Abstract
Objective
We recently reported that murine marrow cultured ex vivo for gamma-retrovirus transduction engrafts ~10 fold less well than fresh marrow upon transplantation into submyeloablated hosts. Here, we evaluated homing efficiency as a potential mechanism for this engraftment disparity, and whether CD26 inhibition with the tripeptide Diprotin A (DipA) would enhance engraftment of ex vivo cultured cells in submyeloablated hosts.
Methods
Homing and engraftment of fresh and ex vivo cultured lineage-negative (lin-) marrow cells in submyeloablated congenic hosts with and without DipA treatment was evaluated. Expression of CXCR4 and CD26 on fresh and cultured lin- marrow cells was compared.
Results
Homing of lin- cells cultured for gamma-retrovirus transduction was ≥3-fold less than that of fresh lin- cells 20 hours after transplantation into submyeloablated hosts. DipA treatment of fresh lin- cells resulted in ≥-fold increased homing and engraftment in submyeloablated hosts. DipA treatment, however, did not significantly improve homing or engraftment of cells undergoing a three-day culture protocol for gamma-retrovirus transduction in submyeloablated hosts. CXCR4 expression on lin- cells was significantly decreased following three days of culture; CXCR4 expression was not significantly altered following overnight culture.
Conclusions
Ex vivo culture of lin- cells for gamma-retroviral transduction downregulates CXCR4 expression and markedly impairs homing and engraftment of murine lin- marrow in submyeloablated hosts. While inhibition of CD26 activity with DipA increases homing and engraftment of fresh lin- cells, DipA treatment does not improve homing and engraftment of cultured lin- marrow cells in submyeloablated congenic hosts.
INTRODUCTION
Submyeloablative (also termed non-myeloablative or reduced-intensity) conditioning for hematopoietic stem cell (HSC) transplantation provides a means for engraftment of allogeneic or autologous donor cells while lessening the potentially severe toxicities caused by myeloablative conditioning. One clinical application of submyeloablative conditioning is transplantation of gene-corrected autologous HSC for the treatment of genetic blood cell diseases. Patients with chronic, non-malignant blood disorders often have infectious or end-organ complications which preclude the use of traditional myeloablative conditioning. Thus, reduced-toxicity conditioning regimens that permit engraftment of sufficient numbers of gene-corrected autologous cells to ameliorate manifestations of the underlying disease may be advantageous.
Unfortunately, numerous preclinical transplantation studies designed to quantify engraftment of gene-marked cells have shown that the ex vivo manipulation necessary for gamma-retroviral transduction severely impairs the engraftment of HSC into submyeloablated hosts. We previously reported that gamma-retrovirus-transduced murine marrow cells acquire an engraftment defect leading to ~three-fold lower engraftment in 160 cGy-conditioned hosts than fresh marrow cells, despite the transduced cell population being enriched for primitive-phenotype marrow cells [1]. More recently, we developed a quantitative submyeloablative competitive repopulation assay and showed that murine marrow cells transduced using two gene-transfer protocols (a standard research protocol and a clinically-applicable approach) engraft ~10-fold less well than freshly-isolated marrow cells in submyeloablated hosts [2]. However, the mechanism(s) responsible for this engraftment defect remains unclear.
The binding of stromal-derived factor-1 (SDF-1) to its receptor CXCR4 has been shown to be important for HSC homing and engraftment in both human xenograft [3, 4] and mouse models [5-8], though the function of this axis may be somewhat different in murine and human cells [5, 8, 9]. Prolonged ex vivo culture, as is needed for gamma-retroviral-mediated gene transfer, has been shown to downregulate CXCR4 levels on HSC [10, 11]. However, ex vivo culture has also been shown to increase the dependence of homing pathways on CXCR4 signaling [5], which may in part explain the decreased engraftment observed with ex vivo expanded HSC. Broxmeyer and colleagues showed that maintenance of SDF-1 function by inhibition of the dipeptidyl peptidase CD26, which cleaves and inactivates SDF-1, leads to enhanced homing and engraftment in ablated murine hosts [12, 13]. Our objective in this study was to determine if impaired homing was a potential mechanism behind the engraftment defect induced by the gamma-retroviral transduction protocol, and to ascertain if inhibition of CD26 using the tripeptide Diprotin A (DipA) could enhance homing and engraftment of fresh and cultured murine marrow cells in submyeloablated hosts.
MATERIALS AND METHODS
Mice
Wild-type C57Bl/6J (Bl/6; CD45.2+) mice were purchased from Jackson Laboratories (Bar Harbor, ME, USA). B6.SJL-PtrcaPep3b/BoyJ (Boy J; CD45.1+) and Bl/6xBoy J F1 (F1; CD45.1+/CD45.2+) mice were obtained from an on-site breeding colony. All mice were maintained under pathogen-free conditions, and were fed autoclaved food and acidified water ad libitum. All protocols were approved by the Institutional Animal Care and Use Committee at Indiana University School of Medicine.
Gamma-retroviral vectors and supernatant production
The construction of gamma-retroviral vectors MFG-EGFP [14] encoding the enhanced green fluorescent protein (GFP) and SFFV-91-LNGFR [15] encoding a truncated human low-affinity nerve growth factor receptor (LNGFR) used in this study have been described previously. For MFG-EGFP, supernatants were generated prior to each transduction experiment by transient plasmid transfection of ecotropic Phoenix packaging cells [16]. For SFFV-91-LNGFR, supernatant was collected from a stable producer cell line. Experiments utilizing ecotropic supernatant were carried out in a biological safety cabinet in a BL-2 laboratory, and transplanted animals were handled under BL-1 guidelines as per approved institutional protocols.
Marrow cell isolation and gamma-retroviral transduction
Gamma-retroviral transduction was performed as previously described [2] as adapted from Li and colleagues [17]. Briefly, lineage depleted marrow was prepared using a mouse Lineage Cell Depletion kit and VarioMACS apparatus (Miltenyi Biotec, Auburn, CA, USA). The lineage-depleted (lin-) cells were prestimulated in serum-free medium (STEMSPAN SF, StemCell Technologies, Vancouver, Canada) supplemented with 100 ng/mL stem cell factor (SCF) and 100 units/mL IL-6 for 48 hours (both from PeproTech, Rocky Hill, NJ, USA), followed by one overnight transduction on gamma-retroviral supernatant-preloaded, RetroNectin (Takara Shuzo, Otsu, Japan)-coated plates.
Analysis of transduced cells
Bulk transduction efficiency was determined immediately after transduction by GFP fluorescence or by staining with anti-LNGFR (Becton-Dickinson (BD)-Pharmingen, San Diego, CA, USA). CD26 and CXCR4 expression was determined using antibodies labeled with fluorescein isothiocyanate or allophycocyanin (BD-Pharmingen). Cells were analyzed using a FACSCalibur instrument and CELLQuest software (BD) as previously described [18]. Because not all the cells which underwent the three-day gene transfer process were transduced, unless otherwise specified only cells expressing a transgene will be referred to as “transduced cells”. The entire donor cell population which underwent culture for gene transfer (which includes transduced and non-transduced cells) will be referred to as “cultured cells”.
DipA treatment of marrow cells
Prior to transplantation for homing and long-term transplant assays, lin- cells were subjected to 15 minutes incubation at room temperature in medium containing 5 mM DipA (Ile-Pro-Ile; Peptides International, Louisville, KY, USA), a CD26 inhibitor previously shown to improve homing and engraftment of treated marrow [13]. Cells were washed once in medium prior to transplantation.
Homing assays
Homing assays were performed by transplanting 106 fresh or cultured lin- marrow cells from Bl/6 or F1 donors into nonirradiated, 300 cGy-, 550 cGy- or 1100 cGy-conditioned (137Cs source, single dose for 300 cGy and 500 cGy, 2 divided doses for 1100 cGy) Boy J hosts by intravenous tail vein injection as previously described [19]. Twenty hours after injection, hosts were sacrificed and marrow was harvested and stained with antibodies against lineage markers (Gr-1, B220 and CD3; phycoerythrin-conjugated), CD45.1-fluorescein isothiocyanate and CD45.2-biotin followed by streptavidin-allophycocyanin (BD-Pharmingen). The lin- population (defined as the ~10% least lin+ cells) was first gated upon, then the percentage of CD45.2+ or CD45.1+/CD45.2+ cells within the lin- fraction were analyzed to determine the fraction of homed donor cells.
Long-term marrow transplants and competitive repopulation experiments
5 × 105-106 fresh or ex vivo cultured lin- cells from Bl/6 donors were transplanted into 300 cGy- or 550 cGy-conditioned Boy J or F1 hosts by tail vein injection. Competitive repopulation assays in 1100 cGy-conditioned hosts were performed as described [2]. Peripheral blood total donor chimerism was determined monthly for 4-6 months post-transplant using antibodies to CD45.1 and CD45.2 [2]. The fraction of donor-derived cells expressing the transgene was determined as described above.
Statistics
All data are represented as the mean ± standard error of the mean (SEM). The data were compared using the unpaired Student's t test, or the Mann-Whitney test if the SEMs were significantly different, using InStat Version 3.05 for Windows (GraphPad Software, San Diego, CA, USA; www.graphpad.com).
RESULTS
Competitive repopulation of cultured and transduced marrow
We previously showed that ex vivo cultur for gamma-retroviral transduction results in decreased HSC engraftment in submyeloablated hosts [1, 18], and developed a novel competitive repopulation assay in submyeloablated hosts to quantify the engraftment defect [2]. Engraftment impairment, however, is not limited to submyeloablated hosts; the long-term repopulating ability of lin- marrow cultured for gamma-retroviral transduction, with or without exposure to gamma-retroviral supernatant, is also ~10-fold lower than that of fresh lin- marrow in ablated hosts (Figure 1).
Figure 1. Ex vivo culture results in markedly impaired long-term repopulating ability in 1100 cGy-conditioned hosts.
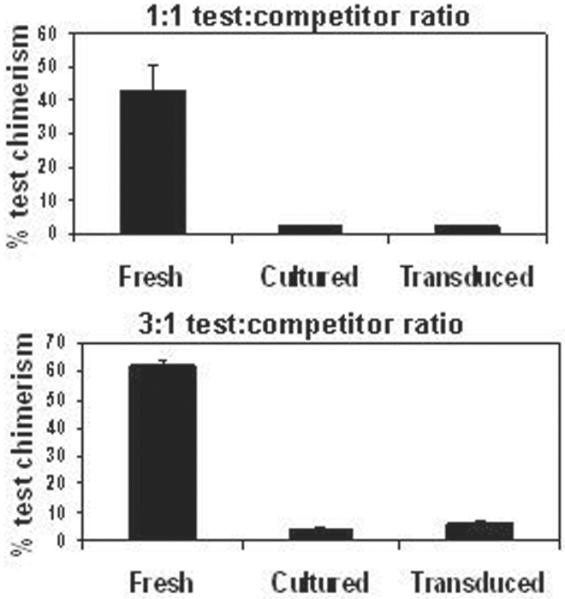
Cultured (i.e., not exposed to gamma-retroviral supernatant) or transduced (i.e., exposed to supernatant) CD45.2+ lin- test cells were mixed with freshly-isolated CD45.1+ lin- competitor cells, and the mixtures were transplanted into 1100 cGy-conditioned Boy J hosts. In the left panel (A), 3 × 105 test cells were mixed with 3 × 105 competitor cells; in the right panel (B), 6 × 105 test cells were mixed with 2 × 105 competitor cells. Test cell chimerism is shown 4-5 months post-transplant from two independent experiments. N = 4 hosts for each parameter. *,P < 0.012 comparing fresh test cell chimerism to that of either cultured or transduced test cells. (Note: A portion of this data was published previously [2]).
Homing efficiency of fresh and cultured marrow ± DipA treatment
Overexpression of CXCR4 on HSC improves homing and engraftment [11, 20]. Moreover, CXCR4 function may be enhanced by the tripeptide DipA, which inhibits CD26 dipeptidase activity on the surface of HSC, thereby increasing local concentrations of SDF-1 and enhancing homing and engraftment [12, 13]. Because studies of engraftment enhancement by DipA to date have used ablated or submyeloablated immunodeficient xenogeneic hosts, we wanted to ascertain if DipA also enhances homing and engraftment in submyeloablated congenic hosts. As an initial step to determine potential mechanisms governing the engraftment defect induced by gamma-retroviral transduction (Fig. 1 and [1, 2, 18]), we determined the homing efficiency of 106 fresh and cultured lin- marrow cells. The effects of DipA treatment on homing of lin- cells in submyeloablated and unconditioned hosts has not been examined. In addition, since homing efficiency may vary with the degree of host conditioning, the homing of fresh lin- cells in unconditioned, 300 cGy-, 550 cGy- and 1100 cGy-conditioned congenic hosts was evaluated with and without treatment with DipA (see homing assay schema, Fig. 2A). Consistent with previously published studies, marrow cells homed more efficiently in unconditioned hosts than in conditioned hosts (Fig. 2B, compare -DipA 0 cGy vs. -DipA 300 cGy, 550 cGy and 1100 cGy). In addition, we now show for the first time that DipA enhances engraftment of fresh lin-cells in congenic hosts conditioned with two submyeloablative regimens, 300 cGy and 550 cGy. However, DipA treatment of fresh lin- cells did not improve homing efficiency in unconditioned hosts (Fig. 2B, compare -DipA 0 cGy vs. +DipA 0 cGy).
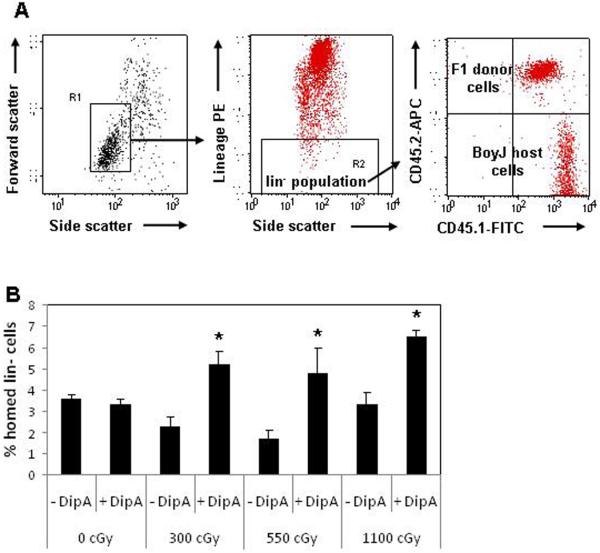
Figure 2. DipA treatment of fresh lin- cells improves homing in submyeloablated hosts.
(A) Dot plots demonstrate the schema used to detect homed F1 donor lin- cells (CD45.1+/CD45.2+) in host Boy J (CD45.1+/CD45.2-) marrow. (B) Fresh F1 lin- cells were isolated, the cell pool was divided, and half of the cells were treated with 5 mM DipA for 15 minutes. Cells were washed and transplanted into BoyJ hosts and analyzed for the fraction of homed cells 20h after transplant. Homing of untreated vs. DipA-treated cells: 0 cGy, 3.3 ± vs. 0.3 vs. 3.6 ± 0.2%; 300 cGy, 2.3 ± 0.4 vs. 5.2 ± 0.6%; 550 cGy, 1.7 ± 0.4 vs. 4.8 ± 1.2%; 1100 cGy, 3.2 ± 0.6 vs. 6.5 ± 0.3%. N = 4-5 hosts/group. *, P ≤ 0.04 comparing DipA-treated to untreated cells for a given radiation dose.
Next, homing in submyeloablated hosts was evaluated using lin- cells from Bl/6 or F1 donors cultured for retroviral transduction using a clinically-relevant gamma-retroviral protocol with vectors expressing GFP or LNGFR as described above. In initial homing experiments in which transduced lin- cells were injected into 300 cGy-conditioned hosts, we could detect only very low (<0.2%) levels of homed cells (data not shown); therefore, subsequent experiments were performed using 550 cGy-conditioned hosts. Homing of cultured lin- marrow was significantly decreased compared to fresh lin- cells (Figure 3A, 6.5 ± 0.8% vs. 1.8 ± 0.2%; P = 0.0006) populations. DipA treatment did not improve homing of cultured lin- cells (1.7 ± 0.2% vs. 1.7 ± 0.1%; Figure 3B). The fraction of cells expressing a transgene was 16.3 ± 3.1% prior to transplantation and 15.2 ± 2.5% after transplantation, indicating that transgene expression did not influence homing of transduced cells.
Figure 3. Cultured lin- cells home less well than fresh in submyeloablated hosts, and DipA does not enhance homing of cultured cells.
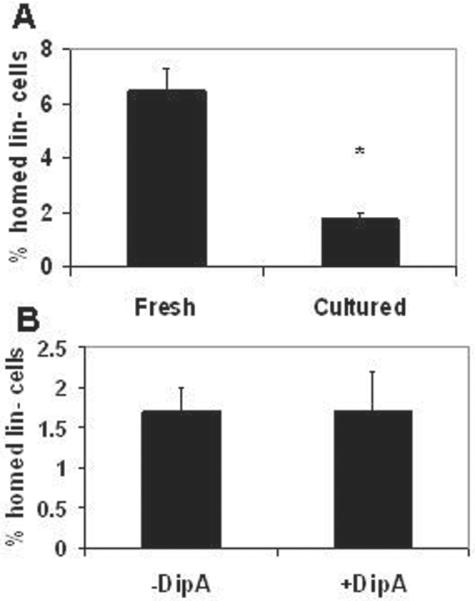
Lin- cells were isolated from Bl/6 (CD45.2+) donors and some cells were cultured for gamma-retroviral transduction as described in the Materials and Methods. (A) 106 fresh or transduced cells were transplanted into 550 cGy-conditioned Boy J (CD45.1+) hosts and analyzed for the fraction of homed CD45.2+ donor cells within the lin- cell populations 20h after transplant. N = 8 hosts per group from 3 independent experiments. *, P = 0.0006.(B) Bl/6 lin- cells were cultured for gamma-retroviral transduction, and half of the cells were treated with DipA before transplantation into 550 cGy-conditioned Boy J hosts for 20h homing analysis. N = 8 hosts per group from 2 independent experiments. The difference between treatment groups was not statistically significant.
Effect of DipA on engraftment in submyeloablated hosts
We previously showed that transplantation of 106 fresh lin- cells into 300 cGy- and 550 cGy-conditioned hosts resulted in donor chimerism of ~30% and ~90%, respectively [2]. Because it would not be feasible to determine if DipA treatment significantly enhanced engraftment in 550 cGy-conditioned hosts due to the very high levels of donor chimerism, we used 300 cGy-conditioned hosts for long-term transplant experiments using fresh lin- cells. We observed higher levels of donor chimerism in 300 cGy-conditioned hosts 4 months after transplantation of DipA-treated compared to untreated fresh lin- cells (40.5 ± 2.3% vs. 16.8 ± 8.8%; P = 0.03; Figure 4A).
Figure 4. DipA treatment significantly improves engraftment of fresh but not cultured lin- cells in submyeloablated hosts.
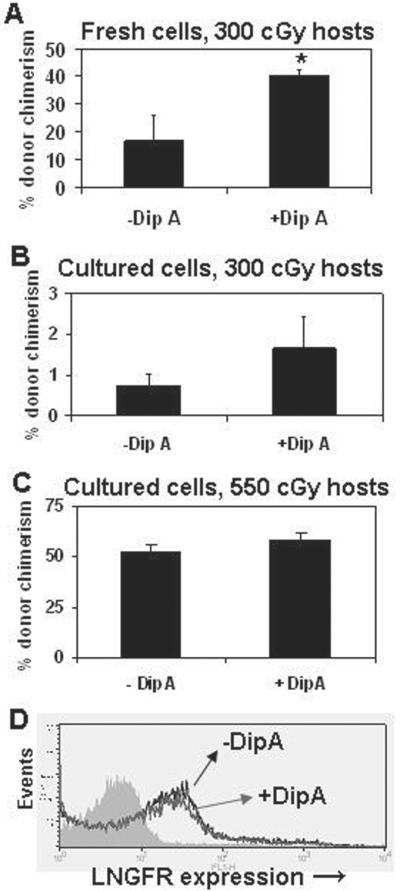
Lin- cells were isolated from Bl/6 donors, and half of the cells were treated with 5 mM DipA for 15 minutes before transplantation of 5 × 105 cells into 300 cGy-conditioned Boy J hosts. Hosts (N = 4) were analyzed for donor chimerism 4 months after transplant. *, P = 0.03. Next, lin- cells were isolated from F1 donors, cultured as for gamma-retroviral transduction, and half of the cells were treated with 5 mM DipA for 15 minutes. Cells were washed and 106 cells were transplanted into either (B) 300 cGy- or (C) 550 cGy-conditioned Bl/6 hosts and analyzed for donor chimerism 4 months after transplant. N = 5 hosts per group in each experiment. Differences between treatment groups were not statistically significant. (D) Expression of LNGFR transgene from 550 cGy-conditioned hosts 4 months post-transplant. The filled histogram is the isotype control, the black line represents LNGFR+ cells from hosts transplanted with lin- cells not treated with DipA, and the gray line represents LNGFR+ cells from hosts transplanted with lin- cells treated with DipA.
We next sought to determine if, despite the lack of improved homing as shown in Fig 3, DipA treatment could improve engraftment of cultured lin- cells, since long-term transplant assays may reveal functional differences not apparent in short-term homing assays. We transplanted 106 DipA-treated or untreated cultured lin- cells into both 300 cGy- and 550 cGy-conditioned congenic hosts: 300 cGy conditioning provides a more stringent test of HSC function and may bring out treatment-related differences which may not be obvious using more intense conditioning [2]. DipA treatment resulted in a small but not statistically significant increase in long-term donor chimerism in 300 cGy-(1.7 ± 0.8% vs. 0.7 ± 0.3%, P = 0.23; Figure 4B) and 550 cGy- (58.4 ± 3.2% vs. 53 ± 2.7%, P = 0.24; Figure 4C) conditioned hosts. We also determined the fraction of gene-marked cells in the 550 cGy-conditioned hosts four months post-transplant. The fraction of engrafted donor cells which expressed LNGFR was similar in pooled blood samples from DipA untreated and treated groups (29% vs. 29.4%; Figure 4D), indicating that engraftment of transduced cells was neither enhanced nor impaired by DipA treatment.
Analysis of CD26 and CXCR4 expression on cultured lin- marrow cells
Multiple groups have demonstrated that expression of adhesion molecules on murine [21-23] and human [10, 11, 24] HSC is modified by ex vivo culture, and that these changes in expression may impact engraftment. Therefore, we examined the expression of CD26 and CXCR4 on the surface of fresh and cultured lin- cells. We found that the three day culture process altered expression of both CD26 and CXCR4 (Figure 5A, B). A subset of transduced cells displayed higher expression of CD26 (Figure 5C), although the mean fluorescence intensity (MFI) was not significantly different (Figure 5E). In contrast, the culture process caused a significant decrease in both the fraction of CXCR4-positive cells (Figure 5D) and the MFI (Figure 5F) upon staining with CXCR4 antibody.
Figure 5. Gamma-retroviral transduction modulates CD26 expression and decreases CXCR4 expression.
CD26 and CXCR4 expression was examined on fresh and transduced lin- cells. (A, B) Histograms showing expression of CD26 and CXCR4, respectively. Filled histograms represent expression on fresh cells; unfilled dark lines represent expression on cultured (cx) cells; unfilled light lines represent isotype (iso) controls. (C, D) Bar graphs showing fraction of cells expressing CD26 and CXCR4, respectively. *, P < 0.0001; †, P = 0.008. (E, F) Bar graphs showing MFI of CD26 and CXCR4, respectively. ‡, P = 0.02. For C-F, filled bars represent fresh cells, unfilled bars represent transduced cells. N = ≥3 independent experiments for all groups.
Homing and engraftment of overnight versus 72h cultured lin- marrow
Finally, we studied the impact of culture time on lin- marrow cell homing and engraftment. Published data suggest that transduction with alternative vectors such as lentiviral vectors, which require only a brief culture period for efficient gene transfer, may lead to higher levels of engraftment [25]. Thus, we performed long-term transplant experiments to directly determine if short (overnight; 12-14h) culture produced higher levels of donor chimerism than the ~72h culture period needed for retroviral transduction. Overnight-cultured lin- cells engrafted significantly better in 550 cGy-conditioned hosts than 72h-cultured cells (Fig. 6A). Given this finding, we examined the homing of overnight-cultured cells with and without DipA treatment in 550 cGy-conditioned hosts (Fig. 6B). Overnight-cultured cells showed intermediate homing efficiency compared to fresh lin- cells (Fig. 2B) and 72h-cultured cells (Fig. 3), but the differences between these groups were not significant. Homing of cells treated with DipA showed a modest but not significant improvement compared to untreated cells (2.6 ± 0.6% vs. 2.3 ± 0.5%, P = 0.68; Fig. 6B). We also examined CXCR4 and CD26 expression on overnight-cultured cells. The fraction of fresh vs. overnight-cultured lin- cells expressing CXCR4 (97.5 ± 1.5% vs. 93 ± 1%) and CD26 (8.8 ± 3.3% vs. 8.8 ± 0.4%; N = 2 independent experiments) was altered less on the overnight-cultured cells than on the 72h-cultured cells (Fig. 5).
Figure 6. Short-term culture leads to improved engraftment in submyeloablated hosts.

Lin- marrow cells were prepared from Bl/6 donors; a portion of the cells were immediately transplanted into 550 cGy-conditioned Boy J hosts (“fresh” group); the rest were cultured overnight prior to transplant (“O/N culture” group). N = 5 hosts each for fresh and cultured cells. Composite donor chimerism data from two independent experiments (N = 14) comprise the 72h culture group. Donor chimerism is shown 4-5 months post-transplant. *, P = 0.022 comparing fresh to O/N cultured lin- cells; †, P < 0.0001 comparing O/N cultured to 72h cultured lin- cells. (B) 20h homing assays of overnight-cultured lin- cells without and with DipA treatment was performed. DipA treatment resulted in a modest but not significant increase in homing efficiency.
DISCUSSION
The CXCR4/SDF-1 axis plays an important role in HSC homing and engraftment [3-8], and has been reviewed extensively elsewhere. In a novel study exploiting this essential molecular relationship, Christopherson et al. [13] showed that increasing local SDF-1 concentrations by inhibiting the dipeptidyl peptidase CD26, which cleaves and inactivates SDF-1, on the surface of HSC with DipA led to ~1.5 to 9-fold increase in homing and engraftment upon transplantation of DipA-treated HSC into ablated mice. Their findings were substantiated in subsequent studies using allogeneic murine marrow [26] and human cord blood [27-29]. The concept that inhibition of CD26 may improve the engraftment of transduced marrow cells was tested by Tian et al. [30]. This group transduced 5-fluorouracil-treated donor marrow with a murine Class I MHC gene and transplanted 5 × 105 DipA treated or untreated cells into ablated hosts. Homing efficiency was not studied, but these authors reported that 12/16 mice receiving DipA-treated cells expressed a mean of ~4% Class I transgene on blood cells, compared to <1% expression in 10 hosts receiving medium-treated cells. All 12 mice with higher levels of Class I antigen expression tolerated allogeneic skin grafts, compared to none of the mice expressing lower levels of antigen.
These data led us to hypothesize that the engraftment of gamma-retrovirus-transduced cells in submyeloablated hosts may also be enhanced by DipA treatment. However, to date the ability of DipA treatment to enhance donor homing and engraftment in submyeloablated hosts has not been reported. This information is important to know because most patients receiving hematopoietic gene therapy will not undergo ablative conditioning [31, 32]. Despite the advantages of reduced toxicity, in submyeloablated hosts a significant fraction of original HSC survive and can repopulate the host, as well as compete with donor cells for marrow niches and engraftment. Furthermore, we previously showed that gamma-retrovirus-transduced HSC acquire an engraftment defect that is apparent upon transplantation into submyeloablated hosts [1, 18] and that transduced cells engraft ~10-fold less well than fresh cells in competitive repopulation assays in submyeloablated hosts [2], and also in ablated hosts (Fig. 1). These data indicate that ex vivo manipulation of marrow cells for gamma-retroviral transduction results in diminished engraftment upon transplantation into mice, regardless of the intensity of the host conditioning, and that the culture process and not the viral transduction itself impairs engraftment.
However, the molecular basis of this engraftment defect is not known. In this study, we examined homing as a potential mechanism underlying the engraftment defect of cultured and/or transduced cells. To provide a context for the work with cultured cells, we demonstrated that DipA-mediated inhibition of CD26 resulted in improved homing and engraftment of fresh donor cells in submyeloablated hosts (Figure 2B). Fresh lin- cells homed more efficiently in unconditioned hosts, as has been observed previously [33-36], but DipA treatment did not enhance homing in unconditioned mice. The reason for this observation remains unclear; we speculate that the CXCR4-SDF-1 gradient is less intense in unconditioned than in conditioned hosts due to the lack of stroma damage, diminishing the effect of the CD26 inhibition. Next, we showed that homing of cultured cells is at least 3-fold less than that of fresh cells in submyeloablated congenic hosts (Figure 3A). However, we did not observe an increase in homing of transduced cells (Figure 3), and found only a minor trend towards improved long-term donor chimerism (Figure 4B, C) with DipA treatment. Thus, we demonstrate that defective homing may be one mechanism by which engraftment of cultured lin- marrow cells is impaired (Fig. 3B), and we extend the results of prior published reports by showing that DipA treatment significantly enhances homing and engraftment of fresh but not gamma-retrovirus-transduced lin- marrow cells in submyeloablated hosts. These data provide definitive evidence that the culture process, and not gamma-retroviral transduction itself, is responsible for impairing homing and engraftment (Figs. 3, 4).
A likely reason for the lack of effect of DipA on the homing and engraftment of cultured cells is that increased competition with residual HSC occurs in submyeloablated hosts, whereas in ablated hosts [30] competition for niches is significantly diminished. It is also possible that the homing defect in transduced cells is more complex than simple perturbation of CXCR4/SDF-1 axis which could not be significantly improved by DipA treatment alone. However, the report by Tian et al. [30] argues against this explanation. We examined expression of CD26 and CXCR4 on transduced cells to determine if the transduction process may alter expression, and therefore function, of these molecules. We found that a greater percentage of transduced cells, compared to fresh cells, expressed CD26 (Figure 5A, C), although the MFI of the entire transduced cell population was not significantly changed (Figure 5E). We also found that CXCR4 expression was markedly decreased on transduced, compared to fresh, lin- cells (Figure 5B, D, F). These data indicate that the transduction protocol used here modulates CD26 expression and decreases CXCR4 expression, either of which may limit any beneficial effect provided by DipA treatment.
Potential means to overcome the homing and engraftment defect induced by gamma-retroviral transduction include different cell manipulations and treatments, such as culture in hypoxic conditions to enhance CXCR4 expression [37], or use of agents like prostaglandin E2 to enhance HSC function [38]. As we noted previously [2], many different cytokine cocktails are successfully used in transduction protocols; alternative cytokine cocktails may have various effects on HSC homing and engraftment. For example, Kassmer et al. [39] recently reported that certain cytokine combinations enhance whereas others inhibit SDF-1-mediated migration of murine HSC. In addition, various cytokines have been recently shown to increase CD26 expression on murine [40] and human [41] HSC, which could again limit the efficacy of DipA treatment. Alternative conditioning regimens may also impact engraftment of transduced HSC. We previously showed that treatment of sublethally-irradiated murine hosts with granulocyte-colony stimulating factor enhances engraftment of fresh and transduced donor cells [19]. Other conditioning regimens, such as low-dose busulfan [32], may produce improved engraftment as well. Finally, transduction with alternative vectors such as lentiviral vectors, which require only a brief ex vivo culture period for efficient gene transfer [25], will likely improve engraftment. We determined that transplantation of lin- cells cultured overnight (as if for lentiviral transduction) results in higher donor chimerism than cells cultured for 3 days as in the gamma-retroviral transduction protocol (Fig. 6A). Overnight-cultured cells homed with an efficiency in between that of fresh and 72h-cultured cells, but DipA treatment did not significantly enhance homing further (Fig. 6B). Moreover, overnight culture modulated CXCR4 and CD26 expression less than 72h culture, which may in part explain the improved homing and engraftment of these cells. These data do not demonstrate a direct correlation between homing efficiency and engraftment in submyeloablated hosts; nonetheless, the engraftment defect caused by the prolonged ex vivo culture for gamma-retroviral transduction may be best overcome using vectors which require only brief ex vivo culture.
In summary, we showed that gamma-retroviral transduction markedly impairs homing of murine lin- marrow in submyeloablated hosts. Since inhibition of CD26 activity with DipA has been shown to enhance donor marrow cell homing and engraftment in ablated hosts, we asked if CD26 inhibition could similarly enhance homing and engraftment in submyeloablated hosts. We demonstrated for the first time that DipA treatment could increase the engraftment of fresh but not cultured marrow cells in submyeloablated, but not unconditioned, hosts. We showed that the transduction protocol used downregulates CXCR4 expression and modulates CD26 expression, which may diminish any positive effects of DipA treatment, but that the impact of culture is tempered in overnight- vs. 72h-cultured cells. These data demonstrate that ex vivo culture for viral transduction modulates expression of key surface antigens important for homing, and provides an explanation as to why DipA did not significantly improve homing of transduced lin- cells.
ACKNOWLEDGMENTS
This work was supported by National Institutes of Health grant K08 HL75253 (to WSG); a Hope Street Kids grant (to KEP); and the Riley Children's Foundation. No financial interest/relationships with financial interest relating to the topic of this article have been declared.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- [1].Goebel WS, Yoder MC, Pech N, Dinauer MC. Donor chimerism and stem cell function in a murine congenic transplantation model following low dose radiation conditioning: Effects of a retroviral-mediated gene transfer protocol and implications for gene therapy. Exp Hematol. 2002;30:1324–1332. doi: 10.1016/s0301-472x(02)00927-x. [DOI] [PubMed] [Google Scholar]
- [2].Wyss BK, Meyers JL, Sinn AL, Cai S, Pollok KE, Goebel WS. A novel competitive repopulation strategy to quantitate engraftmen of ex vivo manipulated murine marrow cells in submyeloablated hosts. Exp Hematol. 2008;36:513–521. doi: 10.1016/j.exphem.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Peled A, Petit I, Kollet O, et al. Dependence of human stem cell engraftment and repopulation of NOD/SCID mice on CXCR4. Science. 1999;283:845–848. doi: 10.1126/science.283.5403.845. [DOI] [PubMed] [Google Scholar]
- [4].Kollet O, Spiegel A, Peled A, et al. Rapid and efficient homing of human CD34+CD38-/lowCXCR4+ stem and progenitor cells to the bone marrow and spleen of NOD/SCID and NOD/SCID/B2mnull mice. Blood. 2001;97:3283–3291. doi: 10.1182/blood.v97.10.3283. [DOI] [PubMed] [Google Scholar]
- [5].Bonig H, Priestley GV, Papayannopoulou T. Hierarchy of molecular-pathway usage in bone marrow homing and its shift by cytokines. Blood. 2006;107:79–86. doi: 10.1182/blood-2005-05-2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Broxmeyer HE, Hangoc G, Cooper S, Campbell T, Ito S, Mantel C. AMD3100 and CD26 modulate mobilization, engraftment, and survival of hematopoietic stem and progenitor cells mediated by the SDF-1/CXCL12-CXCR4 axis. Annals of the New York Academy of Sciences. 2007;1106:1–19. doi: 10.1196/annals.1392.013. [DOI] [PubMed] [Google Scholar]
- [7].Campbell TB, Broxmeyer HE. CD26 inhibition and hematopoiesis: a novel approach to enhance transplantation. Front Biosci. 2008;13:1795–1805. doi: 10.2741/2800. [DOI] [PubMed] [Google Scholar]
- [8].Nie Y, Han YC, Zou YR. CXCR4 is required for the quiescence of primitive hematopoietic cells. J Exp Med. 2008;205:777–783. doi: 10.1084/jem.20072513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Foudi A, Jarrier P, Zhang Y, et al. Reduced retention of radioprotective hematopoietic cells within the bone marrow microenvironment in CXCR4-/- chimeric mice. Blood. 2006;107:2243–2251. doi: 10.1182/blood-2005-02-0581. [DOI] [PubMed] [Google Scholar]
- [10].Denning-Kendall P, Singha S, Bradley B, Hows J. Cytokine expansion culture of cord blood CD34+ cells induces marked and sustained changes in adhesion receptor and CXCR4 expressions. Stem Cells. 2003;21:61–70. doi: 10.1634/stemcells.21-1-61. [DOI] [PubMed] [Google Scholar]
- [11].Brenner S, Whiting-Theobald N, Kawai T, et al. CXCR4-transgene expression significantly improves marrow engraftment of cultured hematopoietic stem cells. Stem Cells. 2004;22:1128–1133. doi: 10.1634/stemcells.2003-0196. [DOI] [PubMed] [Google Scholar]
- [12].Christopherson KW, 2nd, Hangoc G, Broxmeyer HE. Cell surface peptidase CD26/dipeptidylpeptidase IV regulates CXCL12/stromal cell-derived factor-1 alpha-mediated chemotaxis of human cord blood CD34+ progenitor cells. J Immunol. 2002;169:7000–7008. doi: 10.4049/jimmunol.169.12.7000. [DOI] [PubMed] [Google Scholar]
- [13].Christopherson KW, 2nd, Hangoc G, Mantel CR, Broxmeyer HE. Modulation of hematopoietic stem cell homing and engraftment by CD26. Science. 2004;305:1000–1003. doi: 10.1126/science.1097071. [DOI] [PubMed] [Google Scholar]
- [14].Pollok KE, van Der Loo JC, Cooper RJ, et al. Differential transduction efficiency of SCID-repopulating cells derived from umbilical cord blood and granulocyte colony-stimulating factor-mobilized peripheral blood. Hum Gene Ther. 2001;12:2095–2108. doi: 10.1089/10430340152677430. [DOI] [PubMed] [Google Scholar]
- [15].Sadat MA, Pech N, Saulnier S, et al. Long-Term High-Level Reconstitution of NADPH Oxidase Activity in Murine X-Linked Chronic Granulomatous Disease Using a Bicistronic Vector Expressing gp91phox and a DeltaLNGFR Cell Surface Marker. Hum Gene Ther. 2003;14:651–666. doi: 10.1089/104303403321618164. [DOI] [PubMed] [Google Scholar]
- [16].Wahlers A, Schwieger M, Li Z, et al. Influence of multiplicity of infection and protein stability on retroviral vector-mediated gene expression in hematopoietic cells. Gene Ther. 2001;8:477–486. doi: 10.1038/sj.gt.3301426. [DOI] [PubMed] [Google Scholar]
- [17].Li Z, Schwieger M, Lange C, et al. Predictable and efficient retroviral gene transfer into murine bone marrow repopulating cells using a defined vector dose. Exp Hematol. 2003;31:1206–1214. doi: 10.1016/j.exphem.2003.08.008. [DOI] [PubMed] [Google Scholar]
- [18].Goebel WS, Pech N, Meyers JL, Srour EF, Yoder MC, Dinauer MC. A murine model of antimetabolite-based, submyeloablative conditioning for bone marrow transplantation: biologic insights and potential applications. Exp Hematol. 2004;32:1255–1264. doi: 10.1016/j.exphem.2004.08.007. [DOI] [PubMed] [Google Scholar]
- [19].Barese C, Pech N, Dirscherl S, et al. Granulocyte colony-stimulating factor prior to nonmyeloablative irradiation decreases murine host hematopoietic stem cell function and increases engraftment of donor marrow cell. Stem Cells. 2007;25:1578–1585. doi: 10.1634/stemcells.2006-0808. [DOI] [PubMed] [Google Scholar]
- [20].Kahn J, Byk T, Jansson-Sjostrand L, et al. Overexpression of CXCR4 on human CD34+ progenitors increases their proliferation, migration, and NOD/SCID repopulation. Blood. 2004;103:2942–2949. doi: 10.1182/blood-2003-07-2607. [DOI] [PubMed] [Google Scholar]
- [21].Peters SO, Kittler EL, Ramshaw HS, Quesenberry PJ. Ex vivo expansion of murine marrow cells with interleukin-3 (IL-3), IL-6, IL-11, and stem cell factor leads to impaired engraftment in irradiated hosts. Blood. 1996;87:30–37. [PubMed] [Google Scholar]
- [22].Becker PS, Nilsson SK, Li Z, et al. Adhesion receptor expression by hematopoietic cell lines and murine progenitors: modulation by cytokines and cell cycle status. Exp Hematol. 1999;27:533–541. doi: 10.1016/s0301-472x(98)00037-x. [DOI] [PubMed] [Google Scholar]
- [23].Orschell-Traycoff CM, Hiatt K, Dagher RN, Rice S, Yoder MC, Srour EF. Homing and engraftment potential of Sca-1+lin- cells fractionated on the basis of adhesion molecule expression and position in cell cycle. Blood. 2000;96:1380–1387. [PubMed] [Google Scholar]
- [24].Reems JA, Mielcarek M, Torok-Storb B. Differential modulation of adhesion markers with ex vivo expansion of human umbilical CD34+ progenitor cells. Biol Blood Marrow Transplant. 1997;3:133–141. [PubMed] [Google Scholar]
- [25].Mostoslavsky G, Kotton DN, Fabian AJ, Gray JT, Lee JS, Mulligan RC. Efficiency of transduction of highly purified murine hematopoietic stem cells by lentiviral and oncoretroviral vectors under conditions of minimal in vitro manipulation. Mol Ther. 2005;11:932–940. doi: 10.1016/j.ymthe.2005.01.005. [DOI] [PubMed] [Google Scholar]
- [26].Peranteau WH, Endo M, Adibe OO, Merchant A, Zoltick PW, Flake AW. CD26 inhibition enhances allogeneic donor-cell homing and engraftment after in utero hematopoietic-cell transplantation. Blood. 2006;108:4268–4274. doi: 10.1182/blood-2006-04-018986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Christopherson KW, 2nd, Paganessi LA, Napier S, Porecha NK. CD26 inhibition on CD34+ or lineage- human umbilical cord blood donor hematopoietic stem cells/hematopoietic progenitor cells improves long-term engraftment into NOD/SCID/Beta2null immunodeficient mice. Stem Cells Dev. 2007;16:355–360. doi: 10.1089/scd.2007.9996. [DOI] [PubMed] [Google Scholar]
- [28].Campbell TB, Hangoc G, Liu Y, Pollok K, Broxmeyer HE. Inhibition of CD26 in human cord blood CD34+ cells enhances their engraftment of nonobese diabetic/severe combined immunodeficiency mice. Stem Cells Dev. 2007;16:347–354. doi: 10.1089/scd.2007.9995. [DOI] [PubMed] [Google Scholar]
- [29].Kawai T, Choi U, Liu PC, Whiting-Theobald NL, Linton GF, Malech HL. Diprotin A infusion into nonobese diabetic/severe combined immunodeficiency mice markedly enhances engraftment of human mobilized CD34+ peripheral blood cells. Stem Cells Dev. 2007;16:361–370. doi: 10.1089/scd.2007.9997. [DOI] [PubMed] [Google Scholar]
- [30].Tian C, Bagley J, Forman D, Iacomini J. Inhibition of CD26 peptidase activity significantly improves engraftment of retrovirally transduced hematopoietic progenitors. Gene Ther. 2006;13:652–658. doi: 10.1038/sj.gt.3302695. [DOI] [PubMed] [Google Scholar]
- [31].Cavazzana-Calvo M, Hacein-Bey S, de Saint Basile G, et al. Gene therapy of human severe combined immunodeficiency (SCID)-X1 disease. Science. 2000;288:669–672. doi: 10.1126/science.288.5466.669. [DOI] [PubMed] [Google Scholar]
- [32].Ott MG, Schmidt M, Schwarzwaelder K, et al. Correction of X-linked chronic granulomatous disease by gene therapy, augmented by insertional activation of MDS1-EVI1, PRDM16 or SETBP1. Nat Med. 2006;12:401–409. doi: 10.1038/nm1393. [DOI] [PubMed] [Google Scholar]
- [33].Hendrikx PJ, Martens CM, Hagenbeek A, Keij JF, Visser JW. Homing of fluorescently labeled murine hematopoietic stem cells. Exp Hematol. 1996;24:129–140. [PubMed] [Google Scholar]
- [34].Cui J, Wahl RL, Shen T, et al. Bone marrow cell trafficking following intravenous administration. Brit J Haematol. 1999;107:895–902. doi: 10.1046/j.1365-2141.1999.01779.x. [DOI] [PubMed] [Google Scholar]
- [35].Plett PA, Frankovitz SM, Orschell-Traycoff CM. In vivo trafficking, cell cycle activity and engraftment poential of phenotypically-defined primitive hematopoietic cells after transplantation into irradiated or non-irradiated recipients. Blood. 2002;100:3545–3552. doi: 10.1182/blood.V100.10.3545. [DOI] [PubMed] [Google Scholar]
- [36].Collis SJ, Neutzel S, Thompson TL, et al. Hematopoietic progenitor stem cell homing in mice lethally irradiated with ionizing radiation at differing dose rates. Radiation Res. 2004;162:48–55. doi: 10.1667/rr3197. [DOI] [PubMed] [Google Scholar]
- [37].Skinner AM, O'Neill SL, Grompe M, Kurre P. CXCR4 induction in hematopoietic progenitor cells from Fanca-/-, -c-/-, and -d2-/- mice. Exp Hematol. 2008;36:273–282. doi: 10.1016/j.exphem.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].North TE, Goessling W, Walkley CR, et al. Prostaglandin E2 regulates vertebrate haematopoietic stem cell homeostasis. Nature. 2007;447:1007–1011. doi: 10.1038/nature05883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Kassmer SH, Niggemann B, Punzel M, Mieck C, Zanker KS, Dittmar T. Cytokine combinations differentially influence the SDF-1alpha-dependent migratory activity of cultivated murine hematopoietic stem and progenitor cells. Biol Chem. 2008 doi: 10.1515/BC.2008.099. [DOI] [PubMed] [Google Scholar]
- [40].Fukuda S, Bian H, King AG, Pelus LM. The chemokine GRObeta mobilizes early hematopoietic stem cells characterized by enhanced homing and engraftment. Blood. 2007;110:860–869. doi: 10.1182/blood-2006-06-031401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Christopherson KW, 2nd, Uralil SE, Porecha NK, Zabriskie RC, Kidd SM, Ramin SM. G-CSF- and GM-CSF-induced upregulation of CD26 peptidase downregulates the functional chemotactic response of CD34+CECD38- human cord blood hematopoietic cells. Exp Hematol. 2006;34:1060–1068. doi: 10.1016/j.exphem.2006.03.012. [DOI] [PubMed] [Google Scholar]