Abstract
For nearly 100 years, pediatricians have regularly employed oxygen to treat neonatal and childhood diseases. Over this time, it has become clear that oxygen is toxic and that overzealous use can lead to significant morbidity. As we have learned more about the appropriate clinical indications for oxygen therapy, studies at the bench have begun to elucidate the molecular mechanisms by which cells respond to hyperoxia. In this review, we discuss transcription factors whose activity is regulated by oxygen, including Nrf2, AP-1, p53, NF-κB, STAT and CEBP. Special attention is paid to the mechanisms by which hyperoxia affects these transcription factors in the lung. Finally, we identify downstream targets of these transcription factors, with a focus on heme oxygenase-1. A better understanding of how oxygen affects various signaling pathways could lead to interventions aimed at preventing hyperoxic injury.
Oxygen therapy has a long and tortuous history in Neonatology. The pendulum has swung from a liberal use of supplemental oxygen in the early 20th century, to limited application in the 1950’s based on the association with retinopathy of prematurity. Today, clinical studies are focused on addressing which neonatal pathologic states require treatment with oxygen, and what level of oxygen administration is safe. In concert with these clinical studies, much work has been done at the bench to ascertain how oxygen affects gene expression. This is of particular relevance in neonates because changes in gene expression at critical times in development can have long lasting effects and subsequent consequences on lung structure and function. This review will address lessons learned and new insights as to the effects of hyperoxia on pulmonary gene expression.
EVOLUTIONARY PERSPECTIVE
Responses to atmospheric oxygen have evolved in eukaryotes over the last 1.5 billion years (1). The ability of organisms to reduce oxygen to water critically altered cellular metabolism and energy production, but also resulted in the formation of toxic reactive oxygen species via the mitochondrial respiratory chain. These radicals are electron donors, which can damage DNA, RNA, protein and lipids. They can also propagate deleterious reactions throughout cells and tissues resulting in death and apoptosis. In addition, these ROS can alter gene expression by modulating transcription factor activation, which then impact downstream targets. In oxygen breathing animals, only three tissues — the cornea, the skin, and the respiratory tract epithelium - are exposed to 21% oxygen, equivalent to a partial pressure of about 160 mm Hg at sea level. The remaining tissues are exposed to much lower oxygen tensions. The affinity of hemoglobin for oxygen maintains the partial pressure of oxygen in the mitochondria below 0.5 mm Hg, limiting the production of reactive oxygen species and effectively protecting the body from oxygen toxicity (2). Before the advent of the medical use of oxygen, humans were rarely exposed to oxygen tensions that were greater than those in their ambient environment. Thus, it stands to reason that evolution may not have dictated a well-developed response to acute increases in oxygen tension. The notable exception is the transition at birth from the womb to the outside world where we were rapidly shifted from a relative hypoxic environment to relative hyperoxia. Additionally, the lung epithelium is constantly exposed to “relative hyperoxia” compared to other tissues and is further stressed by oxygen therapy.
HISTORICAL PERSPECTIVE
From the time of its discovery in the 1770’s, oxygen has held promise as an elixir for multiple human ailments. Within 10 years of its discovery, Anton Lavoisier applied oxygen to newborn infants requiring resuscitation (3). By the early 1900’s, physicians were administering oxygen to treat cyanosis in premature infants (4). Shortly thereafter, oxygen therapy became widespread in neonatal units, with therapeutic indications ranging from respiratory distress to periodic breathing. However, by the early 1950s, published reports linking oxygen to the pathogenesis of retinopathy of prematurity began to appear, and the use of oxygen was quickly curtailed (5). Nevertheless, physicians were reminded that oxygen was a powerful and life-saving therapy when increased mortality from hyaline membrane disease (6) and the resurgence of cerebral palsy (7) were observed. This demonstrated that both too much and too little oxygen were problematic. Vigorous debates about the appropriate use of oxygen during newborn resuscitation (8) and the proper pulse oximetry saturation goals for premature infants (9) currently rage on. At this time, six multi-center randomized controlled trials are attempting to define optimal oxygen therapy goals for preterm babies (9).
Studies at the bench pair nicely with these clinical trials. Investigators have used multiple in vivo and in vitro models to determine how oxygen affects gene expression and subsequent lung structure and function. Hyperoxia results in alveolar and endothelial cell destruction, fluid leak into the air space, respiratory failure and mortality (10). The lungs of animals exposed to hyperoxia show increased mean linear intercepts, influx of macrophages, extracellular matrix turnover and fibrin deposition (11). During hyperoxia, reactive oxygen species (ROS) are produced both by the electron transport chain in the mitochondria and by the membrane-bound NADPH oxidase (12-15). ROS cause DNA strand breaks and other chromosomal aberrations (16, 17), which stimulate the expression of genes involved in inhibiting cell cycle progression (18). There is clear evidence in animal models that exposure to hyperoxia results in lung morphology similar to that of bronchopulmonary dysplasia (11, 19). These studies serve as important correlates to the ongoing trials involving oxygen therapy for premature infants.
HYPEROXIC GENE REGULATION
Organs, tissues and cells have evolved systems to rapidly respond to changes in their microenvironment. A stimulus, which causes a perturbation, must be detected and translated into a response, which then facilitates a return to the steady state (Figure 1). Receptors, signaling pathways, transcription factors and downstream changes in proteins and metabolic function have evolved for this purpose. Only a few transcription factors that specifically alter gene expression in response to increased oxygen tension have been identified, as well as some direct down-stream targets (see Table 1). These will be discussed below.
Figure 1.
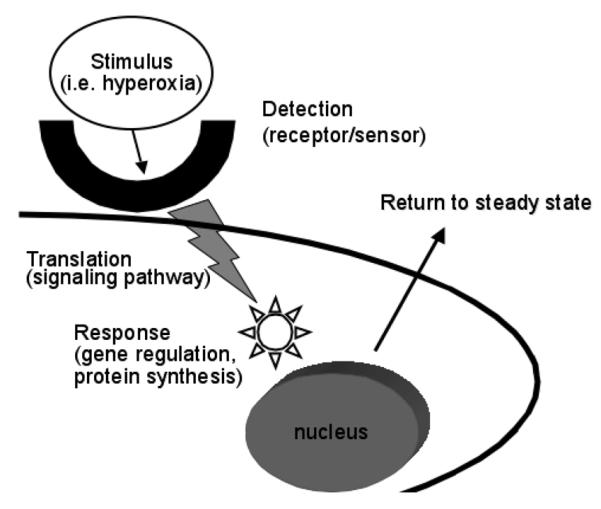
How a stimulus is perceived and how cells respond to return to the steady state. Cellular receptors or sensors detect stimuli such as hyperoxia. This leads to the translation of this signal via signal transduction pathways, which result in transcription factor activation. This then generates a response such as gene regulation and subsequent protein synthesis and a return to the steady state.
Table 1.
Summary of transcription factors regulated by hyperoxia
| Transcription Factor |
Regulated Genes | Protective Effect against Hyperoxia |
References |
|---|---|---|---|
| Nrf2 | ARE-mediated phase 2 detoxifying and antioxidant enzymes (i.e. HO-1) |
yes | (26-30) |
| AP-1 | IL-8 | yes | (34-40) |
| NF-κB | IGFBP2 ICAM-1 IL-6 ENaC p21 |
yes/no | (57, 62-68, 72, 77, 80-88, 95, 104, 107, 108, 111, 113-117, 119-127) |
| STAT | IL-6 | yes | (90, 91) |
| CEBP Proteins | CCSP | yes | (38, 93, 94) |
TRANSCRIPTION FACTORS RESPONSIVE TO HYPEROXIA
Nrf2 (Figure 2)
Figure 2.
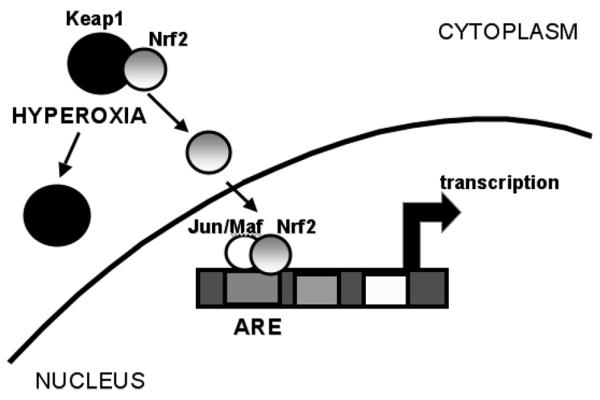
Nrf-2 mediated gene expression. The transcription factor Nrf-2 is sequestered in the cytoplasm bound to Keap1. Upon hyperoxic exposure, it dissociates from Keap1 and can migrate to the nucleus where it forms a complex with Jun or Maf proteins and results in gene activation.
The detoxification of ROS and electrophiles is important to prevent cellular injury. The transcription factor nuclear factor, erythroid 2 related factor 2 (Nrf2) regulates the inducible expression of a group of detoxification enzymes, such as glutathione S-transferase and NAD(P)H:quinone oxidoreductase, via antioxidant response elements (ARE). Under normal circumstances, Nrf2 is retained in the cytoplasm by a repressor protein Kelch-like ECH-associated protein 1 (Keap1). Exposure to xenobiotics and oxidants leads to the dissociation of Nrf2 from Keap1, which allows the free Nrf2 to translocate to the nucleus where it heterodimerizes with c-Jun, an activator protein-1 (AP-1) family protein (20). The consensus binding sequence of Nrf2 shows high similarity to the ARE/electrophile-responsive element (EpRE) sequence previously identified (21-23). Nrf2 can also heterodimerize with small Maf proteins to regulate ARE-mediated gene expression (24). These Maf proteins are so named because of their structural similarity to the founding member, the oncoprotein v-Maf. They include a characteristic basic region linked to a leucine zipper (b-Zip) domain which mediate DNA binding and subunit dimerization respectively (25).
Lung Nrf2 responds to hyperoxia (26). Linkage analysis identified Nrf2 as an important mediator of protection against lung hyperoxic injury (27) and mice deficient in Nrf2 exhibit aggravated lung injury and a lack of upregulation of ARE-mediated phase 2 detoxifying and antioxidant enzymes (28). Further gene array analysis of wild type versus Nrf2 deficient mice revealed discordance in multiple genes, thus identifying potential downstream targets of this important transcription factor (29). In fact, a single nucleotide polymorphism found in the Nrf2 promoter increases the risk of acute lung injury in human subjects (30). This evidence provides an important translational correlate and may lead to the development of therapeutic strategies.
AP-1
Activator protein-1 was first identified as a transcriptional factor that binds to an essential cis-element of the human metallothionein II gene (31). It is composed of fos and jun protein dimers which bind via hydrophobic interactions of their leucine-zipper regions (32). The jun/jun and jun/fos dimers form the AP-1 complex. This transcription factor controls genes involved in cellular proliferation and death in response to various stimuli including hyperoxia. The consensus AP-1-binding site is embedded in the ARE where fos and jun proteins may heterodimerize to Nrf2 in the presence of electrophiles and oxidants as discussed above (33). Blocking AP-1 activation enhances hyperoxia-induced cell death in murine lung epithelial cells (34, 35). One specific target of hyperoxia-induced JNK1/AP-1 activation in A549 cells is the IL-8 promoter (36). This could modulate inflammatory responses with hyperoxic exposure. It is interesting to note that neonatal mice exposed to hyperoxia show no increase in lung AP-1 consensus sequence binding (37) in contrast to their adult counterparts (37, 38). However, in the brain, increased AP-1 consensus sequence binding occurs in the forebrain and hippocampus of both adult and younger rats exposed to hyperoxia (39, 40). These data suggest both maturational differences and tissue specificity of AP-1 activation.
p53
The transcription factor p53 regulates the expression of a large number of target genes including those related to cell cycle arrest, cell death and DNA repair (41). Since its discovery in 1979, p53 has been identified as a tumor suppressor and its role in human cancer has become clearer (42). Under basal conditions, p53 resides in the cytoplasm and is subjected to ubiquitin-mediated proteolysis. However, in response to stimuli such as DNA damage, p53 is phosphorylated, stabilized and enters the nucleus (41). Under conditions of cellular stress, activated p53 initiates growth arrest and induces proapoptotic gene expression (42). Hyperoxia increases p53 gene transcription, protein levels and activity (43-46). In preterm baboons, exposure to hyperoxia results in increased p53 protein levels in airway epithelium (47, 48). However, in p53-/- mice exposed to hyperoxia, lung injury and lethality did not differ from similarly exposed wild-type animals (44, 49). These data indicate that the exact role of p53 in modulating the cellular response to hyperoxia remains to be elucidated.
NF-κB (Figure 3)
Figure 3.
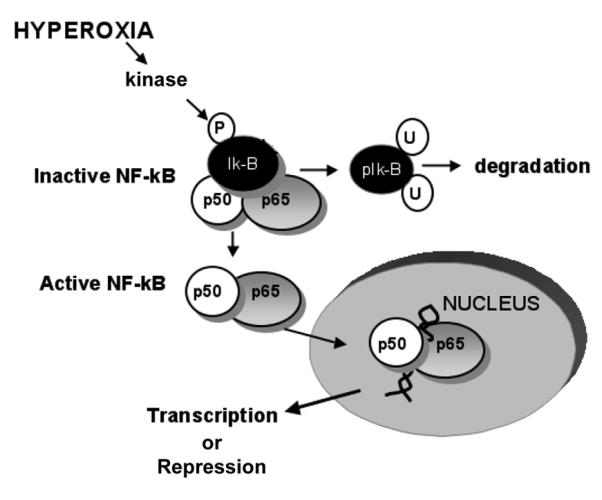
NF-κB mediated gene expression. With hyperoxia, there is phosphorylation (p) of the inhibitory protein IκBα on tyrosine 42. This results in the ubiquination (u) and subsequent degradation of IκBα. This allows for dissociation and nuclear translocation of the active NF-κB complex (p65 and p50 are represented here), binding to consensus sequences on various genes and transcriptional activation or repression of gene expression.
The nuclear factor kappa B (NF-κB) family is composed of highly conserved dimeric proteins, which activate genes that regulate apoptosis, inflammation and oxidative stress (50-52). This factor regulates gene expression and was first described by Baltimore and Sen (53). In quiescent cells, NF-κB dimers remain sequestered in the cytoplasm bound to a member of the IκB family of inhibitory proteins (51). IκBα is the prototypical member of this family and the most well studied. With inflammatory or oxidant stress, IκBα is phosphorylated, resulting in dissociation and unmasking of the nuclear localization sequence of NF-κB (52). Following inflammatory stimuli, such as TNF-α activation, IκBα is phosphorylated on serine 32/36 and degraded through the proteosomal pathway (52). In addition to this canonical pathway, an atypical pathway of NF-κB activation results from specific phosphorylation of IκBα on tyrosine 42 (54). This occurs after stimulation with pervanadate, nerve growth factor, hydrogen peroxide and ischemia-reperfusion (54-56) and, as most recently demonstrated, with hyperoxia (57). This latter pathway represents an intriguing molecular target for modulating the pulmonary response to hyperoxia.
Hyperoxia-induced NF-κB activation appears to be stimulus and cell type specific. It is important to note that NF-κB nuclear translocation and DNA binding can either enhance or suppress target gene expression. The subunit composition of the NF-κB dimer likely confers specificity to the expression of target genes following activation (58). The most abundant NF-κB protein is the p65-p50 dimer (59). The p65 subunit contains a transactivation domain that interacts with other transcription proteins to increase gene expression (60). The p50 subunit lacks this transactivation domain, and can repress transcription when bound to DNA as a p50-p50 homodimer (60, 61). Furthermore, the ability of NF-κB to alter gene expression is affected by post-translational modifications including phosphorylation and acetylation (60).
Nuclear translocation of NF-κB was shown in A549 lung adenocarcinoma cells exposed to hyperoxia-induced but this activation did not protect against cell death (62). Also, in adult mice exposed to hyperoxia, NF-κB activated pro-inflammatory markers in pulmonary lymphocytes (63). Furthermore, in fetal mouse lung explants, hyperoxia-induced NF-κB activation was associated with increased apoptosis which was reversed by blocking NF-κB activation (64). In contrast, inhibition of hyperoxia-induced NF-κB activation accelerated nonapoptotic cell death in primary and transformed lung epithelial cells, resulting in decreased levels of MnSOD (65). Additionally, A549 cells pre-treated with hyperoxia showed less apoptosis following exposure to hydrogen peroxide, an effect reversed by inhibiting NF-κB activation (66). In other examples, NF-κB was not activated in response to hyperoxic exposure (67, 68), suggesting that this signaling pathway is cell specific. The lung contains over forty different cell types (69), and the response to hyperoxia is cell type specific. Endothelial cells are very sensitive to oxygen toxicity, while type II epithelial cells are resistant and proliferate in the recovery phase (70). Furthermore, in the developing lung, exposure to hyperoxia prevents the normal differentiation of type II cells to type I cells in the developing lung (71). Further studies are necessary to fully dissect the specificity and complexity of hyperoxia-induced NF-κB activation. Nevertheless, these findings suggest that interventions to either inhibit or enhance NF-κB activation in hyperoxia could be of therapeutic benefit.
Various clinical interventions can affect NF-κB activation. Adrenalectomized adult mice exposed to hyperoxia had less lung injury and had improved survival due to increased NF-κB activation (72). Glucocorticoids are known to inhibit NF-κB activation (73-76). Thus, hyperoxia-induced NF-κB activation, when not limited by endogenous glucocorticoids, protects the adult lung from oxygen toxicity (72). Interestingly, following glucocorticoid therapy for BPD, cells obtained from tracheobronchial lavage fluid of premature neonates showed inhibition of NF-κB activation (77). Nitric oxide, which may prevent BPD in some infants(78), also inhibits NF-κB activity (79). The clinical implications of these findings remain to be explored in humans.
Of particular interest to pediatricians are the maturational differences found in NF-κB activation. Multiple models have shown increased NF-κB activation in neonates compared to adults following exposure to inflammatory and oxidant stimuli (80-82). In rat fetal alveolar type II cells, NF-κB translocates to the nucleus and binds DNA after hyperoxic exposure (83). This binding peaks soon after birth and gradually decreases postnatally, suggesting that NF-κB regulates genes involved in the transition from the relative hypoxic environment seen in utero (83). This activation may have important downstream effects as shown in hyperoxia exposed fetal lung fibroblasts where NF-κB activation prevented apoptosis through the suppression of pro-apoptotic genes (57). In contrast, this hyperoxic activation of NF-κB was not seen in adult lung fibroblasts (57). In the only published study evaluating hyperoxia-induced NF-κB activation in a neonatal in vivo model, Yang and colleagues showed that hyperoxia-induced NF-κB occurred in the lungs of neonatal but not adult mice (82). This activation was associated with the relative tolerance to hyperoxic injury in the neonatal animals when compared with adults, and this tolerance was reversed when hyperoxia-induced NF-κB activation was inhibited (82). In contrast, clinical studies show that enhanced NF-κB activation is linked to respiratory distress syndrome and an increased risk of developing BPD in preterm infants (84-86). Thus, it is not yet clear whether inhibition of lung NF-κB is beneficial or harmful in human neonates.
The hyperoxic activation of NF-κB has also been investigated in tissues other than the lung. Using a bioluminescent NF-κB reporter mouse line, Dohlen and colleagues showed increased NF-κB activity in the brain after resuscitation with 100% O2 (87). In other studies, hyperoxia without preceding ischemia decreased NF-κB activation in the basal forebrain, with a more pronounced effect in aged versus young mice (88).
It is clear that the NF-κB mediated response to oxygen is influenced by maturation. Whether these changes are beneficial or detrimental remains to be seen. Understanding the maturational differences in hyperoxia-induced NF-κB activation could help guide interventions aimed to modulate this response in neonates.
STAT
Another important transcription factor involved in hyperoxic gene regulation is the signal transducers and activators of transcription protein (STAT). This family of proteins is activated by various cell-surface receptors in response to ligands, including cytokines, growth factors, and peptides (89). Hyperoxic lung injury is attenuated in mice constitutively expressing Stat3 in respiratory epithelial cells (90). Conversely, mice with disruption of Stat3 in respiratory epithelial cells demonstrate exaggerated hyperoxic lung injury and increased expression of pro-inflammatory cytokines including IL-6 (91).
CEBP
The ccat/enhancer binding protein (C/EBP) family of proteins are basic leucine zipper (bZIP) transcription factors that respond to extracellular signals to regulate cell proliferation, differentiation and tissue development (92). C/EBPβand C/EBPconsensus sequence binding was increased in the lungs of young and aged mice exposed to hyperoxia (38). In the mouse exposed to hyperoxia, there is down-regulation of the protective Clara cell secretory protein (CCSP) due to enhanced C/EBPβnuclear translocation and binding to the CCSP promoter (93). These studies are particularly relevant because is required for lung maturation (94).
Other transcription factors regulated by hyperoxia
Acute and chronic exposure to hyperoxia may result in activation of a variety of other transcription factors including cmyc, fos related antigen (Fra)-1, junB, c-fos as well as nerve growth factor (NGF)1-A and —B (95). Furthermore, in the neonatal lung, hyperoxia can cause down-regulation of sox-7 and sox-18 (95). The relevance of these signaling events is not fully clear.
SPECIFIC DOWNSTREAM GENE TARGETS OF HYPEROXIA (See Table 1)
Because transcription factors that are regulated in hyperoxia control a multitude of genes, it would be difficult to list all of these genes. For example, the activation of NF-κB can regulate the expression of over 100 genes. Nevertheless, only a small fraction of NF-κB responsive genes are activated in hyperoxia. Some of the genes regulated by Nrf-2 and NF-κB will be highlighted below.
Nrf-2-regulated genes
Nrf2 binds to the ARE, driving the expression of genes including antioxidants such as glutathione peroxidase, catalase, superoxide dismutase, thiol metabolism-associated detoxifying enzymes such as glutathione-s-transferase and stress-response genes such as heme oxygenase (HO-1), amongst others (25-28). These genes are all highly responsive to hyperoxia. We will focus on HO-1 as an example of an Nrf-2 regulated gene regulated in hyperoxia.
The HO-1 gene encodes for the rate-limiting enzyme in the degradation of heme and the formation of biliverdin, which is subsequently reduced to bilirubin by biliverdin reductase. In recent years, many roles have been identified for this protein and it has been clearly demonstrated that HO-1 is a generalized response to oxidative stress (96). The mouse HO-1 gene is 6.8 kb in length and organized into 4 introns and 5 exons. A promoter sequence is located 28 base pairs (bps) upstream of the transcription initiation site. There is a proximal enhancer (PE) directly upstream of the promoter and there are two more distal enhancers located at 4 kb (DE1) and 10 kb (DE2) upstream of the transcription initiation site. Each enhancer region contains multiple transcription factor binding sites including composite AP-1 and NF-E2 or CREB/ATF sites (see Figure 4) (97-99). Induction of HO-1 in oxidative stress is via Nrf2 and small Maf proteins binding to the ARE (100). Competitive binding between Nrf2 and BTB and CNC homology 1, basic leucine zipper transcription factor 1 (Bach1), at the ARE is important in heme-mediated regulation of HO-1 (101). Several investigators have documented hyperoxic induction of HO-1 in adult mice. However, in the neonatal rodent HO-1 induction is limited. In the neonatal mouse and rat, hyperoxic exposure did not result in a significant increase in HO-1 mRNA as it did in similarly exposed adult (102, 103). In another study, lung HO-1 mRNA only increased after ten days of hyperoxic exposure in neonatal mice (95) whereas this occurred within 24 hours in adult mice (104). There may be some teleological wisdom in not further elevating the levels of HO-1 when they are already quite high at birth and in the neonatal period, especially if this could lead to deleteriously high levels thus aggravating hyperoxic injury (105). We have also observed increased protein levels and DNA binding for Bach1, an inhibitor of HO-1 transcriptional activation, in neonates at baseline and after exposure to hyperoxia compared to adults (103). Typically, Bach1 is degraded in the presence of ROS (106). Enhanced Bach1 expression could ensure that there are sufficient levels for HO-1 gene inhibition in the neonate.
Figure 4.
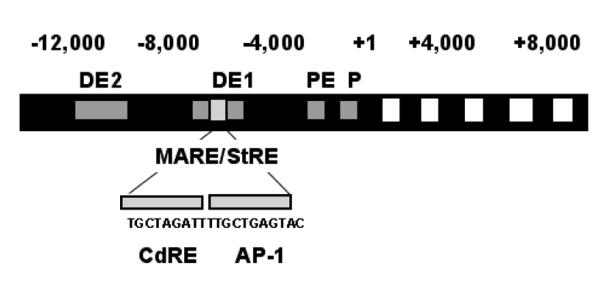
Diagram of the HO-1 gene. Numbers indicate base pairs. There are two distal enhancers (DE). These contain a multiple antioxidant response element/stress response element (MARE/StRE), which has consensus sequence for a cadmium response element (CdRE) as well as an AP-1 binding site. The gene also contains a proximal enhancer (PE) and a promoter (P).
NF-κB regulated genes
The IGF-binding protein (IGFBP)2 promoter has NF-κB consensus sequence binding sites, and both NF-κB consensus sequence binding and IGFBP2-promoter reporter activity increase in response to hyperoxia (107). This binding protein inhibits DNA synthesis and cellular entry into the S-phase, indicating a role for hyperoxia-induced NF-κB activation in modulating oxygen toxicity in the lung. Methylprednisolone treatment inhibits hyperoxia-induced NF-κB activation and downregulates ICAM-1 expression in human pulmonary artery endothelial cells (108), resulting in less neutrophil adhesion to the endothelium. As discussed above, adrenalectomized mice show attenuation of hyperoxic lung injury, and this is associated with preservation of NF-κB activation and induction of IL-6 (72). This cytokine is under the exclusive regulation of NF-κB with inflammation (109, 110). Whether IL-6 is exclusively regulated by NF-κB in response to hyperoxia is not known. Nevertheless, IL-6 is enhanced in the lungs of neonatal and adult mice in response to hyperoxia (111), although this phenomenon is not consistently observed in adult mice (63, 104). The amiloride-sensitive sodium channel, ENac, responsible for sodium and fluid absorption from the alveolar space (112), has an NF-κB binding site (113), and both NF-κB activation and ENaC gene expression increase with relative hyperoxia (114, 115). Furthermore, hyperoxia-induced ENaC expression is prevented with NF-κB blockade (115) in some reports but not others (116, 117).
Cell cycle genes
Another important effect of hyperoxia is the modulation of genes involved in cell cycle regulation. Both acute and chronic exposure to hyperoxia results in upregulation of p21 (95). Of note, NF-κB is known to regulate the expression of p21 in some cells (118). This key inhibitor of cell cycle regulation and cellular proliferation is increased in both the neonatal (119) and adult (120) lung following exposure to hyperoxia. Expression of this protein in response to hyperoxia relies on either TGF-β signaling (121) or p53 activation (122, 123). Upregulation of p21 is protective against hyperoxic injury in both neonatal (124) and adult (125) mice. It is hypothesized that inhibition of cellular proliferation during periods of oxidative stress allows for additional time to repair damaged DNA (126) thus providing cytoprotection.
CONCLUSION
Hyperoxia regulates multiple transcription factors in the lung. These in turn regulate a variety of downstream targets including ARE regulated genes such as HO-1, antioxidant enzymes which are important in the detoxification of electrophiles, as well as genes involved in cell cycle regulation and the inflammatory response. The overall effect of hyperoxia in the lung depends on the maturational stage of the organism. The net effect of hyperoxic lung gene regulation may be both enhanced cytoprotection and worsened lung function. In the neonate where postnatal lung development is crucial to proper alveolar formation, hyperoxic gene regulation may have long-lasting effect on lung structure and function. A further understanding of how hyperoxia affects specific signaling pathways and subsequent gene expression could lead to interventions aimed at preventing hyperoxic injury.
Acknowledgments
Financial Support: This work was supported by the National Institutes of Health (RO-1 HL-58752 P.A.D.) and Pediatric Scientist Development Program (K12-HD00850, C.J.W.).
ABBREVIATIONS
- AP-1
activator protein 1
- ARE
antioxidant response elements
- Bach1
basic leucine zipper transcription factor 1
- BPD
bronchopulmonary dysplasia
- C/EBP
ccat/enhancer binding protein
- ENaC
epithelium sodium channel
- HO-1
heme oxygenase-1
- IκB
inhibitor of κB
- Il-6
interleukin-6
- Keap 1
Kelch-like ECH-associated protein 1
- NF-κB
nuclear factor κB
- Nrf2
nuclear factor, erythroid 2 related factor 2
- ROS
reactive oxygen species
- STAT
signal transducers and activators of transcription protein
REFERENCES
- 1.Raymond J, Segre D. The effect of oxygen on biochemical networks and the evolution of complex life. Science. 2006;311:1764–1767. doi: 10.1126/science.1118439. [DOI] [PubMed] [Google Scholar]
- 2.Richmond S, Goldsmith JP. Air or 100% oxygen in neonatal resuscitation? Clin Perinatol. 2006;33:11–27. doi: 10.1016/j.clp.2005.11.003. v. [DOI] [PubMed] [Google Scholar]
- 3.Silverman WA. A cautionary tale about supplemental oxygen: the albatross of neonatal medicine. Pediatrics. 2004;113:394–396. doi: 10.1542/peds.113.2.394. [DOI] [PubMed] [Google Scholar]
- 4.Robertson AF. Reflections on errors in neonatology: I. The ‘Hands-Off’ years, 1920 to 1950. J Perinatol. 2003;23:48–55. doi: 10.1038/sj.jp.7210842. [DOI] [PubMed] [Google Scholar]
- 5.Silverman WA. Retrolental Fibroplasia: A Modern Parable. Grune and Stratton; New York: 1980. [Google Scholar]
- 6.Avery ME. Recent increase in mortality from hyaline membrane disease. J Pediatr. 1960;57:553–559. doi: 10.1016/s0022-3476(60)80083-2. [DOI] [PubMed] [Google Scholar]
- 7.McDonald AD. Cerebral Palsy in Children of Very Low Birth Weight. Arch Dis Child. 1963;38:579–588. doi: 10.1136/adc.38.202.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saugstad OD, Ramji S, Soll RF, Vento M. Resuscitation of Newborn Infants with 21% or 100% Oxygen: An Updated Systematic Review and Meta-Analysis. Neonatology. 2008;94:176–182. doi: 10.1159/000143397. [DOI] [PubMed] [Google Scholar]
- 9.Tin W, Gupta S. Optimum oxygen therapy in preterm babies. Arch Dis Child Fetal Neonatal Ed. 2007;92:F143–F147. doi: 10.1136/adc.2005.092726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Crapo JD. Morphologic changes in pulmonary oxygen toxicity. Annu Rev Physiol. 1986;48:721–731. doi: 10.1146/annurev.ph.48.030186.003445. [DOI] [PubMed] [Google Scholar]
- 11.Warner BB, Stuart LA, Papes RA, Wispé JR. Functional and pathological effects of prolonged hyperoxia in neonatal mice. Am J Physiol. 1998;275:L110–L117. doi: 10.1152/ajplung.1998.275.1.L110. [DOI] [PubMed] [Google Scholar]
- 12.Brueckl C, Kaestle S, Kerem A, Habazettl H, Krombach F, Kuppe H, Kuebler WM. Hyperoxia-induced reactive oxygen species formation in pulmonary capillary endothelial cells in situ. Am J Respir Cell Mol Biol. 2006;34:453–463. doi: 10.1165/rcmb.2005-0223OC. [DOI] [PubMed] [Google Scholar]
- 13.Parinandi NL, Kleinberg MA, Usatyuk PV, Cummings RJ, Pennathur A, Cardounel AJ, Zweier JL, Garcia JG, Natarajan V. Hyperoxia-induced NAD(P)H oxidase activation and regulation by MAP kinases in human lung endothelial cells. Am J Physiol Lung Cell Mol Physiol. 2003;284:L26–L38. doi: 10.1152/ajplung.00123.2002. [DOI] [PubMed] [Google Scholar]
- 14.Roy S, Khanna S, Bickerstaff AA, Subramanian SV, Atalay M, Bierl M, Pendyala S, Levy D, Sharma N, Venojarvi M, Strauch A, Orosz CG, Sen CK. Oxygen sensing by primary cardiac fibroblasts: a key role of p21(Waf1/Cip1/Sdi1) Circ Res. 2003;92:264–271. doi: 10.1161/01.res.0000056770.30922.e6. [DOI] [PubMed] [Google Scholar]
- 15.Chandel NS, Budinger GR. The cellular basis for diverse responses to oxygen. Free Radic Biol Med. 2007;42:165–174. doi: 10.1016/j.freeradbiomed.2006.10.048. [DOI] [PubMed] [Google Scholar]
- 16.Barazzone C, Horowitz S, Donati Y, Rodriguez I, Piguet PF. Oxygen toxicity in mouse lung: pathways to cell death. Am J Respir Cell Mol Biol. 1998;19:573–581. doi: 10.1165/ajrcmb.19.4.3173. [DOI] [PubMed] [Google Scholar]
- 17.O’Reilly MA. DNA damage and cell cycle checkpoints in hyperoxic lung injury: braking to facilitate repair. Am J Physiol Lung Cell Mol Physiol. 2001;281:L291–L305. doi: 10.1152/ajplung.2001.281.2.L291. [DOI] [PubMed] [Google Scholar]
- 18.Roper JM, Gehen SC, Staversky RJ, Hollander MC, Fornace AJ, Jr, O’Reilly MA. Loss of Gadd45a does not modify the pulmonary response to oxidative stress. Am J Physiol Lung Cell Mol Physiol. 2005;288:L663–L671. doi: 10.1152/ajplung.00355.2004. [DOI] [PubMed] [Google Scholar]
- 19.Bonikos DS, Bensch KG, Ludwin SK, Northway WH. Oxygen toxicity in the newborn. The effect of prolonged 100 per cent O2 exposure on the lungs of newborn mice. Lab Invest. 1975;32:619–635. [PubMed] [Google Scholar]
- 20.Jaiswal AK. Regulation of genes encoding NAD(P)H:quinone oxidoreductases. Free Radic Biol Med. 2000;29:254–262. doi: 10.1016/s0891-5849(00)00306-3. [DOI] [PubMed] [Google Scholar]
- 21.Friling RS, Bensimon A, Tichauer Y, Daniel V. Xenobiotic-inducible expression of murine glutathione S-transferase Ya subunit gene is controlled by an electrophile-responsive element. Proc Natl Acad Sci USA. 1990;87:6258–6262. doi: 10.1073/pnas.87.16.6258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rushmore TH, Morton MR, Pickett CB. The antioxidant responsive element. Activation by oxidative stress and identification of the DNA consensus sequence required for functional activity. J Biol Chem. 1991;266:11632–11639. [PubMed] [Google Scholar]
- 23.Wasserman WW, Fahl WE. Functional antioxidant responsive elements. Proc Natl Acad Sci USA. 1997;94:5361–5366. doi: 10.1073/pnas.94.10.5361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dhakshinamoorthy S, Jaiswal AK. c-Maf negatively regulates ARE-mediated detoxifying enzyme genes expression and anti-oxidant induction. Oncogene. 2002;21:5301–5312. doi: 10.1038/sj.onc.1205642. [DOI] [PubMed] [Google Scholar]
- 25.Motohashi H, Shavit JA, Igarashi K, Yamamoto M, Engel JD. The world according to Maf. Nucleic Acids Res. 1997;25:2953–2959. doi: 10.1093/nar/25.15.2953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cho HY, Reddy SP, Kleeberger SR. Nrf2 defends the lung from oxidative stress. Antioxid Redox Signal. 2006;8:76–87. doi: 10.1089/ars.2006.8.76. [DOI] [PubMed] [Google Scholar]
- 27.Cho HY, Jedlicka AE, Reddy SP, Zhang LY, Kensler TW, Kleeberger SR. Linkage analysis of susceptibility to hyperoxia. Nrf2 is a candidate gene. Am J Respir Cell Mol Biol. 2002;26:42–51. doi: 10.1165/ajrcmb.26.1.4536. [DOI] [PubMed] [Google Scholar]
- 28.Cho HY, Jedlicka AE, Reddy SP, Kensler TW, Yamamoto M, Zhang LY, Kleeberger SR. Role of NRF2 in protection against hyperoxic lung injury in mice. Am J Respir Cell Mol Biol. 2002;26:175–182. doi: 10.1165/ajrcmb.26.2.4501. [DOI] [PubMed] [Google Scholar]
- 29.Cho HY, Reddy SP, Debiase A, Yamamoto M, Kleeberger SR. Gene expression profiling of NRF2-mediated protection against oxidative injury. Free Radic Biol Med. 2005;38:325–343. doi: 10.1016/j.freeradbiomed.2004.10.013. [DOI] [PubMed] [Google Scholar]
- 30.Marzec JM, Christie JD, Reddy SP, Jedlicka AE, Vuong H, Lanken PN, Aplenc R, Yamamoto T, Yamamoto M, Cho HY, Kleeberger SR. Functional polymorphisms in the transcription factor NRF2 in humans increase the risk of acute lung injury. FASEB J. 2007;21:2237–2246. doi: 10.1096/fj.06-7759com. [DOI] [PubMed] [Google Scholar]
- 31.Lee W, Haslinger A, Karin M, Tjian R. Activation of transcription by two factors that bind promoter and enhancer sequences of the human metallothionein gene and SV40. Nature. 1987;325:368–372. doi: 10.1038/325368a0. [DOI] [PubMed] [Google Scholar]
- 32.Angel P, Karin M. The role of Jun, Fos and the AP-1 complex in cell-proliferation and transformation. Biochim Biophys Acta. 1991;1072:129–157. doi: 10.1016/0304-419x(91)90011-9. [DOI] [PubMed] [Google Scholar]
- 33.Gong P, Stewart D, Hu B, Vinson C, Alam J. Multiple basic-leucine zipper proteins regulate induction of the mouse heme oxygenase-1 gene by arsenite. Arch Biochem Biophys. 2002;405:265–274. doi: 10.1016/s0003-9861(02)00404-6. [DOI] [PubMed] [Google Scholar]
- 34.Romashko J, Horowitz S, Franek WR, Palaia TA, Miller EJ, Lin A, Birrer MJ, Scott W, Mantell L. MAPK pathways mediate hyperoxia-induced oncotic cell death in lung epithelial cells. Free Radic Biol Med. 2003;35:978–993. doi: 10.1016/s0891-5849(03)00494-5. [DOI] [PubMed] [Google Scholar]
- 35.Li Y, Arita Y, Koo HC, Davis JM, Kazzaz JA. Inhibition of c-Jun N-terminal kinase pathway improves cell viability in response to oxidant injury. Am J Respir Cell Mol Biol. 2003;29:779–783. doi: 10.1165/rcmb.2003-0087RC. [DOI] [PubMed] [Google Scholar]
- 36.Joseph A, Li Y, Koo HC, Davis JM, Pollack S, Kazzaz JA. Superoxide dismutase attenuates hyperoxia-induced interleukin-8 induction via AP-1. Free Radic Biol Med. 2008;45:1143–1149. doi: 10.1016/j.freeradbiomed.2008.07.006. [DOI] [PubMed] [Google Scholar]
- 37.Yang G, Madan A, Dennery PA. Maturational differences in hyperoxic AP-1 activation in rat lung. Am J Physiol Lung Cell Mol Physiol. 2000;278:L393–L398. doi: 10.1152/ajplung.2000.278.2.L393. [DOI] [PubMed] [Google Scholar]
- 38.Choi AM, Sylvester S, Otterbein L, Holbrook NJ. Molecular responses to hyperoxia in vivo: relationship to increased tolerance in aged rats. Am J Respir Cell Mol Biol. 1995;13:74–82. doi: 10.1165/ajrcmb.13.1.7598940. [DOI] [PubMed] [Google Scholar]
- 39.Tong L, Toliver-Kinsky T, Rassin D, Werrbach-Perez K, Perez-Polo JR. Hyperoxia increases AP-1 DNA binding in rat brain. Neurochem Res. 2003;28:111–115. doi: 10.1023/a:1021656430576. [DOI] [PubMed] [Google Scholar]
- 40.Tong L, Toliver-Kinsky T, Edwards M, Rassin DK, Werrbach-Perez K, Perez-Polo JR. Attenuated transcriptional responses to oxidative stress in the aged rat brain. J Neurosci Res. 2002;70:318–326. doi: 10.1002/jnr.10428. [DOI] [PubMed] [Google Scholar]
- 41.Horn HF, Vousden KH. Coping with stress: multiple ways to activate p53. Oncogene. 2007;26:1306–1316. doi: 10.1038/sj.onc.1210263. [DOI] [PubMed] [Google Scholar]
- 42.Vogelstein B, Lane D, Levine AJ. Surfing the p53 network. Nature. 2000;408:307–310. doi: 10.1038/35042675. [DOI] [PubMed] [Google Scholar]
- 43.O’Reilly MA, Staversky RJ, Stripp BR, Finkelstein JN. Exposure to hyperoxia induces p53 expression in mouse lung epithelium. Am J Respir Cell Mol Biol. 1998;18:43–50. doi: 10.1165/ajrcmb.18.1.2950m. [DOI] [PubMed] [Google Scholar]
- 44.Barazzone C, Horowitz S, Donati YR, Rodriguez I, Piguet PF. Oxygen toxicity in mouse lung: pathways to cell death. Am J Respir Cell Mol Biol. 1998;19:573–581. doi: 10.1165/ajrcmb.19.4.3173. [DOI] [PubMed] [Google Scholar]
- 45.Vaziri H, West MD, Allsopp RC, Davison TS, Wu YS, Arrowsmith CH, Poirier GG, Benchimol S. ATM-dependent telomere loss in aging human diploid fibroblasts and DNA damage lead to the post-translational activation of p53 protein involving poly(ADP-ribose) polymerase. EMBO J. 1997;16:6018–6033. doi: 10.1093/emboj/16.19.6018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shenberger JS, Dixon PS. Oxygen induces S-phase growth arrest and increases p53 and p21(WAF1/CIP1) expression in human bronchial smooth-muscle cells. Am J Respir Cell Mol Biol. 1999;21:395–402. doi: 10.1165/ajrcmb.21.3.3604. [DOI] [PubMed] [Google Scholar]
- 47.Das KC, Ravi D, Holland W. Increased apoptosis and expression of p21 and p53 in premature infant baboon model of bronchopulmonary dysplasia. Antioxid Redox Signal. 2004;6:109–116. doi: 10.1089/152308604771978417. [DOI] [PubMed] [Google Scholar]
- 48.Maniscalco WM, Watkins RH, Roper JM, Staversky R, O’Reilly MA. Hyperoxic ventilated premature baboons have increased p53, oxidant DNA damage and decreased VEGF expression. Pediatr Res. 2005;58:549–556. doi: 10.1203/01.pdr.0000176923.79584.f7. [DOI] [PubMed] [Google Scholar]
- 49.O’Reilly MA, Staversky RJ, Watkins RH, Maniscalco WM, Keng PC. p53-independent induction of GADD45 and GADD153 in mouse lungs exposed to hyperoxia. Am J Physiol Lung Cell Mol Physiol. 2000;278:L552–L559. doi: 10.1152/ajplung.2000.278.3.L552. [DOI] [PubMed] [Google Scholar]
- 50.Baeuerle PA, Baltimore D. NF-kappa B: ten years after. Cell. 1996;87:13–20. doi: 10.1016/s0092-8674(00)81318-5. [DOI] [PubMed] [Google Scholar]
- 51.Hoffmann A, Baltimore D. Circuitry of nuclear factor kappaB signaling. Immunol Rev. 2006;210:171–186. doi: 10.1111/j.0105-2896.2006.00375.x. [DOI] [PubMed] [Google Scholar]
- 52.Janssen-Heininger YM, Poynter ME, Baeuerle PA. Recent advances towards understanding redox mechanisms in the activation of nuclear factor kappaB. Free Radic Biol Med. 2000;28:1317–1327. doi: 10.1016/s0891-5849(00)00218-5. [DOI] [PubMed] [Google Scholar]
- 53.Sen R, Baltimore D. Inducibility of kappa immunoglobulin enhancer-binding protein Nf-kappa B by a posttranslational mechanism. Cell. 1986;47:921–928. doi: 10.1016/0092-8674(86)90807-x. [DOI] [PubMed] [Google Scholar]
- 54.Imbert V, Rupec RA, Livolsi A, Pahl HL, Traenckner EB, Mueller-Dieckmann C, Farahifar D, Rossi B, Auberger P, Baeuerle PA, Peyron JF. Tyrosine phosphorylation of I kappa B-alpha activates NF-kappa B without proteolytic degradation of I kappa B-alpha. Cell. 1996;86:787–798. doi: 10.1016/s0092-8674(00)80153-1. [DOI] [PubMed] [Google Scholar]
- 55.Koong AC, Chen EY, Giaccia AJ. Hypoxia causes the activation of nuclear factor kappa B through the phosphorylation of I kappa B alpha on tyrosine residues. Cancer Res. 1994;54:1425–1430. [PubMed] [Google Scholar]
- 56.Bui NT, Livolsi A, Peyron JF, Prehn JH. Activation of nuclear factor kappaB and Bcl-x survival gene expression by nerve growth factor requires tyrosine phosphorylation of IkappaBalpha. J Cell Biol. 2001;152:753–764. doi: 10.1083/jcb.152.4.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wright CJ, Zhuang T, La P, Yang G, Dennery PA. Hyperoxia-Induced NF-{kappa}B Activation Occurs Via A Maturationally Sensitive Atypical Pathway. Am J Physiol Lung Cell Mol Physiol. 2008 doi: 10.1152/ajplung.90499.2008. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Baldwin AS. The NF-kappa B and I kappa B proteins: new discoveries and insights. Annu Rev Immunol. 1996;14:649–683. doi: 10.1146/annurev.immunol.14.1.649. [DOI] [PubMed] [Google Scholar]
- 59.Hayden MS, Ghosh S. Shared principles in NF-kappaB signaling. Cell. 2008;132:344–362. doi: 10.1016/j.cell.2008.01.020. [DOI] [PubMed] [Google Scholar]
- 60.Chen LF, Greene WC. Shaping the nuclear action of NF-kappaB. Nat Rev Mol Cell Biol. 2004;5:392–401. doi: 10.1038/nrm1368. [DOI] [PubMed] [Google Scholar]
- 61.Ghosh S, May MJ, Kopp EB. NF-kappa B and Rel proteins: evolutionarily conserved mediators of immune responses. Annu Rev Immunol. 1998;16:225–260. doi: 10.1146/annurev.immunol.16.1.225. [DOI] [PubMed] [Google Scholar]
- 62.Li Y, Zhang W, Mantell L, Kazzaz JA, Fein AM, Horowitz S. Nuclear factor-kappaB is activated by hyperoxia but does not protect from cell death. J Biol Chem. 1997;272:20646–20649. doi: 10.1074/jbc.272.33.20646. [DOI] [PubMed] [Google Scholar]
- 63.Shea LM, Beehler C, Schwartz M, Shenkar R, Tuder R, Abraham E. Hyperoxia activates NF-kappaB and increases TNF-alpha and IFN-gamma gene expression in mouse pulmonary lymphocytes. J Immunol. 1996;157:3902–3908. [PubMed] [Google Scholar]
- 64.Dieperink HI, Blackwell TS, Prince LS. Hyperoxia and apoptosis in developing mouse lung mesenchyme. Pediatr Res. 2006;59:185–190. doi: 10.1203/01.pdr.0000196371.85945.3a. [DOI] [PubMed] [Google Scholar]
- 65.Franek WR, Morrow DM, Zhu H, Vancurova I, Miskolci V, Darley-Usmar K, Simms HH, Mantell L. NF-kappaB protects lung epithelium against hyperoxia-induced nonapoptotic cell death-oncosis. Free Radic Biol Med. 2004;37:1670–1679. doi: 10.1016/j.freeradbiomed.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 66.Franek WR, Horowitz S, Stansberry L, Kazzaz JA, Koo HC, Li Y, Arita Y, Davis JM, Mantell AS, Scott W, Mantell L. Hyperoxia inhibits oxidant-induced apoptosis in lung epithelial cells. J Biol Chem. 2001;276:569–575. doi: 10.1074/jbc.M004716200. [DOI] [PubMed] [Google Scholar]
- 67.D’Angio CT, LoMonaco MB, Johnston CJ, Reed CK, Finkelstein JN. Differential roles for NF-kappa B in endotoxin and oxygen induction of interleukin-8 in the macrophage. Am J Physiol Lung Cell Mol Physiol. 2004;286:L30–L36. doi: 10.1152/ajplung.00360.2002. [DOI] [PubMed] [Google Scholar]
- 68.Rahman I, Mulier B, Gilmour PS, Watchorn T, Donaldson K, Jeffery PK, MacNee W. Oxidant-mediated lung epithelial cell tolerance: the role of intracellular glutathione and nuclear factor-kappaB. Biochem Pharmacol. 2001;62:787–794. doi: 10.1016/s0006-2952(01)00702-x. [DOI] [PubMed] [Google Scholar]
- 69.Breeze RG, Wheeldon EB. The cells of the pulmonary airways. Am Rev Respir Dis. 1977;116:705–777. doi: 10.1164/arrd.1977.116.4.705. [DOI] [PubMed] [Google Scholar]
- 70.Crapo JD, Barry BE, Foscue HA, Shelburne J. Structural and biochemical changes in rat lungs occurring during exposures to lethal and adaptive doses of oxygen. Am Rev Respir Dis. 1980;122:123–143. doi: 10.1164/arrd.1980.122.1.123. [DOI] [PubMed] [Google Scholar]
- 71.Yee M, Vitiello PF, Roper JM, Staversky RJ, Wright TW, McGrath-Morrow SA, Maniscalco WM, Finkelstein JN, O’Reilly MA. Type II epithelial cells are critical target for hyperoxia-mediated impairment of postnatal lung development. Am J Physiol Lung Cell Mol Physiol. 2006;291:L1101–L1111. doi: 10.1152/ajplung.00126.2006. [DOI] [PubMed] [Google Scholar]
- 72.Barazzone-Argiroffo C, Pagano A, Juge C, Metrailler I, Rochat A, Vesin C, Donati Y. Glucocorticoids aggravate hyperoxia-induced lung injury through decreased nuclear factor-kappa B activity. Am J Physiol Lung Cell Mol Physiol. 2003;284:L197–L204. doi: 10.1152/ajplung.00239.2002. [DOI] [PubMed] [Google Scholar]
- 73.Scheinman RI, Cogswell PC, Lofquist AK, Baldwin AS., Jr Role of transcriptional activation of I kappa B alpha in mediation of immunosuppression by glucocorticoids. Science. 1995;270:283–286. doi: 10.1126/science.270.5234.283. [DOI] [PubMed] [Google Scholar]
- 74.Auphan N, DiDonato JA, Rosette C, Helmberg A, Karin M. Immunosuppression by glucocorticoids: inhibition of NF-kappa B activity through induction of I kappa B synthesis. Science. 1995;270:286–290. doi: 10.1126/science.270.5234.286. [DOI] [PubMed] [Google Scholar]
- 75.Ray A, Prefontaine KE. Physical association and functional antagonism between the p65 subunit of transcription factor NF-kappa B and the glucocorticoid receptor. Proc Natl Acad Sci USA. 1994;91:752–756. doi: 10.1073/pnas.91.2.752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Heck S, Bender K, Kullmann M, Gottlicher M, Herrlich P, Cato AC. I kappaB alpha-independent downregulation of NF-kappaB activity by glucocorticoid receptor. EMBO J. 1997;16:4698–4707. doi: 10.1093/emboj/16.15.4698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Aghai ZH, Kumar S, Farhath S, Kumar M, Saslow J, Nakhla T, Eydelman R, Strande L, Stahl G, Hewitt C, Nesin M, Rahman I. Dexamethasone suppresses expression of Nuclear Factor-kappaB in the cells of tracheobronchial lavage fluid in premature neonates with respiratory distress. Pediatr Res. 2006;59:811–815. doi: 10.1203/01.pdr.0000219120.92049.b3. [DOI] [PubMed] [Google Scholar]
- 78.Ballard RA, Truog WE, Cnaan A, Martin RJ, Ballard PL, Merrill JD, Walsh MC, Durand DJ, Mayock DE, Eichenwald EC, Null DR, Hudak ML, Puri AR, Golombek SG, Courtney SE, Stewart DL, Welty SE, Phibbs RH, Hibbs AM, Luan X, Wadlinger SR, Asselin JM, Coburn CE. Inhaled nitric oxide in preterm infants undergoing mechanical ventilation. N Engl J Med. 2006;355:343–353. doi: 10.1056/NEJMoa061088. [DOI] [PubMed] [Google Scholar]
- 79.Franek WR, Chowdary YC, Lin X, Hu M, Miller EJ, Kazzaz JA, Razzano P, Romashko J, Davis JM, Narula P, Horowitz S, Scott W, Mantell LL. Suppression of nuclear factor-kappa B activity by nitric oxide and hyperoxia in oxygen-resistant cells. J Biol Chem. 2002;277:42694–42700. doi: 10.1074/jbc.M202623200. [DOI] [PubMed] [Google Scholar]
- 80.Kilpinen S, Henttinen T, Lahdenpohja N, Hulkkonen J, Hurme M. Signals leading to the activation of NF-kappa B transcription factor are stronger in neonatal than adult T lymphocytes. Scand J Immunol. 1996;44:85–88. doi: 10.1046/j.1365-3083.1996.d01-277.x. [DOI] [PubMed] [Google Scholar]
- 81.Vancurova I, Bellani P, Davidson D. Activation of nuclear factor-kappaB and its suppression by dexamethasone in polymorphonuclear leukocytes: newborn versus adult. Pediatr Res. 2001;49:257–262. doi: 10.1203/00006450-200102000-00021. [DOI] [PubMed] [Google Scholar]
- 82.Yang G, Abate A, George AG, Weng YH, Dennery PA. Maturational differences in lung NF-kappaB activation and their role in tolerance to hyperoxia. J Clin Invest. 2004;114:669–678. doi: 10.1172/JCI19300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Haddad JJ, Land SC. O(2)-evoked regulation of HIF-1alpha and NF-kappaB in perinatal lung epithelium requires glutathione biosynthesis. Am J Physiol Lung Cell Mol Physiol. 2000;278:L492–L503. doi: 10.1152/ajplung.2000.278.3.L492. [DOI] [PubMed] [Google Scholar]
- 84.Bourbia A, Cruz MA, Rozycki HJ. NF-kappaB in tracheal lavage fluid from intubated premature infants: association with inflammation, oxygen, and outcome. Arch Dis Child Fetal Neonatal Ed. 2006;91:F36–F39. doi: 10.1136/adc.2003.045807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cao L, Liu C, Cai B, Jia X, Kang L, Speer CP, Sun B. Nuclear factor-kappa B expression in alveolar macrophages of mechanically ventilated neonates with respiratory distress syndrome. Biol Neonate. 2004;86:116–123. doi: 10.1159/000078940. [DOI] [PubMed] [Google Scholar]
- 86.Cheah FC, Winterbourn CC, Darlow BA, Mocatta TJ, Vissers MC. Nuclear factor kappaB activation in pulmonary leukocytes from infants with hyaline membrane disease: associations with chorioamnionitis and Ureaplasma urealyticum colonization. Pediatr Res. 2005;57:616–623. doi: 10.1203/01.PDR.0000156209.37627.82. [DOI] [PubMed] [Google Scholar]
- 87.Dohlen G, Carlsen H, Blomhoff R, Thaulow E, Saugstad OD. Reoxygenation of hypoxic mice with 100% oxygen induces brain nuclear factor-kappa B. Pediatr Res. 2005;58:941–945. doi: 10.1203/01.PDR.0000182595.62545.EE. [DOI] [PubMed] [Google Scholar]
- 88.Toliver-Kinsky T, Rassin D, Perez-Polo JR. NF-kappaB activity decreases in basal forebrain of young and aged rats after hyperoxia. Neurobiol Aging. 2002;23:899–905. doi: 10.1016/s0197-4580(02)00023-4. [DOI] [PubMed] [Google Scholar]
- 89.Levy DE, Darnell JE., Jr Stats: transcriptional control and biological impact. Nat Rev Mol Cell Biol. 2002;3:651–662. doi: 10.1038/nrm909. [DOI] [PubMed] [Google Scholar]
- 90.Lian X, Qin Y, Hossain SA, Yang L, White A, Xu H, Shipley JM, Li T, Senior RM, Du H, Yan C. Overexpression of Stat3C in pulmonary epithelium protects against hyperoxic lung injury. J Immunol. 2005;174:7250–7256. doi: 10.4049/jimmunol.174.11.7250. [DOI] [PubMed] [Google Scholar]
- 91.Hokuto I, Ikegami M, Yoshida M, Takeda K, Akira S, Perl AK, Hull WM, Wert SE, Whitsett JA. Stat-3 is required for pulmonary homeostasis during hyperoxia. J Clin Invest. 2004;113:28–37. doi: 10.1172/JCI200419491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Nerlov C. C/EBPs: recipients of extracellular signals through proteome modulation. Curr Opin Cell Biol. 2008;20:180–185. doi: 10.1016/j.ceb.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 93.Ramsay PL, DeMayo FJ, Hegemier SE, Wearden ME, Smith CV, Welty SE. Clara cell secretory protein oxidation and expression in premature infants who develop bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2001;164:155–161. doi: 10.1164/ajrccm.164.1.2008022. [DOI] [PubMed] [Google Scholar]
- 94.Martis PC, Whitsett JA, Xu Y, Perl AK, Wan H, Ikegami M. C/EBPalpha is required for lung maturation at birth. Development. 2006;133:1155–1164. doi: 10.1242/dev.02273. [DOI] [PubMed] [Google Scholar]
- 95.Wagenaar GT, ter Horst SA, van Gastelen MA, Leijser LM, Mauad T, van der Velden PA, de Heer E, Hiemstra PS, Poorthuis BJ, Walther FJ. Gene expression profile and histopathology of experimental bronchopulmonary dysplasia induced by prolonged oxidative stress. Free Radic Biol Med. 2004;36:782–801. doi: 10.1016/j.freeradbiomed.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 96.Applegate LA, Luscher P, Tyrrell RM. Induction of heme oxygenase: a general response to oxidant stress in cultured mammalian cells. Cancer Res. 1991;51:974–978. [PubMed] [Google Scholar]
- 97.Alam J, Cai J, Smith A. Isolation and characterization of the mouse heme oxygenase-1 gene. Distal 5′ sequences are required for induction by heme or heavy metals. J Biol Chem. 1994;269:1001–1009. [PubMed] [Google Scholar]
- 98.Alam J, Camhi S, Choi AM. Identification of a second region upstream of the mouse heme oxygenase-1 gene that functions as a basal level and inducer-dependent transcription enhancer. J Biol Chem. 1995;270:11977–11984. doi: 10.1074/jbc.270.20.11977. [DOI] [PubMed] [Google Scholar]
- 99.Lee PJ, Alam J, Wiegand GW, Choi AM. Overexpression of heme oxygenase-1 in human pulmonary epithelial cells results in cell growth arrest and increased resistance to hyperoxia. Proc Natl Acad Sci USA. 1996;93:10393–10398. doi: 10.1073/pnas.93.19.10393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Alam J, Stewart D, Touchard C, Boinapally S, Choi AM, Cook JL. Nrf2, a Cap‘n’Collar transcription factor, regulates induction of the heme oxygenase-1 gene. J Biol Chem. 1999;274:26071–26078. doi: 10.1074/jbc.274.37.26071. [DOI] [PubMed] [Google Scholar]
- 101.Sun Z, Andersson R. NF-kappaB activation and inhibition: a review. Shock. 2002;18:99–106. doi: 10.1097/00024382-200208000-00001. [DOI] [PubMed] [Google Scholar]
- 102.Dennery PA, Lee CS, Ford BS, Weng YH, Yang G, Rodgers PA. Developmental expression of heme oxygenase in the rat lung. Pediatr Res. 2003;53:42–47. doi: 10.1203/00006450-200301000-00010. [DOI] [PubMed] [Google Scholar]
- 103.Kassovska-Bratinova S, Yang G, Igarashi K, Dennery PA. Bach1 Modulates Heme Oxygenase-1 Expression in the Neonatal Mouse Lung. Pediatr Res. 2008 doi: 10.1203/PDR.0b013e318191eedc. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Perkowski S, Sun J, Singhal S, Santiago J, Leikauf GD, Albelda SM. Gene expression profiling of the early pulmonary response to hyperoxia in mice. Am J Respir Cell Mol Biol. 2003;28:682–696. doi: 10.1165/rcmb.4692. [DOI] [PubMed] [Google Scholar]
- 105.Suttner DM, Dennery PA. Reversal of HO-1 related cytoprotection with increased expression is due to reactive iron. FASEB J. 1999;13:1800–1809. doi: 10.1096/fasebj.13.13.1800. [DOI] [PubMed] [Google Scholar]
- 106.Igarashi K, Sun J. The heme-Bach1 pathway in the regulation of oxidative stress response and erythroid differentiation. Antioxid Redox Signal. 2006;8:107–118. doi: 10.1089/ars.2006.8.107. [DOI] [PubMed] [Google Scholar]
- 107.Cazals V, Nabeyrat E, Corroyer S, de Keyzer Y, Clement A. Role for NF-kappa B in mediating the effects of hyperoxia on IGF-binding protein 2 promoter activity in lung alveolar epithelial cells. Biochim Biophys Acta. 1999;1448:349–362. doi: 10.1016/s0167-4889(98)00095-0. [DOI] [PubMed] [Google Scholar]
- 108.Suzuki Y, Nishio K, Takeshita K, Takeuchi O, Watanabe K, Sato N, Naoki K, Kudo H, Aoki T, Yamaguchi K. Effect of steroid on hyperoxia-induced ICAM-1 expression in pulmonary endothelial cells. Am J Physiol Lung Cell Mol Physiol. 2000;278:L245–L252. doi: 10.1152/ajplung.2000.278.2.L245. [DOI] [PubMed] [Google Scholar]
- 109.Vanden Berghe W, Vermeulen L, De Wilde G, De Bosscher K, Boone E, Haegeman G. Signal transduction by tumor necrosis factor and gene regulation of the inflammatory cytokine interleukin-6. Biochem Pharmacol. 2000;60:1185–1195. doi: 10.1016/s0006-2952(00)00412-3. [DOI] [PubMed] [Google Scholar]
- 110.Libermann TA, Baltimore D. Activation of interleukin-6 gene expression through the NF-kappa B transcription factor. Mol Cell Biol. 1990;10:2327–2334. doi: 10.1128/mcb.10.5.2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Johnston CJ, Wright TW, Reed CK, Finkelstein JN. Comparison of adult and newborn pulmonary cytokine mRNA expression after hyperoxia. Exp Lung Res. 1997;23:537–552. doi: 10.3109/01902149709039242. [DOI] [PubMed] [Google Scholar]
- 112.Pitkanen O, Tanswell AK, Downey G, O’Brodovich H. Increased Po2 alters the bioelectric properties of fetal distal lung epithelium. Am J Physiol. 1996;270:L1060–L1066. doi: 10.1152/ajplung.1996.270.6.L1060. [DOI] [PubMed] [Google Scholar]
- 113.Otulakowski G, Rafii B, Bremner HR, O’Brodovich H. Structure and hormone responsiveness of the gene encoding the alpha-subunit of the rat amiloride-sensitive epithelial sodium channel. Am J Respir Cell Mol Biol. 1999;20:1028–1040. doi: 10.1165/ajrcmb.20.5.3382. [DOI] [PubMed] [Google Scholar]
- 114.Rafii B, Tanswell AK, Otulakowski G, Pitkanen O, Belcastro-Taylor R, O’Brodovich H. O2-induced ENaC expression is associated with NF-kappaB activation and blocked by superoxide scavenger. Am J Physiol. 1998;275:L764–L770. doi: 10.1152/ajplung.1998.275.4.L764. [DOI] [PubMed] [Google Scholar]
- 115.Haddad JJ, Collett A, Land SC, Olver RE, Wilson SM. NF-kappaB blockade reduces the O2-evoked rise in Na+ conductance in fetal alveolar cells. Biochem Biophys Res Commun. 2001;281:987–992. doi: 10.1006/bbrc.2001.4453. [DOI] [PubMed] [Google Scholar]
- 116.Richard K, Ramminger SJ, Inglis SK, Olver RE, Land SC, Wilson SM. O2 can raise fetal pneumocyte Na+ conductance without affecting ENaC mRNA abundance. Biochem Biophys Res Commun. 2003;305:671–676. doi: 10.1016/s0006-291x(03)00832-5. [DOI] [PubMed] [Google Scholar]
- 117.Baines DL, Ramminger SJ, Collett A, Haddad JJ, Best OG, Land SC, Olver RE, Wilson SM. Oxygen-evoked Na+ transport in rat fetal distal lung epithelial cells. J Physiol. 2001;532:105–113. doi: 10.1111/j.1469-7793.2001.0105g.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hinata K, Gervin AM, Zhang Y Jennifer, Khavari PA. Divergent gene regulation and growth effects by NF-kappa B in epithelial and mesenchymal cells of human skin. Oncogene. 2003;22:1955–1964. doi: 10.1038/sj.onc.1206198. [DOI] [PubMed] [Google Scholar]
- 119.McGrath SA. Induction of p21WAF/CIP1 during hyperoxia. Am J Respir Cell Mol Biol. 1998;18:179–187. doi: 10.1165/ajrcmb.18.2.2964m. [DOI] [PubMed] [Google Scholar]
- 120.O’Reilly MA, Staversky RJ, Watkins RH, Maniscalco WM. Accumulation of p21(Cip1/WAF1) during hyperoxic lung injury in mice. Am J Respir Cell Mol Biol. 1998;19:777–785. doi: 10.1165/ajrcmb.19.5.3200. [DOI] [PubMed] [Google Scholar]
- 121.Corroyer S, Maitre B, Cazals V, Clement A. Altered regulation of G1 cyclins in oxidant-induced growth arrest of lung alveolar epithelial cells. Accumulation of inactive cyclin E-DCK2 complexes. J Biol Chem. 1996;271:25117–25125. doi: 10.1074/jbc.271.41.25117. [DOI] [PubMed] [Google Scholar]
- 122.Helt CE, Rancourt RC, Staversky RJ, O’Reilly MA. p53-dependent induction of p21(Cip1/WAF1/Sdi1) protects against oxygen-induced toxicity. Toxicol Sci. 2001;63:214–222. doi: 10.1093/toxsci/63.2.214. [DOI] [PubMed] [Google Scholar]
- 123.Helt CE, Staversky RJ, Lee YJ, Bambara RA, Keng PC, O’Reilly MA. The Cdk and PCNA domains on p21Cip1 both function to inhibit G1/S progression during hyperoxia. Am J Physiol Lung Cell Mol Physiol. 2004;286:L506–L513. doi: 10.1152/ajplung.00243.2003. [DOI] [PubMed] [Google Scholar]
- 124.McGrath-Morrow SA, Cho C, Soutiere S, Mitzner W, Tuder R. The effect of neonatal hyperoxia on the lung of p21Waf1/Cip1/Sdi1-deficient mice. Am J Respir Cell Mol Biol. 2004;30:635–640. doi: 10.1165/rcmb.2003-0049OC. [DOI] [PubMed] [Google Scholar]
- 125.O’Reilly MA, Staversky RJ, Watkins RH, Reed CK, de Mesy Jensen KL, Finkelstein JN, Keng PC. The cyclin-dependent kinase inhibitor p21 protects the lung from oxidative stress. Am J Respir Cell Mol Biol. 2001;24:703–710. doi: 10.1165/ajrcmb.24.6.4355. [DOI] [PubMed] [Google Scholar]
- 126.O’Reilly MA. Redox activation of p21Cip1/WAF1/Sdi1: a multifunctional regulator of cell survival and death. Antioxid Redox Signal. 2005;7:108–118. doi: 10.1089/ars.2005.7.108. [DOI] [PubMed] [Google Scholar]
- 127.Franek WR, Chowdary YC, Lin X, Hu M, Miller EJ, Kazzaz JA, Razzano P, Romashko J, Davis JM, Narula P, Horowitz S, Scott W, Mantell L. Suppression of nuclear factor-kappa B activity by nitric oxide and hyperoxia in oxygen-resistant cells. J Biol Chem. 2002;277:42694–42700. doi: 10.1074/jbc.M202623200. [DOI] [PubMed] [Google Scholar]


