Abstract
Transdermal drug delivery has made an important contribution to medical practice, but has yet to fully achieve its potential as an alternative to oral delivery and hypodermic injections. First-generation transdermal delivery systems have continued their steady increase in clinical use for delivery of small, lipophilic, low-dose drugs. Second-generation delivery systems using chemical enhancers, non-cavitational ultrasound and iontophoresis have also resulted in clinical products; the ability of iontophoresis to control delivery rates in real time provides added functionality. Third-generation delivery systems target their effects to skin’s barrier layer of stratum corneum using microneedles, thermal ablation, microdermabrasion, electroporation and cavitational ultrasound. Microneedles and thermal ablation are currently progressing through clinical trials for delivery of macromolecules and vaccines, such as insulin, parathyroid hormone and influenza vaccine. Using these novel second- and third-generation enhancement strategies, transdermal delivery is poised to significantly increase impact on medicine.
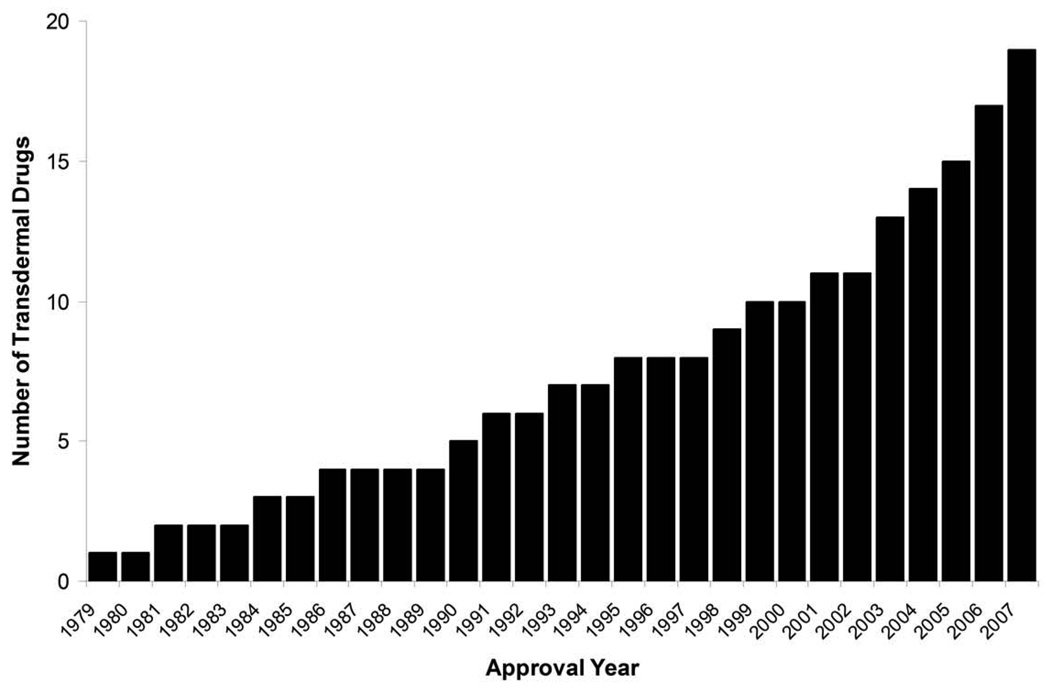
Transdermal delivery represents an attractive alternative to oral delivery of drugs and is poised to provide an alternative to hypodermic injection too1–4. For thousands of years, people have placed substances on the skin for therapeutic effects and, in the modern era, a variety of topical formulations have been developed to treat local indications. The first transdermal system for systemic delivery—a three-day patch that delivers scopolamine to treat motion sickness—was approved for use in the United States in 1979. A decade later, nicotine patches became the first transdermal blockbuster, raising the profile of transdermal delivery in medicine and for the public in general. Today, there are 19 transdermal delivery systems for such drugs as estradiol, fentanyl, lidocaine and testosterone; combination patches containing more than one drug for contraception and hormone replacement; and iontophoretic and ultrasonic delivery systems for analgesia (Table 1, Fig. 1). Between 1979 and 2002, a new patch was approved on average every 2.2 years. Over the past 5 years (2003–2007), that rate has more than tripled to a new transdermal delivery system every 7.5 months. It is estimated that more than one billion transdermal patches are currently manufactured each year.
Table 1.
Transdermal drugs approved by the US FDA.*
| Approval year | Drug | Indication | Product Name | Marketing company |
|---|---|---|---|---|
| 1979 | Scopolamine | Motion sickness | Transderm-Scop | Novartis Consumer Health (Parsippany, NJ) |
| 1981 | Nitroglycerin | Angina pectoris | Transderm-Nitro | Novartis (East Hannover, NJ) |
| 1984 | Clonidine | Hypertension | Catapres-TTS | Boehringer Ingelheim (Ridgefield, CT) |
| 1986 | Estradiol | Menopausal symptoms | Estraderm | Novartis (East Hannover, NJ) |
| 1990 | Fentanyl | Chronic pain | Duragesic | Janssen Pharmaceutica (Titusville, NJ) |
| 1991 | nicotine | Smoking cessation | Nicoderm, Habitrol, ProStep | GlaxoSmithKline (Philadelphia, PA), Novartis Consumer Health (Parsippany, NJ) Elan (Gainesville, GA) |
| 1993 | Testosterone | Testosterone deficiency | Testoderm | Alza, Mountain View, CA |
| 1995 | Lidocaine/epinephrine (iontophoresis) | Local dermal analgesia | Iontocaine | Iomed (Salt Lake City, UT) |
| 1998 | Estradiol/norethidrone | Menopausal symptoms | Combipatch | Novartis (East Hannover, NJ) |
| 1999 | Lidocaine | Post-herpetic neuralgia pain | Lidoderm | Endo Pharmaceuticals (Chadds Ford, PA) |
| 2001 | Ethinyl estradiol/norelgestromin | Contraception | Ortho Evra | Ortho-McNeil Pharmaceutical (Raritan, NJ) |
| 2003 | Estradiol/levonorgestrel | Menopausal symptoms | Climara Pro | Bayer Healthcare Pharmaceuticals (Wayne, NJ) |
| 2003 | Oxybutynin | Overactive bladder | Oxytrol | Watson Pharma (Corona, CA) |
| 2004 | Lidocaine (ultrasound) | Local dermal anesthesia | SonoPrep | Echo Therapeutics (Franklin, MA) |
| 2005 | Lidocaine/tetracaine | Local dermal analgesia | Synera | Endo Pharmaceuticals (Chadds Ford, PA) |
| 2006 | Fentanyl HCl (iontophoresis) | Acute postoperative pain | Ionsys | Alza, Mountain View, CA |
| 2006 | Methylphenidate | Attention deficit hyperactivity disorder | Daytrana | Shire (Wayne, PA) |
| 2006 | Selegiline | Major depressive disorder | Emsam | Bristol-Myers Squibb (Princeton, NJ) |
| 2007 | Rotigotine | Parkinson’s disease | Neupro | Schwarz Pharma (Mequon, WI) |
| 2007 | Rivastigmine | Dementia | Exelon | Novartis (East Hannover, NJ) |
This list includes transdermal patches and delivery systems approved by the FDA. Only the first approved product for a given drug or drug combination administered by a given delivery method is shown. Topical creams, ointments, gels and sprays are not included.
Figure 1.
Cumulative number of transdermal drugs approved by the FDA since the first approval in 1979. There are currently 19 drugs and drug combinations administered by various delivery methods that are approved in the United States (see Table 1). Data were obtained from the FDA Orange Book63.
Transdermal delivery has a variety of advantages compared with the oral route. In particular, it is used when there is a significant first-pass effect of the liver that can prematurely metabolize drugs. Transdermal delivery also has advantages over hypodermic injections, which are painful, generate dangerous medical waste and pose the risk of disease transmission by needle re-use, especially in developing countries5. In addition, transdermal systems are non-invasive and can be self-administered. They can provide release for long periods of time (up to one week). They also improve patient compliance and the systems are generally inexpensive.
Perhaps the greatest challenge for transdermal delivery is that only a limited number of drugs are amenable to administration by this route. With current delivery methods, successful transdermal drugs have molecular masses that are only up to a few hundred Daltons, exhibit octanol-water partition coefficients that heavily favor lipids and require doses of milligrams per day or less1–4. It has been difficult to exploit the transdermal route to deliver hydrophilic drugs; the transdermal deliver of peptides and macromolecules, including new genetic treatment employing DNA or small-interfering RNA (siRNA)6, has posed particular challenges.
Another area of great interest is the delivery of vaccines7. In addition to avoiding hypodermic needles, transdermal vaccine delivery could improve immune responses by targeting delivery to immunogenic Langerhans cells in the skin (Box 1). Given the external placement and patient control over patches, it might also be possible to develop modulated or pulsatile delivery, which could involve feedback control. Indeed, an analgesic patch was recently approved in the United States that uses patient-regulated delivery of fentanyl modulated by electricity to control pain (iontophoresis)8, which has also been launched in Europe.
Box 1.
Rising interest in transdermal vaccines
Transdermal delivery offers compelling opportunities to improve vaccine administration. Although vaccines are typically macromolecules, viral particles, or other large supramolecular constructs, their small (microgram) doses facilitate the possibility of transdermal delivery. Vaccine delivery via the skin is even more attractive because it targets the potent epidermal Langerhans and dermal dendritic cells that may generate a strong immune response at much lower doses than deeper injection7. The most successful vaccine of all time—the smallpox vaccine, which eradicated the disease worldwide—was administered via the skin with the aid of a small needle device to breach the stratum corneum barrier. Although effective, this approach does not provide good control over delivery, which has motivated development of new delivery methods.
Elimination of the need for hypodermic needles further motivates transdermal vaccine development61. In a world where needle reuse kills at least 1.3 million people per year from hepatitis B and AIDS5, needle-free, patch-based vaccination could have large impact. In addition, the possibility of administering vaccine patches by minimally trained personnel or patients themselves could not only facilitate compliance with routine, seasonal and pandemic vaccination needs, but could also expedite vaccination campaigns in developing countries where medical personnel are in short supply. Effective vaccination via the skin may be achieved by increasing skin permeability to the vaccine using the methods discussed in this review. Some of the physical enhancement methods have been shown to have additional adjuvant effects that increase immune response further39, 41. The immune response can also be heightened by adding chemical adjuvants7.
Excitement about this approach is exemplified by completion of phase 3 clinical trails and submission for registration in Europe by Sanofi Pasteur (Paris) and Becton Dickinson (Franklin Lakes, NJ, USA) for their microneedle-based influenza vaccine (Table 2); major investments in Iomai for their transdermal vaccine patch portfolio; and a growing number of academic and industry laboratories engaging in this field of research.
Finally, there is the possibility of not only delivering drugs, but also extracting molecules (analytes) through the skin9. This has already been achieved for glucose monitoring by extracting interstitial fluid using electrical means and is in clinical trials using other approaches, such as ultrasound.
From a global perspective, we propose that advances in transdermal delivery systems can be categorized as undergoing three generations of development from the first generation of systems that produced many of today’s patches by judicious selection of drugs that can cross the skin at therapeutic rates with little or no enhancement; through the second generation that has yielded additional advances for small molecule delivery by increasing skin permeability and driving forces for transdermal transport; to the third generation that will enable transdermal delivery of small molecule drugs, macromolecules (including proteins and DNA) and virus-based/other vaccines through targeted permeabilization of the skin’s stratum corneum.
In this review, we describe the transdermal delivery methods in each generation. We then comment on their current and future potential impact in medicine.
First-generation transdermal delivery systems
The first generation of transdermal delivery systems is responsible for most of the transdermal patches that have thus far been in clinical use. Significant advances in patch technology, and public acceptance, have enabled the recent surge in first-generation transdermal patches reaching the market (Box 2). However, this surge will taper off as drugs with suitable properties for such systems are depleted. First-generation delivery candidates must be low-molecular weight, lipophilic and efficacious at low doses. Usually, their transdermal delivery should be more attractive than oral delivery due to low oral bioavailability, the need or desire for less frequent dosing or steady delivery profiles, or other factors.
Box 2.
Transdermal patch design
In almost all transdermal patch designs, the drug is stored in a reservoir that is enclosed on one side with an impermeable backing and has an adhesive that contacts the skin on the other side62. Some designs employ drug dissolved in a liquid or gel-based reservoir, which can simplify formulations and permit the use of liquid chemical enhancers, such as ethanol. These designs characteristically are composed of four layers: an impermeable backing membrane; a drug reservoir; a semi-permeable membrane that may serve as a rate-limiting barrier; and an adhesive layer. Other designs incorporate the drug into a solid polymer matrix, which simplifies manufacturing. Matrix systems can have three layers, by eliminating the semi-permeable membrane, or just two layers, by incorporating the drug directly into the adhesive.
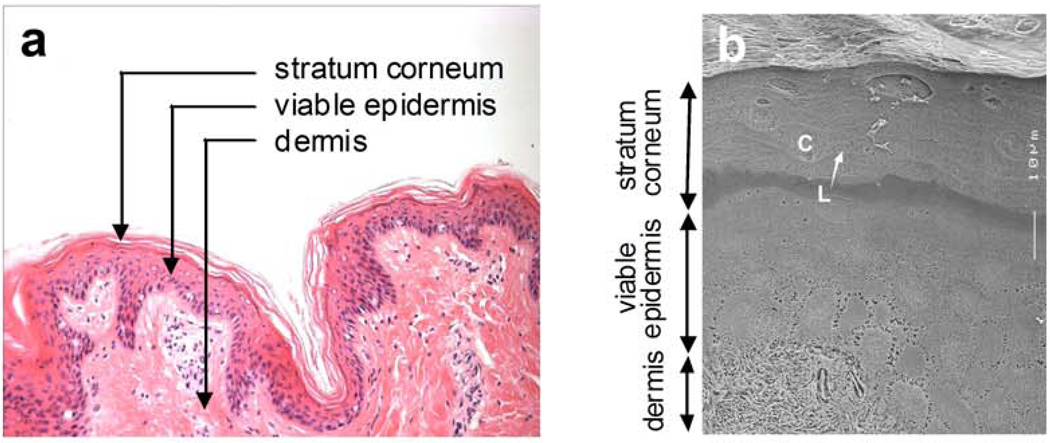
The first-generation approach to transdermal delivery is limited primarily by the barrier posed by skin’s outermost layer called stratum corneum, which is 10 to 20 µm thick (Fig. 2). Underneath this layer is the viable epidermis, which measures 50 to 100 µm and is avascular. Deeper still is the dermis, which is 1–2 mm thick and contains a rich capillary bed for systemic drug absorption just below the dermal–epidermal junction. Closer examination of the stratum corneum barrier reveals a brick and mortar structure, where the bricks represent non-living corneocyte cells composed primarily of cross-linked keratin and the intercellular mortar is a mixture of lipids organized largely in bilayers. Drug transport across the stratum corneum typically involves diffusion through the intercellular lipids via a path that winds tortuously around corneocytes, where hydrophilic molecules travel through the lipid head group regions and lipophilic molecules travel through the lipid tails. This transport pathway is highly constrained by the structural and solubility requirements for solution and diffusion within stratum corneum lipid bilayers.
Figure 2.
Histological structure of mammalian skin. (a) Skin structure. The skin’s outermost layer is the stratum corneum, which is a nonviable tissue that provides most of skin’s barrier properties. The viable epidermis is an epithelial layer that serves to continuously renew the stratum corneum, among other functions. The dermis is a largely fibrous layer that provides skin’s mechanical support, as well as the skin’s vasculature and anchoring of sweat gland and hair follicle appendages. (Image of H&E stained porcine skin provided courtesy of Samantha Andrews, Georgia Institute of Technology). (b) Stratum corneum structure. Drug penetration across the stratum corneum is limited primarily by the lipids organized in bilayer structures (L) that fill the intercellular spaces between corneocytes (C). (Cryo-scanning electron micrograph provided courtesy of Joke Bouwstra, Leiden University and reproduced with permission from ref. 64).
A variation on the traditional transdermal patch of first-generation delivery systems involves no patch at all, but applies a metered liquid spray, gel or other topical formulation to the skin that, upon evaporation or absorption, can drive small lipophilic drugs into the stratum corneum, which in turn serves as the drug reservoir for extended release into the viable epidermis over hours10. For example, testosterone gels have been in use for several years and a transdermal spray has been recently approved for estradiol delivery.
Second-generation transdermal delivery systems
The second generation of transdermal delivery systems recognizes that skin permeability enhancement is needed to expand the scope of transdermal drugs. The ideal enhancer should (i) increase skin permeability by reversibly disrupting stratum corneum structure, (ii) provide an added driving force for transport into the skin and (iii) avoid injury to deeper, living tissues. However, enhancement methods developed in this generation, such as conventional chemical enhancers, iontophoresis and non-cavitational ultrasound, have struggled with the balance between achieving increased delivery across stratum corneum, while protecting deeper tissues from damage. As a result, this second generation of delivery systems has advanced clinical practice primarily by improving small molecule delivery for localized, dermatological, cosmetic and some systemic applications, but has made little impact on delivery of macromolecules1–4.
Conventional chemical enhancers
Recognizing the need to increase skin permeability, second-generation delivery strategies have turned largely to the development of chemical enhancers11,12. This approach is a logical extension of the traditional pharmaceutical toolbox because it primarily involves designing new formulations with chemical excipients. Many effective chemical enhancers disrupt the highly ordered bilayer structures of the intracellular lipids found in stratum corneum by inserting amphiphilic molecules into these bilayers to disorganize molecular packing or by extracting lipids using solvents and surfactants to create lipid packing defects of nanometer dimensions. Hundreds of different chemical enhancers have been studied, including off-the-shelf compounds and others specifically designed and synthesized for this purpose, such as Azone (1-dodecylazacycloheptan-2-one) and SEPA (2-n-nonyl-1,3dioxolane) (Table 2).
Table 2.
Representative transdermal drugs in clinical development
| Drug | Company | Indication | Clinical phase | Delivery technology |
|---|---|---|---|---|
| AB-1001 | Abeille | Nausea and vomiting | Phase 3 | Passive |
| acyclovir | Transport | Herpes labialis | Phase 2 | Iontophoresis |
| buprenorphine | Purdue Pharma | Pain | Phase 3* | Passive |
| fertility hormone | Vyteris / Ferring | Female infertility | Phase 1 | Iontophoresis |
| granisetron | Prostrakan | Nausea and vomiting | Pre-registration | Passive |
| heat-labile enterotoxin of E. coli. | Iomai | Travelers’ diarrhea | Phase 2 | Skin abrasion |
| human growth hormone | TransPharma / Teva | Growth hormone deficiency | Phase 1 | Thermal ablation |
| influenza vaccine | Becton Dickinson / Sanofi-Pasteur | Influenza prophylaxis | Pre-registration | Microneedles |
| insulin | Altea | Diabetes mellitus | Phase 1 | Thermal ablation |
| insulin | Phosphagenics | Diabetes mellitus | Phase 2 | Vesicular carrier |
| ketoprofen | ZARS | Osteoarthritis | Phase 3 | Heat enhancement |
| parathyroid hormone (1–34) | Zosano | Osteoporosis | Phase 2 | Microneedles |
| sufentanil | Durect / Endo | Chronic pain | Phase 2 | Passive |
| testosterone | Acrux / VIVUS | Female sexual dysfunction | Phase 2 | Metered dose transdermal spray |
| testosterone | MacroChem | Male hypogonadism | Phase 2 | Chemical enhancer (SEPA) |
| testosterone | Procter & Gamble / Watson | Hypoactive sexual desire disorder | Pre-registration* | Passive |
| triamcinolone acetonide | Echo Therapeutics | Dermatoses | Pre-registration | Chemical enhancer (AzoneTS) |
Already marketed outside of USA
One challenge of this approach is that increased permeation enhancement, even of small molecules, typically correlates with increased skin irritation. A small subset of these enhancers that increase skin permeability without irritation have been used successfully to deliver small molecules, but have had limited impact on the problem of delivering hydrophilic compounds or macromolecules. Overall, chemical enhancers can increase skin permeability and provide an added driving force for transport by increasing drug partitioning into the skin (thereby increasing the concentration gradient driving diffusion), but the difficulty of localizing their effects to the stratum corneum so as to avoid irritation or toxicity to living cells in the deeper skin has severely constrained their application.
Mitragotri and colleagues13 have suggested guidelines to design chemical enhancers that may increase skin permeability without causing irritation. Using Fourier transform infrared (FTIR) spectroscopy as a screening tool, they proposed that effective and non-irritating enhancers should alter stratum corneum lipid CH2 symmetric stretching (which correlates with increased skin permeability) and avoid changes in stratum corneum protein amide I band absorption (which correlates with skin irritation). These design principles predicted that optimal chemical structures for enhancing drug delivery would be amphiphiles with long, saturated carbon tails or compounds with multiple aromatic rings; the authors went on to validate their predictions experimentally.
Liposomes, dendrimers and microemulsions have also been used as chemical enhancers with supramolecular structure that can not only increase skin permeability, but also increase drug solubilization in the formulation and drug partitioning into the skin14,15. Their supramolecular size generally precludes penetration into the skin and thereby helps localize effects to the stratum corneum. These approaches have found success for enhanced delivery of some small molecules, especially for topical dermatological and cosmetic applications. A highly deformable liposome formulation is currently in clinical trials for insulin delivery (Table 2).
Another transdermal delivery approach that has been applied is the use of prodrugs. Through the addition of a cleavable chemical groups that typically increases drug lipophilicity16, such prodrugs can facilitate the transfer of a drug across the skin. This is accomplished by adding, for example, alkyl side chains with enzymatically cleavable linkers, such as esters or carbonates. One prodrug approach relies on the linkage of either two of the same or two different small molecule drugs to each other by a labile bond, which reduces their hydrophilicity, albeit at the expense of increasing molecular weight17.
Because the prodrug approach is based on altering drug structure, as opposed to skin structure, prodrugs can avoid skin irritation. Even so, advancement of this field has been limited by the complexity of prodrug design, the applicability of the approach only to small molecule drugs and the need to gain US Food and Drug Administration (FDA) approval of the prodrug as a new chemical entity (rather than approval only of the transdermal delivery route for an already approved drug).
Iontophoresis
Iontophoresis has been studied for moto increase transdermal delivery for more than a century by typically applying a continuous low-voltage current18, 19. While there can be increased skin permeability, iontophoresis mainly provides an electrical driving force for transport across stratum corneum. Charged drugs are moved via electrophoresis, while weakly charged and uncharged compounds can be moved by electroosmotic flow of water generated by the preferential movement of mobile cations (e.g., Na+) instead of fixed anions (e.g., keratin) in the stratum corneum20. Because iontophoresis does not primarily change the skin barrier itself, it is mostly applicable to small molecules that carry a charge and some macromolecules up to a few thousand Daltons.
The strongest asset of iontophoresis is that the rate of drug delivery scales with the electrical current, which can be readily controlled by a microprocessor or, in some cases, the patient21. In this way, drug delivery can be turned on and off and even modulated over time to enable complex delivery profiles. However, the maximum current—and therefore the maximum delivery rate—is limited by skin irritation and pain caused by the general inability of iontophoresis to localize its effects to the stratum corneum (Prausnitz22).
Guided by these strengths and weaknesses, current applications emphasize the ability of iontophoresis to provide control over drug dosing, because it scales with the amount of charge (i.e., the product of current and time) delivered to the skin18,19. Iontophoresis is currently used clinically to rapidly deliver lidocaine for local anesthesia23, pilocarpine to induce sweating as part of a cystic fibrosis diagnostic test24 and tap water to treat hyperhydrosis (i.e., excessive sweating)25, as well as extract glucose from the skin for glucose monitoring26. A recently approved iontophoretic patch enables patients to periodically activate the patch to administer a bolus of fentanyl based on their need for pain relief8 (Table 1). In contrast to this costly, microprocessor-controlled system, another recently approved iontophoretic patch involves simply connecting the drug reservoir to a constant-voltage, printed battery that can also have some simple control circuitry and delivers drug until the battery runs out27. Although the drug delivery rate is not as well controlled using this low-cost alternative, the total amount of drug administered is controlled, because the total amount of charge transferred across the skin is limited by the battery capacity. An additional alternative that seeks to achieve a balance between low cost and microprocessor control of delivery involves a single-use iontophoretic system in clinical trials for delivery of acyclovir to treat herpes labialis28 (Table 2).
Non-cavitational ultrasound
Ultrasound was first widely recognized as a skin permeation enhancer when physical therapists discovered that massaging anti-inflammatory agents into the skin using ultrasonic heating probes increased efficacy29,30. Ultrasound is an oscillating pressure wave at a frequency too high for humans to hear. Although some have hypothesized that the pressure gradients and oscillation associated with ultrasound act as a driving force to move drugs into the skin, it appears that the dominant effect is to disrupt stratum corneum lipid structure and thereby increase permeability. The effects of non-cavitational ultrasound on skin permeability have generally been limited to enhancing small, lipophilic compounds. Using more aggressive non-cavitational ultrasound conditions has been limited by associated tissue heating that is not targeted to the stratum corneum and can damage deeper tissue. Under different conditions, ultrasound can also be used to generate cavitational bubble activity, which has different effects and is discussed below.
Third-generation transdermal delivery systems
The third generation of transdermal delivery systems is poised to make significant impact on drug delivery because it targets its effects to the stratum corneum. This targeting enables stronger disruption of the stratum corneum barrier, and thereby more effective transdermal delivery, while still protecting deeper tissues. In this way, novel chemical enhancers, electroporation, cavitational ultrasound and more recently microneedles, thermal ablation and microdermabrasion (Arora, Prausnitz and Mitragotri31) have been shown to deliver macromolecules, including therapeutic proteins and vaccines, across the skin in human clinical trials. These advances were made possible in part by the emergence of technologies to localize effects to the stratum corneum combined with recognition that the safety afforded by localization should make these more aggressive approaches medically acceptable.
Combinations of chemical enhancers
Recent studies have suggested that suitably designed combinations of chemical enhancers can balance trade-offs between enhancement and irritation based on the hypothesis that certain enhancer combinations are especially potent when present at specific, narrow compositions. This approach enables a strategy to target effects that enhance skin permeability in the stratum corneum, but avoids irritation in deeper tissues where the formulation composition becomes diluted or otherwise altered.
Finding such rare combinations is experimentally intensive and therefore benefits from high-throughput screening. Such a study was carried out, examining close to 500 different pairs of chemical enhancers formulated to have more than 5000 compositions32. Dramatically increased enhancement with low skin irritation potential was found, for example, for a combination of sodium laureth sulfate (an anionic surfactant) and phenyl piperazine (a compound with aromatic nitrogen) at concentrations of 0.35 and 0.15 wt%, respectively, in a 1:1 mixture of ethanol and phosphate-buffered saline. In vitro screening results were validated with in vivo delivery of a peptide (leuprolide acetate) to hairless rats. These results suggest that combinations of chemical enhancers may succeed for delivery of macromolecules where individual enhancers have generally failed. Work on this approach continues in industry33.
Biochemical enhancers
Recently, peptides have been examined as enhancers of skin permeability. In one approach, phage display was used to screen a library of peptides, which yielded an 11-amino acid synthetic peptide that increased transdermal delivery of insulin in diabetic rats34. Additional analysis suggested that a pathway via hair follicles was targeted. Work in one of our laboratories (Kim, Ludovice & Prausnitz35) has shown that a natural pore-forming peptide, magainin, can be used to increase skin permeability by a mechanism proposed to target bilayer disruption in stratum corneum lipids and not in deeper tissue35. The magainin was only effective when used in synergistic combination with a surfactant chemical enhancer, which served the dual purpose of increasing skin permeability to the drug as well as increasing penetration of magainin into the stratum corneum. Using a prodrug approach, cyclosporine was covalently attached to a polyarginine-heptamer cell-penetrating peptide, which led to increased topical absorption that inhibited cutaneous inflammation36. In these examples, the highly specific bioactivity enabled by peptide chemistry can enable delivery via targeted routes through the skin.
Electroporation
The use of short, high-voltage pulses is well known as a method to reversibly disrupt cell membranes for gene transfection and other applications. Electroporation has also been shown to disrupt lipid bilayer structures in the skin37,38. Although the electric field applied for milliseconds during electroporation provides an electrophoretic driving force, diffusion through long-lived electropores can persist for up to hours, such that transdermal transport can be increased by orders of magnitude for small model drugs, peptides, vaccines and DNA. Recently, electroporation was shown to deliver a model peptide vaccine into the skin of mice to generate a strong cytotoxic T lymphocyte response39.
Because the stratum corneum electrical resistance is orders of magnitude greater than deeper tissues, the electric field applied during electroporation is initially concentrated in the stratum corneum. However, upon electroporation of stratum corneum lipid bilayers, stratum corneum resistance rapidly and dramatically drops, and the electric field correspondingly distributes to a greater extent into the deeper tissues, which contain sensory and motor neurons. The associated pain and muscle stimulation can be avoided by using closely spaced microelectrodes that constrain the electric field within the stratum corneum40. Although electroporation has been studied extensively in animals, this approach to transdermal delivery has received limited attention in humans thus far due largely to the complexity of device design.
Cavitational ultrasound
In addition to heating, ultrasound is also known to generate cavitation, which is the formation, oscillation and, in some cases, collapse of bubbles in an ultrasonic pressure field. Cavitation is only generated under specific conditions (e.g., low-frequency ultrasound) that differ from those of ultrasonic heating or imaging devices. The opportunity for transdermal drug delivery is that cavitation bubbles concentrate the energy of ultrasound and thereby enable targeted effects at the site of bubble activity30,41. Because bubbles are more difficult to grow and oscillate within densely-packed tissue, cavitation preferentially occurs within the coupling medium (e.g., a hydrogel) between the ultrasound transducer and skin. The expected mechanism of cavitational ultrasound is that bubbles oscillate and collapse at the skin surface, which generates localized shock waves and liquid microjets directed at the stratum corneum42. This disrupts stratum corneum lipid structure and thereby increases skin permeability for up to many hours without damaging deeper tissues. Cavitational ultrasound is not believed to contribute a significant driving force for transport.
Already, cavitational ultrasound has been approved for enhanced delivery of lidocaine through the skin43 and has been studied extensively in animals for delivery of insulin, as well as heparin, tetanus toxoid vaccine and other compounds41. Ultrasound can be applied using hand-held devices, as well as low-profile, cymbal transducers that could be integrated into a patch44. Ultrasound has also been used to extract interstitial fluid glucose for diabetes monitoring45 and to increase skin permeability to small molecules, as well as macromolecules up to tens of kilodaltons. Lasers have similarly been used to increase skin permeability by a related shock-wave mechanism46.
Microneedles
A conceptually straightforward way to selectively permeabilize the stratum corneum is to pierce it with very short needles. Over the past decade, microneedles have been developed as a means to deliver drugs into the skin in a minimally invasive manner47,48. Solid microneedles have been shown to painlessly pierce the skin to increase skin permeability to a variety of small molecules, proteins and nanoparticles from an extended-release patch. Alternatively, drug formulations have been coated on or encapsulated within microneedles for rapid or controlled release of peptides and vaccines in the skin. Hollow microneedles have been used to deliver insulin and vaccines by infusion.
In general, microneedles (i) increase skin permeability by creating micron-scale pathways into the skin, (ii) can actively drive drugs into the skin either as coated or encapsulated cargo introduced during microneedle insertion or via convective flow through hollow microneedles and (iii) target their effects to the stratum corneum, although microneedles typically pierce across the epidermis and into the superficial dermis too (Prausnitz, Gill & Park48).
Several recent advances in microneedle design and formulation are worthy of note. Original fabrication methods involving clean room-based sculpting of silicon-based structures have moved to low-cost manufacturing methods to make microneedles out of metals and polymers commonly found in FDA-approved devices and parenteral formulations. Microneedles have been dip coated with a variety of compounds, including small molecules, proteins, DNA, and virus particles (Gill & Prausnitz49). Microneedles have also been made of water-soluble polymers that encapsulate various compounds within the needle matrix (Lee, Park & Prausnitz50). These microneedles dissolve in the skin over a timescale of minutes and thereby leave no sharp medical waste after use.
Advances have also been made in delivery to humans using microneedles. In a recent study, naltrexone was administered to healthy volunteers whose skin was pretreated with microneedles51. After applying a naltrexone patch, blood levels of naltrexone reached the therapeutic range. Transdermal delivery without microneedle pretreatment yielded naltrexone levels below detection. Microneedle treatment was reported to be painless by the volunteers and was generally well tolerated. Other unpublished human studies have demonstrated delivery of parathyroid hormone from coated microneedles, which have advanced from animal studies through clinical trials (Table 2).
Vaccine delivery using microneedles has also been a focus for research. Animal studies have demonstrated delivery of live attenuated virus, inactivated virus, protein sub-unit, and DNA vaccines against influenza, hepatitis B, Japanese encephalitis and anthrax using solid and hollow microneedles (Prausnitz, Mikszta, Cormier & Andrianov 52). Human studies have demonstrated the reliability of hollow microneedles to achieve intradermal injection without special training53. Administration of influenza vaccine using these microneedles has recently progressed through completion of clinical trials and filing for registration in Europe (Table 2).
Thermal ablation
Thermal ablation selectively heats the skin surface to generate micron-scale perforations in the stratum corneum. Transiently heating the skin’s surface to hundreds of degrees for microseconds to milliseconds localizes heat transfer to the skin surface without allowing heat to propagate to the viable tissues below54,55. This spares these tissues from damage or pain. Mechanistically, thermal ablation may involve rapidly vaporizing water in the stratum corneum, such that the resulting volumetric expansion ablates micron-scale craters in the skin’s surface. More recent studies suggest that temperatures well above the boiling point of water are needed and that other processes, such as tissue combustion, may be at play (Park, Lee, Kim & Prausnitz56).
Animal studies have demonstrated the ability of thermal ablation to deliver a number of different compounds, such as human growth hormone and interferon α-2b (refs. 55,57). Skin heating has been achieved using ohmic microheaters and radio-frequency ablation. The microscopic length scales of localized skin disruption caused by thermal ablation have resulted in the procedure being well tolerated. Unpublished studies report clinical trials for delivery of a number of drugs, including human growth hormone and insulin (Table 2).
Microdermabrasion
A final way to remove the stratum corneum barrier employs abrasion by microdermabrasion or simply using sandpaper. Microdermabrasion is a widely used method to alter and remove skin tissue for cosmetic purposes. This abrasive mechanism, which is related to sand blasting on the microscopic scale, has been shown to increase skin permeability to drugs, including lidocaine and 5-fluorouracil, which suggests possible applications in transdermal drug delivery58. Vaccine delivery across the skin has also been facilitated by skin abrasion using sandpaper59. Initial studies in animals generated strong immune responses to several vaccines when administered topically in combination with a potent adjuvant (i.e., heat-labile enterotoxin of Escherichia coli). More recently, human trials have addressed vaccination against traveler’s diarrhea and influenza (Table 2)
Comparison of transdermal delivery systems
In addition to more than 100 drugs formulated as creams and ointments, there are now 19 drugs or drug combinations administered using FDA-approved transdermal delivery systems (Fig. 1). Most of these first-generation delivery systems rely primarily on appropriate drug properties that permit absorption into the skin without significant skin permeation enhancement. However, advances in the field through second- and third-generation transdermal delivery systems are opening the door to transdermal administration of hydrophilic molecules, macromolecules and vaccines (Table 2).
Most enhancement approaches increase skin permeability without providing an added driving force for transdermal transport. Chemical enhancers are an exception, because they can disrupt stratum corneum structure as well as increase drug solubility and thereby increase the drug concentration-gradient driving force. Microneedles are another exception, because they not only pierce the skin, but can carry drug into the skin via coating and encapsulation using solid microneedles or infusion through hollow needles. Although electrical methods of delivery can affect skin permeability as well as provide an electrical driving force, iontophoresis acts primarily to drive drugs into the skin and electroporation acts largely to disrupt stratum corneum structure. Because iontophoresis provides a transport driving force, it may be especially useful when coupled with another method that increases skin permeability. Such combined enhancement strategies have received previous attention in the literature60.
Successful transdermal delivery is based on achieving a suitable balance between effective delivery and safety to the skin. Some of the third-generation systems rely on the hypothesis that relatively large, micron-scale defects in the stratum corneum should be well tolerated by patients as long as significant damage is not done to living cells in the viable epidermis and dermis. Reports to date suggest that this hypothesis is reasonable, based on data from a growing collection of clinical trials that have advanced through phase 1 safety trials and into phase 2 and 3 studies of efficacy, especially using microneedles and thermal ablation (Table 2). This may not be surprising, given that the skin reliably repairs itself without scarring or infection after being routinely subjected to microscopic defects caused by scrapes, scratches, shaving, hypodermic injection, and other minor mechanical trauma.
Clinical impact relies not only on a transdermal delivery system that administers drugs in a safe and effective manner, but one that is also low-cost and easy to use, given that most transdermal delivery systems are designed for self-administration at home. The various chemical enhancers can be integrated into small, inexpensive patches that patients find convenient. The various physical enhancers may be more effective to deliver macromolecules and vaccines, but are generally driven by hand-held devices that require electrical power. As a result, most physical enhancers rely on relatively costly, re-usable devices that interface with a disposable drug reservoir component. Microneedles are an exception, because they can deliver macromolecules and vaccines, should be inexpensive to manufacture as single-use patches, and do not require a power supply. However, microneedles are also unique in that they are physically invasive, which raises additional safety and sterility considerations.
Future outlook and conclusions
Looking to the future, it is likely that first-generation patch technology will continue to be used for delivery of small molecule drugs with the right set of properties, especially those drugs that are currently administered orally and by injection that are coming off patent. Second-generation chemical enhancers should find continued use as formulation excipients in topical dermatological creams and ointments and some systemic patches for small molecule drugs. They will probably have little impact on delivery of hydrophilic drugs and macromolecules, because the most effective chemical enhancers generally diffuse out of the stratum corneum and irritate deeper tissue. Targeted, third-generation combinations of chemical enhancers and biochemical approaches offer strategies for more targeted enhancement, but are still in early stages of development.
Second-generation physical enhancement using iontophoresis has already made clinical impact, especially for rapid, localized delivery to the skin. Its electronic control over delivery rates gives iontophoresis a special property that can be exploited for patient-controlled dosing and other complex delivery profiles. However, because iontophoresis does not substantially change the skin barrier, it appears unlikely to impact macromolecule or vaccine delivery, unless used in combination with other methods that increase skin permeability. Likewise, non-cavitational ultrasound has found use for transdermal delivery of anti-inflammatories in the context of physical therapy, but does not appear suitable for delivery of large compounds.
Third-generation physical enhancement using cavitational ultrasound and electroporation enhance transdermal delivery by disrupting stratum corneum on the nanometer scale. Cavitational ultrasound has already been approved for transdermal delivery of lidocaine and may be approved in the future for peptides and other small macromolecules. Although effective, applications of cavitational ultrasound may be limited by the need for a sophisticated device that only increases skin permeability at the nanometer scale and thereby may not be broadly applicable to macromolecules and vaccines.
Skin can be disrupted on the micron scale by third-generation physical enhancement using microneedles, thermal ablation and microdermabrasion. These methods have special promise, because they appear broadly capable of delivering not only small molecules, but macromolecules and vaccines as well. Unpublished clinical trails appear to yield promising results, and published data suggest that these methods can be safe and effective. A microneedle product for vaccine delivery has been submitted in Europe for regulatory approval and other microneedle and thermal ablation products are proceeding through advanced clinical trials.
A limitation of diffusing large compounds through micron-scale disruptions is that diffusivity is a strong inverse function of molecular size. Thus, even though, for example, an inactivated virus particle vaccine can easily fit though a micron-sized hole, it may take a long time to diffuse through. When rapid delivery is desirable, it may be preferable to use microneedles that actively drive macromolecules and drugs into the skin or to combine micron-scale disruption with an added driving force, such as iontophoresis.
Overall, transdermal drug delivery offers compelling opportunities to address the low bioavailability of many oral drugs; the pain and inconvenience of injections; and the limited controlled release options of both. Building off the successes of first-generation transdermal patches, second-generation chemical enhancers and iontophoresis are expanding delivery capabilities for small molecules, whereas third-generation physical enhancers (including ultrasound, thermal ablation and microneedles) could enable transdermal delivery of macromolecules and vaccines. These scientific and technological advances that enable targeted disruption of stratum corneum while protecting deeper tissues have brought the field to a new level of capabilities that position transdermal drug delivery for increasingly widespread impact on medicine.
Acknowledgements
We thank Daniel Bucks, Gary Cleary, Robert Gale, Samir Mitragotri and Audra Stinchcomb for helpful discussions. M.R.P. is the Emerson-Lewis Faculty Fellow and works at the Center for Drug Design, Development and Delivery and the Institute for Bioengineering and Bioscience at Georgia Tech. This work was supported in part by the National Institutes of Health.
References
- 1.Guy RH, Hadgraft J, editors. New York: Marcel Dekker; 2003. Transdermal Drug Delivery. [Google Scholar]
- 2.Williams A. London: Pharmaceutical Press; 2003. Transdermal and Topical Drug Delivery. [Google Scholar]
- 3.Prausnitz MR, Mitragotri S, Langer R. Current status and future potential of transdermal drug delivery. Nat Rev Drug Discov. 2004;3:115–124. doi: 10.1038/nrd1304. [DOI] [PubMed] [Google Scholar]
- 4.Bronaugh RL, Maibach HI, editors. Edn. 4th. New York: Marcel Dekker; 2005. Percutaneous Absorption. [Google Scholar]
- 5.Miller MA, Pisani E. The cost of unsafe injections. Bull World Health Organ. 1999;77:808–811. [PMC free article] [PubMed] [Google Scholar]
- 6.Foldvari M, Babiuk S, Badea I. DNA delivery for vaccination and therapeutics through the skin. Curr Drug Deliv. 2006;3:17–28. doi: 10.2174/156720106775197493. [DOI] [PubMed] [Google Scholar]
- 7.Glenn GM, Kenney RT. Mass vaccination: solutions in the skin. Curr Top Microbiol Immunol. 2006;304:247–268. doi: 10.1007/3-540-36583-4_14. [DOI] [PubMed] [Google Scholar]
- 8.Mayes S, Ferrone M. Fentanyl HCl patient-controlled iontophoretic transdermal system for the management of acute postoperative pain. Ann Pharmacother. 2006;40:2178–2186. doi: 10.1345/aph.1H135. [DOI] [PubMed] [Google Scholar]
- 9.Sieg A, Guy RH, Delgado-Charro MB. Noninvasive and minimally invasive methods for transdermal glucose monitoring. Diabetes Technol Ther. 2005;7:174–197. doi: 10.1089/dia.2005.7.174. [DOI] [PubMed] [Google Scholar]
- 10.Morgan TM, Reed BL, Finnin BC. Enhanced skin permeation of sex hormones with novel topical spray vehicles. J Pharm Sci. 1998;87:1213–1218. doi: 10.1021/js980025k. [DOI] [PubMed] [Google Scholar]
- 11.Williams AC, Barry BW. Penetration enhancers. Adv Drug Deliv Rev. 2004;56:603–618. doi: 10.1016/j.addr.2003.10.025. [DOI] [PubMed] [Google Scholar]
- 12.Smith EW, Maibach HI, editors. Boca Raton, FL: Taylor and Francis Group; 2006. Percutaneous Penetration Enhancers. [Google Scholar]
- 13.Karande P, Jain A, Ergun K, Kispersky V, Mitragotri S. Design principles of chemical penetration enhancers for transdermal drug delivery. Proc Natl Acad Sci U S A. 2005;102:4688–4693. doi: 10.1073/pnas.0501176102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kogan A, Garti N. Microemulsions as transdermal drug delivery vehicles. Adv Colloid Interface Sci. 2006;123–126:369–385. doi: 10.1016/j.cis.2006.05.014. [DOI] [PubMed] [Google Scholar]
- 15.Touitou E, Godin B. Enhancement in Drug Delivery. In: Touitou E, Barry B, editors. Boca Raton, FL: CRC Press; 2007. pp. 255–278. [Google Scholar]
- 16.Sloan KB, Wasdo SC, Rautio J. Design for optimized topical delivery: Prodrugs and a paradigm change. Pharm Res. 2006;23:2729–2747. doi: 10.1007/s11095-006-9108-0. [DOI] [PubMed] [Google Scholar]
- 17.Kiptoo PK, Hamad MO, Crooks PA, Stinchcomb AL. Enhancement of transdermal delivery of 6-beta-naltrexol via a codrug linked to hydroxybupropion. J Control Release. 2006;113:137–145. doi: 10.1016/j.jconrel.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 18.Banga AK. London: Taylor & Francis; 1998. Electrically-Assisted Transdermal and Topical Drug Delivery. [Google Scholar]
- 19.Kalia YN, Naik A, Garrison J, Guy RH. Iontophoretic drug delivery. Adv Drug Deliv Rev. 2004;56:619–658. doi: 10.1016/j.addr.2003.10.026. [DOI] [PubMed] [Google Scholar]
- 20.Pikal MJ. The role of electroosmotic flow in transdermal iontophoresis. Adv Drug Deliv Rev. 2001;46:281–305. doi: 10.1016/s0169-409x(00)00138-1. [DOI] [PubMed] [Google Scholar]
- 21.Subramony JA, Sharma A, Phipps JB. Microprocessor controlled transdermal drug delivery. Int J Pharm. 2006;317:1–6. doi: 10.1016/j.ijpharm.2006.03.053. [DOI] [PubMed] [Google Scholar]
- 22.Prausnitz MR. The effects of electric current applied to the skin: a review for transdermal drug delivery. Rev. 1996;18:395–425. [Google Scholar]
- 23.Zempsky WT, Sullivan J, Paulson DM, Hoath SB. Evaluation of a low-dose lidocaine iontophoresis system for topical anesthesia in adults and children: a randomized, controlled trial. Clin Ther. 2004;26:1110–1119. doi: 10.1016/s0149-2918(04)90183-x. [DOI] [PubMed] [Google Scholar]
- 24.Beauchamp M, Lands LC. Sweat-testing: a review of current technical requirements. Pediatr Pulmonol. 2005;39:507–511. doi: 10.1002/ppul.20226. [DOI] [PubMed] [Google Scholar]
- 25.Kreyden OP. Iontophoresis for palmoplantar hyperhidrosis. J Cosmet Dermatol. 2004;3:211–214. doi: 10.1111/j.1473-2130.2004.00126.x. [DOI] [PubMed] [Google Scholar]
- 26.Tamada J, et al. Noninvasive glucose monitoring: comprehensive clinical results. Cygnus Research Team. JAMA. 1999;282:1839–1844. doi: 10.1001/jama.282.19.1839. [DOI] [PubMed] [Google Scholar]
- 27.Chaturvedula A, et al. Dermal, subdermal, and systemic concentrations of granisetron by iontophoretic delivery. Pharm Res. 2005;22:1313–1319. doi: 10.1007/s11095-005-5335-z. [DOI] [PubMed] [Google Scholar]
- 28.Morrel EM, Spruance SL, Goldberg DI. Topical iontophoretic administration of acyclovir for the episodic treatment of herpes labialis: a randomized, double-blind, placebocontrolled, clinic-initiated trial. Clin Infect Dis. 2006;43:460–467. doi: 10.1086/505872. [DOI] [PubMed] [Google Scholar]
- 29.Machet L, Boucaud A. Phonophoresis: efficiency, mechanisms and skin tolerance. Int J Pharm. 2002;243:1–15. doi: 10.1016/s0378-5173(02)00299-5. [DOI] [PubMed] [Google Scholar]
- 30.Wu J, Nyborg W, editors. London: Imperial College Press; 2006. Emerging Therapeutic Ultrasound. [Google Scholar]
- 31.Arora A, Prausnitz MR, Mitragotri S. Micro-scale devices for transdermal drug delivery. Int J Pharm. 2008 doi: 10.1016/j.ijpharm.2008.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Karande P, Jain A, Mitragotri S. Discovery of transdermal penetration enhancers by high-throughput screening. Nat Biotechnol. 2004;22:192–197. doi: 10.1038/nbt928. [DOI] [PubMed] [Google Scholar]
- 33.Kling J, DeFrancesco DeFrancesco, L. The paper trial to commercialization: High-throughput path to acquisition. Nat Biotechnol. 2007;25:1217–1223. doi: 10.1038/nbt1107-1217a. [DOI] [PubMed] [Google Scholar]
- 34.Chen Y, et al. Transdermal protein delivery by a coadministered peptide identified via phage display. Nat Biotechnol. 2006;24:455–460. doi: 10.1038/nbt1193. [DOI] [PubMed] [Google Scholar]
- 35.Kim YC, Ludovice PJ, Prausnitz MR. Transdermal delivery enhanced by magainin pore-forming peptide. J Control Release. 2007;122:375–383. doi: 10.1016/j.jconrel.2007.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rothbard JB, et al. Conjugation of arginine oligomers to cyclosporin A facilitates topical delivery and inhibition of inflammation. Nat Med. 2000;6:1253–1257. doi: 10.1038/81359. [DOI] [PubMed] [Google Scholar]
- 37.Denet AR, Vanbever R, Preat V. Skin electroporation for transdermal and topical delivery. Adv Drug Deliv Rev. 2004;56:659–674. doi: 10.1016/j.addr.2003.10.027. [DOI] [PubMed] [Google Scholar]
- 38.Li S, editor. Totowa, NJ: Humana Press; 2008. Electroporation Protocols: Preclinical and Clinical Gene Medicine. [Google Scholar]
- 39.Zhao YL, et al. Induction of cytotoxic T-lymphocytes by electroporation-enhanced needle-free skin immunization. Vaccine. 2006;24:1282–1290. doi: 10.1016/j.vaccine.2005.09.035. [DOI] [PubMed] [Google Scholar]
- 40.Pliquett U, Weaver JC. Feasibility of an electrode-reservoir device for transdermal drug delivery by noninvasive skin electroporation. IEEE Trans Biomed Eng. 2007;54:536–538. doi: 10.1109/TBME.2006.886828. [DOI] [PubMed] [Google Scholar]
- 41.Ogura M, Paliwal S, Mitragotri S. Low-frequency sonophoresis: Current status and future prospects. Adv Drug Deliv Rev. 2008 doi: 10.1016/j.addr.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 42.Paliwal S, Menon GK, Mitragotri S. Low-frequency sonophoresis: ultrastructural basis for stratum corneum permeability assessed using quantum dots. J Invest Dermatol. 2006;126:1095–1101. doi: 10.1038/sj.jid.5700248. [DOI] [PubMed] [Google Scholar]
- 43.Becker BM, et al. Ultrasound with topical anesthetic rapidly decreases pain of intravenous cannulation. Acad Emerg Med. 2005;12:289–295. doi: 10.1197/j.aem.2004.11.019. [DOI] [PubMed] [Google Scholar]
- 44.Park EJ, Werner J, Smith NB. Ultrasound mediated transdermal insulin delivery in pigs using a lightweight transducer. Pharm Res. 2007;24:1396–1401. doi: 10.1007/s11095-007-9306-4. [DOI] [PubMed] [Google Scholar]
- 45.Chuang H, Taylor E, Davison TW. Clinical evaluation of a continuous minimally invasive glucose flux sensor placed over ultrasonically permeated skin. Diabetes Technol Ther. 2004;6:21–30. doi: 10.1089/152091504322783378. [DOI] [PubMed] [Google Scholar]
- 46.Doukas AG, Kollias N. Transdermal drug delivery with a pressure wave. Adv Drug Deliv Rev. 2004;56:559–579. doi: 10.1016/j.addr.2003.10.031. [DOI] [PubMed] [Google Scholar]
- 47.Sivamani RK, Liepmann D, Maibach HI. Microneedles and transdermal applications. Expert Opin Drug Deliv. 2007;4:19–25. doi: 10.1517/17425247.4.1.19. [DOI] [PubMed] [Google Scholar]
- 48.Prausnitz MR, Gill HS, Park JH. Modified Release Drug Delivery. In: Rathbone MJ, Hadgraft J, Roberts MS, Lane ME, editors. New York: Informa Healthcare; 2008. [Google Scholar]
- 49.Gill HS, Prausnitz MR. Coated microneedles for transdermal delivery. J Control Release. 2007;117:227–237. doi: 10.1016/j.jconrel.2006.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lee JW, Park JH, Prausnitz MR. Dissolving microneedles for transdermal drug delivery. Biomaterials. 2008;29:2113–2124. doi: 10.1016/j.biomaterials.2007.12.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wermeling DP, et al. Microneedles permit transdermal delivery of a skin-impermeant medication to humans. Proc Natl Acad Sci U S A. 2008;105:2058–2063. doi: 10.1073/pnas.0710355105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Prausnitz MR, Mikszta JA, Cormier M, Andrianov AK. Microneedle-based vaccines. Curr Top Microbiol Immunol. doi: 10.1007/978-3-540-92165-3_18. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Laurent PE, et al. Evaluation of the clinical performance of a new intradermal vaccine administration technique and associated delivery system. Vaccine. 2007;25:8833–8842. doi: 10.1016/j.vaccine.2007.10.020. [DOI] [PubMed] [Google Scholar]
- 54.Bramson J, et al. Enabling topical immunization via microporation: a novel method for pain-free and needle-free delivery of adenovirus-based vaccines. Gene Ther. 2003;10:251–260. doi: 10.1038/sj.gt.3301886. [DOI] [PubMed] [Google Scholar]
- 55.Levin G, et al. Transdermal delivery of human growth hormone through RF-microchannels. Pharm Res. 2005;22:550–555. doi: 10.1007/s11095-005-2498-6. [DOI] [PubMed] [Google Scholar]
- 56.Park JH, Lee JW, Kim YC, Prausnitz MR. The effect of heat on skin permeability. Int J Pharm. 2008;359:94–103. doi: 10.1016/j.ijpharm.2008.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Badkar AV, Smith AM, Eppstein JA, Banga AK. Transdermal delivery of interferon alpha-2B using microporation and iontophoresis in hairless rats. Pharm Res. 2007;24:1389–1395. doi: 10.1007/s11095-007-9308-2. [DOI] [PubMed] [Google Scholar]
- 58.Herndon TO, Gonzalez S, Gowrishankar T, Anderson RR, Weaver JC. Transdermal microconduits by microscission for drug delivery and sample acquisition. BMC Med. 2004;2:12. doi: 10.1186/1741-7015-2-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Glenn GM, et al. Transcutaneous immunization with heat-labile enterotoxin: development of a needle-free vaccine patch. Expert Rev Vaccines. 2007;6:809–819. doi: 10.1586/14760584.6.5.809. [DOI] [PubMed] [Google Scholar]
- 60.Mitragotri S. Synergistic effect of enhancers for transdermal drug delivery. Pharm Res. 2000;17:1354–1359. doi: 10.1023/a:1007522114438. [DOI] [PubMed] [Google Scholar]
- 61.Weniger BG, Papania M. In: Vaccines. Edn. 5th. Plotkn S, Orenstein W, Offit P, editors. Philadelphia: Elsevier; 2008. pp. 1357–1392. [Google Scholar]
- 62.Venkatraman S, Gale R. Skin adhesives and skin adhesion. 1. Transdermal drug delivery systems. Biomaterials. 1998;19:1119–1136. doi: 10.1016/s0142-9612(98)00020-9. [DOI] [PubMed] [Google Scholar]
- 63.Food and Drug Administration. Edn. 27th. Rockville, MD: Department of Health and Human Services; 2007. Approved Drug Products with Therapeutic Equivalence Evaluations. [Google Scholar]
- 64.Bouwstra JA, et al. Water distribution and related morphology in human stratum corneum at different hydration levels. J Invest Dermatol. 2003;120:750–758. doi: 10.1046/j.1523-1747.2003.12128.x. [DOI] [PubMed] [Google Scholar]