Abstract
Inflammation plays a critical role in atherogenesis, yet the mediators linking inflammation to specific atherogenic processes remain to be elucidated. One such mediator may be secretory sphingomyelinase (S-SMase), a product of the acid sphingomyelinase gene. The secretion of S-SMase by cultured endothelial cells is induced by inflammatory cytokines, and in vivo data have implicated S-SMase in subendothelial lipoprotein aggregation, macrophage foam cell formation, and possibly other atherogenic processes. Thus, the goal of this study was to seek evidence for S-SMase regulation in vivo during a physiologically relevant inflammatory response. First, wild-type mice were injected with saline or lipopolysaccharide (LPS) as a model of acute systemic inflammation. Serum S-SMase activity 3 h postinjection was increased 2- to 2.5-fold by LPS (P < 0.01). To determine the role of IL-1 in the LPS response, we used IL-1 converting enzyme knockout mice, which exhibit deficient IL-1 bioactivity. The level of serum S-SMase activity in LPS-injected IL-1 converting enzyme knockout mice was ≈35% less than that in identically treated wild-type mice (P < 0.01). In LPS-injected IL-1-receptor antagonist knockout mice, which have an enhanced response to IL-1, serum S-SMase activity was increased 1.8-fold compared with LPS-injected wild-type mice (P < 0.01). Finally, when wild-type mice were injected directly with IL-1β, tumor necrosis factor α, or both, serum S-SMase activity increased 1.6-, 2.3-, and 2.9-fold, respectively (P < 0.01). These data show regulation of S-SMase activity in vivo and they raise the possibility that local stimulation of S-SMase may contribute to the effects of inflammatory cytokines in atherosclerosis.
The inflammatory response is not only an important component in the defense against pathogens, but it is also an important contributor to pathophysiologic processes such as atherosclerosis, autoimmune diseases, and endotoxic shock (1–10). In atherosclerosis, both clinical and biochemical evidence strongly suggest that lesion development, which is initiated by lipoprotein retention and aggregation within the vessel wall (11, 12), can be accelerated by local actions of inflammatory cytokines, notably on endothelial cells (13–17). For example, both lipopolysaccharide (LPS), the mediator of Gram-negative endotoxic shock, and systemic lupus erythematosus have been associated with the development of atherosclerotic lesions (18, 19).
The hallmarks of cytokine biology are pleiotropism and redundancy. Although a number of signaling pathways have been implicated in mediating the cellular effects of inflammatory cytokines, there is still considerable uncertainty about the relative importance of each of these pathways during inflammatory processes in vivo, including atherosclerosis and endotoxic shock (1, 2). One class of signaling pathways that has received considerable attention over the last few years involves the generation of ceramide by the action of cytokine-stimulated sphingomyelinase (SMase) (20, 21). Ceramide generated from SMase activation has been reported to play roles in cytokine-mediated apoptosis, cellular differentiation, and cellular senescence (20, 21), each of which may be important in the inflammatory response. Whereas studies in vitro have suggested roles for several different mammalian SMase activities in various ceramide signaling pathways (20, 21), a product of the acid SMase (ASM) gene has been implicated in several specific processes in vivo by molecular genetic studies using the ASM knockout mouse (22, 23). In particular, ASM knockout mice are relatively resistance to endothelial apoptosis induced by radiation (22) or LPS (23), both of which act through ceramide signaling.
ASM knockout mice on a hypercholesterolemic genetic background also show marked resistance to atherosclerotic lesion development (24). There are two possible scenarios linking ASM gene products to atherosclerosis. First, inflammation plays an important role in atherogenesis (13–17), and, given the evidence cited above, ASM-mediated ceramide signaling may be responsible for certain inflammatory responses in the developing lesion, such as smooth muscle cell proliferation (25) and apoptosis of macrophages and smooth muscle cells (26, 27). Second, one of the products of the ASM gene—secretory SMase (S-SMase; see below)—has been linked directly to extracellular subendothelial lipoprotein aggregation, an event that promotes both retention of lipoproteins in the arterial wall and macrophage foam cell formation (25, 28–39).
The ASM gene encodes a single mRNA and protein precursor that gives rise to both S-SMase and lysosomal SMase (L-SMase) via differential trafficking: if the protein precursor is mannose-phosphorylated, it will be trafficked to lysosomes, whereas if that same precursor is not mannose-phosphorylated, it is trafficked to the secretory pathway, generating S-SMase (40). Both L-SMase and S-SMase are absent in ASM knockout mice and in patients with types A and B Niemann–Pick disease (40, 41). Although defects in ASM-deficient states often are assumed to be caused by an absence of L-SMase (42), it is possible that some of these defects may be the result of the absence of S-SMase, which is the only known extracellular SMase in higher organisms (43). In this light, subendothelial lipoprotein aggregation is an extracellular event. Similarly, most cellular SM is on the external leaflet of the cellular membrane (44), where it would be inaccessible to L-SMase. Moreover, any ceramide generated by L-SMase is unable to escape lysosomes (45), raising doubts about its participation as a second messenger. Furthermore, several cell types involved in atherosclerosis and the inflammatory response are rich sources of S-SMase, such as endothelial cells and macrophage (32, 41). Endothelial cells, in particular, have the highest levels of S-SMase secretion of any cultured cell type tested in vitro (32) and the most intense immunostaining of any cell type within vascular tissue samples (34). Finally, several inflammatory cytokines, such as IL-1β, tumor necrosis factor α (TNF-α), and IFN-γ (46), increase the already high levels of S-SMase secretion from cultured endothelial cells and, importantly, decrease the levels of L-SMase in these cells (32).
Thus, we propose that S-SMase is a key mediator linking the inflammatory response to atherogenesis and possibly other cytokine-mediated events. An essential test of this hypothesis would be to examine the regulation of S-SMase by cytokines in vivo during a physiologically relevant inflammatory response. The goal of this study, therefore, was to determine whether S-SMase activity is regulated in vivo during the course of acute systemic inflammation. Our data show that S-SMase activity in the serum of mice is, indeed, increased by injection of LPS or by the inflammatory cytokines IL-1β and TNF-α. Furthermore, using two induced mutant mouse models, we show that the effect of LPS injection on serum S-SMase levels is mediated in part by the endogenous production of IL-1. These findings, which provide evidence for the regulation of S-SMase in vivo, raise the possibility that local stimulation of S-SMase may play a role in the action of inflammatory cytokines in atherosclerosis.
Experimental Procedures
Animals.
Pathogen-free 20- to 35-g female mice housed in a light-controlled (12-h on/12-h off) and temperature-controlled environment with food and water ad libitum were used. Wild-type C57BL/6J mice were obtained from The Jackson Laboratory. IL-1 converting enzyme (ICE) knockout mice were created, characterized, and provided by BASF Bioresearch (Cambridge, MA; ref. 47). IL-1 receptor antagonist (IL-1ra) knockout mice were created and characterized as described (48). All induced mutant mice and wild-type littermate controls used in this study were bred by at least 10 serial backcrosses onto the C57BL/6 background.
Genotyping.
Tail DNA was isolated and genotyping was performed by PCR. For ICE knockout mice, PCR was performed to distinguish the endogenous from the disrupted ICE locus by using (a) an intronic forward primer complementary to bases 5538–5557 in the published sequence (49) (gaagagatgttacagaagcc), (b) an intronic reverse primer corresponding to bases 6054–6074 (catgcctgaataatgatcacc) (49), and (c) a reverse primer for the insert used to create the disrupted null allele (gcgccttcccctacccgg). Wild-type animals have a single band at about 0.6 kb (a–b reaction), and homozygous knockout animals have a single band at around 0.6 kb (a–c reaction). For IL-1ra knockout mice, PCR was performed to distinguish the wild-type and disrupted IL-1ra alleles by using a sense primer complementary to part of exon II (genomic bases 2149–2171; aaccagctcattgctgggtactta) and an antisense primer corresponding to part of exon III (genomic bases 3175–3198; gcccaagaacacactatgaaggtc) (50). Wild-type animals have a single band at ≈1.1 kb, and homozygous knockout animals have a single band at ≈2.2 kb.
LPS and Cytokine Administration.
Groups of C57BL/6J, ICE knockout, and ICE wild-type mice were given Escherichia coli LPS (055:B5, Sigma) prepared in 0.2 ml of sterile saline and administered i.p. by single injection at a dose of 50 mg/kg. Groups of IL-1ra knockout and IL-1ra wild-type were given 25 mg/kg LPS i.p., also in 0.2 ml of saline. Control groups received 0.2 ml of saline i.p. Animals were studied 3 h after the injections. LPS administration at these doses induces cytokine-mediated systemic inflammation in rodents and is a widely used model of a systemic inflammatory response syndrome (47, 51). In separate experiments, groups of C57BL/6J mice were injected i.p. with human recombinant IL-1β (0.5 mg/kg; R & D Systems), human recombinant TNF-α (0.5 mg/kg; R & D Systems), both, or saline, and animals were studied 1–3 h after the injections. Blood, used to prepare serum for the SMase assay described below, was obtained by either tail bleed or by intraventricular cardiac puncture after the mice were killed; SMase activity was not affected by the mode of blood acquisition.
Serum SMase Assay.
The 200-μl assay mixture consisted of 20 μl of a 1:10 dilution of serum in assay buffer (0.1 M sodium acetate/0.1 mM ZnCl2, pH 5.0), an additional 140 μl of assay buffer, and 40 μl of [N-palmitoyl-9–10-3H]sphingomyelin (50 pmol at 50,000 cpm/pmol) in 0.25 M sucrose/0.1 mM ZnCl2/3% Triton X-100 (final concentration of Triton X-100 in the 200-μl assay mix = 0.6%) (41). The [3H]sphingomyelin was synthesized as described (41, 52, 53). In experiments to demonstrate Zn2+ dependence, 5 mM EDTA was substituted for 0.1 mM ZnCl2 in all components of the 200-μl assay mixture. Assay mixtures were incubated at 37°C for 1 h and then extracted by the method of Bligh and Dyer (54); the lower, organic phase was harvested, evaporated under N2, and fractionated by TLC by using chloroform/methanol (95:5). The ceramide spots were scraped and directly counted to quantify [3H]ceramide.
Statistics.
Unless otherwise indicated, results are given as means ± SEM. For comparisons between a single experimental group and a control, the unpaired, two-tailed t test was used. For comparisons involving several groups simultaneously, ANOVA was used initially. When ANOVA indicated differences among the groups, pairwise comparisons between groups were performed by using the Student–Newman–Keuls q statistic (55).
Results
Induction of S-SMase in Vivo During Acute Systemic Inflammation.
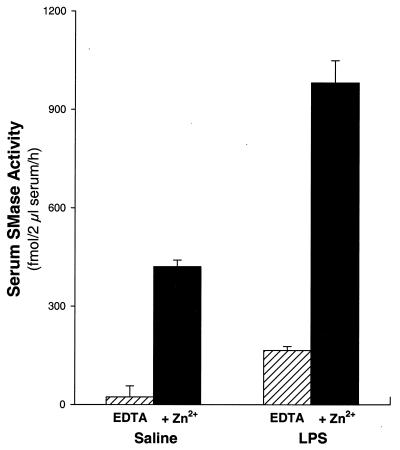
To determine whether S-SMase is regulated in vivo during an acute inflammatory response, we examined the effects of LPS injection on serum levels of this enzyme. The assays were conducted in the absence or presence of Zn2+, because S-SMase activity, but not L-SMase activity, depends on exogenous Zn2+ (40, 41). As shown in Fig. 1, the serum of saline-injected control C57BL/6J mice has measurable Zn2+-dependent SMase activity; in previous experiments, we had shown that this activity is linear within our assay conditions and that there is no detectable SMase activity in the serum of ASM knockout mice. Remarkably, Zn2+-dependent serum SMase activity was increased approximately 2.5-fold (P < 0.01) in C57 mice 3 h after an i.p. injection of E. coli LPS. Note that the additional serum SMase activity in LPS-treated mice was as dependent on exogenous Zn2+ as the activity in saline-injected control mice. Thus, the increase in serum SMase activity by LPS treatment was not caused by the release of L-SMase from damaged cells, but rather by a true increase in S-SMase activity in the serum of mice.
Figure 1.
Serum Zn2+-dependent SMase activity is increased by LPS injection. Wild-type mice were injected i.p. with 50 mg/kg LPS or saline as control, and 3 h later serum was assayed for SMase activity in the presence of EDTA (hatched bars) or Zn2+ (solid bars). There were three mice in each group. Each of the four possible pairwise comparisons between groups in this figure (EDTA vs. Zn2+ in the saline and LPS groups and saline vs. LPS in the EDTA and Zn2+ groups) indicated a significant difference (P < 0.01).
The Role of Inflammatory Cytokines in Inflammation-Induced S-SMase in Vivo.
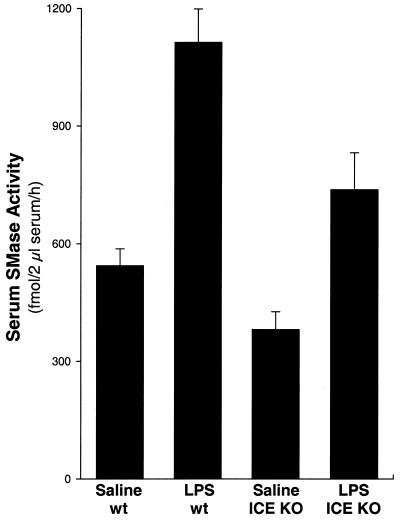
To assess the role of inflammatory cytokines in this effect, we examined ICE knockout mice, which have a 75% reduction in IL-1α immunoactivity, demonstrate no detectable IL-1β secretion, and show partial resistance to endotoxic shock (47). Similar to the data in Fig. 1, Zn2+-dependent serum SMase activity was increased ≈2-fold in LPS-injected wild-type mice (P < 0.01) (Fig. 2). In ICE knockout mice, however, the level of serum S-SMase activity after LPS injection was decreased ≈35% compared with LPS-treated wild-type mice (P < 0.01). Importantly, the difference between serum SMase activity in LPS-injected ICE knockout mice and the basal level of activity (i.e., in saline-injected wild-type mice) did not reach statistical significance. These data indicate that the increase in serum S-SMase activity in response to LPS in vivo is mediated in part through the induction of IL-1.
Figure 2.
LPS-induced serum S-SMase activity partially depends on ICE, an enzyme involved in IL-1 secretion. Wild-type (wt) and ICE knockout (KO) mice were injected i.p. with 50 mg/kg LPS or saline as control, and 3 h later serum was assayed for SMase activity in the presence of Zn2+. There were seven mice in each group. A significant difference (P < 0.01) was found between the following groups: LPS vs. saline (both in wild-type and ICE knockout mice) and LPS ICE knockout vs. LPS wild type.
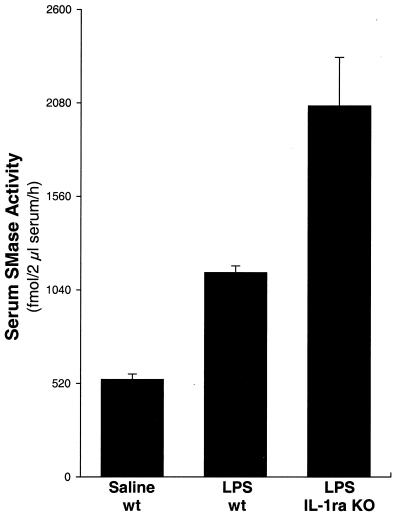
The response of cells and tissues to LPS-induced processes is blunted partly by IL-1ra, an endogenous competitive inhibitor of the type I IL-1 receptor (IL-1RI) (56). To independently confirm the contribution of IL-1 to the LPS-induced increase in serum S-SMase activity, we measured serum S-SMase levels after LPS injection in IL-1ra knockout mice. These animals lack endogenous antagonism to bioactive IL-1 and therefore demonstrate a heightened response to the biological effects of that cytokine, including increased susceptibility to lethal endotoxemia after LPS injection (48). As shown in Fig. 3, the increase in serum S-SMase activity induced by LPS was 1.8-fold greater in the IL-1ra knockouts compared with LPS-injected wild-type mice (P < 0.01). These data, like those in Fig. 2, indicate a substantial role for IL-1 in LPS-induced regulation of S-SMase in vivo during systemic inflammation.
Figure 3.
The absence of IL-1 receptor antagonist enhances serum S-SMase activity after LPS injection. Wild-type (wt) and IL-1ra knockout (KO) mice were injected i.p. with 25 mg/kg LPS, and a group of wild-type mice was injected with saline. Serum was collected 3 h later and assayed for SMase activity in the presence of Zn2+. There were five mice in the saline-wt and LPS-wt groups and three mice in the LPS–IL-1ra KO group. Each of the three possible pairwise comparisons between groups in this figure indicated a significant difference (P < 0.01).
Induction of S-SMase in Vivo by Direct Exposure to Inflammatory Cytokines.
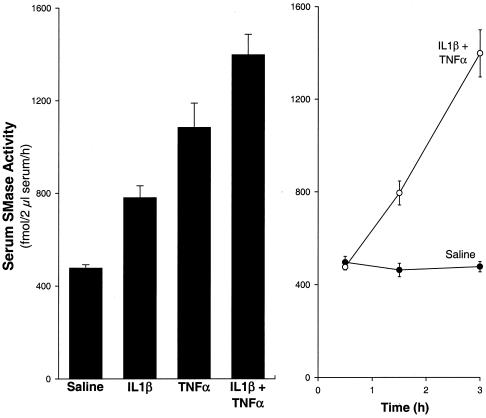
The effects of direct injection of specific cytokines on serum S-SMase activity are shown in Fig. 4A. Injection with either human recombinant IL-1β or human recombinant TNF-α led to ≈2- to 3-fold increases in serum S-SMase activity (P < 0.01), and the effect of these two cytokines given together was partially additive. The induction by the combined cytokines was seen as early as 90 min postinjection and continued to increase in a linear fashion for at least 3 h postinjection (Fig. 4B). Thus, IL-1β is involved in serum S-SMase activity in vivo, but other cytokines, such as TNF-α, also contribute to that effect.
Figure 4.
Serum S-SMase activity is increased by injection with IL-1β and TNF-α. Wild-type mice were injected i.p. with 0.8 mg/kg human recombinant IL-1β, 0.5 mg/kg human recombinant TNF-α, both, or saline. Serum SMase activity in the presence of Zn2+ was assayed 3 h after cytokine or saline injection in A and 0.5–3 h after combined cytokine (○) or saline (●) injection in B. In A, there were seven mice in the saline group and four mice in each of the other groups. Each of the six possible pairwise comparisons between groups in A indicated a significant difference (P < 0.01). In B, there were three mice in each group; the 1.5- and 3-h values from the combined cytokine group were statistically different (P < 0.05) from the respective saline values.
Discussion
Our results show that in an animal model of acute systemic inflammation there is marked S-SMase induction in the circulation. Animals with an induced mutation that increases IL-1 bioactivity had increased S-SMase induction by inflammation, whereas those with a mutation that decreases IL-1 bioactivity had reduced levels of inflammation-induced S-SMase activity. Injection of either recombinant IL-1β or recombinant TNF-α induced S-SMase in vivo. Thus, we show that IL-1 is involved, but not required, for the induction of S-SMase in acute systemic inflammation.
The clinical association between inflammatory events and atherosclerosis has been an area of intense study. However, the mechanism by which inflammatory events lead to specific atherogenic events, such as lipid deposition within the arterial wall, remain to be elucidated. With the burgeoning interest in the role of SMase in key cell-signaling pathways (20, 21) and in atherogenesis (24, 28–31), and the realization that S-SMase may be centrally involved in these processes (22–24, 30–32, 34, 41, 45), elucidation of S-SMase regulation by inflammatory mediators takes on added significance. Tissue culture studies thus far have revealed only two regulatory events: monocyte-to-macrophage differentiation is associated with marked increases in both L-SMase and S-SMase (41), and treatment of cultured human umbilical vein endothelial cells with specific inflammatory cytokines leads to an increase in S-SMase but a decrease in L-SMase (32). Findings with cultured cells, however, often do not apply to realistic circumstances in living organisms. In this study, we have shown that serum S-SMase activity is stimulated by LPS injection, an in vivo model of acute systemic inflammation, and that this effect is mediated by IL-1 and possibly by TNF-α.
The results of this study not only provide evidence for S-SMase regulation in vivo but also raise the possibility that the proposed roles of inflammatory cytokines in atherosclerosis may involve local stimulation of S-SMase. We recently have shown that a product of the ASM gene, most likely S-SMase, is an important mediator of atherogenesis in a mouse model of atherosclerosis, the apolipoprotein E knockout mouse (24). Inflammatory cytokines, including IL-1β and TNF-α, are both present in atherosclerotic lesions and are thought to play important roles in atherogenesis, particularly at the level of the endothelium (13–17). Interestingly, LPS itself may accelerate atherogenesis by enhancing the inflammatory components of developing atherosclerotic lesions (18), and a polymorphism in the gene encoding LPS receptor, CD14, has been associated with acute myocardial infarction in humans (57, 58). Our results suggest that a possible mechanism for the atherogenicity of inflammatory cytokines and LPS is through their ability to stimulate secretion of S-SMase from the overlying endothelium. Because S-SMase is secreted both apically and basolaterally from endothelial cells (32), it is likely that the LPS-induced increase in serum SMase shown herein is paralleled by an increase in subendothelial S-SMase. In this regard, the subendothelium of atherosclerotic lesions has abundant immunostainable extracellular SMase (34). S-SMase in the subendothelium then would induce aggregation of low density lipoprotein (LDL), which, in turn, further promotes LDL retention in the arterial wall and promotes macrophage foam cell formation (24, 28–31). S-SMase is also a prime candidate for the SMase involved in endothelial apoptosis in radiation- and LPS-treated mice (22, 23, 44, 45). Given the data reported herein, we suggest that LPS stimulates the secretion of S-SMase from the endothelium, which then may generate ceramide that acts on endothelial SM in an autocrine and paracrine manner.
Additional levels of regulation almost certainly contribute to the overall physiology of S-SMase. For example, although the enzyme functions optimally at acid pH, we have shown that lesional lipoproteins are modified in the arterial wall in vivo to one or more forms (e.g., via an increase in the SM/phospholipid ratio) that make them susceptible to S-SMase at neutral pH (31, 59). A similar increase in susceptibility may occur to SM in cells in inflammatory and atherosclerotic lesions. Furthermore, S-SMase activity against all substrates increases at low pH (31, 60), and there is evidence that pockets of acidity exist in both atherosclerotic and inflammatory lesions (61–65). Finally, the availability of extracellular zinc may regulate the activity of S-SMase, and there is good evidence that Zn2+ levels rise in both inflammatory and atherosclerotic lesions (66, 67). Indeed, the zinc requirement of S-SMase is similar to that of matrix metalloproteinases (41, 60, 68, 69), and these proteinases have been shown to be active in atherosclerotic lesions (70).
In addition to showing a role for the proinflammatory cytokines IL-1 and TNF-α in the induction of S-SMase by acute systemic inflammation in vivo, this study raises several interesting questions concerning the mechanisms of S-SMase regulation during the inflammatory response, including the cellular source or sources of the induced S-SMase and the intracellular signaling pathways involved. Cultured endothelial cells are by far the most abundant source of S-SMase among the many cell types studied by us, a finding supported by immunohistochemical analysis of animal aortae. Moreover, S-SMase from endothelial cells, but not from macrophages, is induced by cytokines (32, 34). Approximately half of the secretion of S-SMase from cultured endothelial cells is from the apical surface (32). Therefore, secretion of S-SMase from the endothelium into the blood stream is a likely origin of serum S-SMase.
Another key mechanistic issue is the molecular basis of cytokine-induced S-SMase induction. The ASM gene gives rise to a single mRNA and a single protein precursor that is either mannose-phosphorylated, and thus trafficked to lysosomes (L-SMase), or not mannose-phosphorylated, and thus trafficked to the secretory pathway (S-SMase) (40). In cytokine-treated cultured human umbilical vein endothelial cells, cytokines influence the trafficking of the SMase precursor, i.e., away from the lysosomal pathway and into the secretory pathway (32). The simplest model to explain these data is one in which cytokines lead to a partial decrease in the activity of the mannose-phosphorylating enzyme in the cis-Golgi, N-acetylglucosaminyl-1-phosphotransferase (71). This regulatory event would result in diminished mannose phosphorylation and thus reduced lysosomal trafficking and increased secretion of SMase. Future studies are needed to test this hypothesis both in cultured cells and in vivo and, if proven true, to map out the relevant cytokine signaling pathways.
In summary, the data presented in this report demonstrate that S-SMase is regulated in vivo in the setting of a physiologically relevant inflammatory event. These findings, taken into consideration with previous work on the biology of S-SMase and the ASM gene, raise the possibility that local stimulation of S-SMase may play a role in the action of inflammatory cytokines in atherosclerosis.
Acknowledgments
We thank BASF Bioresearch (Cambridge, MA) for providing the ICE knockout mice and Eugene Bak for technical assistance with this project. These studies were supported by National Institutes of Health Grant HL56984 to I.T. and K.J.W. and the National Alliance for Research on Schizophrenia and Depression to M.-L.W.
Abbreviations
- S-SMase
secretory sphingomyelinase
- L-SMase
lysosomal sphingomyelinase
- ASM
acid sphingomyelinase
- LPS
lipopolysaccharide
- ICE
IL-1 converting enzyme
- IL-1ra
IL-1 receptor antagonist
- TNF-α
transforming growth factor α
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.150098097.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.150098097
References
- 1.Cerami A. Clin Immunol Immunopathol. 1992;62:S3–S10. doi: 10.1016/0090-1229(92)90035-m. [DOI] [PubMed] [Google Scholar]
- 2.Kunkel S, Standiford T, Chensue S W, Kasahara K, Strieter R M. Agents Actions. 1991;32,Suppl.:205–218. doi: 10.1007/978-3-0348-7405-2_28. [DOI] [PubMed] [Google Scholar]
- 3.Cope A P. Curr Opin Immunol. 1998;10:669–676. doi: 10.1016/s0952-7915(98)80087-3. [DOI] [PubMed] [Google Scholar]
- 4.Van Noort J M, Amor S. Int Rev Cytol. 1998;178:127–206. doi: 10.1016/s0074-7696(08)62137-3. [DOI] [PubMed] [Google Scholar]
- 5.Warren J S. Clin Lab Med. 1997;17:547–558. [PubMed] [Google Scholar]
- 6.Pajkrt D, van Deventer S J. Curr Top Microbiol Immunol. 1996;216:119–132. doi: 10.1007/978-3-642-80186-0_6. [DOI] [PubMed] [Google Scholar]
- 7.Ulevitch R J, Tobias P S. Annu Rev Immunol. 1995;13:437–457. doi: 10.1146/annurev.iy.13.040195.002253. [DOI] [PubMed] [Google Scholar]
- 8.Parrillo J E. N Engl J Med. 1993;328:1471–1477. doi: 10.1056/NEJM199305203282008. [DOI] [PubMed] [Google Scholar]
- 9.Raetz C R, Ulevitch R J, Wright S D, Sibley C H, Ding A, Nathan C F. FASEB J. 1991;5:2652–2660. doi: 10.1096/fasebj.5.12.1916089. [DOI] [PubMed] [Google Scholar]
- 10.Ross R. N Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 11.Williams K J, Tabas I. Arterioscler Thromb Vasc Biol. 1995;15:551–561. doi: 10.1161/01.atv.15.5.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Williams K J, Tabas I. Curr Opin Lipidol. 1998;9:471–474. doi: 10.1097/00041433-199810000-00012. [DOI] [PubMed] [Google Scholar]
- 13.Mantovani A, Sozzani S, Vecchi A, Introna M, Allavena P. Thromb Haemostasis. 1997;78:406–414. [PubMed] [Google Scholar]
- 14.Libby P, Hansson G K. Lab Invest. 1991;64:5–15. [PubMed] [Google Scholar]
- 15.Hajjar D P, Pomerantz K B. FASEB J. 1992;6:2933–2941. doi: 10.1096/fasebj.6.11.1644257. [DOI] [PubMed] [Google Scholar]
- 16.Hansson G K. Curr Opin Lipidol. 1997;8:301–311. doi: 10.1097/00041433-199710000-00009. [DOI] [PubMed] [Google Scholar]
- 17.Blum A, Miller H I. Isr J Med Sci. 1996;32:1059–1065. [PubMed] [Google Scholar]
- 18.Liao W. J Lab Clin Med. 1999;128:452–460. doi: 10.1016/s0022-2143(96)90042-6. [DOI] [PubMed] [Google Scholar]
- 19.Manzi S, Selzer F, Sutton-Tyrrell K, Fitzgerald S G, Rairie J E, Tracy R P, Kuller L H. Arthritis Rheum. 1999;42:51–60. doi: 10.1002/1529-0131(199901)42:1<51::AID-ANR7>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 20.Kolesnick R N, Kronke M. Annu Rev Physiol. 1998;60:643–665. doi: 10.1146/annurev.physiol.60.1.643. [DOI] [PubMed] [Google Scholar]
- 21.Hannun Y A, Obeid L M. Adv Exp Med Biol. 1997;407:145–149. doi: 10.1007/978-1-4899-1813-0_22. [DOI] [PubMed] [Google Scholar]
- 22.Santana P, Pena L A, Haimovitz-Friedman A, Martin S, Green D, McLoughlin M, Cordon-Cardo C, Schuchman E H, Fuks Z, Kolesnick R. Cell. 1996;86:189–199. doi: 10.1016/s0092-8674(00)80091-4. [DOI] [PubMed] [Google Scholar]
- 23.Haimovitz-Friedman A, Cordon-Cardod C, Bayoumyb S, Garzottob M, McLoughlin M, Gallily R, Edwards C K, III, Schuchman E H, Fuks Z, Kolesnick R. J Exp Med. 1997;186:1831–1841. doi: 10.1084/jem.186.11.1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marathe S, Tribble D L, Kuriakose G, La Belle M, Chu B M, Gong E L, Johns A, Williams K J, Tabas I. Circulation. 1999;100:I–695. (abstr.). [Google Scholar]
- 25.Auge N, Andrieu A, Negre-Salvayre A, Thiers J C, Levade T, Salvayre R. J Biol Chem. 1996;271:19251–19255. doi: 10.1074/jbc.271.32.19251. [DOI] [PubMed] [Google Scholar]
- 26.Mitchinson M J, Hardwick S J, Bennett M R. Curr Opin Lipidol. 1996;7:324–329. doi: 10.1097/00041433-199610000-00011. [DOI] [PubMed] [Google Scholar]
- 27.Kockx M M. Arterioscler Thromb Vasc Biol. 1998;18:1519–1522. doi: 10.1161/01.atv.18.10.1519. [DOI] [PubMed] [Google Scholar]
- 28.Xu X, Tabas I. J Biol Chem. 1991;266:24849–24858. [PubMed] [Google Scholar]
- 29.Tabas I, Li Y, Brocia R W, Wu S W, Swenson T L, Williams K J. J Biol Chem. 1993;268:20419–20432. [PubMed] [Google Scholar]
- 30.Schissel S L, Tweedie-Hardman J, Rapp J H, Graham G, Williams K J, Tabas I. J Clin Invest. 1996;98:1455–1464. doi: 10.1172/JCI118934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schissel S L, Jiang X C, Tweedie-Hardman J, Jeong T S, Camejo E H, Najib J, Rapp J H, Williams K J, Tabas I. J Biol Chem. 1998;273:2738–2746. doi: 10.1074/jbc.273.5.2738. [DOI] [PubMed] [Google Scholar]
- 32.Marathe S, Schissel S L, Yellin M J, Beatini N, Mintzer R, Williams K J, Tabas I. J Biol Chem. 1998;273:4081–4088. doi: 10.1074/jbc.273.7.4081. [DOI] [PubMed] [Google Scholar]
- 33.Oorni K, Kakala J K, Annila A, Ala-Korpela M, Kovanen P T. J Biol Chem. 1998;273:29127–29134. doi: 10.1074/jbc.273.44.29127. [DOI] [PubMed] [Google Scholar]
- 34.Marathe S, Kuriakose G, Williams K J, Tabas I. Arterioscler Thromb Vasc Biol. 1999;19:2648–2658. doi: 10.1161/01.atv.19.11.2648. [DOI] [PubMed] [Google Scholar]
- 35.Slotte J P, Bierman E L. Biochem J. 1988;250:653–658. doi: 10.1042/bj2500653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fournier N, Paul J L, Atger V, Cogny A, Soni T, de la Llera-Moya M, Rothblat G, Moatti N. Arterioscler Thromb Vasc Biol. 1997;17:2685–2691. doi: 10.1161/01.atv.17.11.2685. [DOI] [PubMed] [Google Scholar]
- 37.Jian B, de la Llera-Moya M, Royer L, Rothblat G, Francone O, Swaney J B. J Lipid Res. 1997;38:734–744. [PubMed] [Google Scholar]
- 38.Zhao Y, Sparks D L, Marcel Y L. Biochemistry. 1996;35:16510–16518. doi: 10.1021/bi961622t. [DOI] [PubMed] [Google Scholar]
- 39.Hashizume T, Kageura T, Sato T. Biochem Mol Biol Int. 1998;44:489–496. doi: 10.1080/15216549800201512. [DOI] [PubMed] [Google Scholar]
- 40.Schissel S L, Keesler G A, Schuchman E H, Williams K J, Tabas I. J Biol Chem. 1998;273:18250–18259. doi: 10.1074/jbc.273.29.18250. [DOI] [PubMed] [Google Scholar]
- 41.Schissel S L, Schuchman E H, Williams K J, Tabas I. J Biol Chem. 1996;271:18431–18436. doi: 10.1074/jbc.271.31.18431. [DOI] [PubMed] [Google Scholar]
- 42.Brady R O. In: Sphingomyelin Lipidoses. Stanbury J B, Wyngarden J B, Fredrickson D S, Goldstein J L, Brown M S, editors. New York: McGraw–Hill; 1983. pp. 831–841. [Google Scholar]
- 43.Tabas I. Chem Phys Lipids. 1999;102:131–139. doi: 10.1016/s0009-3084(99)00080-8. [DOI] [PubMed] [Google Scholar]
- 44.Merrill A H, Jones D D. Biochim Biophys Acta. 1990;1044:1–12. doi: 10.1016/0005-2760(90)90211-f. [DOI] [PubMed] [Google Scholar]
- 45.Chatelut M, Leruth M, Harzer K, Dagan A, Marchesini S, Gatt S, Salvayre R, Courtoy P, Levade T. FEBS Lett. 1998;426:102–106. doi: 10.1016/s0014-5793(98)00325-1. [DOI] [PubMed] [Google Scholar]
- 46.Zetterstrom M, Sundgren-Andersson A K, Ostlund P, Bartfai T. Ann NY Acad Sci. 1998;856:48–52. doi: 10.1111/j.1749-6632.1998.tb08311.x. [DOI] [PubMed] [Google Scholar]
- 47.Li P, Allen H, Banerjee S, Franklin S, Herzog L, Johnston C, McDowell J, Paskind M, Rodman L, Salfeld J, et al. Cell. 1995;80:401–411. doi: 10.1016/0092-8674(95)90490-5. [DOI] [PubMed] [Google Scholar]
- 48.Hirsch E, Irikura V M, Paul S M, Hirsh D. Proc Natl Acad Sci USA. 1996;93:11008–11013. doi: 10.1073/pnas.93.20.11008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Casano F J, Rolando A M, Mudgett J S, Molineaux S M. Genomics. 1994;20:474–481. doi: 10.1006/geno.1994.1203. [DOI] [PubMed] [Google Scholar]
- 50.Zahedi K A, Uhlar C M, Rits M, Prada A E, Whitehead A S. Cytokine. 1994;6:1–9. doi: 10.1016/1043-4666(94)90001-9. [DOI] [PubMed] [Google Scholar]
- 51.Morrison D C, Duncan R L, Jr, Goodman S A. Prog Clin Biol Res. 1985;189:81–99. [PubMed] [Google Scholar]
- 52.Sripada P K, Maulik P R, Hamilton J A, Shipley G G. J Lipid Res. 1987;28:710–718. [PubMed] [Google Scholar]
- 53.Ahmad T Y, Sparrow J T, Morrisett J D. J Lipid Res. 1985;26:1160–1165. [PubMed] [Google Scholar]
- 54.Bligh E G, Dyer W J. Can J Biochem Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 55.Glantz S A. Primer of Biostatistics. New York: McGraw—Hill; 1992. [Google Scholar]
- 56.Dinarello C A. Blood. 1996;87:2095–2147. [PubMed] [Google Scholar]
- 57.Unkelbach K, Gardemann A, Kostrzewa M, Philipp M, Tillmanns H, Haberbosch W. Arterioscler Thromb Vasc Biol. 1999;19:932–938. doi: 10.1161/01.atv.19.4.932. [DOI] [PubMed] [Google Scholar]
- 58.Kane J P, Havel R J. Circulation. 1999;99:3210–3212. doi: 10.1161/01.cir.99.25.3210. [DOI] [PubMed] [Google Scholar]
- 59.Jeong T S, Schissel S L, Tabas I, Pownall H J, Tall A R, Jiang X C. J Clin Invest. 1998;101:905–912. doi: 10.1172/JCI870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Spence M W, Byers D M, Palmer F B, Cook H W. J Biol Chem. 1989;264:5358–5363. [PubMed] [Google Scholar]
- 61.Menkin V. Am J Pathol. 1934;10:193–210. [PMC free article] [PubMed] [Google Scholar]
- 62.Smith E B. Adv Exp Med Biol. 1979;115:245–297. [Google Scholar]
- 63.Maroudas A, Weinberg P D, Parker K H, Winlove C P. Biophys Chem. 1988;32:257–270. doi: 10.1016/0301-4622(88)87012-1. [DOI] [PubMed] [Google Scholar]
- 64.Tapper H, Sundler R. Biochem J. 1992;281:245–250. doi: 10.1042/bj2810245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Silver I A, Murrills R J, Etherington D J. Exp Cell Res. 1988;175:266–276. doi: 10.1016/0014-4827(88)90191-7. [DOI] [PubMed] [Google Scholar]
- 66.Milanino R, Marrella M, Gasperini R, Pasqualicchio M, Velo G. Agents Actions. 1993;39:195–209. doi: 10.1007/BF01998974. [DOI] [PubMed] [Google Scholar]
- 67.Mondis S. Biol Trace Element Res. 1989;22:251–256. doi: 10.1007/BF02916613. [DOI] [PubMed] [Google Scholar]
- 68.Donangelo C M, Chang G W. Clin Chim Acta. 1981;113:201–206. doi: 10.1016/0009-8981(81)90154-6. [DOI] [PubMed] [Google Scholar]
- 69.Sorbi D, Fadly M, Hicks R, Alexander S, Arbeit L. Kidney Int. 1993;44:1266–1272. doi: 10.1038/ki.1993.378. [DOI] [PubMed] [Google Scholar]
- 70.Galis Z S, Sukhova G K, Lark M W, Libby P. J Clin Invest. 1994;94:2493–2503. doi: 10.1172/JCI117619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kornfeld S. FASEB J. 1987;1:462–468. doi: 10.1096/fasebj.1.6.3315809. [DOI] [PubMed] [Google Scholar]