Abstract
Novel C-seco-taxoids were synthesized from 10-deacetylbaccatin III and their potencies evaluated against drug-sensitive and drug-resistant cancer cell lines. The drug-resistant cell lines include ovarian cancer cell lines resistant to cisplatin, topotecan, adriamycin and paclitaxel overexpressing class III β-tubulin, A2780TC1 and A2780TC3. The last two cell lines were selected through chronic exposure of A2780wt to paclitaxel and Pgp blocker cyclosporine. All novel C-seco-taxoids exhibited remarkable potency against A2780TC1 and A2780TC3 cell lines, and no cross resistance to cisplatin- and topotecan-resistant cell lines, A2780CIS and A2780TOP. Four of those C-seco-taxoids exhibit much higher activities than IDN5390 against paclitaxel-resistant cell lines, A2780ADR, A2780TC1 and A2780TC3. SB-CST-10202 possesses the best all-round high potencies across different drug-resistant cell lines. Molecular modeling studies, including molecular dynamics simulations, on the drug-protein complexes of class I and III β-tubulins were performed to identify possible cause of the remarkable potency of these C-seco-taxoids against paclitaxel-resistant cell lines overexpressing class III β-tubulin.
Paclitaxel and docetaxel represent two of the most important chemotherapeutic drugs currently used for the treatment of ovarian cancer, breast cancer, melanoma, non-small cell lung cancer and Kaposi’s sarcoma. Upon prolonged exposure to antitumor agents, cancer cells develop mechanisms of drug-resistance, thus becoming progressively less sensitive to the effect of drugs. Higher doses are then required to achieve the same efficacy with consequent increase in systemic toxicity to normal tissues.
A great effort has been devoted to overcome these limitations, by studying in detail the mechanisms of drug-resistance and by identifying novel and highly specific anticancer drugs. Nevertheless, an increasingly prominent mechanism of drug resistance, which has not been successfully addressed yet, is related to the overexpression of specific tubulin isotypes.1–4 Tubulin dimers, derived from different β isotypes have different behaviors in vitro in regard to assembly, dynamics, conformation and ligand binding.1,2,5–7 In analogy, microtubules with altered β-tubulin isotype composition have different dynamics in response to paclitaxel.8
The equilibrium between growing and shortening of microtubule ends is defined as dynamic instability.9 The effect of paclitaxel on the dynamic instability of microtubules depends on its concentration levels. At sub-stoichiometric levels, paclitaxel is able to suppress shortening rates, but it does not affect growing rate of polymer mass. At stoichiometric levels with respect to tubulin, paclitaxel can almost completely suppress microtubule dynamics.10 Derry and coworkers reported that αβ III microtubules are more dynamic than αβII or αβIV and higher concentrations of paclitaxel were required to suppress their dynamic polymerization.8
In vertebrates there are 6 and 7 isotypes of α and β tubulins, respectively.11,12 Most of the amino acid mutations are concentrated at the carboxyl-terminal and a lower level of diversity is present in the amino terminal region.13 The analysis of β-tubulin genes in different species has shown that class I β-tubulin is the major isotype, expressed in all tissues of mice, rats and human.
Class III isotype is characterized by a higher level of heterogeneity and the expression of this class has been observed in chordate or chicken brain and identified as a minor neuronal isotype. However, Kavallaris has recently reported that paclitaxel-resistant epithelial ovarian tumor cell lines overexpress two brain-specific β-tubulin isotypes, Hβ4 (class III) and H5β (class IVa), which normally are not expressed in epithelial tissues.3
The clinical relevance of the overexpression of class III β-tubulin on paclitaxel-resistance has been demonstrated in a recent review on the mechanism of action of ixabepilone, an epothilone aza-analogue.14
Ferlini et al. have reported that C-seco-taxoid IDN 5390, is up to 8-fold more potent than paclitaxel against the inherently drug-resistant cell line OVCAR3 and paclitaxel-resistant human ovarian adenocarcinoma cell lines, A2780TC1 and A2780TC3.15 The last two cell lines are selected through chronic exposure of A2780wt to paclitaxel and Pgp blocker cyclosporine. Structural analysis of the three cell lines revealed the overexpression of the class III β-tubulin mRNA level, without significant changes in the levels of class I, IVa and IVb.15,16,17
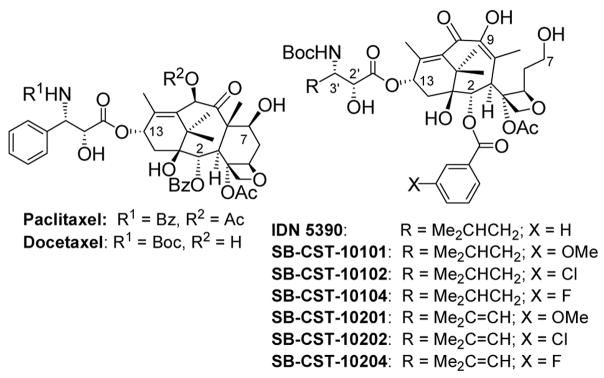
To further explore the unique activity of C-seco-taxoids, against drug-resistant cell lines overexpressing class III β-tubulin, we designed and synthesized several new analogues of IDN 5390 with modifications at the C2 and C3′ positions.
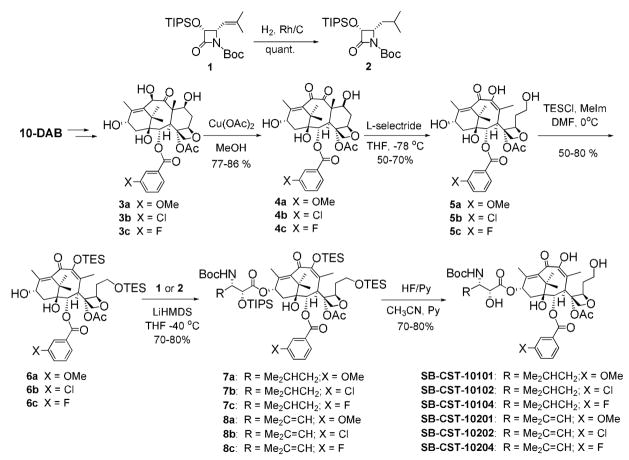
Since it has been shown in the SAR study on paclitaxel and taxoids that the introduction of a substituent to the meta-position of the C2 benzoyl moiety increases potency,18–20 we introduced methoxy, chloro and fluoro groups to this position of the IDN 5390 structure (figure 1). Also, an isobutenyl (Me2C=CH) group was introduced to the C3′ position in addition to an isobutyl (Me2CHCH2) group, which is the C3′ substituent of IDN 5390. Six analogues of IDN 5390 (Scheme 1) were synthesized using the β-Lactam Synthon Method,21 through Ojima-Holton coupling21–24 of modified C-seco-10-deacetylbaccatins 6a–c with β-lactams 1 and 2. Attempts to replace the C2 benzoyl moiety of a C-seco-baccatin with a substituted benzoyl group failed.25 Accordingly, it was necessary to modify the C2 position of 10-deacetylbaccatin (10-DAB) before the cleavage of the C-ring. Thus, C2-modified 10-deacetylbaccatins 3a–c were prepared first.19,20 Then, 3a–c were oxidized at C10 using Appendino’s protocol.26 The resulting 4a–c (a mixture of epimers at C7) was treated with L-selectride at −78 °C in THF to afford C-seco-baccatins 5a–c (Scheme 1).
Figure 1.
Scheme 1.
Two hydroxyl groups at C7 and C9 were protected as TES ethers to give 7,9-diTES-C-seco-baccatins 6a–c. The Ojima-Holton coupling of 6a–c with β-lactam 127 afforded 7,9,2′-protected C-seco-taxoids 7a–c in good yields. Subsequent deprotection with HF-pyridine afforded C-seco-taxoids SB-CST-10201, SB-CST-10202 and SB-CST-10204.
In a similar manner, C3′-isobutyl analogues, SB-CST-10101, SB-CST-10102 and SB-CST-10104, were synthesized from 4-isobutyl-β-lactam 228 and 6a–c via 8a–c.
It should be noted that, due to the flexibility of the C-seco-taxoid, a rather slow dynamic conformational equilibrium is present at room temperature, which results in broad NMR signals. Accordingly, it was necessary to increase the temperature to 130 °C to accelerate the equilibrium and obtain sharp 1H NMR spectra for characterization, as reported by Appendino et al.29
Novel C-seco-taxoids were evaluated for their cytotoxicity against several human ovarian adenocarcinoma cell lines: A2780wt (drug-sensitive wild-type), A2780CIS, A2780TOP, A2780ADR (resistant to cisplatin, topotecan, and adriamycin/doxorubicin, respectively), and A2780TC1 and A2780TC3 (resistant to both paclitaxel and cyclosporine A). The drug resistance in the A2780ADR cell line is based on MDR, while that in the A2780TC1 and A2780TC3 cell lines is caused by the overexpression of class III β-tubulin subunit and other possible mutations.
Thus, the activity of these new C-seco-taxoids is of particular interest. Results are summarized in Table 1.
Table 1.
IC50 (nM)a of C-Seco-Taxoids
| R | X | A2780wtb | A2780CISc | A2780TOPd | A2780ADRe | A2780TC1f | A2780TC3f | |
|---|---|---|---|---|---|---|---|---|
| Paclitaxelg | 1.7±1.2 | 2.2±0.2 | 7.2±1.5 | 1,239±265 | 10,027±3,195 | 17,800±5,499 | ||
| IDN 5390 | Me2CHCH2 | H | 17.4±1.5 | 16.8±3.1 | 27.5±5.1 | 2,617±1,028 | 2,060±344 | 2,237±471 |
| SB-CST-10101 | Me2CHCH2 | OMe | 6.8±3.6 | 4.3±1.0 | 3.5±1.6 | 356±74 | 1,121±454 | 211±50 |
| SB-CST-10102 | Me2CHCH2 | Cl | 5.5±3.3 | 4.1±1.8 | 4.6±1.3 | 604±246 | 1,801±988 | 385±6.0 |
| SB-CST-10104 | Me2CHCH2 | F | 11.1±8.4 | 11.8±1.0 | 12.8±3.5 | 3,726±198 | 1,497±31 | 460±128 |
| SB-CST-10201 | Me2C=CH | OMe | 4.6±3.2 | 5.7±2.4 | 2.6±1.9 | 386±181 | 1,057±185 | 490±212 |
| SB-CST-10202 | Me2C=CH | Cl | 4.6±0.4 | 4.0±0.1 | 1.7±0.4 | 452±28 | 751±11 | 357±126 |
| SB-CST-10204 | Me2C=CH | F | 6.1±0.6 | 4.9±0.2 | 6.9±0.8 | 2,218±588 | 4,454±1,391 | 745±60 |
Concetration of drug which inhibits cell growth by 50% (72 h continuous exposure).
Human ovarian adenocarcinoma cell line (wild type).
Cisplatin resistant cell line.
Topotecan-resistant cell line.
Adriamycin(doxorubicin)-resistant cell line.
paclitaxel-resistant cell line overexpressing class III β-tubulin.
IC50 data are taken from Ref. 15 for comparison.
As Table 1 shows, all new C-seco-taxoids possess remarkable potency against the paclitaxel-resistant cell line, A2780TC3, i.e., the most drug-resistant cell line for paclitaxel in this series (24–84 times more potent than paclitaxel and 3–11 times more potent than IDN 5390). The resistance factor for this cell line, i.e., IC50 (A2780TC3)/IC50 (A2780wt), is 10,470 for paclitaxel, but it is only 31 for SB-CST-10101. For comparison, IDN 5390 exhibits 8.0 times higher potency than paclitaxel with a resistance factor of 129 against the same cell line. These results are quite impressive by taking into account the fact that the only structural difference between IDN 5390 and these C-seco-taxoids is one substitution at the meta position of the C2-benzoate moiety of the C-seco-taxoid molecule. These C-seco-taxoids also exhibit 2.3–13 times higher potency than paclitaxel against the A2780TC1 cell line, and more potent than IDN5390 except SB-CST-10204. The resistance factor of paclitaxel for this cell line is 5,898, but that of SB-CST-10202 is 163.
The potencies of these C-seco-taxoids against A2780ADR (Pgp-based MDR cell line) are mixed. While SB-CST-10101, SB-CST-10102, SB-CST-10201, and SB-CST-10202 (X = OMe or Cl) possess 2.1–3.5 times and 4.3–7.4 times higher potency than paclitaxel and IDN5390, respectively, SB-CST-10104 and SB-CST-10204 (X = F) are less potent than paclitaxel. Thus, it appears that fluorine substitution at this position makes these C-seco-taxoids better substrate for Pgp efflux. The potencies of these C-seco-taxoids against A2780CIS and A2780TOP do not show any appreciable cross resistance, as anticipated.
The C3′-substitutent of C-seco-taxoids, i.e., a 3′-isobutyl or 3′-isobutenyl group, shows some effect on potency, but without a consistent trend. For example, SB-CST-10204 (C3′ = isobutenyl; X = F) exhibits higher potency than SB-CST-10104 (C3′ = isobutyl; X = F) against A2780wt, A2780CIS, A2780TOP and A2780ADR. However, the reversal of this SAR is observed against A2780TC1 and A2780TC3 in which the class III β-tubulin is overexpressed. In contrast, SB-CST-10202 (C3′ = isobutenyl; X = Cl) exhibits consistently higher potency than SB-CST-10102 (C3′ = isobutyl; X = Cl) against all cell lines examined.
Overall, SB-CST-10202 (C3′ = isobutenyl; X = Cl) appears to be the best drug candidate with all-round high potencies across different drug-resistant cell lines.
To investigate differences in the interactions of taxane molecules with class I and III β-tubulins, we also performed computational analyses. Following up recent molecular modeling studies of paclitaxel and IDN5390 in human class I and III β-tubulin,15,30 we employed molecular dynamics (MD) simulation to predict the binding conformation of C-seco-taxoids in class I and III β-tubulins. A cryo-EM crystal structure of bovine brain tubulin (1JFF)31 was used as the template to create 3-D class I and III β-tubulin models, TubB1 and TubB3, respectively.32 The protein sequences were obtained from SwissProt (TubB1 code: TBB5-HUMAN, P07437; TubB3 code: TBB3-HUMAN, Q13509)33 and aligned using the CLUSTALX program.12,34 A standard comparative modeling procedure was used by replacing the side chains of the template with the antechamber program in the AMBER9 package, followed by energy minimization.35
Since C-seco-taxoids are very flexible, attempted direct docking with the DOCK® program failed to provide reasonable binding conformations.36 Thus, C-seco-taxoid molecules were manually docked into the proteins with the InsightII® 2000 program, based on the conformation of paclitaxel in 1JFF.31
The complexes were solvated in a 8-Å truncated octahedron of TIP3P explicit water.37 The solvated complexes were then equilibrated by carrying out a short minimization, which was followed by a simulation with a restrain on protein backbones from 0 to 1.4 ns and without restrain in the following 3.9 ns (totally 5.3 ns). The coordinates were recorded every 10 ps wherein temperature, density, total energy and root mean square deviation (rmsd) were monitored.35
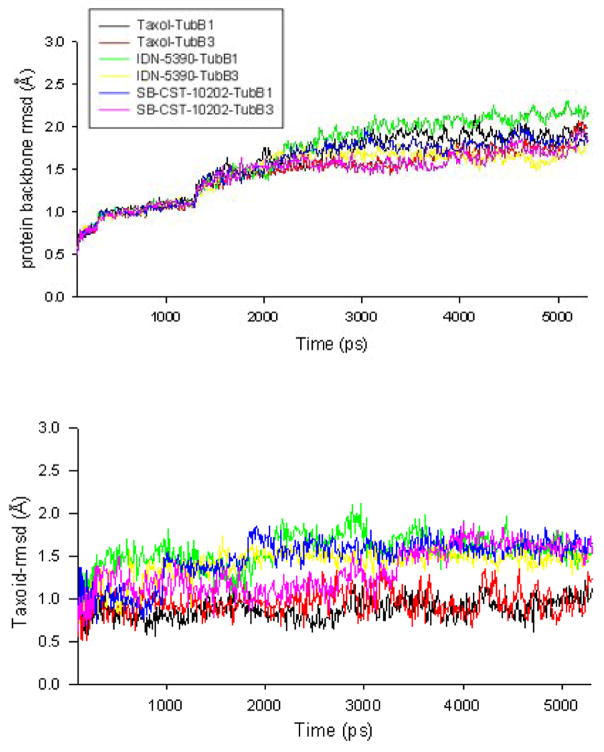
The rmsd values of the backbone of the protein (TubB1 or TubB3) – paclitaxel/C-seco-taxoid complexes are shown in Figure 2A, which indicate the conformational changes in the backbone of the two proteins. As Figure 2A shows, the rmsd values of the tubulin backbones increased gradually, until it reached equilibrium after ~3000 ps. The largest rmsd value of the backbones of class I and III β-tubulins was less than 2.2 Å. Because of the flexibility of C-seco-taxoid molecules, the rmsd values of IDN-5390 and SB-CST-10202 (~1.5 Å) were higher than those of paclitaxel (~1.0 Å) (Figure 2B).
Figure 2.
Figurer 2A. rmsd of β-tubulin class I and III
Figure 2B. rmsd of taxoids
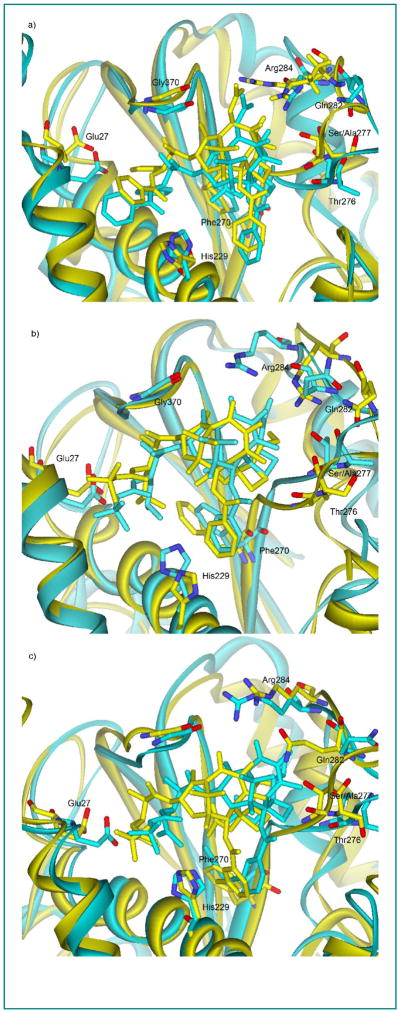
Snapshots of the binding conformation of paclitaxel and C-seco-taxoids in TubB1 and TubB3 at the end of the simulations (5.3 ns) are shown in Figure 3. The overall protein structures of TubB1 and TubB3 are similar, but the M-loop of TubB3 (cyan) has higher flexibility than that of TubB1 (yellow), which is very likely to be caused by the loss of a H-bonding between Ser277 and Ser280 in TubB1 by the mutation of Ser277 to Ala277 in TubB3.31 As Figures 3 shows, there is an appreciable change in the relative position of paclitaxel in TubB3 as compared to that of paclitaxel in TubB1 towards M-loop (Figure 3a), i.e., the paclitaxel in TubB3 is closer to the M-loop, most likely due to the higher flexibility of the M-loop in TubB3. The same positional shift is observed for the C-seco-taxoids (Figures 3b and 3c). Our molecular modeling analysis indicates that the two mutations (Cys241Ser and Ser277Ala) in the binding site do not have direct interaction with paclitaxel and C-seco-taxoids. However, a couple of H-bonding interactions appear to be critical for the difference in the binding mode of paclitaxel and C-seco-taxoids in the class I β-tubulin (TubB1) and the class III β-tubulin (TubB3). The C7-OH of paclitaxel can form a H-bond with Arg284, but this H-bond would be substantially weakened by increased flexibility of M-loop in TubB3. Thus, the binding of paclitaxel to the class III β-tublin should be less favorable than that to the class I β-tubulin. On the other hand, the flexible C7-OH of C-seco-taxoids can form a H-bond with Gln282 or Arg284. This H-bonding would not be affected by the increased flexibility of M-loop in the class III β-tubulin, allowing C-seco-taxoids to keep high affinity to the class III β-tubulin. Our analysis also indicates a favorable interaction of His229 with the meta-substituent of the C2-benzoate moiety in both TubB1 and TubB3.
Figure 3.
Snapshots of paclitaxel (a), IDN-5390 (b) and SB-CST-10202 (c) in TBB1 (yellow) and TBB3 (cyan)
As shown in Table 1, the meta-substituent of the C2-benzoate moiety plays a crucial role in the enhancement of the potency of C-seco-taxoid against cancer cell lines overexpressing class III β-tubulin. The result strongly indicates that the the C2-benzoate moiety bearing a meta-substituent has enhanced its interaction with His229 in both class I and III β-tubulins. Especially, the introduction of OMe or Cl to this position is crucial for the markedly higher potency of SB-CST-10202 and other C-seco-taxoids. The result makes a sharp contrast to the discussions on the negligible role of His229 in the previously reported molecular modeling studies on IDN5109 and paclitaxel.15
In summary, this study has demonstrated that the meta-substitution of the C2-benzoate moiety of C-seco-taxoids with appropriate groups can remarkably increase the interaction of C-seco-taxoids with the class III β-tubulin to overcome paclitaxel-resistance, due to the over-expression of this particular subclass of β-tubulin. SB-CST-10202 was identified as the most promising drug candidate, exhibiting all-round high potencies across different drug-resistant cell lines. Molecular modeling study on the tubulin-bound conformations of paclitaxel, IDN5 390 and SB-CST-10202 in TBB1 and TBB3 showed considerable difference in their conformations between the two β-tubulin subclasses. This study strongly supported the importance of hydrophobic interaction between the C2-benzoyl moiety and His227.
Further studies on the SAR of C-seco-taxoids to overcome various types of drug resistances are actively in progress in these laboratories.
Supplementary Material
Acknowledgments
This research was supported by grants from the National Cancer Institute (CA 103314 to I.O.) and Indena, SpA (to I.O.). Authors would like to thank Professor Carlos Simmerling, SUNY at Stony Brook for his valuable advice on the molecular modeling study and Dr. Ramesh Gupta, ChemMaster International, for his technical advice.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Banerjee A, Roach MC, Trcka P, Luduena RF. J Biol Chem. 1992;267:5625–5630. [PubMed] [Google Scholar]
- 2.Panda D, Miller HP, Banerjee A, Luduena RF, Wilson L. Proc Natl Acad Sci USA. 1994;91:11358. doi: 10.1073/pnas.91.24.11358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kavallaris M, Kuo DS, Burkhart CA, Regl DL, Norris MD, Haber M, Horwitz SB. J Clin Invest. 1997;100:1282. doi: 10.1172/JCI119642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sullivan KF. Ann Rev Cell Dev Biol. 1988;4:687. doi: 10.1146/annurev.cb.04.110188.003351. [DOI] [PubMed] [Google Scholar]
- 5.Banerjee A, Luduena RF. J Biol Chem. 1992;267:13335. [PubMed] [Google Scholar]
- 6.Sharma J, Luduena RF. J Prot Chem. 1994;13:165. doi: 10.1007/BF01891975. [DOI] [PubMed] [Google Scholar]
- 7.Schwarz PM, Liggins JR, Luduena RF. Biochemistry. 1998;37:4687. doi: 10.1021/bi972763d. [DOI] [PubMed] [Google Scholar]
- 8.Derry WB, Wilson S, Khan IA, Luduena RF, Jordan MA. Biochemistry. 1997;36:3554. doi: 10.1021/bi962724m. [DOI] [PubMed] [Google Scholar]
- 9.Schiff PB, Horwitz SB. Nature. 1979;277:665. doi: 10.1038/277665a0. [DOI] [PubMed] [Google Scholar]
- 10.Horwitz SB. J Nat Prod. 2004;67:136. doi: 10.1021/np0304464. [DOI] [PubMed] [Google Scholar]
- 11.Little M, Seehaus T. Comp Biochem Physiol, Part B: Biochem Mol Biol. 1988;90B:655. doi: 10.1016/0305-0491(88)90320-3. [DOI] [PubMed] [Google Scholar]
- 12.Huzil JT, Luduena RF, Tuszynski J. Nanotechnology. 2006;17:S90. doi: 10.1088/0957-4484/17/4/014. [DOI] [PubMed] [Google Scholar]
- 13.Sullivan KF, Cleveland DV. Proc Natl Acad Sci USA. 1986;83:4327. doi: 10.1073/pnas.83.12.4327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dumontet C, Jordan MA, Lee FFY. Mol Cancer Ther. 2009;8:17. doi: 10.1158/1535-7163.MCT-08-0986. [DOI] [PubMed] [Google Scholar]
- 15.Ferlini C, Raspaglio G, Mozzetti S, Cicchillitti L, Filippetti F, Gallo D, Fattorusso C, Campiani G, Scambia G. Cancer Res. 2005;65:2397. doi: 10.1158/0008-5472.CAN-04-3065. [DOI] [PubMed] [Google Scholar]
- 16.Haber M, Burkhart CA, Regl DL, Madafiglio J, Norris MD, Horwitz SB. J Biol Chem. 1995;270:31269. doi: 10.1074/jbc.270.52.31269. [DOI] [PubMed] [Google Scholar]
- 17.Kavallaris M, Burkhart CA, Horwitz SB. Brit J Cancer. 1999;80:1020. doi: 10.1038/sj.bjc.6690507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chaudhary AG, Gharpure MM, Rimoldi JM, Chordia MD, Gunatilaka AAL, Kingston DGI, Grover S, Lin CM, Hamel E. J Am Chem Soc. 1994;116:4097. [Google Scholar]
- 19.Ojima I, Wang T, Miller ML, Lin S, Borella C, Geng X, Pera P, Bernacki RJ. Bioorg Med Chem Lett. 1999;9:3423. doi: 10.1016/s0960-894x(99)00629-0. [DOI] [PubMed] [Google Scholar]
- 20.Ojima I, Chen J, Sun L, Borella CP, Wang W, Miller ML, Lin S, Geng X, Kuznetsova L, Qu C, Gallager D, Zhao X, Zanardi I, Xia S, Horwitz SB, Mallen-StClair J, Guerriero JL, Bar-Sagi D, Veith JM, Pera P, Bernacki RJ. J Med Chem. 2008;51:3203. doi: 10.1021/jm800086e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ojima I. Acc Chem Res. 1995;28:383. [Google Scholar]
- 22.Ojima I, Habus I, Zhao M, Zucco M, Park YH, Sun CM, Brigaud T. Tetrahedron. 1992;48:6985. [Google Scholar]
- 23.Ojima I, Slater JC, Michaud E, Kuduk SD, Bounaud PY, Vrignaud P, Bissery MC, Veith JM, Pera P, Bernacki RJ. J Med Chem. 1996;39:3889. doi: 10.1021/jm9604080. [DOI] [PubMed] [Google Scholar]
- 24.Holton RA, Biediger RJ, Boatman PD. In: Taxol: Science and applications. Suffness M, editor. CRC Press; New York: 1995. pp. 97–121. [Google Scholar]
- 25.Appendino G, Bettoni P, Noncovich A, Sterner O, Fontana G, Bombardelli E, Pera P, Bernacki RJ. J Nat Prod. 2004;67:184. doi: 10.1021/np0303456. [DOI] [PubMed] [Google Scholar]
- 26.Appendino G, Jakupovic J, Cravotto G, Enriú R, Varese M, Bombardelli E. Tetrahedron Lett. 1995;36:3233. [Google Scholar]
- 27.Ojima I, Slater JC, Kuduk SD, Takeuchi CS, Gimi RH, Sun CM, Park YH, Pera P, Veith JM, Bernacki RJ. J Med Chem. 1997;40:267. doi: 10.1021/jm960563e. [DOI] [PubMed] [Google Scholar]
- 28.Ojima I, Sun CM, Park YH. J Org Chem. 1994;59:1249. [Google Scholar]
- 29.Appendino G, Noncovich A, Bettoni P, Dambruoso P, Sterner O, Fontana G, Bombardelli E. Eur J Org Chem. 2003:4422. [Google Scholar]
- 30.Magnani M, Ortuso F, Soro S, Alcaro S, Tramontano A, Botta M. FEBS J. 2006;273:3301. doi: 10.1111/j.1742-4658.2006.05340.x. [DOI] [PubMed] [Google Scholar]
- 31.Löwe J, Li H, Downing KH, Nogales E. J Mol Biol. 2001;313:1045. doi: 10.1006/jmbi.2001.5077. [DOI] [PubMed] [Google Scholar]
- 32.The 1JFF protein contains 426 amino acid residues and the structure of the C-terminus is missing.
- 33.The class I and class III β-tubulins have been recently re-sequenced.22,24 The protein annotated as the class V β-tubuin in the SwissProt (code: TBB5-HUMAN, P07437), for some reason, actually corresponds to the sequence of the class I β-tubulin.22,24 The protein coded as TBB3-HUMAN, Q13509 in the SwissProt correctly corresponds to the class III β-tublin.22,24 The previously reported molecular modeling study by Ferlini et al.14 used an incorrect class-III tubulin sequence (Arg277 should be Ala277), partly due to the inconsistent annotations in the SwissProt.
- 34.Higgins DG, Thompson JD, Gibson TJ, Russell FD. Method Enzymol. 1996;266:383. doi: 10.1016/s0076-6879(96)66024-8. [DOI] [PubMed] [Google Scholar]
- 35.Case DA, Cheatham TE, III, Darden T, Gohlke H, Luo R, Merz KM, Jr, Onufriev A, Simmerling C, Wang B, Woods RJ. J Comput Chem. 2005;26:1668. doi: 10.1002/jcc.20290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.http://dock.compbio.ucsf.edu/DOCK_6/index.htm.
- 37.Jorgensen WL, Chandrasekhar J, Madura JD, Impey RW, Klein ML. J Chem Phys. 1983;79:926. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.