrophages [25, 97–99]. Dextran-coated MNPs induce differentiation of monocytes into macrophages. While proinflammatory signaling many manifest macrophage activation, it may mediate MNP-induced macrophage cytotoxicity. Given that particle clearance by macrophages is size-dependent, ultrasmall nanoparticles can escape phagocytosis and lavage within the macrophages [79], promoting activation of oxidative stress and ROS-mediated redox-sensitive transcription via NF-κB and AP-1 [100], proinflammatory TNF-α/p38 signaling and apoptosis [99]. In fact, these properties have translated into therapeutic «macrophage-suicide» approaches of nanocarrier systems for macrophage-driven diseases of bacterial infection, atherosclerosis, rheumatoid arthritis and neuroinflammation [55, 80]. However, the limited selectivity of such designs to macrophages due to often similar cell surface composition in other immunocompetent cells, such as Schwann cells and microglia in the nervous system, as well as the adverse effects of macrophage destruction on local and central immunity, should be considered in the overall scheme of nanotoxicity. Inhibition of p38 MAPK by SB-239063 reduces uptake of MNPs by aortic macrophages after their i.v. administration [101], providing a potential strategy to modulate MNP toxicity in vivo.
Based on the multi-factorial relationship of MNPs with macrophages, engineering strategies to increase the ability of MNPs to evade macrophages have to be accompanied by thorough evaluation of their toxic potential associated with relevant routes of delivery and identification of target cells of their internalization. Intrinsic to their enhanced reactive surface area, propensity to cross tissue and cell barriers, and ability to lavage within cells, nano-sized materials can compromise functions of cell membranes, mitochondria, nuclear membranes and DNA [14, 78].
Oxidative stress: A paradigm for nanotoxicity
Iron oxide MNPs are believed to induce redox cycling and catalytic chemistry via The Fenton Reaction [H2O2 + Fe2+ → Fe3+ + HO− + HO*] [14], the most prevalent source of reactive oxygen species (ROS) in biological systems. In fact, uncoated magnetite nanoparticles are significantly cytotoxic [28]. But the mechanism of cytotoxicity of the fully oxidized (Fe2O3) maghemite MNPs is not directly explained by the Fenton chemistry. Furthermore, ROS generation leading to oxidative stress (OS) is associated with the nanotoxicity of non-metal nanoparticles as well [13–15, 78, 102]. While the mechanisms by which nanoparticles generate ROS are still largely unknown, it is hypothesized that disruption of the well-structured electronic configuration of the nano-sized material surface creates reactive electron donor or acceptor sites, leading to the formation of superoxide radicals [14]. As a multistage process, nanoparticle-induced ROS activation induces defense anti-oxidant response elements by the transcription factor Nrf-2, leading to increased expression of over 200 phase II antioxidant enzymes, such as heme oxygenase 1 (HO-1), superoxide dismutase, etc. (tier I OS) [99]. If damage proceeds, protective systems are superseded by mitogen-activated protein kinase (MAPK) and NF-κB-activated intracellular signaling, resulting in pro-inflammatory cytokine, chemokine and matrix metalloproteinase (MMP) release (tier II OS), leading to apoptosis (tier III OS) [14]. Current studies on mechanisms of nanotoxicity overwhelmingly point to OS-mediated activation of proinflammatory cytokines and MAPK signaling pathways in respiratory and gastrointestinal tracts, blood cells, skin and the nervous system, as carefully reviewed [14, 77, 79, 103]. A continuum of molecular events from tier I to III OS manifested through increased HO-1 and JNK-mediated TNF-α production leading to apoptosis [99]. Studies into the effects of NP size, shape and state of dispersion indicate a clear correlation between these physiochemical characteristics with cytotoxicity [104] and the cellular proinflammatory response [105].
Tier II OS can manifest in vivo through immune cell infiltration, mediated by the actions of proinflammatory cytokines and MMPs [106]. For example, ROS controls MMP activity by two distinct mechanisms: MAPK-induced overexpression of MMP gene and directly through structural oxidative modification of thiol residues on inactive pro-MMPs, resulting in release of the zinc-binding domain and MMP activation [107]. In Figure 3 we summarize our hypothesis of ROS-induced MMP activity in the nervous system leading to increased blood-brain-barrier (BBB) permeability and neuronal damage [108–110]. In support of this hypothesis, MMPs are proposed to mediate (and present a highly sensitive measure for) macrophage activation in response to nanoparticles [55]. Internalization of chitosan-coated MNPs promote invasive potential of cells into the 3-D-porous scaffolds via MMP action [30]. MNPs are believed to promote cytotoxicity of human fibroblasts through the action of proinflammatory cytokines and MMPs [111], which are considered to be useful biomarkers for metal toxicity [112]. In the context of AMNP-induced macrophage recruitment into the nerve, depicted in Figure 2, it is worth noting that cytokines and MMPs are important modulators of macrophage recruitment into the nerve [113, 114]. Together, these studies support a model in which MNPs induce ROS to promote MMP-mediated degradation of the blood-brain and blood-nerve barriers to promote recruitment of macrophages into the nervous system (Figure 3).
Figure 3. A proposed mechanism of MNP-induced macrophage recruitment into neuronal tissues.
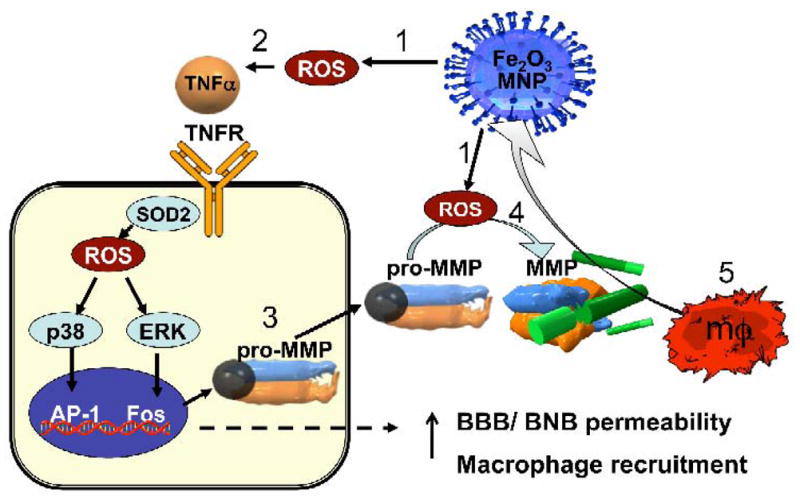
(1) Exposure to cytotoxic MNPs stimulated the formation of ROS in resident cells. (2) ROS promotes expression and release of proinflammatory cytokines, such as TNF-α. Through its two receptors (TNFR), TNF-α activates p38 and ERK mitogen-activated protein kinases pathways to (3) induce the expression of matrix metalloproteinases (MMPs) in its inactive, pro-MMP form. In addition, (4) ROS can directly promote MMP activation from pro-form. MMPs are the only enzymes in the body capable of degrading blood-brain and blood-nerve barriers (BBB/BNB), which (5) promotes infiltration of circulating macrophages (mΦ) into neuronal tissues. MNP size and surface chemistry determines the mechanisms and the target cells of MNP internalization, as well as extent of neurotoxicity of MNPs.
Metals and MNPs in neurodegeneration
While iron oxide is considered safe, imbalance in its homeostasis has known toxic implications to many organ systems [115]. As the abundant, redox-active metal, iron facilitates the generation of free radicals in the brain [116, 117] and excess iron is associated with multiple neurodegenerative disorders, including multiple sclerosis, Alzheimer’s and Parkinson’s diseases [73]. We have mentioned that nano-scale iron oxide deposits have been identified in neurological tissues using SQUID magnetometry in the brains of Alzheimer’s patients [74] and basal ganglia of neuroferritinopathy patients [75]. These iron nanoparticles are believed to be biogenic in nature and linked to ferritin, a large (12 nm) natural ferric oxide phosphate storage protein [15, 74, 118]. However, evidence of brain accumulation of MNPs after passive exposure through inhalation [81] calls for confirmation of the source of MNP deposits in the brains of neurodegeneration patients. It is particularly important due to the ability of MNPs to penetrate through skin [82] and cross blood-brain barrier after exposure through inhalation [81] and by intraperitoneal delivery [119]. Studies on the neurotoxicity of MNPs and identifying their safe formulations are required prior to their therapeutic use as an iron chelating therapy for neurodegenerative disorders [76] and drug conjugates (e.g., glial derived neurotrophic factor) as a therapy for drug addiction [120].
Current studies of MNP toxicity
Given the variety of MNP formulations and their biomedical applications, the limited number of studies evaluating their toxicity is astounding. The findings on iron oxide range from cytotoxicity in vitro [121–123] to transient and acute in vivo toxicity [119, 124–126] to unremarkable changes in vivo [27, 56, 119, 127]. In relevance to the journal’s Theme Issue of nanoparticle toxicity, in this section we refer to toxic properties of iron oxide and other metal and metal oxide MNPs.
MNP cytotoxicity to non-immunogenic cells in vitro is increasingly being noted, in addition to that in macrophages reviewed above. Several metal oxide nanoparticles of 20–45 nm produced dose-dependent apoptosis and membrane function in mouse neuro-2A cells that were measured by membrane leakage of lactate dehydrogenase and MTT reduction [128]. In studying the capacity of AMNPs to extend neurite outgrowth under magnetic force, we observed de-differentiation and cell death of neuron-like PC12 cells with increasing concentration of iron oxide [42]. Although DMSA itself (used for coating) did not cause toxicity and is a known non-toxic BBB-crossing therapeutic chelator of metal [129], it presents an efficient cellular internalization system of iron-oxide MNPs [37–39], resulting in intracellular overload with iron. Because target intracellular compartments of iron oxide MNPs include mitochondria and nucleus, increase in oxidative stress-related changes and gene expression can occur. In fact, MNPs of various compositions, sizes and even at mild exposures can activate pro-inflammatory and ROS-induced cell signaling, as well as interference with mitochondrial energy production in cultured cells [105, 111, 122, 123, 128, 130, 131]. However, of several MNPs comparatively tested, iron oxide MNPs were shown to be the safest, producing cytotoxic changes at levels of 100 μg/ml or higher [122, 128, 131]. Reduced cytotoxicity of oxide nanoparticles is associated with increased particle solubility and modification of surface chemistry [121].
In vivo toxicity data on MNPs is hopeful, demonstrating no long-term implications of their use when administered at clinically relevant concentrations via relevant routes. Several reports found iron to accumulate in tissues, but with unremarkable histological changes in vital organs, concluding safety of the respective formulations [27, 56, 119, 127]. Although MNP deposits were detectable in the prostates of prostate cancer patients after a year of magnetic hyperthermia therapy, no signs of systemic toxicity were found [132]. The median lethal dose (LD50) of dextran-coated magnetite MNPs is very high at 400 mg/kg in rats, with cytotoxic effects on peritoneal cells, lymphocytes and neutrophils [133, 134]. Hemangiectasia and leukocyte infiltration observed after single subcutaneous administration of dextran-coated MNPs almost disappeared 72 h after their administration [125]. Signs of oxidative stress, evident in spleen and to a lesser degree kidney and liver of rats after intravenous administration of oleic acid-pluronic-coated iron oxide MNPs, gradually declined after 3 days of administration [126]. As mentioned, MNP surface chemistry that ensures high efficiency of intracellular MNP uptake or effective evasion of the RES is likely to increase their toxic potential [3].
Although the studies on MNP toxicity are limited in number, they point at the influence of MNP composition, size, dispersibility, surface chemistry and the regimens of their administration in the toxic outcomes, the factors expected to influence nanotoxicity [14]. Next, we will outline the influences of testing and study design on nanotoxicity data.
Intricacies of nanotoxicity testing and data analyses
The necessity, purpose and directions in the formation of the new discipline of nanotoxicology have been discussed in detail [14, 15], and MNPs bear no exception. Physiochemical characteristics of nanoparticles (size, shape, surface chemistry, solubility) each influence their toxic potential [104]. Particokinetics and dosimetry approaches to nanotoxicity assessment encompass measures for dose of exposure (mass administered, media mass, surface area), delivered dose (number per cell or cm3), and cellular dose (internalized mass) [135]. With many parameters to assess, the demand for toxicity evaluation exceeds current capabilities of the research field. Thus, prioritization of the physicochemical parameters of clinical relevance to the target organ groups is imperative. E.g., for skin, that includes NP dissolution, size and partition coefficient, whereas for brain, hydrophobicity and surface chemistry, relating to possibility to cross barriers, size and shape and chemical composition are more relevant [136].
It has been suggested that study designs center only on clinically relevant target cells and include in-depth mechanistic analyses of the hierarchical process of oxidative stress (OS) [14]. For tier I OS, ROS species generation and measures of anti-oxidative HO-1 have been proposed as useful measures of OS-specific nanotoxicity [99]. As mentioned already, activation of proinflammatory signaling and MMPs have been adopted by many studies and represent reliable measures of tier II OS. It has been shown, however, that measures of MMP-9 provide higher sensitivity when cytokine measures fail [55]. Overall, the use of multiple assays is important to avoid false-positive or false-negative results [137]. Tier III OS can be identified by activation of pro-apoptotic pathways [14, 99]. Thus, whether or not activation of tier I OS would indicate ROS-induced changes of nanotoxicity, determination of cell apoptosis of tier III OS will help understand the extent of cytotoxicity. In vivo, the cell source for specific changes could be identified by dual-labeling of OS-specific antigens and cell specific markers using confocal microscopy. Caution in the interpretation of mechanistic studies is important and a consideration that low-level ROS signaling contributes to normal cellular redox signaling [138] and cytokine-protease activation may relate to the body’s defense reaction in immobilizing immune cells.
For in vivo nanotoxicity assessment, cell viability and mechanistic OS measures should be supplemented by evaluation of organ-specific toxicity, such as renal filtration or blood-brain barrier breakdown [139, 140]. The biokinetics of nanoparticles through confirmed and potential routes of exposure involves multiple pathways of their translocation prior to clearance [15], particularly as nanoparticle binding to proteins generates complexes that are more mobile and pervasive through tissues normally inaccessible [14]. The time-course of nanotoxicity measures may help reconcile studies demonstrating safety of MNPs [27, 56, 119] with those of transient and acute in vivo toxicity [119, 124]. Direct comparison of in vivo and in vitro pulmonary nanotoxicity using the same assays showed little correlation [141], as NP biodistribution and RES clearance both factor in their in vivo toxicity. Toxicity of MNPs in normal and pathologic tissues may also differ due to the differences in their cell composition and phenotypic status (e.g., availability of specific receptors to mediate MNP endocytosis), and should be addressed independently.
Some toxicity data may not be specific to nanoparticles per se, but relate to the increased substance quantities and accumulation, which could be adjusted with adoption of appropriate concentration standards [136]. Since most substances become toxic at high doses, it is important that nanotoxicity studies incorporate doses at the anticipated human exposures [78]. Toxicity could also be qualitatively different based on size, surface chemistry or specific interaction [136]. When nanotoxicity is observed, the role of each component of the composite structure should be evaluated as control factors, if possible (e.g., its vehicle or coating material). For example, polyakylcyaniacrylate-coated MNPs (220 nm, with 10–20 nm cores) showed an LD50 of 245 mg/kg, however, a similar LD50 was observed in polyakylcyaniacrylate (not MNP-based) particles [142].
Conclusions
Potential benefits of nanotechnology in medicine are inimitable owing to refined, highly targeted, blood-brain barrier-crossing drug delivery and imaging platforms, unique transfection, labeling, bioseparation, as well as analytical and tissue engineering approaches. The versatility of superparamagnetic iron oxide MNPs is owed to their capability to respond to an external magnetic field and be functionalized with bioactive agents at the same time and/or differentially. Challenges to the rapidly advancing field of magnetic nanotechnology include: the reduced magnetization modalities with certain advanced surface modifications; the distances between the target site and magnetic field; the potential dissociation of the functionalized domain (e.g., therapeutic peptide) with the magnetic core; and the agglomeration of particles in magnetic fields, promoting embolization or other tissue toxicities [3]. Capitalizing on the agglomeration properties of MNPs in tissues has led to therapeutic strategies of localized cytotoxicity, such as tumor targeting and macrophage-suicide approaches. However, potentially toxic implications for MNP overload to healthy tissues should be considered. So should engineering approaches to increase MNP ability to evade macrophages. Increasingly surfacing data on MNP toxicity should not be ignored. A rising chorus of government, industry, academia and environmentalists is calling for studies on toxicity of nanoparticles [13, 14, 78, 143] that are already abundantly used in consumer areas that do not require rigorous toxicity assessments, such as cosmetics [144]. Complex issues of regulatory legislature is highly dependent upon the availability of relevant toxicity studies [80]. As the demand for nanotoxicity assessment increases, clinical relevance and mechanistic approaches should be prioritized [14, 79, 136]. The coalescence of bioengineering, biomedical and toxicology disciplines will foster development of relevant strategies to engineer advanced magnetic nanoparticle formulations and nanodevices with biocompatible interfaces.
Table 1.
Key abbreviations used in the article
| AMNP | anionic (DMSA-coated) MNP |
| Au-MNP | gold-coated MNP |
| BBB | blood-brain barrier |
| BNB | blood-nerve barrier |
| BrdU | 5-bromo-2-deoxyuridine |
| CNS | central nervous system |
| DMSA | dimercaptosuccinic acid |
| HO-1 | heme-oxygenase 1 |
| i.v. | intravenous injection |
| MMP | matrix metalloproteinase |
| MNP | magnetic nanoparticles |
| NP | nanoparticle |
| OS | oxidative stress |
| RES | reticuloendothelial system |
| ROS | reactive oxygen species |
| SQUID | superconducting quantum interference device |
| TEM | transmission electron microscopy |
| TNF-α | tumor necrosis factor alpha |
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Whitesides GM. The ‘right’ size in nanobiotechnology. Nat Biotechnol. 2003;21(10):1161–5. doi: 10.1038/nbt872. [DOI] [PubMed] [Google Scholar]
- 2.Gupta AK, Gupta M. Synthesis and surface engineering of iron oxide nanoparticles for biomedical applications. Biomaterials. 2005;26(18):3995–4021. doi: 10.1016/j.biomaterials.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 3.Gupta AK, et al. Recent advances on surface engineering of magnetic iron oxide nanoparticles and their biomedical applications. Nanomed. 2007;2(1):23–39. doi: 10.2217/17435889.2.1.23. [DOI] [PubMed] [Google Scholar]
- 4.McCarthy JR, et al. Targeted delivery of multifunctional magnetic nanoparticles. Nanomed. 2007;2(2):153–67. doi: 10.2217/17435889.2.2.153. [DOI] [PubMed] [Google Scholar]
- 5.Duguet E, et al. Magnetic nanoparticles and their applications in medicine. Nanomed. 2006;1(2):157–68. doi: 10.2217/17435889.1.2.157. [DOI] [PubMed] [Google Scholar]
- 6.Ozdemir V, et al. Shifting emphasis from pharmacogenomics to theragnostics. Nat Biotechnol. 2006;24(8):942–6. doi: 10.1038/nbt0806-942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arbab AS, Liu W, Frank JA. Cellular magnetic resonance imaging: current status and future prospects. Expert Rev Med Devices. 2006;3(4):427–39. doi: 10.1586/17434440.3.4.427. [DOI] [PubMed] [Google Scholar]
- 8.Sosnovik DE, Weissleder R. Emerging concepts in molecular MRI. Curr Opin Biotechnol. 2007;18(1):4–10. doi: 10.1016/j.copbio.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 9.Bulte JW, et al. Magnetodendrimers allow endosomal magnetic labeling and in vivo tracking of stem cells. Nat Biotechnol. 2001;19(12):1141–7. doi: 10.1038/nbt1201-1141. [DOI] [PubMed] [Google Scholar]
- 10.Weissleder R, et al. In vivo magnetic resonance imaging of transgene expression. Nat Med. 2000;6(3):351–5. doi: 10.1038/73219. [DOI] [PubMed] [Google Scholar]
- 11.Kircher MF, et al. In vivo high resolution three-dimensional imaging of antigen-specific cytotoxic T-lymphocyte trafficking to tumors. Cancer Res. 2003;63(20):6838–46. [PubMed] [Google Scholar]
- 12.Kircher MF, et al. Intracellular magnetic labeling with CLIO-Tat for efficient in vivo tracking of cytotoxic T cells by MR imaging. Radiology. 2002;225:453–453. [Google Scholar]
- 13.Donaldson K, et al. Nanotoxicology. Occup Environ Med. 2004;61(9):727–8. doi: 10.1136/oem.2004.013243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nel A, et al. Toxic potential of materials at the nanolevel. Science. 2006;311(5761):622–7. doi: 10.1126/science.1114397. [DOI] [PubMed] [Google Scholar]
- 15.Oberdorster G, Oberdorster E, Oberdorster J. Nanotoxicology: an emerging discipline evolving from studies of ultrafine particles. Environ Health Perspect. 2005;113(7):823–39. doi: 10.1289/ehp.7339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Powell MC, Kanarek MS. Nanomaterial health effects--Part 2: Uncertainties and recommendations for the future. Wmj. 2006;105(3):18–23. [PubMed] [Google Scholar]
- 17.Powell MC, Kanarek MS. Nanomaterial health effects--Part 1: Background and current knowledge. Wmj. 2006;105(2):16–20. [PubMed] [Google Scholar]
- 18.Moore MN. Do nanoparticles present ecotoxicological risks for the health of the aquatic environment? Environ Int. 2006 doi: 10.1016/j.envint.2006.06.014. [DOI] [PubMed] [Google Scholar]
- 19.Lu AH, Salabas EL, Schuth F. Magnetic nanoparticles: synthesis, protection, functionalization, and application. Angew Chem Int Ed Engl. 2007;46(8):1222–44. doi: 10.1002/anie.200602866. [DOI] [PubMed] [Google Scholar]
- 20.Huber DL. Synthesis, properties, and applications of iron nanoparticles. Small. 2005;1(5):482–501. doi: 10.1002/smll.200500006. [DOI] [PubMed] [Google Scholar]
- 21.Tartaj P, et al. The preparation of magnetic nanoparticles for applications in biomedicine. Journal of Physics D-Applied Physics. 2003;36(13):R182–97. [Google Scholar]
- 22.Ito A, et al. Medical application of functionalized magnetic nanoparticles. J Biosci Bioeng. 2005;100(1):1–11. doi: 10.1263/jbb.100.1. [DOI] [PubMed] [Google Scholar]
- 23.Goya GF, Berquo TS, Fonseca FC. Static and dynamic magnetic properties of spherical magnetite nanoparticles. J Appl Phys. 2003;94(5):3520–3528. [Google Scholar]
- 24.Park J, et al. Magnetic iron oxide nanoworms for tumor targeting and imaging. Advanced Materials. 2008;20:1630–1635. doi: 10.1002/adma.200800004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dobrovolskaia MA, McNeil SE. Immunological properties of engineered nanomaterials. Nat Nano. 2007;2:469–478. doi: 10.1038/nnano.2007.223. [DOI] [PubMed] [Google Scholar]
- 26.Wilhelm C, et al. Deformation of intracellular endosomes under a magnetic field. European Biophysics Journal with Biophysics Letters. 2003;32(7):655–660. doi: 10.1007/s00249-003-0312-0. [DOI] [PubMed] [Google Scholar]
- 27.Muldoon LL, et al. Imaging, distribution, and toxicity of superparamagnetic iron oxide magnetic resonance nanoparticles in the rat brain and intracerebral tumor. Neurosurgery. 2005;57(4):785–96. doi: 10.1093/neurosurgery/57.4.785. discussion 785–96. [DOI] [PubMed] [Google Scholar]
- 28.Gupta AK, Curtis AS. Surface modified superparamagnetic nanoparticles for drug delivery: interaction studies with human fibroblasts in culture. J Mater Sci Mater Med. 2004;15(4):493–6. doi: 10.1023/b:jmsm.0000021126.32934.20. [DOI] [PubMed] [Google Scholar]
- 29.Maruoka S, et al. Biocompatibility of polyvinylalcohol gel as a vitreous substitute. Curr Eye Res. 2006;31(7–8):599–606. doi: 10.1080/02713680600813854. [DOI] [PubMed] [Google Scholar]
- 30.Sasaki T, et al. Magnetic nanoparticles for improving cell invasion in tissue engineering. J Biomed Mater Res A. 2007 doi: 10.1002/jbm.a.31724. [DOI] [PubMed] [Google Scholar]
- 31.Daniel MC, Astruc D. Gold nanoparticles: assembly, supramolecular chemistry, quantum-size-related properties, and applications toward biology, catalysis, and nanotechnology. Chem Rev. 2004;104(1):293–346. doi: 10.1021/cr030698+. [DOI] [PubMed] [Google Scholar]
- 32.Eustis S, el-Sayed MA. Why gold nanoparticles are more precious than pretty gold: noble metal surface plasmon resonance and its enhancement of the radiative and nonradiative properties of nanocrystals of different shapes. Chem Soc Rev. 2006;35(3):209–17. doi: 10.1039/b514191e. [DOI] [PubMed] [Google Scholar]
- 33.Chen M, et al. Gold-coated iron nanoparticles for biomedical applications. J Appl Phys. 2003;93(10):7551–7553. [Google Scholar]
- 34.Lin J, et al. Gold-coated iron (Fe@Au) nanoparticles: synthesis, characterization, and magnetic field-induced self assembly. J Solid State Chem. 2001;159:26–31. [Google Scholar]
- 35.Santra S, et al. Synthesis and characterization of silica-coated iron oxide nanoparticles in microemulsion: the effect of non-ionic surfactants. Langmuir. 2001;17:2900–2906. [Google Scholar]
- 36.Tartaj P, Gonzalez-Carreno T, Serna CJ. Single-step nanoengineering of silica coated maghemite hollow spheres with tunable magnetic properties. Adv Mater. 2001;13:1620–1624. [Google Scholar]
- 37.Wilhelm C, et al. Intracellular uptake of anionic superparamagnetic nanoparticles as a function of their surface coating. Biomaterials. 2003;24(6):1001–1011. doi: 10.1016/s0142-9612(02)00440-4. [DOI] [PubMed] [Google Scholar]
- 38.Wilhelm C, Gazeau F, Bacri JC. Magnetophoresis and ferromagnetic resonance of magnetically labeled cells. European Biophysics Journal with Biophysics Letters. 2002;31(2):118–125. doi: 10.1007/s00249-001-0200-4. [DOI] [PubMed] [Google Scholar]
- 39.Wilhelm C, et al. Interaction of anionic superparamagnetic nanoparticles with cells: Kinetic analyses of membrane adsorption and subsequent internalization. Langmuir. 2002;18(21):8148–8155. [Google Scholar]
- 40.Bertorelle F, et al. Fluorescence-modified superparamagnetic nanoparticles: intracellular uptake and use in cellular imaging. Langmuir. 2006;22(12):5385–91. doi: 10.1021/la052710u. [DOI] [PubMed] [Google Scholar]
- 41.Wilhelm C, Gazeau F. Universal cell labelling with anionic magnetic nanoparticles. Biomaterials. 2008;29(22):3161–74. doi: 10.1016/j.biomaterials.2008.04.016. [DOI] [PubMed] [Google Scholar]
- 42.Pisanic TR, 2nd, et al. Nanotoxicity of iron oxide nanoparticle internalization in growing neurons. Biomaterials. 2007;28(16):2572–81. doi: 10.1016/j.biomaterials.2007.01.043. [DOI] [PubMed] [Google Scholar]
- 43.Josephson L, et al. High-efficiency intracellular magnetic labeling with novel superparamagnetic-tat peptide conjugates. Bioconjugate Chemistry. 1999;10(2):186–191. doi: 10.1021/bc980125h. [DOI] [PubMed] [Google Scholar]
- 44.Lewin M, et al. Tat peptide-derivatized magnetic nanoparticles allow in vivo tracking and recovery of progenitor cells. Nature Biotechnology. 2000;18(4):410–414. doi: 10.1038/74464. [DOI] [PubMed] [Google Scholar]
- 45.Zhao M, et al. Differential conjugation of tat peptide to superparamagnetic nanoparticles and its effect on cellular uptake. Bioconjugate Chemistry. 2002;13(4):840–844. doi: 10.1021/bc0255236. [DOI] [PubMed] [Google Scholar]
- 46.Moore A, et al. Measuring transferrin receptor gene expression by NMR imaging. Biochim Biophys Acta. 1998;1402(3):239–49. doi: 10.1016/s0167-4889(98)00002-0. [DOI] [PubMed] [Google Scholar]
- 47.Berry CC, et al. The influence of transferrin stabilised magnetic nanoparticles on human dermal fibroblasts in culture. Int J Pharm. 2004;269(1):211–25. doi: 10.1016/j.ijpharm.2003.09.042. [DOI] [PubMed] [Google Scholar]
- 48.Montet X, et al. Nanoparticle imaging of integrins on tumor cells. Neoplasia. 2006;8(3):214–22. doi: 10.1593/neo.05769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Berry CC, Curtis ASG. Functionalisation of magnetic nanoparticles for applications in biomedicine. Journal of Physics D-Applied Physics. 2003;36(13):R198–206. [Google Scholar]
- 50.Pankhurst QA, et al. Applications of magnetic nanoparticles in biomedicine. Journal of Physics D-Applied Physics. 2003;36(13):R167–81. [Google Scholar]
- 51.Jordan A, et al. Endocytosis of dextran and silan-coated magnetite nanoparticles and the effect of intracellular hyperthermia on human mammary carcinoma cells in vitro. Journal of Magnetism and Magnetic Materials. 1999;194(1–3):185–196. [Google Scholar]
- 52.Jordan A, et al. Inductive heating of ferrimagnetic particles and magnetic fluids: physical evaluation of their potential for hyperthermia. Int J Hyperthermia. 1993;9(1):51–68. doi: 10.3109/02656739309061478. [DOI] [PubMed] [Google Scholar]
- 53.Ito A, et al. Heat-inducible TNF-alpha gene therapy combined with hyperthermia using magnetic nanoparticles as a novel tumor-targeted therapy. Cancer Gene Ther. 2001;8(9):649–54. doi: 10.1038/sj.cgt.7700357. [DOI] [PubMed] [Google Scholar]
- 54.Hogemann D, et al. Improvement of MRI probes to allow efficient detection of gene expression. Bioconjug Chem. 2000;11(6):941–6. doi: 10.1021/bc000079x. [DOI] [PubMed] [Google Scholar]
- 55.Chellat F, et al. Therapeutic potential of nanoparticulate systems for macrophage targeting. Biomaterials. 2005;26(35):7260–75. doi: 10.1016/j.biomaterials.2005.05.044. [DOI] [PubMed] [Google Scholar]
- 56.Dunning MD, et al. Superparamagnetic iron oxide-labeled Schwann cells and olfactory ensheathing cells can be traced in vivo by magnetic resonance imaging and retain functional properties after transplantation into the CNS. J Neurosci. 2004;24(44):9799–810. doi: 10.1523/JNEUROSCI.3126-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Brooks H, Lebleu B, Vives E. Tat peptide-mediated cellular delivery: back to basics. Adv Drug Deliv Rev. 2005;57(4):559–77. doi: 10.1016/j.addr.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 58.Maxwell DJ, et al. Fluorophore-conjugated iron oxide nanoparticle labeling and analysis of engrafting human hematopoietic stem cells. Stem Cells. 2008;26(2):517–24. doi: 10.1634/stemcells.2007-0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tucker BA, Rahimtula M, Mearow KM. A procedure for selecting and culturing subpopulations of neurons from rat dorsal root ganglia using magnetic beads. Brain Res Brain Res Protoc. 2005;16(1–3):50–7. doi: 10.1016/j.brainresprot.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 60.Weber C, Falkenhagen D. Specific blood purification by means of antibody-conjugated magnetic microspheres. In: Hafeli U, et al., editors. Scientific and clinical applications of magnetic carriers. Plenum Press; New York: 1997. pp. 371–378. [Google Scholar]
- 61.Nam JM, Thaxton CS, Mirkin CA. Nanoparticle-based bio-bar codes for the ultrasensitive detection of proteins. Science. 2003;301(5641):1884–6. doi: 10.1126/science.1088755. [DOI] [PubMed] [Google Scholar]
- 62.Kreuter J. Nanoparticulate systems for brain delivery of drugs. Adv Drug Deliv Rev. 2001;47(1):65–81. doi: 10.1016/s0169-409x(00)00122-8. [DOI] [PubMed] [Google Scholar]
- 63.Schroeder U, et al. Nanoparticle technology for delivery of drugs across the blood-brain barrier. J Pharm Sci. 1998;87(11):1305–7. doi: 10.1021/js980084y. [DOI] [PubMed] [Google Scholar]
- 64.Gupta AK, Gupta M. Cytotoxicity suppression and cellular uptake enhancement of surface modified magnetic nanoparticles. Biomaterials. 2005;26(13):1565–1573. doi: 10.1016/j.biomaterials.2004.05.022. [DOI] [PubMed] [Google Scholar]
- 65.Torchilin VP, V, Trubetskoy S. Which polymers can make nanoparticulate drug carriers long-circulating. Adv Drug Del Rev. 1995;16(2–3):141–155. [Google Scholar]
- 66.Scherer F, et al. Magnetofection: enhancing and targeting gene delivery by magnetic force in vitro and in vivo. Gene Ther. 2002;9(2):102–9. doi: 10.1038/sj.gt.3301624. [DOI] [PubMed] [Google Scholar]
- 67.Krotz F, et al. Magnetofection potentiates gene delivery to cultured endothelial cells. J Vasc Res. 2003;40(5):425–34. doi: 10.1159/000073901. [DOI] [PubMed] [Google Scholar]
- 68.Krotz F, et al. Magnetofection--a highly efficient tool for antisense oligonucleotide delivery in vitro and in vivo. Mol Ther. 2003;7(5 Pt 1):700–10. doi: 10.1016/s1525-0016(03)00065-0. [DOI] [PubMed] [Google Scholar]
- 69.Mykhaylyk O, et al. Generation of magnetic nonviral gene transfer agents and magnetofection in vitro. Nat Protoc. 2007;2(10):2391–411. doi: 10.1038/nprot.2007.352. [DOI] [PubMed] [Google Scholar]
- 70.Xu HH, Smith DT, Simon CG. Strong and bioactive composites containing nano-silica-fused whiskers for bone repair. Biomaterials. 2004;25(19):4615–26. doi: 10.1016/j.biomaterials.2003.12.058. [DOI] [PubMed] [Google Scholar]
- 71.Sokolov K, et al. Real-time vital optical imaging of precancer using anti-epidermal growth factor receptor antibodies conjugated to gold nanoparticles. Cancer Res. 2003;63(9):1999–2004. [PubMed] [Google Scholar]
- 72.Liu M, et al. Self-assembled magnetic nanowire arrays. Appl Phys Lett. 2007;90(103105):1–3. [Google Scholar]
- 73.Doraiswamy PM, Finefrock AE. Metals in our minds: therapeutic implications for neurodegenerative disorders. Lancet Neurol. 2004;3(7):431–4. doi: 10.1016/S1474-4422(04)00809-9. [DOI] [PubMed] [Google Scholar]
- 74.Hautot D, et al. Preliminary evaluation of nanoscale biogenic magnetite in Alzheimer’s disease brain tissue. Proc Biol Sci. 2003;270(Suppl 1):S62–4. doi: 10.1098/rsbl.2003.0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hautot D, et al. Preliminary observation of elevated levels of nanocrystalline iron oxide in the basal ganglia of neuroferritinopathy patients. Biochim Biophys Acta. 2007;1772(1):21–5. doi: 10.1016/j.bbadis.2006.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Liu G, et al. Nanoparticle iron chelators: A new therapeutic approach in Alzheimer disease and other neurologic disorders associated with trace metal imbalance. Neurosci Lett. 2006;406(3):189–93. doi: 10.1016/j.neulet.2006.07.020. [DOI] [PubMed] [Google Scholar]
- 77.Unfried K, et al. Cellular responses to nanoparticles: Target structures and mechanisms. Nanotoxicology. 2007;1(1):52–71. [Google Scholar]
- 78.Oberdorster G, Stone V, Donaldson K. Toxicology of nanoparticles: A historical perspective. Nanotoxicology. 2007;1(1):2–25. [Google Scholar]
- 79.Oberdorster G, et al. Principles for characterizing the potential human health effects from exposure to nanomaterials: elements of a screening strategy. Part Fibre Toxicol. 2005;2:8. doi: 10.1186/1743-8977-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Vega-Villa KR, et al. Clinical toxicities of nanocarrier systems. Adv Drug Deliv Rev. 2008;60(8):929–38. doi: 10.1016/j.addr.2007.11.007. [DOI] [PubMed] [Google Scholar]
- 81.Kwon JT, et al. Body distribution of inhaled fluorescent magnetic nanoparticles in the mice. J Occup Health. 2008;50(1):1–6. doi: 10.1539/joh.50.1. [DOI] [PubMed] [Google Scholar]
- 82.Baroli B, et al. Penetration of metallic nanoparticles in human full-thickness skin. J Invest Dermatol. 2007;127(7):1701–12. doi: 10.1038/sj.jid.5700733. [DOI] [PubMed] [Google Scholar]
- 83.Brown DM, et al. Calcium and ROS-mediated activation of transcription factors and TNF-alpha cytokine gene expression in macrophages exposed to ultrafine particles. Am J Physiol Lung Cell Mol Physiol. 2004;286(2):L344–53. doi: 10.1152/ajplung.00139.2003. [DOI] [PubMed] [Google Scholar]
- 84.Moss OR, V, Wong A. When nanoparticles get in the way: impact of projected area on in vivo and in vitro macrophage function. Inhal Toxicol. 2006;18(10):711–6. doi: 10.1080/08958370600747770. [DOI] [PubMed] [Google Scholar]
- 85.Moore A, Weissleder R, Bogdanov A., Jr Uptake of dextran-coated monocrystalline iron oxides in tumor cells and macrophages. J Magn Reson Imaging. 1997;7(6):1140–5. doi: 10.1002/jmri.1880070629. [DOI] [PubMed] [Google Scholar]
- 86.Storm G, et al. Surface modification of nanoparticles to oppose uptake by the mononuclear phagocyte system. Adv Drug Del Rev. 1995;17:31–48. [Google Scholar]
- 87.Yamazaki M, Ito T. Deformation and instability in membrane structure of phospholipid vesicles caused by osmophobic association: mechanical stress model for the mechanism of poly(ethylene glycol)-induced membrane fusion. Biochemistry. 1990;29(5):1309–14. doi: 10.1021/bi00457a029. [DOI] [PubMed] [Google Scholar]
- 88.Zhang Y, Kohler N, Zhang M. Surface modification of superparamagnetic magnetite nanoparticles and their intracellular uptake. Biomaterials. 2002;23(7):1553–61. doi: 10.1016/s0142-9612(01)00267-8. [DOI] [PubMed] [Google Scholar]
- 89.Billotey C, et al. Cell internalization of anionic maghemite nanoparticles: Quantitative effect on magnetic resonance imaging. Magnetic Resonance in Medicine. 2003;49(4):646–654. doi: 10.1002/mrm.10418. [DOI] [PubMed] [Google Scholar]
- 90.von Zur Muhlen C, et al. Superparamagnetic iron oxide binding and uptake as imaged by magnetic resonance is mediated by the integrin receptor Mac-1 (CD11b/CD18): implications on imaging of atherosclerotic plaques. Atherosclerosis. 2007;193(1):102–11. doi: 10.1016/j.atherosclerosis.2006.08.048. [DOI] [PubMed] [Google Scholar]
- 91.Raynal I, et al. Macrophage endocytosis of superparamagnetic iron oxide nanoparticles: mechanisms and comparison of ferumoxides and ferumoxtran-10. Invest Radiol. 2004;39(1):56–63. doi: 10.1097/01.rli.0000101027.57021.28. [DOI] [PubMed] [Google Scholar]
- 92.Stoll G, Jander S. The role of microglia and macrophages in the pathophysiology of the CNS. Prog Neurobiol. 1999;58(3):233–47. doi: 10.1016/s0301-0082(98)00083-5. [DOI] [PubMed] [Google Scholar]
- 93.Weber R, et al. MRI detection of macrophage activity after experimental stroke in rats: new indicators for late appearance of vascular degradation? Magn Reson Med. 2005;54(1):59–66. doi: 10.1002/mrm.20532. [DOI] [PubMed] [Google Scholar]
- 94.Brochet B, et al. Early macrophage MRI of inflammatory lesions predicts lesion severity and disease development in relapsing EAE. Neuroimage. 2006;32(1):266–74. doi: 10.1016/j.neuroimage.2006.03.028. [DOI] [PubMed] [Google Scholar]
- 95.Gorantla S, et al. Quantitative magnetic resonance and SPECT imaging for macrophage tissue migration and nanoformulated drug delivery. J Leukoc Biol. 2006;80(5):1165–74. doi: 10.1189/jlb.0206110. [DOI] [PubMed] [Google Scholar]
- 96.Jander S, Schroeter M, Saleh A. Imaging inflammation in acute brain ischemia. Stroke. 2007;38(2 Suppl):642–5. doi: 10.1161/01.STR.0000250048.42916.ad. [DOI] [PubMed] [Google Scholar]
- 97.Renwick LC, et al. Increased inflammation and altered macrophage chemotactic responses caused by two ultrafine particle types. Occup Environ Med. 2004;61(5):442–7. doi: 10.1136/oem.2003.008227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Fang C, et al. In vivo tumor targeting of tumor necrosis factor-alpha-loaded stealth nanoparticles: effect of MePEG molecular weight and particle size. Eur J Pharm Sci. 2006;27(1):27–36. doi: 10.1016/j.ejps.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 99.Xia T, et al. Comparison of the abilities of ambient and manufactured nanoparticles to induce cellular toxicity according to an oxidative stress paradigm. Nano Lett. 2006;6(8):1794–807. doi: 10.1021/nl061025k. [DOI] [PubMed] [Google Scholar]
- 100.Albrecht C, Borm PJ, Unfried K. Signal transduction pathways relevant for neoplastic effects of fibrous and non-fibrous particles. Mutat Res. 2004;553(1–2):23–35. doi: 10.1016/j.mrfmmm.2004.06.015. [DOI] [PubMed] [Google Scholar]
- 101.Morris JB, et al. p38 MAPK inhibition reduces aortic ultrasmall superparamagnetic iron oxide uptake in a mouse model of atherosclerosis: MRI assessment. Arterioscler Thromb Vasc Biol. 2008;28(2):265–71. doi: 10.1161/ATVBAHA.107.151175. [DOI] [PubMed] [Google Scholar]
- 102.Borm PJ, et al. The potential risks of nanomaterials: a review carried out for ECETOC. Part Fibre Toxicol. 2006;3:11. doi: 10.1186/1743-8977-3-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Medina C, et al. Nanoparticles: pharmacological and toxicological significance. Br J Pharmacol. 2007;150(5):552–8. doi: 10.1038/sj.bjp.0707130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Powers KW, et al. Characterization of the size, shape, and state of dispersion of nanoparticles for toxicological studies. Nanotoxicology. 2007;1(1):42–51. [Google Scholar]
- 105.Monteiller C, et al. The pro-inflammatory effects of low-toxicity low-solubility particles, nanoparticles and fine particles, on epithelial cells in vitro: the role of surface area. Occup Environ Med. 2007;64(9):609–15. doi: 10.1136/oem.2005.024802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Page-McCaw A, Ewald AJ, Werb Z. Matrix metalloproteinases and the regulation of tissue remodelling. Nat Rev Mol Cell Biol. 2007;8(3):221–33. doi: 10.1038/nrm2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Nelson KK, Melendez JA. Mitochondrial redox control of matrix metalloproteinases. Free Radic Biol Med. 2004;37(6):768–84. doi: 10.1016/j.freeradbiomed.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 108.Jian Liu K, Rosenberg GA. Matrix metalloproteinases and free radicals in cerebral ischemia. Free Radic Biol Med. 2005;39(1):71–80. doi: 10.1016/j.freeradbiomed.2005.03.033. [DOI] [PubMed] [Google Scholar]
- 109.Rosenberg GA. Matrix metalloproteinases in neuroinflammation. Glia. 2002;39(3):279–91. doi: 10.1002/glia.10108. [DOI] [PubMed] [Google Scholar]
- 110.Kieseier BC, Hartung HP, Wiendl H. Immune circuitry in the peripheral nervous system. Curr Opin Neurol. 2006;19(5):437–45. doi: 10.1097/01.wco.0000245365.51823.72. [DOI] [PubMed] [Google Scholar]
- 111.Naveau A, et al. Phenotypic study of human gingival fibroblasts labeled with superparamagnetic anionic nanoparticles. J Periodontol. 2006;77(2):238–47. doi: 10.1902/jop.2006.050064. [DOI] [PubMed] [Google Scholar]
- 112.Cammarota M, et al. Matrix metalloproteinases and their inhibitors as biomarkers for metal toxicity in vitro. Toxicol In Vitro. 2006;20(7):1125–32. doi: 10.1016/j.tiv.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 113.Myers RR, Campana WM, Shubayev VI. The role of neuroinflammation in neuropathic pain: mechanisms and therapeutic targets. Drug Discov Today. 2006;11(1–2):8–20. doi: 10.1016/S1359-6446(05)03637-8. [DOI] [PubMed] [Google Scholar]
- 114.Shubayev VI, et al. TNFalpha-induced MMP-9 promotes macrophage recruitment into injured peripheral nerve. Mol Cell Neurosci. 2006;31(3):407–15. doi: 10.1016/j.mcn.2005.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Gurzau ES, Neagu C, Gurzau AE. Essential metals--case study on iron. Ecotoxicol Environ Saf. 2003;56(1):190–200. doi: 10.1016/s0147-6513(03)00062-9. [DOI] [PubMed] [Google Scholar]
- 116.Bush AI. Metals and neuroscience. Curr Opin Chem Biol. 2000;4(2):184–91. doi: 10.1016/s1367-5931(99)00073-3. [DOI] [PubMed] [Google Scholar]
- 117.Ke Y, Ming Qian Z. Iron misregulation in the brain: a primary cause of neurodegenerative disorders. Lancet Neurol. 2003;2(4):246–53. doi: 10.1016/s1474-4422(03)00353-3. [DOI] [PubMed] [Google Scholar]
- 118.Dobson J. Gene therapy progress and prospects: magnetic nanoparticle-based gene delivery. Gene Ther. 2006;13(4):283–7. doi: 10.1038/sj.gt.3302720. [DOI] [PubMed] [Google Scholar]
- 119.Kim JS, et al. Toxicity and tissue distribution of magnetic nanoparticles in mice. Toxicol Sci. 2006;89(1):338–47. doi: 10.1093/toxsci/kfj027. [DOI] [PubMed] [Google Scholar]
- 120.Green-Sadan T, et al. Glial cell line-derived neurotrophic factor-conjugated nanoparticles suppress acquisition of cocaine self-administration in rats. Exp Neurol. 2005;194(1):97–105. doi: 10.1016/j.expneurol.2005.01.020. [DOI] [PubMed] [Google Scholar]
- 121.Brunner TJ, et al. In vitro cytotoxicity of oxide nanoparticles: comparison to asbestos, silica, and the effect of particle solubility. Environ Sci Technol. 2006;40(14):4374–81. doi: 10.1021/es052069i. [DOI] [PubMed] [Google Scholar]
- 122.Hussain SM, et al. In vitro toxicity of nanoparticles in BRL 3A rat liver cells. Toxicol In Vitro. 2005;19(7):975–83. doi: 10.1016/j.tiv.2005.06.034. [DOI] [PubMed] [Google Scholar]
- 123.Peters K, et al. Metallic nanoparticles exhibit paradoxical effects on oxidative stress and pro-inflammatory response in endothelial cells in vitro. Int J Immunopathol Pharmacol. 2007;20(4):685–95. doi: 10.1177/039463200702000404. [DOI] [PubMed] [Google Scholar]
- 124.Gajdosikova A, et al. Acute toxicity of magnetic nanoparticles in mice. Neuro Endocrinol Lett. 2006;27(Suppl 2):96–9. [PubMed] [Google Scholar]
- 125.Yu Z, et al. Acute toxicity and irritation of water-based dextran-coated magnetic fluid injected in mice. J Biomed Mater Res A. 2008;85(3):582–7. doi: 10.1002/jbm.a.31189. [DOI] [PubMed] [Google Scholar]
- 126.Jain TK, et al. Biodistribution, clearance, and biocompatibility of iron oxide magnetic nanoparticles in rats. Mol Pharm. 2008;5(2):316–27. doi: 10.1021/mp7001285. [DOI] [PubMed] [Google Scholar]
- 127.Ma HL, et al. Magnetic targeting after femoral artery administration and biocompatibility assessment of superparamagnetic iron oxide nanoparticles. J Biomed Mater Res A. 2008;84(3):598–606. doi: 10.1002/jbm.a.31346. [DOI] [PubMed] [Google Scholar]
- 128.Jeng HA, Swanson J. Toxicity of metal oxide nanoparticles in mammalian cells. J Environ Sci Health A Tox Hazard Subst Environ Eng. 2006;41(12):2699–711. doi: 10.1080/10934520600966177. [DOI] [PubMed] [Google Scholar]
- 129.Blanusa M, et al. Chelators as antidotes of metal toxicity: therapeutic and experimental aspects. Curr Med Chem. 2005;12(23):2771–94. doi: 10.2174/092986705774462987. [DOI] [PubMed] [Google Scholar]
- 130.Long TC, et al. Titanium dioxide (P25) produces reactive oxygen species in immortalized brain microglia (BV2): implications for nanoparticle neurotoxicity. Environ Sci Technol. 2006;40(14):4346–52. doi: 10.1021/es060589n. [DOI] [PubMed] [Google Scholar]
- 131.Gojova A, et al. Induction of inflammation in vascular endothelial cells by metal oxide nanoparticles: effect of particle composition. Environ Health Perspect. 2007;115(3):403–9. doi: 10.1289/ehp.8497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Johannsen M, et al. Morbidity and quality of life during thermotherapy using magnetic nanoparticles in locally recurrent prostate cancer: results of a prospective phase I trial. Int J Hyperthermia. 2007;23(3):315–23. doi: 10.1080/02656730601175479. [DOI] [PubMed] [Google Scholar]
- 133.Lacava LM, et al. Long-term retention of dextran-coated magetite nanoparticles in the liver and spleen. J Magn Magn Mater. 2004;272–276:2434–2435. [Google Scholar]
- 134.Lacava ZGM, et al. Biological effects of magnetic fluids: toxicity studies. Journal of Magnetism and Magnetic Materials. 1999;201(1–3):431–434. [Google Scholar]
- 135.Teeguarden JG, et al. Particokinetics in vitro: dosimetry considerations for in vitro nanoparticle toxicity assessments. Toxicol Sci. 2007;95(2):300–12. doi: 10.1093/toxsci/kfl165. [DOI] [PubMed] [Google Scholar]
- 136.Warheit DB, et al. Testing strategies to establish the safety of nanomaterials: conclusions of an ECETOC workshop. Inhal Toxicol. 2007;19(8):631–43. doi: 10.1080/08958370701353080. [DOI] [PubMed] [Google Scholar]
- 137.Worle-Knirsch JM, Pulskamp K, Krug HF. Oops they did it again! Carbon nanotubes hoax scientists in viability assays. Nano Lett. 2006;6(6):1261–8. doi: 10.1021/nl060177c. [DOI] [PubMed] [Google Scholar]
- 138.Barthel A, Klotz LO. Phosphoinositide 3-kinase signaling in the cellular response to oxidative stress. Biol Chem. 2005;386(3):207–16. doi: 10.1515/BC.2005.026. [DOI] [PubMed] [Google Scholar]
- 139.Harry GJ, Tiffany-Castiglioni E. Evaluation of neurotoxic potential by use of in vitro systems. Expert Opin Drug Metab Toxicol. 2005;1(4):701–13. doi: 10.1517/17425255.1.4.701. [DOI] [PubMed] [Google Scholar]
- 140.Stern ST, McNeil SE. Nanotechnology safety concerns revisited. Toxicol Sci. 2008;101(1):4–21. doi: 10.1093/toxsci/kfm169. [DOI] [PubMed] [Google Scholar]
- 141.Sayes CM, Reed KL, Warheit DB. Assessing toxicity of fine and nanoparticles: comparing in vitro measurements to in vivo pulmonary toxicity profiles. Toxicol Sci. 2007;97(1):163–80. doi: 10.1093/toxsci/kfm018. [DOI] [PubMed] [Google Scholar]
- 142.Kante B, et al. Toxicity of polyalkylcyanoacrylate nanoparticles I: Free nanoparticles. J Pharm Sci. 1982;71(7):786–90. doi: 10.1002/jps.2600710716. [DOI] [PubMed] [Google Scholar]
- 143.Service RF. Nanotechnology. Calls rise for more research on toxicology of nanomaterials. Science. 2005;310(5754):1609. doi: 10.1126/science.310.5754.1609. [DOI] [PubMed] [Google Scholar]
- 144.Nohynek GJ, et al. Grey goo on the skin? Nanotechnology, cosmetic and sunscreen safety. Crit Rev Toxicol. 2007;37(3):251–77. doi: 10.1080/10408440601177780. [DOI] [PubMed] [Google Scholar]


