Abstract
Increase in intracellular Ca2+ due to voltage-gated Ca2+ (CaV) channel opening represents an important trigger for a number of second-messenger mediated effects ranging from neurotransmitter release to gene activation. Ca2+ entry occurs through the principal pore-forming protein, but several ancillary subunits are known to more precisely tune ion influx. Among them, the CaVβ subunits are perhaps the most important given that they largely influence the biophysical and pharmacological properties of the channel. Notably, several functional features may be associated with specific structural regions of the CaVβ subunits emphasizing the relevance of intramolecular domains in the physiology of these proteins. In the current report, we show that CaVβ3 contains two PEST motifs and undergoes Ca2+-dependent degradation which can be prevented by the specific calpain inhibitor calpeptin. Using mutant constructs lacking the PEST motifs, we present evidence that they are necessary for the cleavage of CaVβ3 by calpain. Furthermore, the deletion of the PEST sequences did not affect the binding of CaVβ3 to the ionconducting CaV2.2 subunit, and when expressed in HEK-293 cells, the PEST motif-deleted CaVβ3 significantly increased whole-cell current density and retarded channel inactivation. Consistent with this observation, calpeptin treatment of HEK-293 cells expressing wild-type CaVβ3 resulted in an increase in current amplitude. Together, these findings suggest that calpainmediated CaVβ3 proteolysis may be an essential process for Ca2+ channel functional regulation.
Keywords: Blotting, Western; methods; Calcium; metabolism; Calcium Channels; chemistry; genetics; metabolism; Calcium Signaling; physiology; Calcium-Binding Proteins; antagonists & inhibitors; metabolism; Cell Line; Dipeptides; pharmacology; Dose-Response Relationship, Radiation; Electric Stimulation; methods; Humans; Immunoprecipitation; methods; Membrane Potentials; drug effects; physiology; radiation effects; Microfilament Proteins; antagonists & inhibitors; metabolism; Mutation; physiology; Patch-Clamp Techniques; methods; Protein Conformation; Protein Subunits; Recombinant Fusion Proteins; chemistry; genetics; metabolism; Time Factors; Transfection; methods
Keywords: Beta subunit, Ca2+ channels, MAGUK proteins, PEST sequences, calpain, HEK-293 cells
Introduction
The major function of the voltage-gated Ca2+ (CaV) channels is to convert changes in membrane potential into an intracellular calcium (Ca2+i) signal. Transient rises of Ca2+i trigger or regulate diverse intracellular events, including metabolic processes, muscle contraction, secretion of hormones and neurotransmitters, cell differentiation and gene expression. Several types of CaV channels have been characterized and designated L, N, P/Q, R, and T. These channel types can be grouped into two major functional classes: high voltage- and low voltage-activated channels (HVA and LVA, respectively). The HVA CaV channel permeation pathway is formed by its α1 subunit, which is encoded by a family of 7 genes (Catterall et al., 2003). The current through these channels may be modulated by distinct structural modifications including the association with auxiliary subunits: the disulfide-linked CaVα2δ, the intracellular CaVβ and the transmembrane CaVγ subunits, which also represent gene families (Arikkath and Campbell, 2003).
Among the auxiliary proteins, the CaVβ subunit plays a crucial role in the formation and behavior of all functional HVA CaV channels. Four different types of CaVβ subunits (β1 to β4) have been identified, each with multiple splicing variants (Walker and De Waard, 1998; Arikkath and Campbell, 2003; Dolphin, 2003). The CaVβ proteins do not cross the plasma membrane, but can directly interact with the CaVα1 subunit and are important for trafficking and expression of the kinetic properties of the channel (Walker and De Waard, 1998; Arikkath and Campbell, 2003; Dolphin, 2003). The physiological importance of the CaVβ subunits is demonstrated by the severe phenotypes of mutant and knockout mice (Gregg et al., 1996; Burgess et al., 1997; McEnery et al., 1999; Ball et al., 2002). In-depth understanding of how the CaVβ auxiliary subunits modulate the activity of the ion-conducting CaVα1 subunits is essential for insights into the operation of HVA CaV channels in both normal and disease states.
Detailed structural modeling of CaVβ subunits has proposed five discrete domains homologous to the membrane-associated guanylate kinase (MAGUK) protein family. Structural models have been proposed for the type 3 src-homology (SH3), and guanylate kinase (GK)-like domains (Hanlon et al., 1999; Opatowsky et al., 2003; 2004; McGee et al., 2004; Takahashi et al., 2004; Chen et al., 2004;). SH3 and GK domains are both necessary to recapitulate full modulatory effects of CaVβ on the pore-forming CaVα1 protein (Takahashi et al., 2004; McGee et al., 2004). Likewise, the C-terminus of CaVβ is associated to membrane targeting properties (Bogdanov et al., 2000) and the N-terminus appears to be involved in the regulation of channel inactivation (Olcese et al., 1994; Restituito et al., 2000; Stotz et al., 2004). Site-directed mutagenesis of conserved serine residues in consensus sites for protein kinase phosphorylation suggests a role of phosphorylation in tuning the functional properties and pharmacological sensitivities of the CaVβ subunits (De Waard et al., 1994; Gerhardstein et al., 1999; Kohn et al., 2003).
These studies point to the importance of intramolecular domains in the physiology of the CaVβ subunits. In the current report, we show that CaVβ3 contains two PEST-like sequences (potential signals for rapid protein degradation), one in the SH3 domain and another in the C-terminal end of the protein. These sequences are sensitive in vitro to low concentrations of calpain (a Ca2+-dependent protease). We further found that CaVβ3 mutants lacking the PEST sequences induced an increase in whole-cell Ca2+ current amplitude compared to wild-type CaVβ3, and caused a change in channel inactivation kinetic properties. Our findings suggest that CaVβ3 proteolytic cleavage may be an essential process for Ca2+ channel functional regulation.
Materials and Methods
Cell culture and recombinant CaV channel expression
Human embryonic kidney (HEK-293) cells were grown in DMEM-high glucose supplemented with 10% horse serum, 2 mM L-glutamine, 110 mg/l sodium pyruvate and 50 μg/ml gentamycin, at 37°C in a 5% CO2/95% air humidified atmosphere. After splitting the cells on the previous day and seeding at ~60% confluency, cells were transfected using the Lipofectamine Plus reagent (Gibco BRL) with 1.2 μg plasmid cDNA encoding the rabbit brain N-type Ca2+ channel CaV2.2 pore-forming subunit (formerly α1B; GenBank accession number D14157) (Fujita et al., 1993) in combination with 1.2 μg cDNA coding the rat brain CaVα2δ-1 (M86621) (Kim et al., 1992), and 1.2 μg cDNA of the rat brain CaVβ3 (M88751) (Castellano et al., 1993) or its mutants (see below). For electrophysiology, 0.36 μg of a plasmid cDNA encoding the green fluorescent protein (GFP; Green-Lantern; Gibco/BRL) was added to the transfection mixture to select positively transfected cells.
Deletions of CaV β3 PEST regions
The different deletions in the CaVβ3 subunit were obtained by the QuikChange XL-mutagenesis kit QCM (Stratagene), following a two stage PCR protocol for deletions (Wang and Malcolm, 2001). The PEST regions 1 and 2 (amino acid residues 24 to 37 and 397 to 411, respectively) were subjected to deletion through duplex oligonucleotides that comprised the adjacent nucleotidic sequences to these regions. The forward oligonucleotides were 5′-GTTCAGCCGACTCTACACCAGAGAGTGCCCGGCGAGAAGTGG-3′ and 5′-GAGGAGCATTCACCCCTGGAGCAGGCCTGGACCGGATCTTCACAG-3′ for PEST1 and PEST2, respectively. Reverse oligonucleotides were complementary to these sequences. In step I of the procedure, two extension reactions were performed in separate tubes, one containing the forward primer and the other including the reverse and complementary primer. Five polymerization cycles were conducted at 95°C 30 s, 55°C 1 min, and 68°C 14 min. After that, both reactions were mixed and the standard QCM procedure continued for 16 cycles. In addition to the single PEST deletions, a double deletion cDNA clone was obtained from the first plasmid harboring the PEST1 deletion in combination with the duplex oligonucleotides for the PEST2 deletion. Deletions were confirmed by either restriction endonuclease analysis (using endonucleases HindIII and XbaI) or automatic sequencing using an ABI PRISM 310 sequence analyzer (Perkin-Elmer Applied Biosystems) and primers for the T7 and SP6 promoters.
In vitro transcription and translation
In vitro transcription/translation assays were performed using the TNT™ Quick Coupled transcription/translation system kit (Promega). Briefly, 2 μg of plasmid DNA was added to 41 μl of TNT™ quick master Mix containing 1 μl of [35S]-methionine (1000 Ci/mmol) at 2.5 mCi/ml (Amersham Pharmacia Biotech) to a final volume of 50 μl and incubated at 30ºC for 120 min. Proteins were subjected to SDS-PAGE (see below) and labeled proteins were detected by film exposure for 48 h.
SDS-PAGE and Western blotting
Microsomes from transfected HEK-293 cells were obtained as described elsewhere (Felix et al., 1997; Gurnett et al., 1997), and proteins were separated on 10% sodium dodecyl sulfate (SDS)- polyacrylamide gels according to the method of Laemmli (1970). Samples were heated at 90°C for 5 min and 100 μg of protein/slot were loaded on gels. Proteins were blotted onto nitrocellulose membranes and were developed with enhanced chemiluminescence as previously described (Felix et al., 1997; Gurnett et al., 1997). The anti-CaVβ3 specific antibody was a sheep polyclonal antibody (1:1000 dilution; Sh0049), and the secondary antibody was a rabbit anti-sheep IgG horseradish peroxidase (Zymed) used at a dilution of 1:4000.
Pull-down experiments
As mentioned earlier, [35S]-labelled proteins (wild-type β3, β3ΔP1, β3ΔP2 and β3ΔP1–2) were expressed in vitro using a coupled transcription/translation system as indicated by the manufacturer (TNT™ Kit, Promega). For pull-down assays, 75 μl of hydrated glutathione-agarose beads (Sigma) were incubated respectively with 20 μg of GST fused to AID2.2 (Alpha1 Interaction Domain of Cav2.2; Sandoz et al., 2001) or of GST alone for 2 hours at 4°C. In order to saturate non specific free sites, GST-AID2.2 and GST beads were incubated with 0.1 mg/ml BSA overnight at 4°C. [35S]-labelled proteins were incubated with the beads for 1 h at room temperature. The beads were then washed with PBS three times and the proteins, bound to beads, were eluted in denaturing buffer and analyzed on sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) by autoradiography.
Electrophysiology
Forty eight hours after transfection, cells expressing the GFP reporter gene were subjected to the whole-cell mode of the patch clamp technique (Hamill et al., 1981). In brief, Ba2+ currents through Ca2+ channels were recorded with an Axopatch 200B amplifier (Axon Instruments) and acquired on-line using a Digidata 1320A interface with pClamp8 software (Axon Instruments). After establishing the whole-cell mode, capacitive transients were canceled with the amplifier. Currents were obtained from a holding potential (HP) of −80 mV and by applying test pulses every 20 s. Leak and residual capacitance currents were subtracted on-line by a P/4 protocol. Current signals were filtered at 2 kHz (internal 4 pole Bessel filter) and digitized at 5.71 kHz. Membrane capacitance (Cm) was determined as described previously (Avila et al., 2004) and used to normalize currents. The bath recording solution contained (in mM) 10 BaCl2, 125 TEA-Cl, 10 HEPES and 10 glucose (pH 7.3). The internal solution consisted of (in mM) 110 CsCl, 5 MgCl2, 10 EGTA, 10 HEPES, 4 Na-ATP and 0.1 GTP (pH 7.3). Experiments were performed at room temperature (~25°C).
Pulse chase and immunoprecipitation experiments
6 cm diameter dishes with 40% confluent HEK-293 cells were transfected with cDNA encoding the wild-type CaVβ3 or its ΔPEST mutants. 24 hours later, the protein labeling condition was set by incubating the cells for 30 min in methionine- and cysteine-free DMEM supplemented with 5% fetal calf serum (FCS; starvation period). Labeling was induced by adding 500 μCi [35S]-L-methionine and 2 mM L-cysteine to each plate for 40 min at 37°C. To remove radioactive media, dishes were washed with PBS. Subsequently, normal DMEM media supplemented with 10% FCS was added to plates (except the t = 0 sample, which represents the start time of the chase). At t = 24 h, all plates were washed with ice-cold PBS. Cells were then scraped in 10 ml ice-cold PBS and transferred to a tube to remove the supernatant by centrifugation. Labeled cells were lysed with 1 ml PBS supplemented with 0.5% triton X-100 and a cocktail of protease inhibitors (complete, Mini, EDTA-free, Roche). Lysates were sonicated and centrifuged at 1,500 rpm for 15 min. The supernatants were used for immunoprecipitation experiments. A total of 200 μg proteins of each sample were incubated with anti-CaVβ3 polyclonal IgG for 1 hr at room temperature. This polyclonal antibody was described elsewhere (Bichet et al., 2000), but was raised against the full-length sequence of CaVβ3. Subsequently, the CaVβ3-IgG complex were immobilized by Protein A sepharose beads. Eluted proteins were then loaded on a 12% SDS-PAGE. After protein separation, the gel was treated for 30 min with a fixation solution (50% methanol, 10% acetic acid) and 30 min with a solution of 10% glycerol, before a 24 hr autoradiography exposure.
Data analysis
The data are given as mean ± S.E. Statistical differences between two means were determined by Student’s t tests (P<0.05). Current inactivation was fitted with single exponential equations of the form: A ×exp(−t/τ) + c, where A is the initial amplitude (pA), t is time (ms), τ is the time constant for inactivation and c is a constant.
Results
Although important progresses in the structure-function relationship of the CaVβ subunits have been made recently (Richards et al., 2004), almost nothing is known about the mechanism and determinants of recognition for proteolytic degradation of these proteins. In this context, PEST domains are short sequences (10–60 residues) enriched in proline (P), glutamic/aspartic (E), serine (S) and threonine (T) residues, found in many short-lived eukaryotic proteins that play a role in their degradation (Rechsteiner and Rogers, 1996). Using the PESTFind algorithm (available at the URL https://emb1.bcc.univie.ac.at/toolbox/) we found one conserved PEST sequence (N-terminal; PEST1) and one variable (C-terminal; PEST2) PEST sequence in the CaVβ proteins. In the rat brain CaVβ3 subunit, the PEST1 region comprises amino acid residues 24 to 37, while PEST2 consists of amino acid residues 397 to 411 (Fig. 1A). PEST1 scored +9.47 and PEST2 +11.52, respectively. These findings prompted us to investigate the importance of the PEST sequences in the susceptibility of CaVβ3 to proteolytic degradation. It is worth mentioning that the CaVβ3 subunit is the major CaVβ constituent of the CaV2.2 channel complex (Scott et al., 1996), which plays a pivotal role in regulating neurotransmitter release and in controlling endocrine secretion.
Fig. 1.

Identification and deletion of the PEST-like sequences in the CaVβ3 subunit. A, Schematic representation of the functional domains of CaVβ3 and the putative PEST sequences. Two potential PEST regions with scores of +9.47 (residues 24–37) and +11.52 (residues 397–411) were found in the amino acid sequence of CaVβ3 when analyzed with the PESTFind software. SH3 denotes a type 3 src-homology and GK-like indicate a guanylate kinase domain. B, Autoradiogram of in vitro translated [35S]-methionine-labeled wild-type (β3) and PEST deletion mutants of the CaVβ3 subunit (ΔP1, ΔP2, ΔP1–2) resolved by SDS-PAGE. 5 μl of each translation reaction were run per lane. C, Western blot analysis of membranes from untransfected HEK-293 cells (−) or cells expressing the wild-type (β3) and the CaVβ3 PEST deletion mutants (ΔP1, ΔP2, ΔP1–2).
By using a two stages PCR protocol (see Methods section), we first created two single (ΔP1 and ΔP2) and one double (ΔP1–2) PEST domain deletions in the CaVβ3 sequence. The plasmids containing the full-length sequence and the constructs harboring the deletions were initially examined using a cell-free transcription/translation system. All the cDNA clones directed the synthesis in vitro of polypeptides of the expected molecular weights (Fig. 1B). Next, the wild-type CaVβ3 and its three PEST-deleted versions were expressed in HEK-293 cells and analyzed by immunoblotting using polyclonal antibodies directed against a fusion protein of the C- terminus of CaVβ3 (Liu et al., 1996; Scott et al., 1996). This analysis revealed a single immunoreactive protein band with a molecular mass of ~58 kDa in microsomes from transfected cells, which corresponds to the full-length CaVβ3 (Fig. 1C). The antibodies also recognized the PEST-truncated proteins by showing shifts in the mobility from 58 kDa down to ~50–55 kDa on SDS-PAGE 10% gels (Fig. 1C). These findings, combined with the lack of endogenous CaVβ3 subunit, make the HEK-293 cell line a good cell model to investigate the importance of the PEST sequences in CaVβ3 function.
We next questioned whether the PEST domains of CaVβ3 were molecular substrates for in vitro degradation by Ca2+-dependent endogenous proteases. This was tested through a comparative analysis of Ca2+-mediated proteolysis using the CaVβ3 mutant lacking both PEST regions (ΔP1–2) and the full-length CaVβ3 subunit as substrates. Figure 2A shows that recombinant CaVβ3 expressed in HEK-293 cells was partially cleaved by endogenous proteases in the presence of CaCl2. Though significant degradation was observed, proteolysis was not complete; this could be explained by the fact that besides Ca2+ no other agent was used to induce proteolysis. In addition, endogenous molecules that activate proteolytic activity (by reducing the Ca2+ requirement, for instance), may be lacking in the cell homogenates employed in these assays. Hence, when Ca2+ was present, the specific anti-CaVβ3 antibody detected additional bands that should correspond to CaVβ3 fragments following the cleavage of the full-length CaVβ3 by Ca2+-dependent endogenous proteases. As expected, this proteolytic break-down of CaVβ3 was prevented by adding EDTA to the incubation buffer used for the experiments. Unlike the full-length CaVβ3 subunit, the ΔP1–2 mutant was stable in the presence of Ca2+ and did not undergo proteolysis in the EDTA-containing buffer (Fig. 2A). Likewise, examination of gels stained with Coomassie Blue provided initial evidence that a ~58,000-Da polypeptide (presumably the CaVβ) is susceptible to Ca2+/calpain-induced proteolysis. Hence, microsomes of HEK-293 cells expressing the CaVβ3 subunit were analyzed on 10% SDS-PAGE stained with Coomassie Blue. As shown in Figure 2B, a 20 min treatment of the microsomes with μ-calpain produced a dose-dependent change in the levels of the ~58 kDa protein in the gels. In contrast, the levels of the ~58 kDa polypeptide were unaffected when the Ca2+ chelator EDTA or when the calpain inhibitor calpeptin were included in the assay. These data corroborate that the HEK-293 cell line possesses basal calpain activity (Shimada et al., 2005), and suggest that the wild-type CaVβ3 subunit is a target of this protease.
Fig. 2.
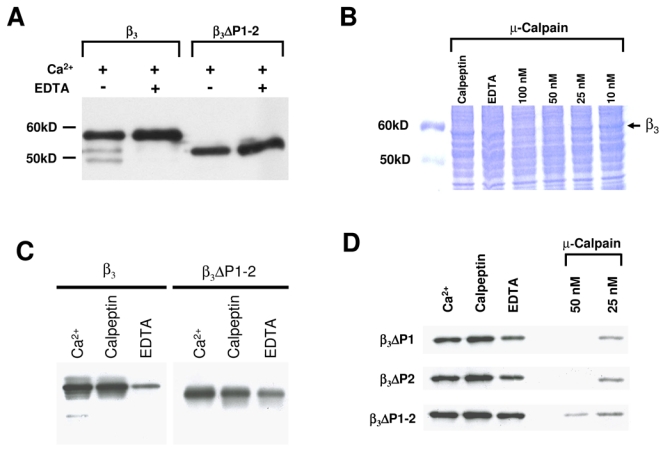
The CaVβ3 subunit is cleaved by Ca2+-dependent proteases. A, Recombinant CaVβ3 ΔP1–2 protein is more stable than the wild-type CaVβ3 to endogenous Ca2+-dependent proteases. Lanes 1 and 2 show the proteolytic breakdown of wild-type CaVβ3 (β3) heterologously expressed in HEK- 293 cells. 200 μg of microsomes were incubated with Ca2+ (750 μM) at 30°C for 20 min in absence or presence of EDTA (1.5 mM). No proteolytic degradation was observed (lanes 3–4) when the PEST-like regions were deleted (ΔP1–2). B, Coomassie-stained SDS-PAGE in a 10% resolving gel using Laemmli buffer system. Lane 1, control; lanes 2–7, microsomes from HEK- 293 cells expressing the wild-type CaVβ3 incubated for 20 min at 30°C with Ca2+, EDTA or calpeptin or exposed to increasing concentrations of μ-calpain. C, Degradation of the CaVβ3 subunits in the presence of Ca2+ and a calpain protease inhibitor. CaVβ3 subunits were incubated at 30°C for 20 min with 750 μM Ca2+, 180 nM of calpeptin or 1.5 mM EDTA. The full-length (β3) or the double PEST deletion mutant (ΔP1–2) are indicated. D, ΔPEST mutant CaVβ3 proteins were incubated for 20 min at 30°C with Ca2+, EDTA or calpeptin as indicated above or exposed to two increasing doses of μ-calpain as listed (in the presence of 750 μM Ca2+). In each case, one representative of at least two independent degradation experiments of wild-type and mutant CaVβ3 proteins is presented.
We next sought to determine whether the PEST regions found in CaVβ3 were substrates of calpain. To this end, proteolysis experiments of wild-type and mutant CaVβ3 proteins were conducted according to a protocol described elsewhere (Shumway et al., 1999). Briefly, calpain activity was indirectly determined by assessing the extent of Ca2+-induced degradation of CaVβ3 ΔP1–2 mutant subunits compared to the wild-type protein degradation (Fig. 2C). Inhibitors such as EDTA and calpeptin were used in the assay to ascertain the specificity of calpain activity. As described above, incubation with Ca2+ (750 μM) caused degradation of CaVβ3 which was prevented in the presence of EDTA or calpeptin. On the other hand, examination of the CaVβ3 ΔP1–2 mutant by Western blot evidenced that this protein does not undergo endogenous Ca2+/calpain-induced proteolytic cleavage, illustrating the importance of the PEST sequences (Fig. 2C).
As a more direct test of the contributing role of the PEST domains as calpain substrates, we examined the proteolytic profile of the CaVβ3 subunit mutants in which the PEST sequences had been removed. First, CaVβ3 was produced by in vitro translation in the rabbit reticulocyte lysate, then mixed with increasing concentrations of calpain in the presence of Ca2+ to activate the protease. The results indicated that the in vitro translated CaVβ3 protein is indeed susceptible to exogenous calpain breakdown in a dose- and Ca2+-dependent manner. Notably, calpain concentrations of ~10 nM were sufficient to significantly degrade the in vitro translated CaVβ3, and this effect was prevented when EDTA (consistent with the Ca2+ requirement of calpain) or the calpain inhibitor calpeptin were present. Of note, these experiments were performed adding exogenous calpain, in contrast to those in which the protein is degraded showing two additional bands (where proteolysis was mediated by endogenous Ca2+ dependent proteases; Fig. 2A). We next questioned whether either the N- or the C-terminal PEST domains of CaVβ3 were molecular determinants for the efficiency of in vivo degradation by calpain. Recombinant CaVβ3 proteins lacking amino acid residues 24 to 37 (ΔP1) and 397 to 411 (ΔP1–2) expressed in HEK-293 cells were subjected to increasing concentrations of μ-calpain and probed by Western blot. Though at a concentration of 90 nM (in the presence of 750 μM Ca2+), the ΔP1–2 mutant proteins were almost completely degraded, proteolytic breakdown of these proteins seemed to be reduced in comparison to the full-length CaVβ3 at equal concentrations of calpain (not shown). Despite the fact that the internal concentration is unknown, we surmise that a low concentration of the protease, in conjunction with the over-expression of our protein, explains that the effect is only partial. In contrast, when exogenous calpain is added, the proteolysis seems to be more potent. These results suggest that the PEST sequences within the CaVβ3 subunit promote its degradation by μ-calpain. In line with this, when the double PEST deletion mutant protein (ΔP1–2) was incubated with calpain, we detected a significant decline in proteolysis relative to that of the single mutants. Following incubation with 50 nM calpain some of the input protein still remained (Fig. 2D). Taken together, these data suggest that deletion of the PEST sequences produced CaVβ3 subunits that were more resistant to cleavage by calpain than the full-length protein and provide evidence that the extent of proteolysis depends on the presence of the PEST sequences.
In order to exclude the possibility that the reduced proteolytic sensitivity shown by the ΔP1–2 mutant may be the result of gross structural alterations in the CaVβ3 protein caused by the amino acids deletion, we next tested whether the ΔPEST mutated versions of CaVβ3 could directly associate with the CaVα1 subunit through the Alpha1 Interaction Domain (AID), the main channel structure involved in the CaVα1-CaVβ subunit interaction. To this end, we used a GST-fusion protein encoding a short fragment of the intracellular I-II loop of the CaV2.2 subunit carrying the AID region (GST-AID2.2), and compared its binding to the in vitro synthesized [35S]-β3 wild-type and mutant proteins (Fig. 3). As can be seen, all of the [35S]-CaVβ3 subunits maintained the ability to bind the GST-AID2.2 fusion protein indicating that the deletions in the PEST regions do not affect the association of these subunits to the purified GST fusion protein.
Fig. 3.
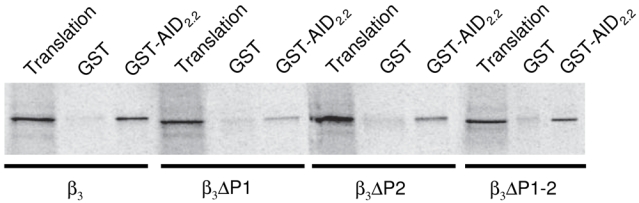
Deletion of the PEST-like sequences did not alter the specific binding of the mutant CaVβ3 to the CaVα1 subunit. Determination of CaVβ3 binding to the AID of the CaV2.2 subunit. Capacity of the wild-type and mutant [35S]-CaVβ3 subunits to interact with the fusion protein GST-AID2.2 was assayed by SDS-PAGE and autoradiography. Translation represents the equivalent volume of in vitro translation of [35S]-CaVβ3 used in the binding assays.
To extend these findings, we next investigated the functional repercussion of the PEST deletions in CaVβ3 by electrophysiological recording. The whole-cell mode of the patch clamp technique was used to study the macroscopic Ba2+ currents (IBa) through recombinant N-type CaV channels (composed of CaV2.2 and α2δ-1) in HEK-293 cells transiently expressing wild-type CaVβ3 or its mutants. Figure 4A shows representative current traces recorded during depolarizing voltage steps to +10 mV from a holding potential of −80 mV. Control experiments carried out using cells transfected with the wild-type CaVβ3 showed that the average current density (peak current amplitude divided by the respective value of Cm) was -141 ± 24 pA/pF. Recordings performed in cells transfected with the PEST deletions revealed an up-regulation of the macroscopic Ba2+ current. As can be seen in Figure 4B, IBa density measured at +10 mV was significantly increased (~60%) in cells expressing the double PEST deletion (ΔP1–2). Similar results, with nearly the same level of IBa up-regulation, were also observed in cells expressing the single PEST deletions (ΔP1 and ΔP2).
Fig. 4.
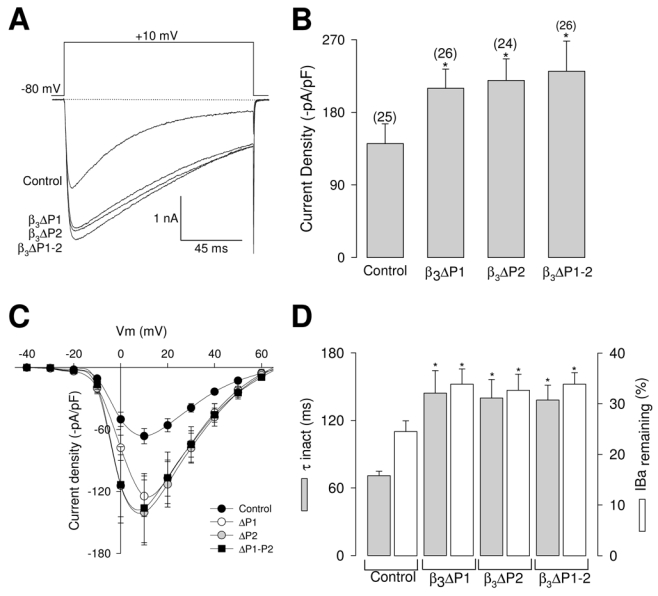
Deletion of the PEST-like sequences alters whole-cell IBa in HEK-293 cells. A, superimposed representative IBa recordings obtained from HEK-293 cells co-expressing neuronal recombinant CaV2.2/α2δ-1 channels and the wild-type CaVβ3 subunit (control) or its PEST deletion mutants (ΔP1, ΔP2 and ΔP1–2) in response to 140 ms test pulses to +10 mV from the holding potential of −80 mV. B, Comparison of peak IBa densities in CaV2.2/α2δ-1 channels coexpressed with wild-type CaVβ3 or the PEST deficient mutants. Data are expressed as mean ± S.E., and the number of recorded cells is indicated in parentheses. Statistical significance was determined by Student’s t-test (*, P<0.05). C, IBa density-voltage relationship averages for HEK- 293 cells co-expressing neuronal recombinant CaV2.2/α2δ-1 channels and the wild-type CaVβ3 subunit (control; filled circles, n = 6) or its single (ΔP1; open circles, n = 7; and ΔP2 gray circles, n = 6) and double PEST deletion mutants (ΔP1–2; filled squares, n = 7). Currents were elicited by eleven 140 ms depolarizing pulses between −40 and +60 mV in 10 mV increments from a holding potential of −80 mV. D, Comparison of inactivation time constants (τinact; gray bars) and percentage of inactivated channels at the end of a test pulse to +10 mV (open bars) for IBa through CaV2.2/α2δ-1 channels co-expressing the wild-type CaVβ3 subunit or its ΔPEST mutants. The time course of inactivation was typically best fit with a monoexponential function. Bars represent mean ± S.E. values of 25 cells in each condition. Statistically significant results are shown by the asterisk (t test; P<0.05).
The above described data are further illustrated in Figure 4C, which shows the IBa density as a function of the voltage step in transfected cells. These current density-voltage relationships indicate that IBa is activated at potentials positive to >−20 mV, and reach its peak at potentials close to +10 mV. The stimulatory effects of PEST deletions on current densities were observed at almost all potentials explored. Interestingly, we noticed that PEST deletions also altered the macroscopic kinetic properties. Normalized currents obtained from either control or cells expressing CaVβ3 mutant subunits showed that the temporal course of the current traces was different (Fig. 4A). Though neither the time to peak nor the time constant for the activation of the current were apparently modified (data not shown), the time constant for the inactivation (τinact) and the percentage of current remaining after 140 ms activating pulses were significantly different between control and cells expressing the mutant subunits (Fig. 4D). It is worth mentioning that the use of Ba2+ as the charge carrier in these experiments may reduce Ca2+- dependent inactivation. Together, these results suggest that the PEST sequences may be important for determining also the effects of CaVβ subunits on channel voltage-dependent inactivation properties. Alternatively, it is also possible that kinetic modifications may be induced by PEST1 deletion through a non specific structural alterations of the CaVβ3 subunit. On the other hand, the PEST2 region is less likely to be involved in such a non specific effect since previous studies have demonstrated that the third variable region (V3) of the protein (where PEST2 is located) may not be necessary for inactivation (Wittemann et al., 2000; Opatowsky et al., 2003).
Given that the deletion of the PEST sequences in CaVβ3 resulted in mutant proteins more resistant to calpain cleavage (Fig. 2) that enhanced functional expression levels of CaV channels (Fig. 4C), we speculated that the calpain system may be responsible for the regulated degradation of the CaVβ3 subunit in vivo. Hence, the observed increase in current density through CaV channels containing mutant subunits may be the result of an increased stability of these proteins in intact cells. If this was the case, inhibition of calpain activity in vivo would decrease CaVβ3 wild-type turnover enhancing the availability of CaVβ3 and promoting the trafficking of the channels to the plasma membrane. Therefore, in order to see whether calpain degrades CaVβ3 under physiological conditions, we treated the HEK-293 cell line cultures with a specific calpain inhibitor. Traces in Figure 5A exemplify representative records of membrane currents through recombinant channels of the CaV2.2/α2δ-1 class co-expressing wild-type CaVβ3 obtained in untreated (control) HEK-293 cells and in cells exposed to calpeptin (25 μM). As can be seen, there was no significant change in whole cell IBa in cells treated for 3 h with the inhibitor. In contrast, longer exposure (6 h) to calpeptin resulted in a significant increase in IBa density (Fig. 5B). On the other hand, there was no significant effect on IBa density in HEK-293 cells expressing recombinant channels that included the CaVβ3 PEST mutant subunits after calpeptin treatment (Figs. 5C and D), consistent with the idea that calpain proteolysis affects CaVβ3 activity through the presence of PEST sequences. Taken as a whole, the results presented above suggest that calpain may be a physiological regulator of CaVβ3 protein turnover.
Fig. 5.
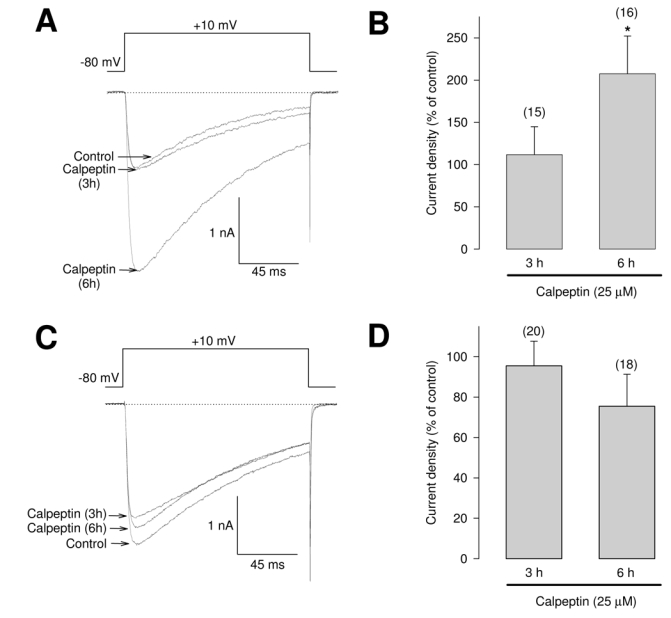
Changes in the functional expression of recombinant N-type (CaV2.2/α2δ-1/β3) Ca2+ channels in HEK-293 cells treated with the specific calpain inhibitor calpeptin. A, Superimposed IBa traces recorded in untreated (control) cells expressing CaV2.2/α2δ-1/β3 channels and cells exposed to calpeptin (25 μM at 37°C for 3–6 h). The currents were elicited by 140 ms voltage steps to +10 mV from a holding potential of −80 mV. B, Summary histogram of IBa densities obtained from cells after 3 or 6 h of exposure to calpeptin. Densities were calculated on dividing peak current amplitudes elicited from voltage steps of −80 to +10 mV, by the whole-cell capacitance. C, Representative superimposed IBa traces in HEK-293 cells co-expressing CaV2.2/α2δ-1 and the mutant ΔP1–2 β3 subunit recorded as in A. D, Histogram of IBa densities obtained from calpeptin-treated cells as listed. Data are expressed as mean ± S.E., and the number of recorded cells is indicated in parentheses. Statistically significant results are shown by the asterisk (t test; P<0.05).
Lastly, as mentioned earlier, our data showed that the deletion of PEST like sequences decreases the degradation of CaVβ3 subunit in vitro (Fig. 2), we therefore aimed to reproduce these results in vivo. In order to determine whether the PEST region deletions affected protein stability, half-lives of the wild-type CaVβ3 subunit and its mutants were measured in metabolic pulse-chase experiments (Fig. 6). In these experiments, HEK-293 cells transiently transfected with the cDNA encoding the CaVβ3 subunits were labeled for 30 min with [35S]-methionine/cysteine and chased with unlabeled methionine and cysteine. After 24 h of chase, proteins were immunoprecipited with a polyclonal anti-β3 raised against the full-length CaVβ3 sequence to reveal most of the proteolytic fragments. As can be seen, the wild-type protein was synthesized as a band with a molecular mass of ~58 kDa which underwent progressive degradation (~85% was degraded after 24 h). This result is consistent with the idea that proteins containing PEST sequences usually have short half lives, and is also consistent with our finding that CaVβ3 may be the target of proteolytic breakdown under physiological conditions. With the ΔPEST mutants, however, less degradation occurred during the 24 h of chase (~28, ~32 and 45% for ΔP1–2, ΔP1 and ΔP2, respectively). This finding suggests that the elevated surface expression of the channels containing the CaVβ3 mutant proteins may be related with enhanced protein halflife.
Fig. 6.
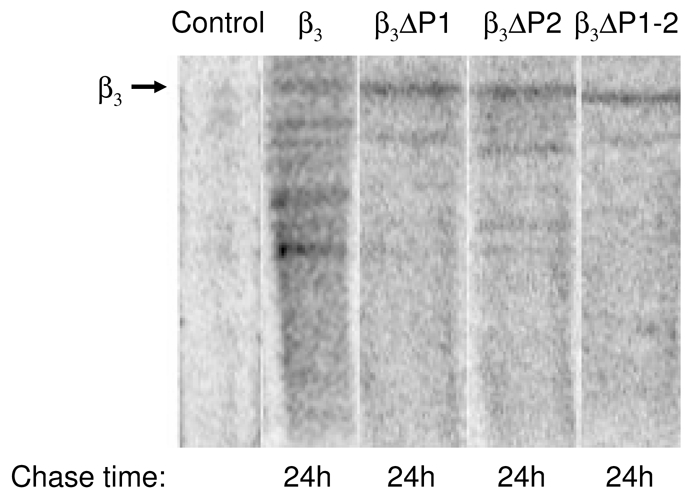
The ΔPEST mutations have an increased protein half-life. Wild type CaVβ3 subunit and its ΔPEST mutants half-lives were determined in HEK-293 cells 24 h after transfection. Cells were pulse-labeled for 40 min. at 37ºC with 500 μCi/μl [35S]-L-containing media. After the pulse, radioactive media was replaced by complete medium; cells were lysed after a 24 h chase period in complete media, and analyzed by SDS-PAGE. An immunoprecipication with a polyclonal anti-β3 IgG was performed to purify CaVβ3 variants and their proteolytic fragments. Lane 1 shows the control precipitation (incubation of protein A sepharose beads with protein extracts containing [35S]-wild-type CaVβ3). Lanes 2, 3, 4 and 5 represent the endogenous proteolytic degradation of the wild-type CaVβ3 subunit and its PEST mutations Δ P1, ΔP2 and ΔP1–2 after immunoprecipitation by the anti-β3 IgGs.
Discussion
A number of proteins related to the metabolism or functions of intracellular Ca2+ have been reported to be substrates for calpain including CaV channels. Initially, the demonstration that the CaVαl subunit was sensitive to calpain suggested a possible mechanism for regulation of CaV channel function. In the skeletal muscle, the C-terminal domain of the CaVα1.1 subunit is sensitive to proteolysis by μ-calpain (De Jongh et al., 1994). This proteolytic cleavage is thought to remove a major site of phosphorylation providing a mechanism for modifying the cAMP-dependent regulation of L-type Ca2+ channels. In addition, calpain proteolysis has been shown to affect the functional activity of CaV channels. In patch clamp studies, Ca2+ currents decline progressively due to “rundown” which depends on the intracellular Ca2+ concentration. This led to the proposal that CaV channels could be degraded by a Ca2+-dependent protease. Interestingly, Ca2+ current rundown in myocytes has been shown to be accelerated by calpain and retarded by the physiological calpain inhibitor calpastatin, suggesting that these proteins may be involved in the regulation of channel activity and/or turnover (Belles et al., 1988; Romanin et al., 1991).
At first glance, the first PEST region which is highly conserved across species and CaVβ subunits appears of particular interest. The last few amino acids of this region contribute to an alpha helix that precedes the beta strands of the SH3 domain in several crystal structures of CaVβ2a (Opatowsky et al., 2004; Van Petegem et al., 2004). Hence, if conserved sequence mediates conserved function, the findings for CaVβ3 might be generalized to the entire family of CaVβ subunits. In addition, as we documented in the Results section, both PEST regions showed modulatory effects on the functional expression of neuronal recombinant CaV channels.
In the present work, we show that calpain proteolysis may also affect the CaVβ3 auxiliary subunit, providing a novel mechanism for modifying the regulation of CaV channels. As mentioned earlier, it has been suggested that calpain may cleave proteins near regions containing PEST sequences (Rechsteiner and Rogers, 1996). It is proposed that these regions increase the local Ca2+ concentration and, in turn, activate calpain. Analysis of the CaVβ3 sequence using the PEST-Find computer program revealed two PEST-like domains in the protein (Fig. 1A). Notably, though the presence of the PEST regions in the sequences of the CaVβ subunits and their possible roles in subunit degradation was suggested initially several years ago (Ruth et al., 1989; Perez- Reyes et al., 1992), their physiological relevance remain virtually unexplored. It is worth mentioning that proteins containing PEST sequences typically have short half lives (~2 h) in intact cells compared with most other proteins (>24 hours). In these proteins, removal or disruption of the PEST sequence increases the half life of the protein while insertion or creation of a new PEST sequence within a PEST sequence free protein decreases this half life. Interestingly, it has been shown that the recombinant CaVβ3 subunit, when expressed alone in a mammalian cell line, is rapidly turned over (2–6 h) (Bogdanov et al., 2000). In contrast to what we observed for the recombinant CaVβ3 used in this study (Fig. 6), which is consistent with a rapid turnover of the protein, it has been noted that the half-life of native CaVβ subunits is about 50 h (Berrow et al., 1995). Though the reason for this discrepancy is unknown, it is possible that the association of the native CaVβ subunits with membrane bound proteins increases their stability.
It is worth mentioning also that proteolytic cleavage within a PEST sequence may not serve for protein degradation only. In this regard, an exciting possibility is that proteolytical cleavage of the full length CaVβ3 may result in the generation of short forms of the protein with potential physiological actions. This is particularly important after the identification of several novel fully functional short variants of the CaVβ1 and CaVβ2 subunits (Foell et al., 2004; Harry et al., 2004; Cohen et al., 2005). In this context, our work might suggest that in addition to the splice isoforms generated through the genetically encoded deletions of specific regions in the CaVβ gene, smaller functional variants could be also formed by post-transcriptional processing of the protein.
On the other hand, the increase in Ca2+ current amplitude induced by transfection of CaVβ3 mutant subunits lacking the PEST regions in the HEK-293 cells suggest that the ΔPEST mutants are more stable than the wild-type protein. In this scenario, the availability of CaVβ3 would be enhanced which may reverse the inhibition imposed by the endoplasmic reticulum (ER) retention signal to the CaVα1 subunit facilitating the cell surface expression of the CaV channel complex. Indeed, PEST deletion seemed to make the ΔPEST mutant proteins less susceptible to calpain cleavage (Fig. 2).
In addition, expression studies have shown that gating as well as regulation of high voltage-activated CaV channels is for a large part determined by the interaction between the CaVα1 and the CaVβ subunits. Though a range of functional effects has been identified for CaVβ, one of the most important actions of this protein is to facilitate the trafficking of the CaVα1 subunit to the plasma membrane, partly by its ability to mask the ER retention signal in CaVα1 (Bichet et al., 2000). However, the CaVβ subunits can also affect the biophysical properties of CaV channels by changing the rates of activation and deactivation by voltage as well as by altering the rate of voltage-induced inactivation, the inhibition by G protein βγ dimers, and/or the coupling of voltage sensing to pore opening (Birnbaumer et al., 1998; Walker and De Waard, 1998; Arikkath and Campbell, 2003; Dolphin, 2003).
In particular, diverse studies have revealed that CaVβ subunits have a marked effect on voltage-dependent inactivation of CaV channels, a key mechanism that contributes to the precise control of Ca2+ entry into cells. Whilst the CaVα1 subunit contains inherent determinants of inactivation, association with different CaVβ subunits determines their overall inactivation rate (Birnbaumer et al., 1998; Walker and De Waard, 1998; Dolphin, 2003). Though the precise mechanisms of voltage-dependent inactivation are not well understood, it is clear that subunit composition differentially affects the inactivation properties of CaV channels. In general, wholecell patch clamp studies indicate that co-expression of CaVβ1b, β2a, and β4 with CaVα1 subunits do not modify the inactivation rate noticeably, whereas CaVβ3 markedly enhances inactivation (Dolphin, 2003). Interestingly, our functional studies on the effects of the PEST sequences using CaVβ3 mutant subunits suggested a role for these regions in the regulation of neuronal N-type recombinant CaV channel activity by inactivation (Fig. 4D).
In response to membrane depolarization, control CaV channels quickly activate followed by rapid inactivation (Fig. 4A). In contrast, the channels containing mutant CaVβ3 subunits lacking the PEST regions displayed slower inactivation kinetics, resulting in a much smaller fraction of inactivated channels at the end of the test pulse (Fig. 4D). In each case, the halfactivation potentials closely aligned with those observed in the presence of the wild-type CaVβ3 subunit, and therefore, any putative effects of the mutant subunits on inactivation kinetics would unlikely be due to altered voltage dependence of activation gating. Interestingly, a variable region (V1) found at the N-terminal of CaVβ3 comprised of a short 14-amino acid stretch has been recently reported as a critical site for voltage-induced inactivation (Stotz et al., 2004). In addition, a second variable region (V2) in combination with one of the conserved domains of the protein can also contribute to regulate CaV2.2 inactivation (Stotz et al., 2004). However, the PEST1 sequence in CaVβ3 is not located in a variable region of the protein, suggesting the presence of numerous domains in CaVβ3 capable of conferring rapid inactivation kinetics.
Although diverse studies with CaVβ subunits suggest that some of their effects on channel activity require phosphorylation (Dolphin, 2003) little is known regarding the role of this process on CaV channel inactivation. By using the NetPhos 2.0 software (available at the URL http://www.cbs.dtu.dk/services/NetPhos/) which produces predictions for serine, threonine and tyrosine phosphorylation sites in eukaryotic proteins (Blom et al., 1999), we found numerous phosphorylation sites for protein kinases on the CaVβ3 PEST-like sequences. Proteolysis of CaVβ3 by calpain would remove the phosphorylation consensus sites and the potential regulation of CaV channel inactivation through phosphorylation at these sites would also be abolished. Further studies are necessary to define whether the protein kinase-mediated regulation of the CaVβ3 subunit may be directly involved in the regulation of the inactivation process.
The biochemical and electrophysiological studies described in the present study show that the CaVβ3 auxiliary subunit is sensitive to μ-calpain digestion within its PEST-like regions and suggest that this enzyme may play a critical role in regulating CaVβ3 turnover. In addition, the results provide evidence that the CaVβ3 PEST-like sequences might be regulatory segments that influence the voltage-dependent inactivation properties of CaV channels. Lastly, if conserved sequence mediates conserved function, it would be interesting to investigate whether the findings for CaVβ3 could be generalized to the entire family of CaVβ subunits.
Acknowledgments
This work was supported by grants from Conacyt and The Miguel Aleman Foundation to RF. Doctoral (A.S.) and postdoctoral (N.O.) fellowships from Conacyt are gratefully acknowledged. We are in debt with Drs. B.A. Adams (Utah State University) and K.P. Campbell (University of Iowa) for their generous gift of the cDNA clones and antibodies. We thank G. Aguilar for assistance with DNA sequencing as well as A. Andrade and L. Escobedo for expert technical assistance.
References
- Arikkath J, Campbell KP. Auxiliary subunits: essential components of the voltage-gated calcium channel complex. Curr Opin Neurobiol. 2003;13:298–307. doi: 10.1016/s0959-4388(03)00066-7. [DOI] [PubMed] [Google Scholar]
- Avila G, Sandoval A, Felix R. Intramembrane charge movement associated with endogenous K+ channel activity in HEK-293 cells. Cell Mol Neurobiol. 2004;24:317–330. doi: 10.1023/B:CEMN.0000022765.52109.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball SL, Powers PA, Shin HS, Morgans CW, Peachey NS, Gregg RG. Role of the β2 subunit of voltage-dependent calcium channels in the retinal outer plexiform layer. Invest Ophthalmol Vis Sci. 2002;43:1595–1603. [PubMed] [Google Scholar]
- Belles B, Hescheler J, Trautwein W, Blomgren K, Karlsson JO. A possible physiological role of the Ca-dependent protease calpain and its inhibitor calpastatin on the Ca current in guinea pig myocytes. Pflugers Arch. 1988;412:554–556. doi: 10.1007/BF00582548. [DOI] [PubMed] [Google Scholar]
- Berrow NS, Campbell V, Fitzgerald EM, Brickley K, Dolphin AC. Antisense depletion of β-subunits modulates the biophysical and pharmacological properties of neuronal calcium channels. J Physiol. 1995;482:481–491. doi: 10.1113/jphysiol.1995.sp020534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bichet D, Cornet V, Geib S, Carlier E, Volsen S, Hoshi T, Mori Y, De Waard M. The I-II loop of the Ca2+ channel α1 subunit contains an endoplasmic reticulum retention signal antagonized by the β subunit. Neuron. 2000;25:177–190. doi: 10.1016/s0896-6273(00)80881-8. [DOI] [PubMed] [Google Scholar]
- Birnbaumer L, Qin N, Olcese R, Tareilus E, Platano D, Costantin J, Stefani E. Structures and functions of calcium channel β subunits. J Bioenerg Biomembr. 1998;30:357–375. doi: 10.1023/a:1021989622656. [DOI] [PubMed] [Google Scholar]
- Blom N, Gammeltoft S, Brunak S. Sequence and structure-based prediction of eukaryotic protein phosphorylation sites. J Mol Biol. 1999;294:1351–1362. doi: 10.1006/jmbi.1999.3310. [DOI] [PubMed] [Google Scholar]
- Bogdanov Y, Brice NL, Canti C, Page KM, Li M, Volsen SG, Dolphin AC. Acidic motif responsible for plasma membrane association of the voltage-dependent calcium channel β1b subunit. Eur J Neurosci. 2000;12:894–902. doi: 10.1046/j.1460-9568.2000.00981.x. [DOI] [PubMed] [Google Scholar]
- Burgess DL, Jones JM, Meisler MH, Noebels JL. Mutation of the Ca2+ channel β subunit gene Cchb4 is associated with ataxia and seizures in the lethargic (lh) mouse. Cell. 1997;88:385–392. doi: 10.1016/s0092-8674(00)81877-2. [DOI] [PubMed] [Google Scholar]
- Castellano A, Wei X, Birnbaumer L, Perez-Reyes E. J Biol Chem. 1993;268:3450–3455. [PubMed] [Google Scholar]
- Catterall WA. Structure and regulation of voltage-gated Ca2+ channels. Annu Rev Cell Dev Biol. 2000;16:521–555. doi: 10.1146/annurev.cellbio.16.1.521. [DOI] [PubMed] [Google Scholar]
- Catterall WA, Striessnig J, Snutch TP, Perez-Reyes E. International Union of Pharmacology. XL. Compendium of voltage-gated ion channels: calcium channels. Pharmacol Rev. 2003;55:579–581. doi: 10.1124/pr.55.4.8. [DOI] [PubMed] [Google Scholar]
- Chen YH, Li MH, Zhang Y, He LL, Yamada Y, Fitzmaurice A, Shen Y, Zhang H, Tong L, Yang J. Structural basis of the α1-β subunit interaction of voltage-gated Ca2+ channels. Nature. 2004;429:675–680. doi: 10.1038/nature02641. [DOI] [PubMed] [Google Scholar]
- Cohen RM, Foell JD, Balijepalli RC, Shah V, Hell JW, Kamp TJ. Unique modulation of L-type Ca2+ channels by short auxiliary β1d subunit present in cardiac muscle. Am J Physiol Heart Circ Physiol. 2005;288:H2363–H2374. doi: 10.1152/ajpheart.00348.2004. [DOI] [PubMed] [Google Scholar]
- De Jongh KS, Colvin AA, Wang KK, Catterall WA. Differential proteolysis of the fulllength form of the L-type calcium channel α1 subunit by calpain. J Neurochem. 1994;63:1558–1564. doi: 10.1046/j.1471-4159.1994.63041558.x. [DOI] [PubMed] [Google Scholar]
- De Waard M, Pragnell M, Campbell KP. Ca2+ channel regulation by a conserved beta subunit domain. Neuron. 1994;13:495–503. doi: 10.1016/0896-6273(94)90363-8. [DOI] [PubMed] [Google Scholar]
- Dolphin AC. β subunits of voltage-gated calcium channels. J Bioenerg Biomembr. 2003;35:599–620. doi: 10.1023/b:jobb.0000008026.37790.5a. [DOI] [PubMed] [Google Scholar]
- Felix R, Gurnett CA, De Waard M, Campbell KP. Dissection of functional domains of the voltage-dependent Ca2+ channel α2δ subunit. J Neurosci. 1997;15:6884–6891. doi: 10.1523/JNEUROSCI.17-18-06884.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foell JD, Balijepalli RC, Delisle BP, Yunker AM, Robia SL, Walker JW, McEnery MW, January CT, Kamp TJ. Molecular heterogeneity of calcium channel beta-subunits in canine and human heart: evidence for differential subcellular localization. Physiol Genomics. 2004;17:183–200. doi: 10.1152/physiolgenomics.00207.2003. [DOI] [PubMed] [Google Scholar]
- Fujita Y, Mynlieff M, Dirksen RT, Kim MS, Niidome T, Nakai J, Friedrich T, Iwabe N, Miyata T, Furuichi T, Furutama D, Mikoshiba K, Mori Y, Beam KG. Neuron. 1993;10:585–598. doi: 10.1016/0896-6273(93)90162-k. [DOI] [PubMed] [Google Scholar]
- Gerhardstein BL, Puri TS, Chien AJ, Hosey MM. Identification of the sites phosphorylated by cyclic AMP-dependent protein kinase on the β2 subunit of L-type voltagedependent calcium channels. Biochemistry. 1999;38:10361–10370. doi: 10.1021/bi990896o. [DOI] [PubMed] [Google Scholar]
- Gregg RG, Messing A, Strube C, Beurg M, Moss R, Behan M, Sukhareva M, Haynes S, Powell JA, Coronado R, Powers PA. Absence of the β subunit (cchb1) of the skeletal muscle dihydropyridine receptor alters expression of the α1 subunit and eliminates excitation-contraction coupling. Proc Natl Acad Sci USA. 1996;93:13961–13966. doi: 10.1073/pnas.93.24.13961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurnett CA, Felix R, Campbell KP. Extracellular interaction of the voltage-dependent Ca2+ channel α2δ and α1 subunits. J Biol Chem. 1997;272:18508–18512. doi: 10.1074/jbc.272.29.18508. [DOI] [PubMed] [Google Scholar]
- Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high resolution current recording from cells and cell-free membrane patches. Pflügers Archiv. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hanlon MR, Berrow NS, Dolphin AC, Wallace BA. Modelling of a voltage-dependent Ca2+ channel β subunit as a basis for understanding its functional properties. FEBS Lett. 1999;445:366– 370. doi: 10.1016/s0014-5793(99)00156-8. [DOI] [PubMed] [Google Scholar]
- Harry JB, Kobrinsky E, Abernethy DR, Soldatov NM. New short splice variants of the human cardiac CaVβ2 subunit: redefining the major functional motifs implemented in modulation of the CaV1.2 channel. J Biol Chem. 2004;279:46367–46372. doi: 10.1074/jbc.M409523200. [DOI] [PubMed] [Google Scholar]
- Kim HL, Kim H, Lee P, King RG, Chin H. Rat brain expresses an alternatively spliced form of the dihydropyridine-sensitive L-type Ca2+ channel α2 subunit. Proc Natl Acad Sci. 1992;89:3251–3255. doi: 10.1073/pnas.89.8.3251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohn AB, Roberts-Misterly JM, Anderson PA, Khan N, Greenberg RM. Specific sites in the Beta Interaction Domain of a schistosome Ca2+ channel β subunit are key to its role in sensitivity to the anti-schistosomal drug praziquantel. Parasitology. 2003;127 (Pt 4):349–356. doi: 10.1017/s003118200300386x. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Liu H, De Waard M, Scott VE, Gurnett CA, Lennon VA, Campbell KP. Identification of three subunits of the high affinity omega-conotoxin MVIIC-sensitive Ca2+ channel. J Biol Chem. 1996;271:13804–13810. [PubMed] [Google Scholar]
- McEnery MW, Copeland TD, Vance CL. Altered expression and assembly of N-type calcium channel α1B and β subunits in epileptic lethargic (lh/lh) mouse. J Biol Chem. 1998;273:21435– 21438. doi: 10.1074/jbc.273.34.21435. [DOI] [PubMed] [Google Scholar]
- McGee AW, Nunziato DA, Maltez JM, Prehoda KE, Pitt GS, Bredt DS. Calcium channel function regulated by the SH3-GK module in beta subunits. Neuron. 2004;42:89–99. doi: 10.1016/s0896-6273(04)00149-7. [DOI] [PubMed] [Google Scholar]
- Olcese R, Qin N, Schneider T, Neely A, Wei X, Stefani E, Birnbaumer L. The amino terminus of a calcium channel beta subunit sets rates of channel inactivation independently of the subunit’s effect on activation. Neuron. 1994;13:1433–1438. doi: 10.1016/0896-6273(94)90428-6. [DOI] [PubMed] [Google Scholar]
- Opatowsky Y, Chomsky-Hecht O, Kang MG, Campbell KP, Hirsch JA. The voltagedependent calcium channel β subunit contains two stable interacting domains. J Biol Chem. 2003;278:52323–52332. doi: 10.1074/jbc.M303564200. [DOI] [PubMed] [Google Scholar]
- Opatowsky Y, Chen CC, Campbell KP, Hirsch JA. Structural analysis of the voltagedependent calcium channel β subunit functional core and its complex with the α1 interaction domain. Neuron. 2004;42:387–399. doi: 10.1016/s0896-6273(04)00250-8. [DOI] [PubMed] [Google Scholar]
- Perez-Reyes E, Castellano A, Kim HS, Bertrand P, Baggstrom E, Lacerda AE, Wei XY, Birnbaumer L. Cloning and expression of a cardiac/brain β subunit of the L-type calcium channel. J Biol Chem. 1992;25:1792–1797. [PubMed] [Google Scholar]
- Rechsteiner M, Rogers SW. PEST sequences and regulation by proteolysis. Trends Biochem Sci. 1996;21:267–271. [PubMed] [Google Scholar]
- Restituito S, Cens T, Barrere C, Geib S, Galas S, De Waard M, Charnet P. The β2a subunit is a molecular groom for the Ca2+ channel inactivation gate. J Neurosci. 2000;20:9046–9052. doi: 10.1523/JNEUROSCI.20-24-09046.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards MW, Butcher AJ, Dolphin AC. Ca2+ channel β-subunits: structural insights AID our understanding. Trends Pharmacol Sci. 2004;25:626–632. doi: 10.1016/j.tips.2004.10.008. [DOI] [PubMed] [Google Scholar]
- Romanin C, Grösswagen P, Schindler H. Calpastatin and nucleotides stabilise cardiac calcium channel activity in excised patches. Pfiugers Arch. 1991;418:86–92. doi: 10.1007/BF00370456. [DOI] [PubMed] [Google Scholar]
- Ruth P, Rohrkasten A, Biel M, Bosse E, Regulla S, Meyer HE, Flockerzi V, Hofmann F. Primary structure of the beta subunit of the DHP-sensitive calcium channel from skeletal muscle. Science. 1989;245:1115–1118. doi: 10.1126/science.2549640. [DOI] [PubMed] [Google Scholar]
- Sandoz G, Bichet D, Cornet V, Mori Y, Felix R, De Waard M. Distinct properties and differential beta subunit regulation of two C-terminal isoforms of the P/Q-type Ca2+-channel α1A subunit. Eur J Neurosci. 2001;14:987–997. doi: 10.1046/j.0953-816x.2001.01728.x. [DOI] [PubMed] [Google Scholar]
- Scott VE, De Waard M, Liu H, Gurnett CA, Venzke DP, Lennon VA, Campbell KP. β subunit heterogeneity in N-type Ca2+ channels. J Biol Chem. 1996;271:3207–3212. doi: 10.1074/jbc.271.6.3207. [DOI] [PubMed] [Google Scholar]
- Shimada M, Mahon MJ, Greer PA, Segre GV. The receptor for parathyroid hormone and parathyroid hormone-related peptide is hydrolyzed and its signaling properties are altered by directly binding the calpain small subunit. Endocrinology. 2005;146:2336–2344. doi: 10.1210/en.2004-1637. [DOI] [PubMed] [Google Scholar]
- Shumway SD, Maki M, Miyamoto S. The PEST domain of IκBα is necessary and sufficient for in vitro degradation by μ-calpain. J Biol Chem. 1999;274:30874–30881. doi: 10.1074/jbc.274.43.30874. [DOI] [PubMed] [Google Scholar]
- Stotz SC, Barr W, McRory JE, Chen L, Jarvis SE, Zamponi GW. Several structural domains contribute to the regulation of N-type calcium channel inactivation by the beta 3 subunit. J Biol Chem. 2004;279:3793–3800. doi: 10.1074/jbc.M308991200. [DOI] [PubMed] [Google Scholar]
- Takahashi SX, Miriyala J, Colecraft HM. Membrane-associated guanylate kinase-like properties of β-subunits required for modulation of voltage-dependent Ca2+ channels. Proc Natl Acad Sci USA. 2004;101:7193–7198. doi: 10.1073/pnas.0306665101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Petegem F, Clark KA, Chatelain FC, Minor DL., Jr Structure of a complex between a voltage-gated calcium channel β-subunit and an α-subunit domain. Nature. 2004;429:671–675. doi: 10.1038/nature02588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker D, De Waard M. Subunit interaction sites in voltage-dependent Ca2+ channels: role in channel function. Trends Neurosci. 1998;21:148–154. doi: 10.1016/s0166-2236(97)01200-9. [DOI] [PubMed] [Google Scholar]
- Wang W, Malcolm BA. Two-stage polymerase chain reaction protocol allowing introduction of multiple mutations, deletions, and insertions, using QuikChange site-directed mutagenesis. Methods Mol Biol. 2002;182:37–43. doi: 10.1385/1-59259-194-9:037. [DOI] [PubMed] [Google Scholar]
- Wittemann S, Mark MD, Rettig J, Herlitze S. Synaptic localization and presynaptic function of calcium channel beta 4-subunits in cultured hippocampal neurons. J Biol Chem. 2000;275:37807–37814. doi: 10.1074/jbc.M004653200. [DOI] [PubMed] [Google Scholar]


