Abstract
The membrane-bound atrial natriuretic peptide receptor (GCA) catalyzes the formation of cGMP from GTP in response to natriuretic peptide hormones. Previous structural studies have focused on the extracellular hormone binding domain of this receptor whereas its intracellular domain has not yet been amenable to such studies. We report here the baculovirus expression and purification of the GCA intracellular domain construct GCAID comprising the complete intracellular region which includes the kinase-homology domain, coiled-coil region, and catalytic cyclase domain. The intracellular domain was enzymatically characterized in terms of guanylyl cyclase activity and the effects of ATP, manganese, and Triton X-100. Our results indicate that the activity of the intracellular domain of the ANP receptor is about 2 fold less active compared to a truncated cyclase domain construct lacking the kinase-like domain that was also expressed and purified. In addition, unlike the full length receptor, the intracellular domain could not be activated by Triton-X100/Mn2+ or its activity stimulated by ATP. These data therefore indicate that the major part of the transition from the basal state to the fully, ANP/ATP-dependent, activated state as well its stimulation/enhancement by Triton-X100/Mn2+ requires the full length receptor. These receptor insights could aid in the development of novel therapeutics as the GCA receptor is a key drug target for cardiovascular diseases.
Keywords: Guanylyl cyclase, atrial natriuretic peptide receptor, second messenger cGMP signaling, purification and enzymatic characterization, kinase-like domain
Introduction
Guanylyl cyclases (GC) are receptors that catalyze the formation of the second messenger cGMP from GTP in response to cellular signals. The atrial natriuretic peptide receptor A (GCA) is one of the best studied GCs and can be activated by atrial natriuretic peptide (ANP) or brain natriuretic peptide (BNP) hormones leading to vasodilation, diuresis and natriuresis among other effects with the net result of lowering blood pressure levels [1]. GCA is therefore a key drug target for treating a number of cardiovascular diseases such as congestive heart failure.
GCA can be topologically dissected into the following regions - the large extra-cellular ligand binding domain (ECD), a transmembrane (TM) helix, an intracellular kinase homology domain (KHD), a coiled-coil (CC) region, and the C-terminal guanylyl cyclase (GC) domain [2]. GCA is a pre-dimerized homo-dimer [3] with a strong dimerization role for the intracellular CC domain [4]; a homologous region in a related receptor was also found to be important for regulation/dimerization [5]. The crystal structure of the GCA ECD [6] and the hormone bound ECDs structure [7] provided structural insights into understanding of the role played by ECD in hormone recognition and relay of signaling information. However, structural insights into the intracellular domains (ID) are still lacking and how the ECD communicates with the GC domain in this process remains unknown. The KHD is situated centrally in between the signal-receiving ECD/TM half and the output catalytic CC/GC half of the GCA receptor and therefore likely plays a key role in the communication between the two halves of GCA.
In addition to the lack of intracellular domain structural insights, the precise role of the KHD and ATP is of debate with various, sometimes opposite, conclusions ranging from the KHD being inhibitory or regulatory, and ATP being either critical for activation or important for enzyme stabilization and/or sustaining activated receptor [8–12]. These studies are often done in cell lines with either transient or stably expressed receptor, assays done either in whole cells or with membrane preparation. While intracellular domain truncations of GCA have previously been expressed [21] and the catalytic domain of GCA has been purified and characterized [13], its full length intracellular domain has not yet been purified and characterized. To therefore investigate the role of GCA’s KHD and ATP in a more isolated state, and also in preparation for structural studies, we over-expressed and purified the entire GCA intracellular domain. Although we realize the shortcomings of the divide-and-conquer method of not working with intact receptor, our ability to obtain purified intracellular domain allowed us to probe important questions regarding certain reported receptor enzymatic characteristics and ascribe these to either being inherent to the (isolated) intracellular domain, or to the full length receptor.
Materials and Methods
Recombinant Protein expression
A full length construct of GCA in vector pCMV3 was used for PCR amplification. GCAID was cloned into pFastbac-1 as a PCR product and corresponds to the entire intracellular GCA comprising amino acids 465–1029. Primers used: 5′cggggatccatgcatcatcatcatcatcatggtatgcagctggaaaaggagctggtc3′ and 5′gaagatcttcatcaacctcgagtgctacatcc3′ yielding a 6xHis-tag at the N-terminus; plasmid was sequenced to confirm the entire sequence. Recombinant bacmid DNA using DH10bac was generated (Bac-to-Bac, Invitrogen) and used to transfect Sf-9 cells using Cellfectin reagent to produce recombinant baculoviruses. For protein production, suspension cultures of High-Five were infected at a rate of 5–10 MOI at a cell density of 1.0–1.2 × 106 cells/ml. Cells were harvested after 72h of infection. A shorter version without the KHD domain called GCACC+GC, comprising amino acids 776–1029, was generated using the primers 5′cacaggatccatatgaacagcagcaacatcctg3′ and 5′acagaattctcatcagcctcgagtgctaca3′ and subcloned into pET28a (Novagen) using restriction sites NdeI and EcoRI. The GCACC+GC containing fragment was subsequently subcloned into the pFastbac1 vector using sites BamHI and EcoRI yielding a 6-His tag at the N-terminus followed by a thrombin cleavage site.
Protein purification
Cells containing recombinant protein were centrifuged and washed in saline phosphate buffer. Cell lysis was carried out by sonication in 50mM sodium phosphate pH 8.0 containing 40mM imidazole, 300mM NaCl, 1mM DTT, 10% glycerol, 5μg/ml DNAseI, 10μg/ml RNAse, and protease inhibitor cocktail (Sigma, USA). Following sonication, NP-40 was added to a final concentration of 1% and the lysates were incubated at 4°C with gentle rocking for 1h. Cell debris was removed by centrifugation at 30,000g for 30min. Washed Ni-NTA beads (Qiagen, USA) were then added to the supernatants and incubated for 2h at 4°C while rocking. Beads were centrifuged, packed into a 50ml column and washed 3x with lysis buffer with 1% NP-40 followed by 5x washing in large volumes (up to 15 times) of the same buffer without NP-40. Elution of the protein was carried out in a buffer containing 50mM sodium phosphate pH 8.0, 250mM imidazole, 300mM NaCl, 1mM DTT, 10% glycerol and the protease inhibitor cocktail. Immediately following elution, EDTA was added to the protein to a final concentration of 10mM. The protein was subsequently buffer exchanged into 20mM Tris-Cl pH8.0, 1mM DTT, and 10% glycerol prior to MonoQ anion exchange chromatography using a NaCl gradient. Final step of purification involved size exclusion chromatography in a Superdex 200 10–30 column (GE health sciences) in a buffer containing 20mM Tris-Cl, pH 8.0, 1mM DTT, 150mM NaCl and 10% glycerol. Protein concentrations were measured by Bradford method using BSA as a standard.
In vitro guanylyl cyclase assay
Assay for guanylyl cyclase activity was carried out in 50mM Tris-Cl pH 7.4 containing 1mM isobutyl methyl xanthine (IBMX), 5mM creatine phosphate, and 20μg/assay creatine phosphokinase, similar to [14]. Either MgCl2 or MnCl2 was used as metal cofactor. Unless otherwise mentioned, GTP concentration was maintained at 1mM, MgCl2 at 5mM (or MnCl2 at 2mM). Reactions were carried out in 0.1ml final volume at 37°C for 10min. After 10min, 0.4ml of 50mM sodium acetate pH 4.5 was added to terminate the reaction followed by 3min incubation in a boiling water bath. Samples were centrifuged before being aliquoted for cGMP measurements. Quantification of cGMP produced was carried out by a microtiter plate based immune adsorption method using the commercially available cGMP EIA kit (Cayman). Concentration of cGMP was measured against a standard curve of samples with known concentration. Each dilution was assayed in duplicate. A standard work sheet provided by the manufacturers was used for final calculation of cGMP concentration using Microsoft Excel.
Statistical analysis
Graphpad Prism (USA) was used for fitting enzyme kinetic curves. cGMP production as a function of substrate concentration was fitted to Hill equation –
where, S0.5 is the Km equivalent for cooperative enzyme kinetics, nH is the Hill coefficient and Vmax maximum enzyme velocity. For curves that obtained an nH close to 1.0, a Michaelis-Menten equation was used for fitting.
Results and Discussion
We have purified and enzymatically characterized the GCAID domain to allow comparison against results obtained from full length GCA receptors as well as against results from a published shorter intracellular domain construct comprised of only the catalytic region [13]. Such a truncated catalytic GCA construct, containing the CC + GC regions, was also expressed and purified in this study to allow comparisons under identical buffer conditions and to carry out additional experiments. Both constructs were expressed as N-terminal 6-His fusion proteins in High-Five cells and purified via affinity Ni-NTA chromatography followed by anion exchange and subsequent size exclusion chromatography. Both GCAID and GCACC+GC eluted on the size exclusion column close to that of their predicted dimeric state (data not shown). Fig. 1 confirms the purity and molecular weights of both proteins after they were purified. This purification involved cell culture volumes of around 5L and resulted in a yield of around ~0.75mg/L and ~0.3mg/L for purified GCACC+GC and GCAID constructs, respectively. To ensure complete removal of the cell-lysis detergent NP40, excessive washing of the Ni-NTA beads bound to the His-tagged proteins was carried out as well as the two subsequent chromatograph steps. The construct GCACC+GC contained a cleavable his-tag; its removal did not affect the gel elution profile nor activity (data not shown). On the other hand, the construct GCAID did not have a cleavable His-tag. We tried to excise the His-tag by treating with dipeptidyl aminopeptidase, DAPase (QIAGEN, USA). However, under our experimental conditions this reaction could not be reproducibly controlled. Due to the above reasons, and that His-tagged proteins often can be crystallized, further (enzymatic) studies were carried out with the His-tagged proteins.
Figure 1.
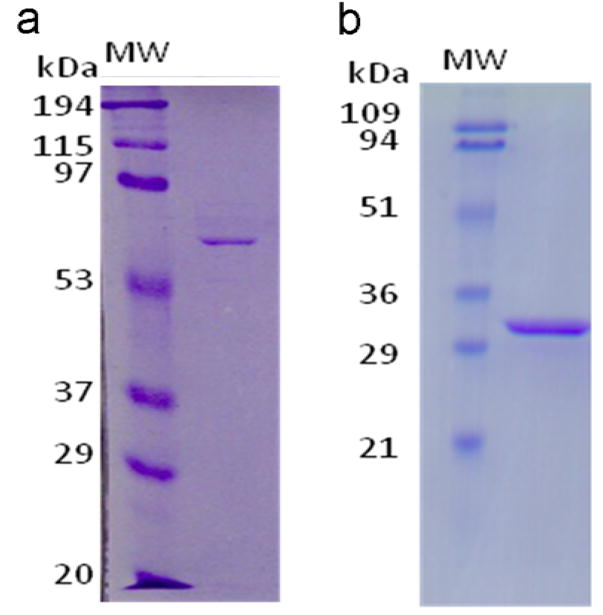
SDS-PAGE of purified GCA constructs. Purified GCAID (a) and GCACC+GC (b) and were resolved on a 10% and 12% polyacrylamide gels, respectively. Gels were stained using Coomassie blue (R250). MW: Molecular weight standards as indicated.
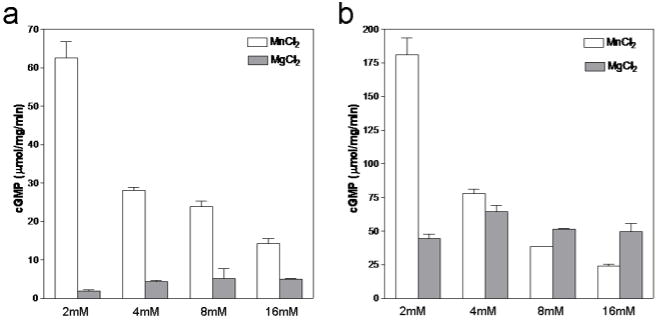
All the guanylyl cyclases studied so far have shown considerably higher catalytic activity in the presence of Mn2+ ions compared to Mg2+. To systematically compare the effects of these ions, we measured guanylyl cyclase activity at increasing concentrations of MgCl2 and MnCl2 while the GTP concentration was kept constant at 1mM. As shown in Fig. 2, GCAID (and GCACC+GC) has increased guanylyl cyclase activity at lower Mn2+ concentrations compared to Mg2+. However, at higher Mn2+ concentrations, the activity of both constructs decreases whereas higher Mg2+ concentrations does not seem to have an effect on activity. Such a negative effect on activity by increased Mn2+ concentrations had been also noted previously in an intact membrane guanylyl cyclase from mouse parotid [15], GCA [16], and adenylate cyclase [17]. Interestingly, the GCAID and GCACC+GC protein constructs seem to have different relative Mn2+ inhibitory effects (the activity for GCACC+GC dropped 7.4-fold and for GCAID only 4.4-fold when the [Mn2+] was increased from 2 to 16mM). This suggests that the KHD might provide a protective influence on the extent of Mn2+ inhibition perhaps via interactions of the KHD with the CC and/or GC domain. Alternatively, the effects of the weakly inhibitory presence of the KHD (~2 fold) and the inhibitory high Mn2+ concentrations are perhaps not additive: GCACC+GC lacks the KHD and is thus in a less inhibited state that can thus perhaps be inhibited to a larger extent by high Mn concentrations compared to GCAID. One possible way for Mn2+ and/or KHD to inhibit the GC domain is perhaps via affecting the closed-to-open transition of the active site that is speculated to occur upon substrate binding [18].
Figure 2.
Effect of Mg2+ and Mn2+ ion concentration on GC activity. GCAID (a) and GCACC+GC (b) were assayed for guanylyl cyclase activity in the presence of increasing concentrations of either Mg2+ (grey bars) or Mn2+ (white bars). GTP concentration was kept constant at 1mM. The enzymes were incubated in the presence of the appropriate ion along with all other components of the assay except GTP for 10min on ice. The reaction were carried out at 37°C for 10min and initiated by addition of GTP. Values presented are average of two experiments performed in duplicates.
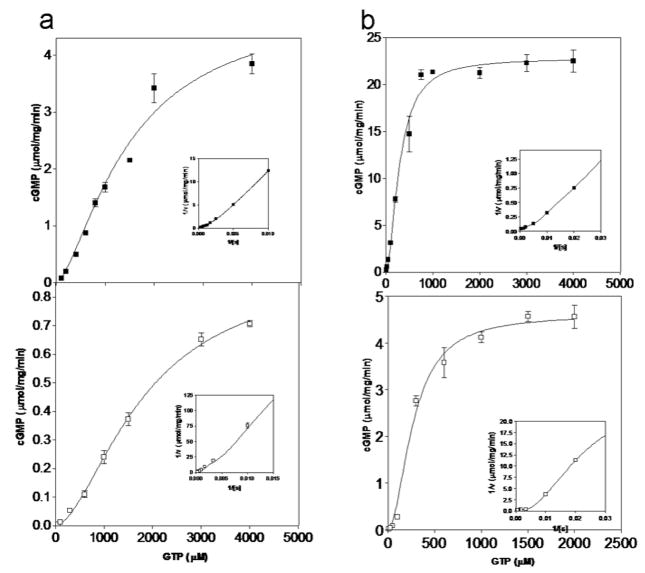
We also measured cGMP formation as a function of GTP concentration in the presence of Mg2+ or Mn2+ (Fig. 3 and Table 1). With Mg2+ as cofactor, the enzymes had similar S0.5 values of 1.4 and 1.8mM for GCACC+GC and GCAID, respectively while GCACC+GC displayed about 5 fold higher Vmax. Both GCAID and GCACC+GC clearly followed cooperative curves and their NH (Hill coefficient) values were 1.7 and 1.5, respectively (Fig. 3; Table 1). Higher catalytic activity with the presence of Mn2+ was due to a drop in the S0.5 values for both constructs. Like with Mg2+, in the presence of Mn2+ the Vmax of GCACC+GC was about 5 fold higher than that of GCAID. Both the enzymes displayed higher levels of cooperativity in the presence of Mn2+ (Fig. 3) which are in agreement with previous reports. Note that the NH value for the GCACC+GC is very close to 1.4 previously published for a similar GC-A cyclase domain construct [13]; our obtained S0.5 and Vmax values for the shorter construct are also in the same range as previously published values yet do differ somewhat more (~4–5 fold) which is likely attributed to the assay condition differences [13] since our conditions are more similar to [14].
Figure 3.
Kinetic analysis of purified GCA deletion mutants. In vitro guanylyl cyclase assays were carried for GCAID (open squares) and GCACC+GC (closed squares) out with increasing concentrations of GTP in the presence of either 10mM MgCl2 (a) or 6mM MnCl2 (b). Average values of duplicate measurements are plotted along with standard deviations. Curves represent the fitting of data points to the Hill equation in an iterative manner using GraphPad Prism, that were used to determine kinetic parameters summarized in Table 1. Inset: Double reciprocal transformation of the data points obtained.
Table 1.
Kinetic parametersa of cGMP formation by purified GCA constructs.
| Protein | S0.5 (mM) | Vmax (μmol/mg/min) | NH |
|---|---|---|---|
| GCACC+GC +Mg2+b | 1.49±0.3 | 4.9±0.72 | 1.5±0.2 |
| GCAID +Mg2+b | 1.81±0.17 | 1.03±0.64 | 1.7±0.3 |
| GCACC+GC +Mn2+c | 0.292±0.09 | 22.8±3.79 | 1.78±0.1 |
| GCAID +Mn2+ c | 0.267±0.1 | 4.6±0.57 | 1.9±0.3 |
Values presented are averages of three experiments.
MgCl2 concentrations were kept constant at 10mM
MnCl2 concentrations were kept constant at 6mM
The Vmax values obtained for GCAID allow comparison against reported values for its full length and truncated catalytic domain, including our purified GCACC+GC construct. As noted above in the presence of Mg2+, GCAID had about 5 fold less activity compared to GCACC+GC. These activity values are calculated per mg of protein, as standard in the field, and since the shorter construct is about half the molecular weight of the larger one, normalizing for the mass of the protein molecules yielded only a 2.2 fold decrease in cyclase activity in the presence of KHD with Mg2+ (67 and 150 mol/mol/min for GCAID and GCACC+GC, respectively). This indicates that the presence of the KHD domain in the larger GCAID construct has a modest negative effect on the cyclase activity of its adjacent cyclase domain. This 2.2 fold decrease is in agreement with a previous study which, although not having measured specific activity, it did plot activity measurements of individual size-exclusion fractions of similar GC-A constructs as ours and estimations of relative protein levels via a Western blot (Figure 2 in Wilson & Chinkers[4]). Since no comparative analysis of this figure was given in the original manuscript, we estimated the relative total activity under the Wilson & Chinkers Figure 2 elution peak for each of their constructs by enlarging and printing out this figure and cutting out the peak and measuring the weight of the paper on a microbalance. This old-fashioned method suggested that, similar to our results, the presence of the KHD domain in GC-A leads to about a ~1.5–2.2 fold decrease in activity keeping in mind that expression levels are similar or even slightly less for the full length intracellular domain as indicated by their Western. When comparing GCACC+GC’s activity to reported values for full length hormone-activated GCA purified from either natural or recombinant sources, in their fully activated states, the truncate domain is either 3.6 or 19.9 fold less active (reported activities are 4.2 and 23.1 μmol cGMP/min/mg protein for recombinant affinity purified 130 kDa GCA [19] and GCA purified from bovine adrenocortical cells [20], respectively which corresponds to 546 and 3003 mol/mol/min, respectively). The fact that there is quite a spread in reported full length GCA activities makes it difficult to draw definitive conclusions regarding the activation state of our constructs with respect to activated full length GCA. Nevertheless, our data therefore indicate that the major part of the transition from the basal state to the fully, ANP/ATP-dependent, activated state requires the full length receptor, not the mere presence of a KHD. In addition, our data points to that GCA’s KHD has likely only a minor role directly suppressing GCA’s activity in the basal state even though deletion of this KHD in full length receptor had been shown to lead to constitutive activation [11]. Our results are in agreement with that a different region of GC-A has also been implicated in possibly keeping the receptor in the low activity basal state, the ECD(/TM) region [21]. This previous study disrupting the ECD dimer interface indicated that the ECD/TM region had a role in forcing GCA in an inactive conformation [21] yet an additional inhibitory role for the KHD could not be ruled out. Therefore, our data showing a minor inhibitory effect of KHD on cyclase activity can be used to hypothesize that KHD is likely involved in relaying structural (distance/rotation) changes from the ECD/TM to the CC/GC. In addition to a role relaying structural (distance/rotation) changes and a minor inhibitory role, GCA’s KHD is also the likely site for ATP binding [12;22;23] and harbors the regulatory phosphorylation sites [24] thus suggesting that the KHD region is also a control point of extra-cellular to intra-cellular domain communication.
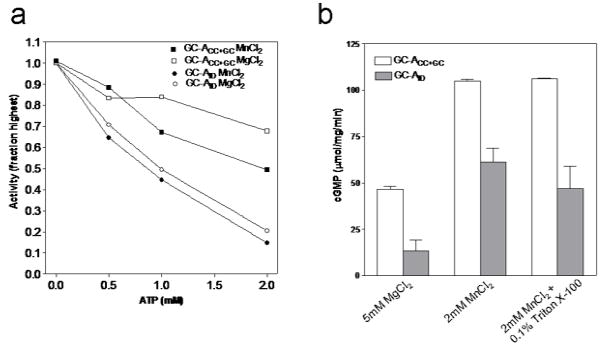
Several lines of evidence presented in the literature have indicated that ATP is required for maximal hormone dependent activation of GCA [19;22;25]. In an intact receptor, release of the inhibitory effect of KHD on GC and a drop in affinity of ECD for the hormones were proposed to be the events related to ATP binding [26]. A recent study reporting that ATP is not an obligatory requirement for NPR-A and -B activation disputes the above model and demonstrates that one of the reasons for ATP dependent activity enhancement is stabilization of the phosphorylated KHD [9]. In addition, a drop in Km for GTP has been shown to be associated with ATP stimulated cyclase activity. To explore the ATP effect, we measured cyclase activity of GCAID and GCACC+GC with varying ATP concentrations (Fig. 4a). Unlike in the full length receptor, ATP actually decreased the cyclase activity in receptors lacking the ECD/TM. Probably the inhibition of guanylyl cyclase activity by ATP is indicative of competition for the active site since ATP is known to bind to and inhibit a GCA guanylyl cyclase domain construct with an IC50 of 3.3mM [13]. Note that the inhibitory effect of 2mM ATP on the activity of GCACC+GC is however about 3-fold less compared to GCAID (for both Mg an Mn, see Figure 4) suggesting a potentially interesting KHD-dependent difference between these two constructs. This suggests that the KHD present in our purified GCAID proteins is capable of binding ATP and that this ATP binding modulates cyclase activity under ATP inhibitory conditions (although this KHD effect could also still be indirect via perhaps increasing ATP’s inhibitory affinity for the GC domain). The even stronger inhibition of GCAID by ATP is in contrast with ATP potentiation (in the presence of ANP) of full length GCA receptor activity indicating that ATP potentiation requires the presence of other structural elements in addition to the KHD although ATP binding itself is likely occurring within the KHD domain. Note also that ATP by itself does have little effect on full length GCA activity as it requires ANP.
Figure 4.
Effect of ATP and Triton X-100 on guanylyl cyclase activity intracellular domain variants. A) In vitro guanylyl cyclase activities for GCACC+GC (squares) and GCAID (circles) were monitored in the presence of 4mM MnCl2 (closed symbols) or 10mM MgCl2 (open symbols) with different concentrations of ATP. Enzymes were incubated with all the reaction components except GTP for 10min on ice. Catalysis was initiated by addition of GTP and allowed to continue for 10min at 37°C. Activity values are shown as percentage of activity obtained in the absence of ATP. Data presented is the representative of two experiments. B) Purified GCACC+GC (white bars) and GCAID (grey bars) were subjected to in vitro guanylyl cyclase assay in the presence of 5mM MgCl2, 2mM MnCl2 or 2mM Mncl2 and 0.1% triton X-100. Values shown are averages of three experiments each measurement carried out in duplicates.
The ability to obtain purified intracellular domain variants of GCA allowed us to probe the mechanism of GCA activation by Triton/Mn2+. The full length GCA receptor is known to be able to be activated by Triton/Mn2+ independent of ANP [27] yet a remaining question is whether constructs lacking the ECD/TM regions can be stimulated as well by Triton/Mn2+. Fig. 4b compares the activities of GCAID (and the truncated version) in the presence of MnCl2 and Triton X-100. Our results point to no increase in cGMP production when Triton X-100 was added to the reaction in the presence of Mn2+. This observation indicates that the ECD/TM region is needed for the Triton X-100/Mn2+ stimulation of GCA. A possible Triton X-100/Mn2+ GCA activation mechanism could involve having the Triton interact with the TM helices via co-insertion in a Triton X-100 micelle and as such cause juxtapositioning of the TM helices leading to activation. This would be analogous to GCA activation by ANP juxtapositioning the juxtamembrane ECD regions, or forcing a cross-link of the extracellular juxtamembrane regions via a artificial disulfide bond [28;29]. Since our constructs lack the TM (and ECD) region, no such Triton-dependent effect is observed. Additionally, it may be argued that our constructs represent the close to constitutively active state of the receptor with maximal activity; any further enhancement of activity by detergent may thus not be conducive.
In summary, we have successfully expressed, purified, and characterized the intracellular domain construct of the GCA receptor. This protein and the truncated GCACC+GC could be useful for structural studies aimed at understanding the molecular basis of GCA receptor functioning. In addition we demonstrate that, when expressed in insect cells, the KHD alone is insufficient for bringing about the full extend of receptor inhibition in the absence of the TM+ECD indicating that the ECD (and TM) may therefore play crucial roles in generating the basal activation state of GCA. We do realize that phosphorylation could also have an effect on our results and the precise phosphorylation state of our constructs is uncertain although we have attempted to dephosphorylate them, finding no change in activity nor electrophoretic mobility. Phosphorylation is a key requirement for GC-A receptor activitation yet it could perhaps also be important for bringing about the KHD inhibition in the basal state of the GC-A receptor. Additional studies of these intracellular domain constructs in either the phosphorylated or dephosphorylated state will need to await the identification of the phosphatases and kinases of the natriuretic peptide receptors.
Acknowledgments
We thank Dr. Lincoln Potter for the cDNA of the ANP receptor. We acknowledge the AHA (SDG 0335159N) and NIH (R01 HL075329) for funding.
Footnotes
Declaration of interest
FvdA receives additional funding from Wyeth and Bayer for projects not involving natriuretic peptide receptors.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Garbers DL, Chrisman TD, Wiegn P, et al. Membrane guanylyl cyclase receptors: an update. Trends Endocrinol Metab. 2006;17:251–258. doi: 10.1016/j.tem.2006.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Padayatti PS, Pattanaik P, Ma X, van den Akker F. Structural insights into the regulation and activation mechanism of mammalian guanylyl cyclases. Pharmacol Ther. 2004;104:83–99. doi: 10.1016/j.pharmthera.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 3.Chinkers M, Wilson EM. Ligand-independent oligomerization of natriuretic peptide receptors. Identification of heteromeric receptors and a dominant negative mutant. J Biol Chem. 1992;267:18589–18597. [PubMed] [Google Scholar]
- 4.Wilson EM, Chinkers M. Identification of sequences mediating guanylyl cyclase dimerization. Biochemistry. 1995;34:4696–4701. doi: 10.1021/bi00014a025. [DOI] [PubMed] [Google Scholar]
- 5.Ramamurthy V, Tucker C, Wilkie SE, Daggett V, Hunt DM, Hurley JB. Interactions within the coiled-coil domain of RetGC-1 guanylyl cyclase are optimized for regulation rather than for high affinity. J Biol Chem. 2001;276:26218–26229. doi: 10.1074/jbc.M010495200. [DOI] [PubMed] [Google Scholar]
- 6.van den Akker F, Zhang X, Miyagi M, Huo X, Misono KS, Yee VC. Structure of the dimerized hormone-binding domain of a guanylyl-cyclase- coupled receptor. Nature. 2000;406:101–104. doi: 10.1038/35017602. [DOI] [PubMed] [Google Scholar]
- 7.Ogawa H, Qiu Y, Ogata CM, Misono KS. Crystal structure of hormone-bound atrial natriuretic peptide receptor extracellular domain: Rotation mechanism for transmembrane signal transduction. J Biol Chem. 2004;279:28625–28631. doi: 10.1074/jbc.M313222200. [DOI] [PubMed] [Google Scholar]
- 8.Antos LK, Potter LR. Adenine nucleotides decrease the apparent Km of endogenous natriuretic peptide receptors for GTP. Am J Physiol Endocrinol Metab. 2007 doi: 10.1152/ajpendo.00321.2007. [DOI] [PubMed] [Google Scholar]
- 9.Antos LK, Abbey-Hosch SE, Flora DR, Potter LR. ATP-independent activation of natriuretic peptide receptors. J Biol Chem. 2005;280:26928–26932. doi: 10.1074/jbc.M505648200. [DOI] [PubMed] [Google Scholar]
- 10.Burczynska B, Duda T, Sharma RK. ATP signaling site in the ARM domain of atrial natriuretic factor receptor guanylate cyclase. Mol Cell Biochem. 2007;301:93–107. doi: 10.1007/s11010-006-9400-7. [DOI] [PubMed] [Google Scholar]
- 11.Chinkers M, Garbers DL. The protein kinase domain of the ANP receptor is required for signaling. Science. 1989;245:1392–1394. doi: 10.1126/science.2571188. [DOI] [PubMed] [Google Scholar]
- 12.Joubert S, Jossart C, McNicoll N, De Lean A. Atrial natriuretic peptide-dependent photolabeling of a regulatory ATP-binding site on the natriuretic peptide receptor-A. FEBS J. 2005;272:5572–5583. doi: 10.1111/j.1742-4658.2005.04952.x. [DOI] [PubMed] [Google Scholar]
- 13.Joubert S, McNicoll N, De Lean A. Biochemical and pharmacological characterization of P-site inhibitors on homodimeric guanylyl cyclase domain from natriuretic peptide receptor-A. Biochem Pharmacol. 2007;73:954–963. doi: 10.1016/j.bcp.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 14.Vijayachandra K, Guruprasad M, Bhandari R, Manjunath UH, Somesh BP, Srinivasan N, Suguna K, Visweswariah SS. Biochemical characterization of the intracellular domain of the human guanylyl cyclase C receptor provides evidence for a catalytically active homotrimer. Biochemistry. 2000;39:16075–16083. doi: 10.1021/bi0013849. [DOI] [PubMed] [Google Scholar]
- 15.Durham JP. Guanylate cyclase: assay and properties of the particulate and supernatant enzymes in mouse parotid. Eur J Biochem. 1976;61:535–544. doi: 10.1111/j.1432-1033.1976.tb10048.x. [DOI] [PubMed] [Google Scholar]
- 16.Ivanova K, Heim JM, Gerzer R. Kinetic characterization of atrial natriuretic factor-sensitive particulate guanylate cyclase. Eur J Pharmacol. 1990;189:317–326. doi: 10.1016/0922-4106(90)90125-h. [DOI] [PubMed] [Google Scholar]
- 17.Bellorin-Font E, Tamayo J, Martin KJ. Regulation of PTH receptor-adenylate cyclase system of canine kidney: influence of Mn2+ on effects of Ca2+, PTH, and GTP. Am J Physiol. 1982;242:F457–F462. doi: 10.1152/ajprenal.1982.242.5.F457. [DOI] [PubMed] [Google Scholar]
- 18.Rauch A, Leipelt M, Russwurm M, Steegborn C. Crystal structure of the guanylyl cyclase Cya2. Proc Natl Acad Sci U S A. 2008;105:15720–15725. doi: 10.1073/pnas.0808473105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wong SK, Ma CP, Foster DC, Chen AY, Garbers DL. The guanylyl cyclase-A receptor transduces an atrial natriuretic peptide/ATP activation signal in the absence of other proteins. J Biol Chem. 1995;270:30818–30822. doi: 10.1074/jbc.270.51.30818. [DOI] [PubMed] [Google Scholar]
- 20.Takayanagi R, Inagami T, Snajdar RM, Imada T, Tamura M, Misono KS. Two distinct forms of receptors for atrial natriuretic factor in bovine adrenocortical cells. Purification, ligand binding, and peptide mapping. J Biol Chem. 1987;262:12104–12113. [PubMed] [Google Scholar]
- 21.Qiu Y, Ogawa H, Miyagi M, Misono KS. Constitutive activation and uncoupling of the atrial natriuretic peptide receptor by mutations at the dimer interface. Role of the dimer structure in signalling. J Biol Chem. 2004;279:6115–6123. doi: 10.1074/jbc.M310225200. [DOI] [PubMed] [Google Scholar]
- 22.Marala RB, Sitaramayya A, Sharma RK. Dual regulation of atrial natriuretic factor-dependent guanylate cyclase activity by ATP. FEBS Lett. 1991;281:73–76. doi: 10.1016/0014-5793(91)80361-6. [DOI] [PubMed] [Google Scholar]
- 23.Duda T, Sharma RK. Two membrane juxtaposed signaling modules in ANF-RGC are interlocked. Biochem Biophys Res Commun. 2005;332:149–156. doi: 10.1016/j.bbrc.2005.04.102. [DOI] [PubMed] [Google Scholar]
- 24.Potter LR, Hunter T. Phosphorylation of the kinase homology domain is essential for activation of the A-type natriuretic peptide receptor. Mol Cell Biol. 1998;18:2164–2172. doi: 10.1128/mcb.18.4.2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chinkers M, Singh S, Garbers DL. Adenine nucleotides are required for activation of rat atrial natriuretic peptide receptor/guanylyl cyclase expressed in a baculovirus system. J Biol Chem. 1991;266:4088–4093. [PubMed] [Google Scholar]
- 26.Potter LR, Hunter T. Guanylyl cyclase-linked natriuretic peptide receptors: structure and regulation. J Biol Chem. 2001;276:6057–6060. doi: 10.1074/jbc.R000033200. [DOI] [PubMed] [Google Scholar]
- 27.Potter LR, Garbers DL. Dephosphorylation of the guanylyl cyclase-A receptor causes desensitization. J Biol Chem. 1992;267:14531–14534. [PubMed] [Google Scholar]
- 28.Labrecque J, Deschenes J, McNicoll N, De Léan A. Agonistic induction of a covalent dimer in a mutant of natriuretic peptide receptor-A documents a juxtamembrane interaction that accompanies receptor activation. J Biol Chem. 2001;276:8064–8072. doi: 10.1074/jbc.M005550200. [DOI] [PubMed] [Google Scholar]
- 29.Huo X, Abe T, Misono KS. Ligand binding-dependent limited proteolysis of the atrial natriuretic peptide receptor: juxtamembrane hinge structure essential for transmembrane signal transduction. Biochemistry. 1999;38:16941–16951. doi: 10.1021/bi9919448. [DOI] [PubMed] [Google Scholar]