Abstract
Niche localized HGF plays an integral role in G0 exit and the return to mitotic activity of adult skeletal muscle satellite cells. HGF actions are regulated by MET initiated intracellular signaling events that include recruitment of SHP2, a protein tyrosine phosphatase. The importance of SHP2 in HGF-mediated signaling was examined in myoblasts and primary cultures of satellite cells. Myoblasts stably expressing SHP2 (23A2-SHP2) demonstrate increased proliferation rates by comparison to controls or myoblasts expressing a phosphatase-deficient SHP2 (23A2-SHP2DN). By comparison to 23A2 myoblasts, treatment of 23A2-SHP2 cells with HGF does not further increase proliferation rates and 23A2-SHP2DN myoblasts are unresponsive to HGF. Importantly, the effects of SHP2 are independent of downstream ERK1/2 activity as inclusion of PD98059 does not blunt the HGF induced proliferative response. SHP2 function was further evaluated in primary satellite cell cultures. Ectopic expression of SHP2 in satellite cells tends to decrease proliferation rates and siSHP2 causes an increase the percentage of dividing myogenic cells. Interestingly, treatment of satellite cells with high concentrations of HGF (50 ng/ml) inhibits proliferation, which can be overcome by knockdown of SHP2. From these results, we conclude that HGF signals through SHP2 in myoblasts and satellite cells to directly alter proliferation rates.
Keywords: satellite cell, myoblast, proliferation, HGF, SHP2, Pax7
INTRODUCTION
Satellite cells are the resident stem cells of postnatal skeletal muscle tissue [1]. In adults, these cells are located adjacent to the muscle fiber in a quiescent state and become mitotically active in response to tissue growth and repair stimuli [2]. Upon reentry into G1, satellite cells proliferate and differentiate in a manner analogous to their embryonic myoblast counterparts. The transition period between G0 and G1 of the cell cycle, referred to as activation, is regulated by locally produced growth factors. Niche factors participating in activation include Notch ligands, nitric oxide and hepatocyte growth factor (HGF) [3–7]. Negative mediators of activation remain poorly defined but may include growth and differentiation factor 8 (GDF8) [8].
Initial experiments defining the activation period of satellite cells revealed the existence of a protein in crude preparations of crushed skeletal muscle that caused fiber-associated satellite cells to emerge from the quiescent state [9]. The requisite factor proved to be HGF, a mitogen for several cell types [10]. Treatment of satellite cells with HGF stimulated activation and subsequent proliferation in vitro [11]. Suppression of HGF activity with an antibody prevents autocrine HGF-mediated activation. Injection of HGF into sites of damaged skeletal muscle causes an increase in the numbers of mitotically active satellite cells within the lesion [6]. And, the growth factor is released from the fibers during periods of stretch exercise [12–14]. Thus, HGF is scrutinized for its positive effects on satellite cell biology with possible therapeutic application for muscle damage and disease.
HGF initiates signals via docking with its transmembrane localized, tyrosine kinase receptor, MET [15]. MET is critical for normal limb muscle formation as it denotes the migratory myoblast population [16]. Mice genetically devoid of MET display normal specification of myoblasts but the cells remain at the proximal limb border failing to migrate and populate the muscle beds. Gab1, an adaptor protein with no inherent kinase activity, orchestrates the intracellular signals underlying the migratory response. Gab1 recruitment to the MET kinase domain is necessary for emigration of myoblasts from the dermamyotome [17]. Mice lacking Gab1 contain fewer and smaller muscles within both the fore and hind limbs. The docking protein mediates the migratory actions of myoblasts by interaction with SHP2, a protein tyrosine phosphatase. Disruption of Gab1:SHP2 interactions duplicates the defective migratory patterns found in mice devoid of MET or Gab1 [18].
The role of SHP2 during myogenesis remains poorly defined. Genetic ablation of SHP2 results in mice with mesoderm defects prior to specification of the myogenic cell populations [19]. Conditional ablation of SHP2 in nestin-expressing (nestinSHP2(−/−)) cells causes neural defects that result in early neonatal lethality [20]. Interestingly, nestinSHP2(−/−) mice exhibit a reduction in muscle mass that phenotypically resembles that of Pax7(−/−) mice [21]. Nestin is expressed in quiescent satellite cells thus, suggesting that the phosphatase may participate in aspects of satellite cell biology [22]. The objective of these experiments was to examine SHP2 expression in satellite cells and provide insight into the importance of the protein during myogenesis. Results presented herein demonstrate that SHP2 limits satellite cell proliferation with no apparent effects on differentiation. Knockdown of SHP2 increases the number of cycling cells independent of ERK1/2 actions. Importantly, SHP2 is a key intracellular mediator of the repressive actions of HGF on satellite cell cycle transit. Elevated concentrations of HGF cause cell cycle exit that are overcome in the absence of SHP2. We conclude that HGF both positively and negatively effects activation in satellite cells and its inhibitory activities are mediated through SHP2.
MATERIALS AND METHODS
Satellite cell isolation and cell culture
Adult Balb-C female mice were euthanized by CO2 inhalation followed by cervical dislocation according to the University of Florida Institutional Animal Care and Use Committee guidelines. Hindlimb muscle groups were harvested from adult mice and connective tissue was removed. The tissue was finely minced and digested with pronase E (1.5 mg/ml in sterile phosphate buffered saline [PBS]) for 60 minutes at 37 C. The proteinase was removed by centrifugation of the tissue slurry at 800 × G for 10 minutes. An equal volume of PBS was added to the tissue, vortexed for 5 minutes and centrifuged at 400 × G for 10 minutes. The process was repeated for a total of four times with the supernatants retained after each centrifugation. The final cell slurry was passaged sequentially through 70 μm and 40 μm sieves to remove debris. The cells were collected by centrifugation and seeded in tissue cultureware or stored frozen in liquid nitrogen.
Satellite cells were routinely cultured in high glucose Dulbecco’s Eagle medium supplemented with 10% horse serum, 1% penicillin-streptomycin and 0.5% gentamicin on entactin/collagen/laminin-coated plates (ECL; Invitrogen). 23A2 embryonic mouse myoblasts were cultured on gelatin-coated tissue cultureware in basal Eagle medium supplemented with 15% fetal bovine serum, 1% antibiotic-antimycotic and 0.2% gentamicin. Stable cell lines (23A2-SHP2-WT, -SHP2-CA, -SHP2-DN) were cultured in growth media supplemented with 400 μg/ml geneticin (G418, Invitrogen). Differentiation of myoblasts was induced by culture in low glucose DMEM supplemented with 2% horse serum and 1% penicillin-streptomycin.
Plasmids and cell transfection
Mammalian expression plasmids contained the cDNA inserts SHP2-WT, coding for wild-type mouse SHP2, SHP2-CA, coding for mouse SHP2 with a substitution at glutamic acid 76 to alanine (E76A) creating a constitutive active phosphatase and SHP2-DN, coding for a catalytically inactive mutant of SHP2 created by substitution of cysteine 459 with serine (C463S). The mutant SHP2 cDNAs were created by PCR-mediated mutatgenesis using wild-type mouse SHP2 cDNA as the template and Accuprime DNA polymerase. cDNA fidelity was verified by DNA sequencing of both strands. cDNA inserts were cloned in frame with a multimerized FLAG epitope to create CMV-SHP2 plasmids. Double-stranded DNA coding for a small interfering RNA to SHP2 was created with 5-CAGGAGCTGAAATACGACG, corresponding to mouse SHP2 nt637–655, and 5-CCCAAAAAGAGTTACATTG, corresponding to nt1080–1101 of mouse SHP2 mRNA, and the complimentary DNA. The double stranded cDNAs were inserted into pSIREN-RetroQ-ZsGreen (Clontech Laboratories) for siRNA transcription from the human U6. All plasmids were purified using Qiagen Maxi purification columns followed by dialysis against 10 mM TRIS, pH 8.0. Myogenic cells were transiently transfected in growth media using FuGene6 (Roche) with 3 volumes reagent and 2 μg plasmid DNA.
Western blot
Total cell lysates were harvested by direct lysis in SDS-PAGE sample buffer. Protein lysates representing an equivalent number of cells were separated through 10% polyacrylamide gels and transferred to nitrocellulose. Membranes were blocked for 30 minutes with 10% nonfat dry milk in TRIS-buffered saline containing 0.1% Tween20 (TBST). Subsequently, blots were incubated with primary antibodies diluted in 1% nonfat dry milk in TBST. Antibodies included anti-tubulin (1:5000; Abcam), anti-ERK1/2 and anti-phosphoERK1/2 (1:1000, Cell Signaling), anti-SHP2 and anti-phosphoSHP2 (1:1000, Cell Signaling), anti-myogenin (1:1000; F5D, Developmental Studies Hybridoma Bank, University of Iowa, IA) and anti-FLAG (1:200, M2, SigmaAldrich). Blots were washed extensively with TBST prior to incubation with the appropriate peroxidase-labeled secondary antibody. Immune complexes were visualized by chemiluminescence (ECL, Amersham) and autoradiography.
Immunocytochemistry
Cells were fixed with fresh 4% paraformaldehyde in PBS for 15 minutes at room temperature. Cells were permeabilized and nonspecific binding sites were blocked with 5% horse serum in PBS containing 0.1% Triton X100. Cells were further incubated overnight at 4 C in mouse anti-Pax7 (1:4 hybridoma supernatant, Developmental Studies Hybridoma Bank), mouse anti-PCNA (1:50, PC10, Santa Cruz Biotech), mouse anti-MyoD (1:30, 5.8A, Vector Laboratories), mouse anti-myosin heavy chain (1:5 hybridoma supernatant, MF20, Developmental Studies Hybridoma Bank) and rabbit anti-Ki67 (1:200, SP6, Novus). After exhaustive washing with PBS, the cells were incubated with goat anti-mouse AlexaFluor488 or AlexaFluor568 (1:200, Invitrogen) or donkey anti-rabbit AlexaFluor488 or AlexaFluor568 (1:200, Invitrogen) for 40 minutes at room temperature. Total nuclei were detected with Hoechst 33342 (10 μg/ml in PBS). Immune complexes were visualized with a Nikon TE2000 inverted phase microscope equipped with epifluorescence. Representative images were captured with a CoolPix CCD camera and assembled and analyzed with NIS-Elements software (Nikon).
Statistical Analysis
All data were analyzed by ANOVA followed by t-test using Statistical Analysis System (SAS Institute Inc., Cary, NC). Results were presented as the mean ± SEM. A p-value <0.05 was considered to be significant.
RESULTS
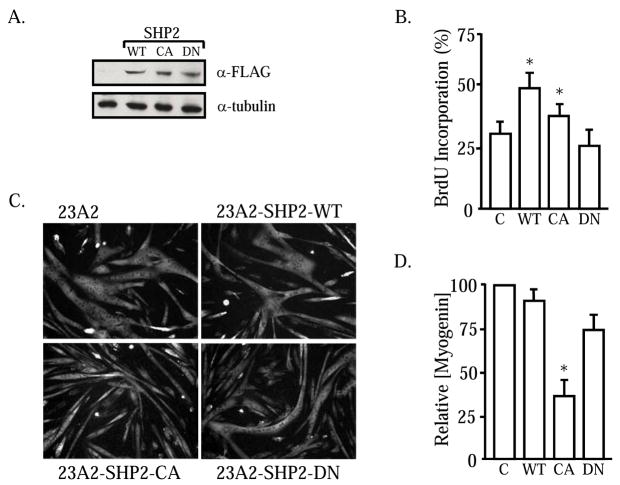
MET initiation of downstream Gab1:SHP2 signals is necessary for limb formation and myoblast migration during development [17, 18, 23, 24]. Because MET signaling stimulates satellite cell activation and migration [3, 25, 26], we examined the importance of SHP2 in mediating HGF effects on these cells. As a first step, stable 23A2 myoblast cell lines were created that over-express wildtype SHP2 (SHP2-WT), constitutive active SHP2 (SHP2-CA) or a phosphatase defective SHP2 (SHP2-DN). 23A2 myoblasts were selected because they contain a high percentage (approx. 30%) of satellite cell progenitors (Pax7+:MRF+) [27]. Initial Western blot analysis reveals that all of the SHP2 cell lines express abundant amounts of the FLAG-tagged fusion phosphatase of the correct size (Figure 1A). The proliferative effects of the mutant SHP2 proteins were evaluated by BrdU pulse labeling (Figure 1B). In brief, the SHP2-expressing myoblasts were seeded at equal cell density in growth media. Two hours prior to fixation, the subconfluent cells were pulsed with BrdU for the immunodetection of proliferating cells. Approximately 30% of the 23A2 control cells are traversing S-phase when maintained as subconfluent monolayers in growth media. 23A2-SHP2-DN incorporate BrdU at rates comparable to control cells suggesting that SHP2 is not required for proliferation. By contrast, both 23A2-SHP2-WT and 23A2-SHP2-CA exhibit an increased percentage of cells synthesizing DNA arguing that the phosphatase elicits a positive response on proliferation. The effects of sustained SHP2 activity on myofiber formation was examined (Figure 1C). Confluent 23A2-SHP2 expressing myoblasts were cultured for 48 hours in differentiation permissive media. In all instances, large multinucleated myofibers formed that express the contractile protein, myosin heavy chain (MyHC). Induction of the full myogenic program was evaluated further by Western analysis for myogenin, the requisite MRF for differentiation. Densitometric analysis of the blots reveals that neither SHP2-WT nor SHP2-DN affect myogenin protein production (Figure 1D). Interestingly, 23A2-SHP2-CA demonstrate a substantial reduction in the amounts of myogenin protein. From these results, we conclude that SHP2 may participate in myoblast proliferation and elicits differential effects on myoblast differentiation.
Figure 1. SHP2 stimulates proliferation in myoblasts.
23A2 myoblasts were stably transfected with plasmids coding for SHP2-WT, SHP2-CA or SHP2-DN. Total cellular protein lysates were examined by Western blot for FLAG-tagged SHP2 expression (A). Subconfluent cultures of 23A2, 23A2-SHP2-WT, 23A2-SHP2-CA and 23A2-SHP2-DN myoblasts were pulsed for 2 hrs with BrdU to measure the numbers of cells traversing S-phase (B). Myoblasts expressing SHP2-WT and SHP2-CA contain a higher percentage of proliferating cells (*; P<0.05). The aforementioned myogenic cells were induced to differentiate for 48 hours and immunostained for myosin heavy chain (C) or lysed for Western blot-scanning densitometry analysis of myogenin expression. All cell types form large, multinucleated myofibers but myogenin content is lower in 23A2-SHP2-CA (*; P<0.05).
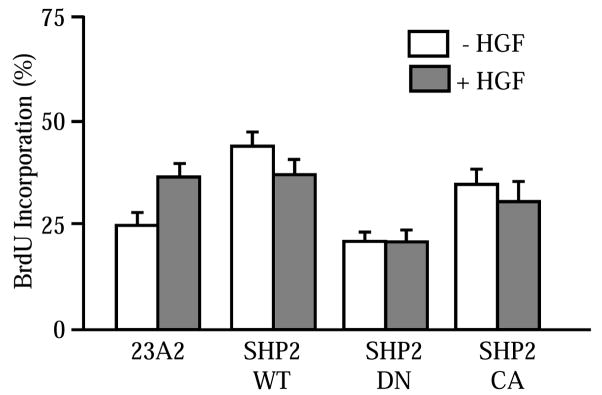
HGF stimulates satellite cell proliferation in vitro [3, 11, 12, 28]. The interplay between SHP2 and HGF was examined in the mutant cell lines. 23A2, 23A2-SHP-WT, -SHP2-CA and –SHP-DN myoblasts were culture in reduced serum media supplemented with HGF for 48 hours. The cells were treated with BrdU for two hours prior to fixation and immunodetection of the thymidine analog. As expected, HGF stimulates myoblast proliferation as evidenced by an increase in the percentage of cell progressing through S-phase (Figure 2). A higher percentage of 23A2-SHP2-WT and 23A2-SHP2-CA myoblasts are proliferating than controls and this number is not further improved by HGF treatment. 23A2-SHP2-DN myoblasts divide at rates comparable to parental 23A2 myoblasts. However, treatment with HGF does not improve proliferation. These results indicate that SHP2 may be the chief mediator of HGF-induced myoblast proliferation.
Figure 2. HGF induced proliferation requires SHP2.
Control and 23A2 myoblasts expressing SHP2-WT, SHP2-CA or SHP2-DN were treated with HGF (10 ng/ml) for 48 hours prior to measurement of the numbers of BrdU immunopositive cells. HGF induces proliferation in control cells that is blocked by SHP2-DN (P<0.05). Means and SEMs of a minimum of three independent experiments are shown.
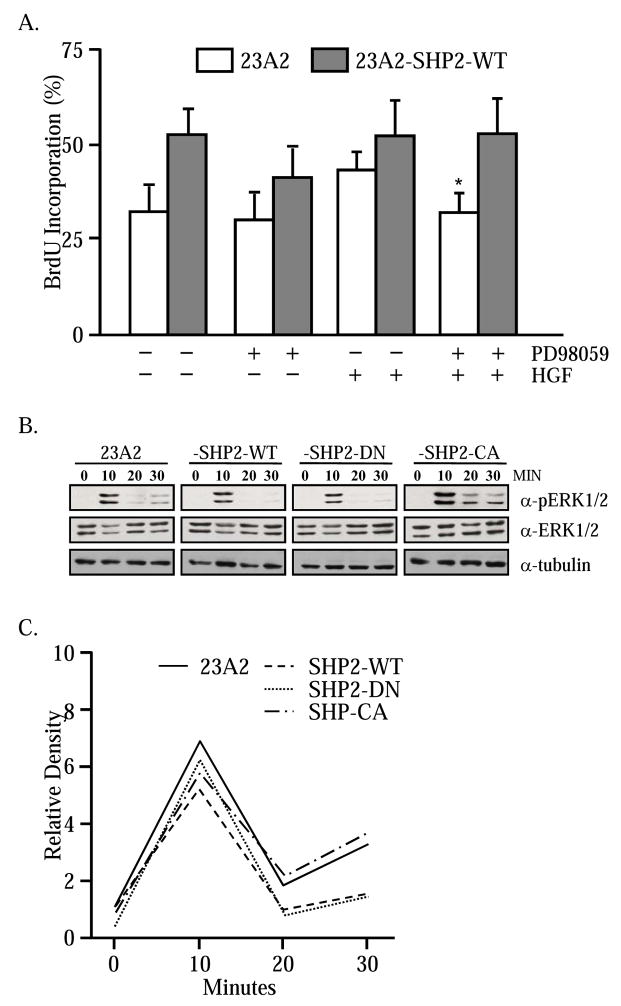
SHP2 prolongs the activity of downstream ERK1/2 [29, 30]. The requirement of ERK1/2 in the SHP2 response was further explored in 23A2-SHP2-WT myoblasts. In brief, 23A2 and 23A2-SHP-WT myoblasts were cultured in low-serum media supplemented with HGF and PD98059, a chemical inhibitor of MEK1/2, or an equivalent volume of vehicle-only. After 48 hours, proliferation was assessed by BrdU incorporation as described throughout. As expected, more mitotically active myoblasts are present in the 23A2-SHP2-WT cultures (Figure 3A). Treatment with the MEK inhibitor causes a reduction in the percentage of dividing SHP2-expressing myoblasts. Supplementation of the culture media with HGF does not affect 23A2-SHP2-WT proliferation rates. Importantly, inhibition of MEK/ERK activity in conjunction with HGF treatment has no effect on 23A2-SHP2-WT proliferation. The ability of SHP2 to compensate for the loss of MEK/ERK signaling argues that the two signaling systems are independent of one another in myogenic cells. The ERK-independent actions of SHP2 are further supported by examination of the ERK1/2 activation profile in response to HGF treatment. Subconfluent 23A2, -SHP2-WT, -SHP2-CA and –SHP2-DN myoblasts were washed with protamine sulfate to remove ECM-bound growth factors and cultured for 60 minutes in serum-free medium. Subsequently, the cells were treated with HGF and lysed over time for assessment of ERK activity. Western blot for phosphorylated ERK1/2 demonstrates that all cells direct a robust response to HGF at 10 minutes and the amounts of phosphoERK1/2 are severely diminished, or completely absent, by 20 minutes post-stimulus (Figure 3B). Semi-quantification of the relative amounts of phosphoERK1/2 demonstrates no differences in the kinase activity profile in response to HGF (Figure 3C).
Figure 3. HGF and SHP2 stimulate proliferation independent of ERK1/2.
The myogenic cell lines were treated for 48 hours with HGF (10 ng/ml) in the presence or absence of 25 μM PD98059. Cells were pulsed with BrdU (2 hrs) and the numbers of BrdU immunopositive cells were measured (A). PD98059 inhibited HGF-induced proliferation in control cells but not 23A2-SHP2-WT cells (*; P<0.05). The ability of HGF to stimulate ERK1/2 was examined in the respective cell lines by Western blot (B). A transient peak of phosphoERK1/2 is present within 10 minutes. The relative intensities of the phosphoERK1/2 bands was normalized to tubulin content and plotted (C). Neither SHP2-WT nor SHP2-CA extend the phosphoERK1/2 signal.
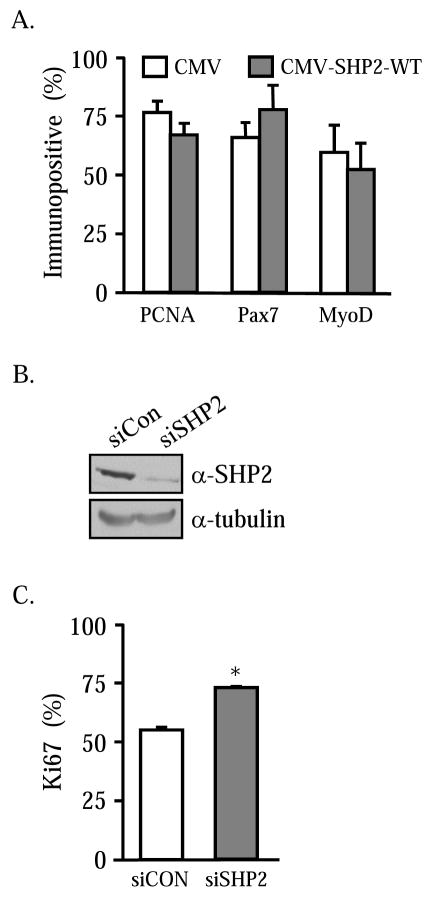
The role of SHP2 was further evaluated in primary cultures of mouse satellite cells. Satellite cells were isolated from adult mice and cultured en mass for 3 days prior to transfection with plasmid encoding SHP2-WT. After 48 hours, the cells were fixed and proliferating cells enumerated following immunocytochemical detection of PCNA in the SHP2 expressing cells. Results indicate that unlike 23A2 myoblasts, satellite cell mitotic activity is unaffected by ectopic SHP2 (Figure 4A). Importantly, the phosphatase does not interfere with the identity of the satellite cells. No differences in the number of Pax7 or MyoD immunopositive cells were evident. These results argue that primary satellite cells are not equivalent to 23A2 myoblasts with regard to SHP2-mediated signaling events. To better understand the importance of SHP2 in primary satellite cells, the relative amounts of the phosphatase were reduced. Small interfering RNAs directed against SHP2 were created and evaluated for knockdown efficiency. 23A2 myoblasts were transiently transfected with pSilencer-siSHP2 or the scrambled control, pSilencer-siCon. Transfection efficiency exceeds 50% in these cells (data not shown). After 48 hours, total cell lysates were harvested and analyzed by Western for SHP2. As shown in Figure 4B, 23A2 myoblasts express SHP2 and the endogenous phosphatase levels are not affected by the scrambled control siRNA. By contrast, ectopic expression of siSHP2 causes a substantial reduction in SHP2 protein content. No differences were observed in tubulin levels indicating equivalent amounts of total protein and cell numbers. Subsequently, the mouse siSHP2 cDNA was cloned into a lentiviral vector that constitutively expresses GFP. Primary satellite cells were transiently transfected with the vector coding for siSHP2 or siCon. After 24 hours the GFP-expressing cells were evaluated for the presence or absence of Ki67, a proliferation marker. Approximately 60% of the satellite cells expressing the scrambled control siRNA were mitotically active (Figure 4C). Satellite cells ectopically expressing siSHP2 demonstrate a higher percentage of proliferating cells. Thus, it appears that SHP2 represents a putative block to optimal proliferation in satellite cells.
Figure 4. SHP2 does not affect satellite cell proliferation.
Satellite cells were transiently transfected with CMV or CMV-SHP2-WT and a GFP-expression plasmid. After 48 hours, the cells were immunostained for the proliferation marker, Ki67. The numbers of GFP-expressing cells that contain Ki67 were enumerated (A). pSirenRetroQ-siCon and –siSHP2 were transfected into 23A2 myoblasts and evaluated by Western blot for SHP2 content after 48 hours (B). The expression plasmids were transfected into satellite cells. After 48 hours, the GFP expressing cells were examined for Ki67 expression (C). A reduction in SHP2 protein causes an increase in proliferation (P<0.05).
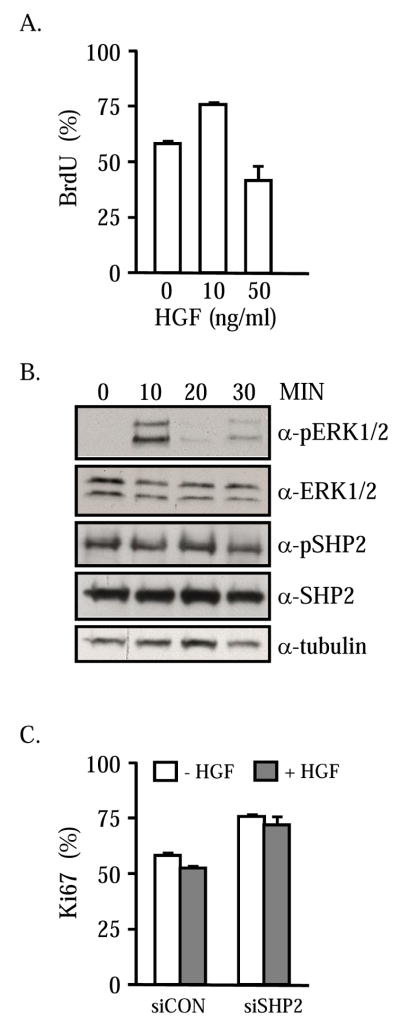
Strong ERK1/2 activity prevents proliferation in myoblasts and induces a reversible quiescent state [27, 31]. Because HGF can induce the MEK/ERK signaling axis in satellite cells [32], we examined the effects of the growth factor on cell cycle transition. Semiconfluent cultures of satellite cells were treated with 10 or 50 ng/ml HGF for 48 hours in low-serum media. Two hours prior to termination, the cells were pulsed with BrdU. The numbers of BrdU-incorporating cells and total cell numbers were quantified. As expected, the lower concentration of HGF readily stimulated cell proliferation as indicated by an increase in the percentage of cells incorporating BrdU (Figure 5A). The higher concentration of HGF caused a significant reduction in the numbers of satellite cells progressing through S-phase. The ability of high concentrations of HGF to induce ERK and SHP2 were evaluated by Western blot. Total cell lysates were harvested at 10-minute intervals following treatment with 50 ng/ml HGF (Figure 5B). A robust increase in phosphoERK1/2 is evident within 10 minutes of HGF supplementation followed by a decline to basal activity within 30 minutes. By contrast, SHP2 is present in a phosphorylated form prior to HGF treatment and the amounts of phosphoSHP2 do not vary across the experimental time frame. No differences in total SHP2, ERK1/2 or tubulin content are apparent. These results demonstrate that SHP2 is present but does not appear to prolong the HGF-mediated ERK1/2 signal. The role of SHP2 in the HGF-imposed block to cell cycle progression was further explored by knockdown of the phosphatase levels. Primary satellite cell cultures were transfected with a lentiviral vector constitutively expressing GFP and siCon or siSHP2. After 24 hours, the cells were further incubated in low-serum media supplemented with 50 ng/ml of HGF or an equivalent volume of vehicle-only. GFP expressing satellite cells were evaluated for Ki67 expression after 48 hours of growth factor treatment. Knockdown of SHP2 results in an increase in the numbers of proliferating cells, as shown previously (Figure 5C). Treatment with 50 ng/ml HGF did not reduce the percentage of proliferating cells by comparison to satellite cells transfected with the scrambled control. The numbers of proliferating siSHP2 cells remain unchanged in the presence of the inhibitory concentration of HGF suggesting that the phosphatase participates in the HGF-induced growth arrest of satellite cells.
Figure 5. HGF induced arrest is mediated by SHP2.
Satellite cells were treated with 10 or 50 ng/ml HGF for 48 hours prior to measurement of proliferation. The percentage of cells incorporating BrdU is shown (A). Treatment of satellite cells with 50 ng/ml HGF does not cause a sustained increase in phosphoERK1/2 or change the relative amount of phosphoSHP2 (B). Cells were transiently transfected with pSirenRetroQ-siCon or –siSHP2 and treated for 48 hours with 50 ng/ml HGF. The percentage of GFP expressing cells containing Ki67 was measured (C). The inhibitory effect of HGF is overcome by removal of SHP2.
DISCUSSION
The importance of HGF for satellite cell activation is indisputable. However, the role of the growth factor during deactivation and the return to G0 is novel and intriguing. HGF is an autocrine factor synthesized and released during the period of rapid proliferation and subsequent self-renewal of the population making it present at the correct time and location [3, 10, 33, 34]. Injection of HGF into a site of muscle damage results in an increase in the numbers of MyoD-expressing satellite cells and a reduction in fiber formation [6]. These results support widely documented in vitro evidence that HGF stimulates proliferation and inhibits differentiation. After 48 hours in culture, satellite cells en mass are considered activated and fully proliferative [35, 36]. This is reflected in our results whereby a low-dose HGF stimulus causes a significant increase in the percentage of satellite cells incorporating the thymidine analog. By contrast, a higher concentration of HGF (50 ng/ml) diminishes satellite cell proliferation. The ability of HGF to alter satellite cell activity as a consequence of signal intensity is supportive of a dual role for the growth factor. In our model, autocrine HGF is present at low concentrations during the initial stages of skeletal muscle damage such that satellite cell activation and subsequent proliferation occur. As the numbers of active satellite cells increase, the local concentration of HGF becomes sufficient to induce cell cycle exit and a return to the quiescent state. This same model can be extrapolated to times of normal skeletal muscle growth and exercise-induced hypertrophy. In this scenario, the individual satellite cell responds to the autocrine factor to divide once with one cell returning to G0 and the daughter fusing into the adjacent fiber. In addition to encouraging a G0-like state, high concentrations of HGF may protect the satellite cell from precocious differentiation. Immunocytochemical detection of myogenin reveals no increase in the numbers of differentiated cells (data not shown) arguing that the HGF-imposed G0 is not permissive to terminal differentiation. It remains to be determined if high HGF concentrations can elicit similar effects in the presence of a strong differentiation inducing signal such as that supplied by IGF-I.
SHP2 is a protein tyrosine phosphatase that is recruited to the MET kinase domain via the docking protein, Gab1 [15]. Either direct or indirect interaction of Gab1:SHP2 is sufficient to promote myoblast migration into the developing limbs of mice [17, 18, 24]. The importance of SHP2 to satellite cell biology is not limited to migratory actions but appears to include cell cycle kinetics. Satellite cells in vitro express the protein and loss of SHP2 causes an increase in the numbers of proliferating cells. Ectopic expression of SHP2 also tends to decrease proliferation as indicated by a reduction in the numbers of PCNA expressing satellite cells. Of importance, loss of SHP2 prevents the inhibitory actions of high concentrations of HGF. These results support a model whereby HGF signals through SHP2 to inhibit proliferation and impose a quiescent program on the satellite cell. In many cell types, SHP2 activities include a prolonged ERK1/2 response [29, 30]. By contrast, the inhibitory actions of HGF on satellite cell proliferation as mediated through SHP2 are independent of sustained ERK1/2 phosphorylation. High concentrations of HGF cause an initial rise in phosphoERK1/2 that is absent within 20 minutes post-stimulus. The downstream SHP2 targets in satellite cells remain unknown but may include p190RhoGAP. SHP2 associates with p190RhoGAP and dephosphorylation of the GTPase activating protein allows for the activation of RhoA in C2C12 myoblasts [37]. RhoA can phosphorylate and activate phosphotidylinositol 3-kinase (PI3K) and downstream Akt to promote myoblast survival [38]. In addition, constitutive expression of RhoA in C2 myoblasts impedes both MyoD expression and muscle fiber formation. HGF/MET activation of RhoA is integral to epithelial cell migration and invasion [39, 40]. Future pursuits may include an examination of HGF’s ability to initiate Rho-dependent satellite cell proliferation and growth arrest.
Coincident with a role in cell cycle kinetics, SHP2 also is integral to stem cell biology and self-renewal. In trophoblasts, SHP2 protects the putative stem cell from apoptosis during early embryogenesis [41]. Mouse SHP2(−/−) blastocysts contain a trophectoderm layer that fails to implant into the uterine wall and lacks differentiated giant cells. Importantly, trophectoderm-derived stem cell lines cannot be established in vitro in the absence of a functional SHP2. In a similar manner, targeted deletion of SHP2 in neural progenitor cells compromises FGF2-mediated self-renewal through repression of Bmi-1 expression, a polycomb protein implicit in self-renewal of multiple stem cell lineages [20, 42]. By contrast, others report that FGF signaling through FRS2a and SHP2 is critical for neural stem and progenitor cell proliferation in vitro but dispensible for self-renewal [43]. The absolute requirement of a functional SHP2 for continuous expansion of stem cells is further complicated by reports that deletion of the phosphatase in ES cells results in improved rates of secondary embryoid body formation, a measure of self-renewal [44]. SHP2 null ES aggregates exhibited reduced expression of MyoD and myogenin suggesting that fewer skeletal myoblasts were formed and differentiated by comparison to wildtype. It is unknown if a Pax3, Pax3:Myf5 or Pax7 expressing myogenic population forms in SHP2(−/−) embryoid bodies. Each of these populations can yield a MyoD-expressing myoblast [45, 46]. Treatment of satellite cells with HGF, which signals through downstream SHP2, tended to increase the numbers of Pax7 immunopositive cells and reduce the percentage of cells expressing MyoD. Satellite cells defined as Pax7+ and Myf5/MyoD- are capable of asymmetric self-renewal in vitro and repopulation of the niche following transplantation into the tibialis anterior muscle [47]. A shift toward a population enriched for Pax7+/MyoD- cells suggests that SHP2 is important for satellite cell self-renewal. Conditional ablation of SHP2 in MyoD and/or Pax7 expressing cells will assist in defining the role of the phosphatase during adult myogenesis.
The involvement of SHP2 during myofiber formation is context dependent with the phosphatase participating in both promotional and inhibitory signals [48, 49]. SHP2 activity increases during the transition from mononucleated myoblast to multinucleated myofiber [37]. However, ectopic expression of constitutive active SHP2 represses C2C12 muscle fiber formation [48]. Ablation of SHP2 in satellite cells in vitro delays myogenesis resulting in small neofibers with fewer than 5 myonuclei thus, supporting a role for the phosphatase during myoblast fusion [50]. Our results indicate a temporal role of SHP2 for induction of biochemical and phenotypic differentiation. Given that SHP2 activity increases during fiber formation, our results are suggestive that sustained activity accelerates 23A2-SHP2-CA muscle fiber maturation as denoted by a reduction in myogenin expression with no effect on morphological parameters. The correct timing of SHP2 activity may underlie the divergent responses of immortal and primary myoblasts. In data not shown, relative amounts of SHP2 are greater in primary satellite cells than in 23A2 myoblasts. Primary satellite cells are notorious for spontaneous differentiation even in the presence of high fetal bovine serum concentrations (10–15%). In this scenario, the elevated SHP2 may predispose the cells to accelerated myogenesis under conditions that normal suppress C2C12 and 23A2 myogenesis. The reasons for divergent behavior of myoblasts to a common growth factor or intracellular signaling intermediate are unknown. However, we provide evidence that conventional myoblast lines are not duplicitous of satellite cells and caution against direct extrapolation of results. During the course of spontaneous immortalization, myoblasts may have acquired a refractile nature to signals that would disrupt cell cycle kinetics or invoke quiescence in a primary myogenic cell. A phosphoproteomic assessment of HGF-induced signaling intermediates that underlie opposing phenotypic responses in the two myogenic cell types will provide insight into both the effects of signal intensity and cell immortalization.
In summary, we provide evidence that HGF participates in satellite cell G0 entry through a mechanism that involves SHP2. Knockdown of SHP2 improves proliferation rates and ectopic SHP2 inhibits satellite cell cycle kinetics. Importantly, SHP2 causes divergent effects on cell proliferation that is dependent upon the myogenic background. Manipulation of SHP2 activity may allow for the creation of myogenic cells that are more suited for engraftment into compromised skeletal muscle.
Acknowledgments
This work was supported by NIH-NIAMSD R01-AR048830 to S.E.J. S.A.R is supported by a USDA- National Needs Fellowship. J.L. is supported by a University of Florida Alumni Fellowship.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Dhawan J, Rando TA. Stem cells in postnatal myogenesis: molecular mechanisms of satellite cell quiescence, activation and replenishment. Trends Cell Biol. 2005;15:666–673. doi: 10.1016/j.tcb.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 2.Wozniak AC, Kong J, Bock E, Pilipowicz O, Anderson JE. Signaling satellite-cell activation in skeletal muscle: markers, models, stretch, and potential alternate pathways. Muscle & nerve. 2005;31:283–300. doi: 10.1002/mus.20263. [DOI] [PubMed] [Google Scholar]
- 3.Allen RE, Sheehan SM, Taylor RG, Kendall TL, Rice GM. Hepatocyte growth factor activates quiescent skeletal muscle satellite cells in vitro. Journal of cellular physiology. 1995;165:307–312. doi: 10.1002/jcp.1041650211. [DOI] [PubMed] [Google Scholar]
- 4.Anderson JE. A role for nitric oxide in muscle repair: nitric oxide-mediated activation of muscle satellite cells. Molecular biology of the cell. 2000;11:1859–1874. doi: 10.1091/mbc.11.5.1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Conboy IM, Rando TA. The regulation of Notch signaling controls satellite cell activation and cell fate determination in postnatal myogenesis. Developmental cell. 2002;3:397–409. doi: 10.1016/s1534-5807(02)00254-x. [DOI] [PubMed] [Google Scholar]
- 6.Miller KJ, Thaloor D, Matteson S, Pavlath GK. Hepatocyte growth factor affects satellite cell activation and differentiation in regenerating skeletal muscle. Am J Physiol Cell Physiol. 2000;278:C174–C181. doi: 10.1152/ajpcell.2000.278.1.C174. [DOI] [PubMed] [Google Scholar]
- 7.Wozniak AC, Anderson JE. Nitric oxide-dependence of satellite stem cell activation and quiescence on normal skeletal muscle fibers. Dev Dyn. 2007;236:240–250. doi: 10.1002/dvdy.21012. [DOI] [PubMed] [Google Scholar]
- 8.McCroskery S, Thomas M, Maxwell L, Sharma M, Kambadur R. Myostatin negatively regulates satellite cell activation and self-renewal. The Journal of cell biology. 2003;162:1135–1147. doi: 10.1083/jcb.200207056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bischoff R. A satellite cell mitogen from crushed adult muscle. Developmental biology. 1986;115:140–147. doi: 10.1016/0012-1606(86)90235-6. [DOI] [PubMed] [Google Scholar]
- 10.Tatsumi R, Anderson JE, Nevoret CJ, Halevy O, Allen RE. HGF/SF is present in normal adult skeletal muscle and is capable of activating satellite cells. Developmental biology. 1998;194:114–128. doi: 10.1006/dbio.1997.8803. [DOI] [PubMed] [Google Scholar]
- 11.Sheehan SM, Tatsumi R, Temm-Grove CJ, Allen RE. HGF is an autocrine growth factor for skeletal muscle satellite cells in vitro. Muscle & nerve. 2000;23:239–245. doi: 10.1002/(sici)1097-4598(200002)23:2<239::aid-mus15>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 12.Tatsumi R, Hattori A, Ikeuchi Y, Anderson JE, Allen RE. Release of hepatocyte growth factor from mechanically stretched skeletal muscle satellite cells and role of pH and nitric oxide. Molecular biology of the cell. 2002;13:2909–2918. doi: 10.1091/mbc.E02-01-0062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tatsumi R, Liu X, Pulido A, Morales M, Sakata T, Dial S, Hattori A, Ikeuchi Y, Allen RE. Satellite cell activation in stretched skeletal muscle and the role of nitric oxide and hepatocyte growth factor. American journal of physiology. 2006;290:C1487–1494. doi: 10.1152/ajpcell.00513.2005. [DOI] [PubMed] [Google Scholar]
- 14.Tatsumi R, Sheehan SM, Iwasaki H, Hattori A, Allen RE. Mechanical stretch induces activation of skeletal muscle satellite cells in vitro. Exp Cell Res. 2001;267:107–114. doi: 10.1006/excr.2001.5252. [DOI] [PubMed] [Google Scholar]
- 15.Rosario M, Birchmeier W. How to make tubes: signaling by the Met receptor tyrosine kinase. Trends in cell biology. 2003;13:328–335. doi: 10.1016/s0962-8924(03)00104-1. [DOI] [PubMed] [Google Scholar]
- 16.Bladt F, Riethmacher D, Isenmann S, Aguzzi A, Birchmeier C. Essential role for the c-met receptor in the migration of myogenic precursor cells into the limb bud. Nature. 1995;376:768–771. doi: 10.1038/376768a0. [DOI] [PubMed] [Google Scholar]
- 17.Sachs M, Brohmann H, Zechner D, Muller T, Hulsken J, Walther I, Schaeper U, Birchmeier C, Birchmeier W. Essential role of Gab1 for signaling by the c-Met receptor in vivo. The Journal of cell biology. 2000;150:1375–1384. doi: 10.1083/jcb.150.6.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schaeper U, Vogel R, Chmielowiec J, Huelsken J, Rosario M, Birchmeier W. Distinct requirements for Gab1 in Met and EGF receptor signaling in vivo. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:15376–15381. doi: 10.1073/pnas.0702555104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saxton TM, Henkemeyer M, Gasca S, Shen R, Rossi DJ, Shalaby F, Feng GS, Pawson T. Abnormal mesoderm patterning in mouse embryos mutant for the SH2 tyrosine phosphatase Shp-2. The EMBO journal. 1997;16:2352–2364. doi: 10.1093/emboj/16.9.2352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ke Y, Zhang EE, Hagihara K, Wu D, Pang Y, Klein R, Curran T, Ranscht B, Feng GS. Deletion of shp2 in the brain leads to defective proliferation and differentiation in neural stem cells and early postnatal lethality. Molecular and cellular biology. 2007;27:6706–6717. doi: 10.1128/MCB.01225-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Seale P, Sabourin LA, Girgis-Gabardo A, Mansouri A, Gruss P, Rudnicki MA. Pax7 is required for the specification of myogenic satellite cells. Cell. 2000;102:777–786. doi: 10.1016/s0092-8674(00)00066-0. [DOI] [PubMed] [Google Scholar]
- 22.Day K, Shefer G, Richardson JB, Enikolopov G, Yablonka-Reuveni Z. Nestin-GFP reporter expression defines the quiescent state of skeletal muscle satellite cells. Developmental biology. 2007;304:246–259. doi: 10.1016/j.ydbio.2006.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saxton TM, Ciruna BG, Holmyard D, Kulkarni S, Harpal K, Rossant J, Pawson T. The SH2 tyrosine phosphatase shp2 is required for mammalian limb development. Nature genetics. 2000;24:420–423. doi: 10.1038/74279. [DOI] [PubMed] [Google Scholar]
- 24.Schaeper U, Gehring NH, Fuchs KP, Sachs M, Kempkes B, Birchmeier W. Coupling of Gab1 to c-Met, Grb2, and Shp2 mediates biological responses. The Journal of cell biology. 2000;149:1419–1432. doi: 10.1083/jcb.149.7.1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yamada M, Sankoda Y, Tatsumi R, Mizunoya W, Ikeuchi Y, Sunagawa K, Allen RE. Matrix metalloproteinase-2 mediates stretch-induced activation of skeletal muscle satellite cells in a nitric oxide-dependent manner. The international journal of biochemistry & cell biology. 2008;40:2183–2191. doi: 10.1016/j.biocel.2008.02.017. [DOI] [PubMed] [Google Scholar]
- 26.Nishimura T, Nakamura K, Kishioka Y, Kato-Mori Y, Wakamatsu J, Hattori A. Inhibition of matrix metalloproteinases suppresses the migration of skeletal muscle cells. Journal of muscle research and cell motility. 2008;29:37–44. doi: 10.1007/s10974-008-9140-2. [DOI] [PubMed] [Google Scholar]
- 27.Reed SA, Ouellette SE, Liu X, Allen RE, Johnson SE. E2F5 and LEK1 translocation to the nucleus is an early event demarcating myoblast quiescence. Journal of cellular biochemistry. 2007;101:1394–1408. doi: 10.1002/jcb.21256. [DOI] [PubMed] [Google Scholar]
- 28.Gal-Levi R, Leshem Y, Aoki S, Nakamura T, Halevy O. Hepatocyte growth factor plays a dual role in regulating skeletal muscle satellite cell proliferation and differentiation. Biochimica et biophysica acta. 1998;1402:39–51. doi: 10.1016/s0167-4889(97)00124-9. [DOI] [PubMed] [Google Scholar]
- 29.Neel BG, Gu H, Pao L. The ‘Shp’ing news: SH2 domain-containing tyrosine phosphatases in cell signaling. Trends in biochemical sciences. 2003;28:284–293. doi: 10.1016/S0968-0004(03)00091-4. [DOI] [PubMed] [Google Scholar]
- 30.Mohi MG, Neel BG. The role of Shp2 (PTPN11) in cancer. Current opinion in genetics & development. 2007;17:23–30. doi: 10.1016/j.gde.2006.12.011. [DOI] [PubMed] [Google Scholar]
- 31.Wang X, Thomson SR, Starkey JD, Page JL, Ealy AD, Johnson SE. Transforming growth factor beta1 is up-regulated by activated Raf in skeletal myoblasts but does not contribute to the differentiation-defective phenotype. The Journal of biological chemistry. 2004;279:2528–2534. doi: 10.1074/jbc.M306489200. [DOI] [PubMed] [Google Scholar]
- 32.Halevy O, Cantley LC. Differential regulation of the phosphoinositide 3-kinase and MAP kinase pathways by hepatocyte growth factor vs. insulin-like growth factor-I in myogenic cells. Experimental cell research. 2004;297:224–234. doi: 10.1016/j.yexcr.2004.03.024. [DOI] [PubMed] [Google Scholar]
- 33.Tatsumi R, Allen RE. Active hepatocyte growth factor is present in skeletal muscle extracellular matrix. Muscle & nerve. 2004;30:654–658. doi: 10.1002/mus.20114. [DOI] [PubMed] [Google Scholar]
- 34.Jennische E, Ekberg S, Matejka GL. Expression of hepatocyte growth factor in growing and regenerating rat skeletal muscle. The American journal of physiology. 1993;265:C122–128. doi: 10.1152/ajpcell.1993.265.1.C122. [DOI] [PubMed] [Google Scholar]
- 35.Beauchamp JR, Heslop L, Yu DS, Tajbakhsh S, Kelly RG, Wernig A, Buckingham ME, Partridge TA, Zammit PS. Expression of CD34 and Myf5 defines the majority of quiescent adult skeletal muscle satellite cells. The Journal of cell biology. 2000;151:1221–1234. doi: 10.1083/jcb.151.6.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zammit PS, Golding JP, Nagata Y, Hudon V, Partridge TA, Beauchamp JR. Muscle satellite cells adopt divergent fates: a mechanism for self-renewal? The Journal of cell biology. 2004;166:347–357. doi: 10.1083/jcb.200312007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kontaridis MI, Eminaga S, Fornaro M, Zito CI, Sordella R, Settleman J, Bennett AM. SHP-2 positively regulates myogenesis by coupling to the Rho GTPase signaling pathway. Molecular and cellular biology. 2004;24:5340–5352. doi: 10.1128/MCB.24.12.5340-5352.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reuveny M, Heller H, Bengal E. RhoA controls myoblast survival by inducing the phosphatidylinositol 3-kinase-Akt signaling pathway. FEBS letters. 2004;569:129–134. doi: 10.1016/j.febslet.2004.05.035. [DOI] [PubMed] [Google Scholar]
- 39.Miao H, Nickel CH, Cantley LG, Bruggeman LA, Bennardo LN, Wang B. EphA kinase activation regulates HGF-induced epithelial branching morphogenesis. The Journal of cell biology. 2003;162:1281–1292. doi: 10.1083/jcb.200304018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kitajo H, Shibata T, Nagayasu H, Kawano T, Hamada J, Yamashita T, Arisue M. Rho regulates the hepatocyte growth factor/scatter factor-stimulated cell motility of human oral squamous cell carcinoma cells. Oncology reports. 2003;10:1351–1356. [PubMed] [Google Scholar]
- 41.Yang W, Klaman LD, Chen B, Araki T, Harada H, Thomas SM, George EL, Neel BG. An Shp2/SFK/Ras/Erk signaling pathway controls trophoblast stem cell survival. Developmental cell. 2006;10:317–327. doi: 10.1016/j.devcel.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 42.Pardal R, Molofsky AV, He S, Morrison SJ. Stem cell self-renewal and cancer cell proliferation are regulated by common networks that balance the activation of proto-oncogenes and tumor suppressors. Cold Spring Harbor symposia on quantitative biology. 2005;70:177–185. doi: 10.1101/sqb.2005.70.057. [DOI] [PubMed] [Google Scholar]
- 43.Yamamoto S, Yoshino I, Shimazaki T, Murohashi M, Hevner RF, Lax I, Okano H, Shibuya M, Schlessinger J, Gotoh N. Essential role of Shp2-binding sites on FRS2alpha for corticogenesis and for FGF2-dependent proliferation of neural progenitor cells. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:15983–15988. doi: 10.1073/pnas.0507961102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Qu CK, Feng GS. Shp-2 has a positive regulatory role in ES cell differentiation and proliferation. Oncogene. 1998;17:433–439. doi: 10.1038/sj.onc.1201920. [DOI] [PubMed] [Google Scholar]
- 45.Bajard L, Relaix F, Lagha M, Rocancourt D, Daubas P, Buckingham ME. A novel genetic hierarchy functions during hypaxial myogenesis: Pax3 directly activates Myf5 in muscle progenitor cells in the limb. Genes & development. 2006;20:2450–2464. doi: 10.1101/gad.382806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Relaix F, Rocancourt D, Mansouri A, Buckingham M. A Pax3/Pax7-dependent population of skeletal muscle progenitor cells. Nature. 2005;435:948–953. doi: 10.1038/nature03594. [DOI] [PubMed] [Google Scholar]
- 47.Kuang S, Kuroda K, Le Grand F, Rudnicki MA. Asymmetric self-renewal and commitment of satellite stem cells in muscle. Cell. 2007;129:999–1010. doi: 10.1016/j.cell.2007.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kontaridis MI, Liu X, Zhang L, Bennett AM. Role of SHP-2 in fibroblast growth factor receptor-mediated suppression of myogenesis in C2C12 myoblasts. Molecular and cellular biology. 2002;22:3875–3891. doi: 10.1128/MCB.22.11.3875-3891.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Koyama T, Nakaoka Y, Fujio Y, Hirota H, Nishida K, Sugiyama S, Okamoto K, Yamauchi-Takihara K, Yoshimura M, Mochizuki S, Hori M, Hirano T, Mochizuki N. Interaction of scaffolding adaptor protein Gab1 with tyrosine phosphatase SHP2 negatively regulates IGF-I-dependent myogenic differentiation via ERK1/2 signaling pathway. The Journal of biological chemistry. 2008 doi: 10.1074/jbc.M803907200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fornaro M, Burch PM, Yang W, Zhang L, Hamilton CE, Kim JH, Neel BG, Bennett AM. SHP-2 activates signaling of the nuclear factor of activated T cells to promote skeletal muscle growth. The Journal of cell biology. 2006;175:87–97. doi: 10.1083/jcb.200602029. [DOI] [PMC free article] [PubMed] [Google Scholar]