Abstract
The influence of caloric restriction (CR) on hepatic sorbitol-metabolizing enzyme activities was investigated in young and old mice. Aldose reductase and sorbitol dehydrogenase activities were significantly lower in old CR mice than in old controls. Young CR mice showed decreased aldose reductase activity and a trend towards decreased sorbitol dehydrogenase when compared to controls. Metabolites of the pathway, namely sorbitol, glucose and fructose were decreased by CR in young and old mice. Pyruvate levels were decreased by CR in both young and old mice, while lactate decreased only in old CR. Malate levels increased in old CR but remained unchanged in young CR, when compared with controls. Accordingly, the lactae/pyruvate and malate/pyruvate ratios in young and old CR mice were increased, indicating increased NADH/NAD and NADPH/NADP redox couples, respectively. The results indicate that decreased glucose levels under CR conditions lead to decreased sorbitol pathway enzyme activities and metabolite levels, and could contribute to the beneficial effects of long-term CR through decreased sorbitol levels and NADPH sparing.
Keywords: Aldose reductase, sorbitol dehydrogenase, polyol, glucose, fructose, redox state
INTRODUCTION
Sorbitol is a sugar alcohol which is widely used as a food sweetener and is produced as a result of glucose reduction. In glucose metabolism, the sorbitol (polyol) pathway is considered to be of minor importance; however, it is implicated in increased oxidative stress and plays a critical role in the pathogenesis of diabetes (Chung et al. 2003; Jay et al. 2006). Under normal physiological conditions, metabolism of glucose proceeds through its phosphorylation by hexokinase/glucokinase, with about 3% of the glucose being metabolized by the polyol pathway (Gonzalez et al. 1984; Ugochukwa and Figgers 2006). However, under pathophysiological conditions such as hyperglycemia, the total amount of glucose metabolized by the polyol pathway can be as much as one-third of the total metabolized glucose (Chung et al. 2003; Gonzalez et al. 1984; Jay et al. 2006), which leads to an overproduction of the polyol pathway products, namely sorbitol and fructose (Balendiran and Rajkumar 2005).
The polyol pathway consists of two enzymes, aldose reductase (AR) (EC 1.1.1.21) and sorbitol dehydrogenase (SDH) (EC 1.1.1.14), with the former requiring NADPH while the latter NAD for their corresponding reactions (for reviews see El-Kabbani et al. 2004; Jeffery and Jörnvall 1988). Expression of AR is known to be up-regulated by oxidative stress, resulting in the depletion of NADPH stores, which is also required for the activity of glutathione reductase and the regeneration of GSH (Ugochukwa and Figgers 2006). Increased AR activity leads to increased levels of sorbitol, which accumulate due to cellular membrane impermeability (Iwata et al. 1990), producing hyperosmotic effects that result in cellular swelling, leading to various diabetic complications such as retinopathy, cataract, nephropathy and neuropathy (Gabbay 1973; Kador et al.1985).
Sorbitol dehydrogenase, a member of the alcohol dehydrogenase superfamily, is the second enzyme of the polyol pathway and plays a critical role in diabetic complications (Jeffery and Jörnvall 1988). Increased oxidation of sorbitol to fructose has also been linked closely to vascular dysfunction (Tilton et al.1995). The enzyme has been shown to be inactivated by glycation, with large amounts of glycated enzyme found in the liver under diabetic conditions (Hoshi et al. 1996). The enzyme is abundant in liver, kidney, and testis, while lower amounts are found in lens, brain and neuronal axons (Jeffery and Jörnvall 1988). The presence of SDH in liver is responsible for the conversion of dietary sorbitol to fructose for further metabolism, while in kidneys it may play a role in the regulation of osmotic pressure by modulating sorbitol levels (Lee et al. 1985).
Caloric restriction (CR), without malnutrition, has been shown to consistently delay pathophysiological changes and diseases and extend maximum life span in various organisms (Sohal and Weindruch 1996; Weindruch and Walford 1988). The successful life-extending effects of CR can only be achieved without inducing deficiency of any specific nutrient (Weindruch and Walford 1988). However, after years of extensive research into CR, the mechanism(s) responsible for its beneficial effects remain unresolved. Previously, we have shown the long-term effects of CR on various metabolic pathways (Hagopian et al. 2003a; Hagopian et al. 2003b; Hagopian et al. 2004; Hagopian et al. 2005a; Hagopian et al. 2005b;Hagopian et al. 2008). We hypothesize that under CR conditions, sorbitol pathway enzymes will show decreased activities due to the reduced levels of glucose, as shown previously (Hagopian et al., 2003a). This would lead to decreased sorbitol pathway metabolite levels, which play a major role in the complications associated with pathophysiological conditions, such as diabetes. This would also result in the sparing of NADPH, which becomes available to other reductive pathways. Therefore, in this study, we investigated the activities of the sorbitol pathway enzymes and the levels of its metabolites, as well as several other metabolites, with the aim of establishing how CR influences these enzyme activities and metabolite levels.
MATERIALS AND METHODS
Chemicals
Chemicals and reagents used in this work were purchased from Sigma Chemical Company (St. Louis, MO) or Roche diagnostics corporation (Indianapolis, IN). The protein assay kit was from Bio-Rad (Hercules, CA) and BSA standards were from Pierce (Rockford, IL).
Animals
Male C57Bl/6J mice were purchased from Charles River Laboratories (Wilmington, MA) at one month of age and housed singly, at 23°C and with a 12 h light/12 h dark light cycle. Animals were maintained in accordance with institutional and federal guidelines governing animal experimentation. The mice were fed ad libitum a non-purified diet, PLI 5001 (Purina Laboratories, St. Louis, MO) for one month and at two months of age they were assigned either to the control or CR group and fed semi-purified diets, as described elsewhere (Pugh et al. 1999). Control and CR mice had daily caloric intake of 12 kcal and 9 kcal per animal, respectively. At the time of sacrifice, young animals were three months of age (one month on either the control or CR diet) while old animals were 30 months of age (28 months on either the control or CR diet). These age groups were selected because three month old mice represented young adults who were sexually mature and outside the peak period of rapid growth while the 30 month old mice represented older animals.
Tissue preparation
All mice were sacrificed, after an overnight fast, and livers harvested between 9:00–10:00am. Livers were rapidly freeze-clamped in situ and placed immediately in liquid nitrogen and powdered, using a mortar and pestle, under liquid nitrogen. All powders were placed in cryogenic tubes and stored in liquid nitrogen for future use.
Enzyme activity assays
Powdered livers stored in liquid nitrogen were removed as rapidly as possible from the cryogenic tubes and placed in an ice-cold glass homogenizer, weighed and homogenized at a 1:10 ratio (w/v), centrifuged and the supernatants saved for assays. The sorbitol (polyol) metabolizing enzymes aldose reductase (EC 1.1.1.21) and sorbitol dehydrogenase (EC1.1.1.14) were assayed as described before (Gaynes and Watkins 1989). All assays were performed at 30°C, using a Perkin Elmer Lambda 25 UV/VIS spectrophotometer set at 340nm and equipped with a Peltier heating control. Enzyme activities were expressed as μmol/min/mg protein using an extinction coefficient of 6.22mM−1.cm−1.
Liver metabolite measurements
Powders stored in liquid nitrogen were removed as rapidly as possible from the cryogenic tubes, weighed and homogenized in ice-cold 1M perchloric acid using motor-driven, glass-Teflon homogenizers kept on ice during the homogenization. The homogenates were centrifuged and supernatants removed and neutralized with 1M KOH, and the levels of malate (Hagopian et al.2004), fructose (Hagopian et al. 2005a), sorbitol (Beutler 1984), and glucose, lactate and pyruvate (Hagopian et al. 1991) were determined. All metabolites were expressed as μmol/g wet weight.
Other methods
Protein concentrations were determined with the Bio-Rad protein assay kit, using BSA as the standard. Statistical comparisons were performed using Student’s t test where values of P < 0.05 were taken as statistically significant.
RESULTS
Sorbitol (Polyol) pathway enzyme activities
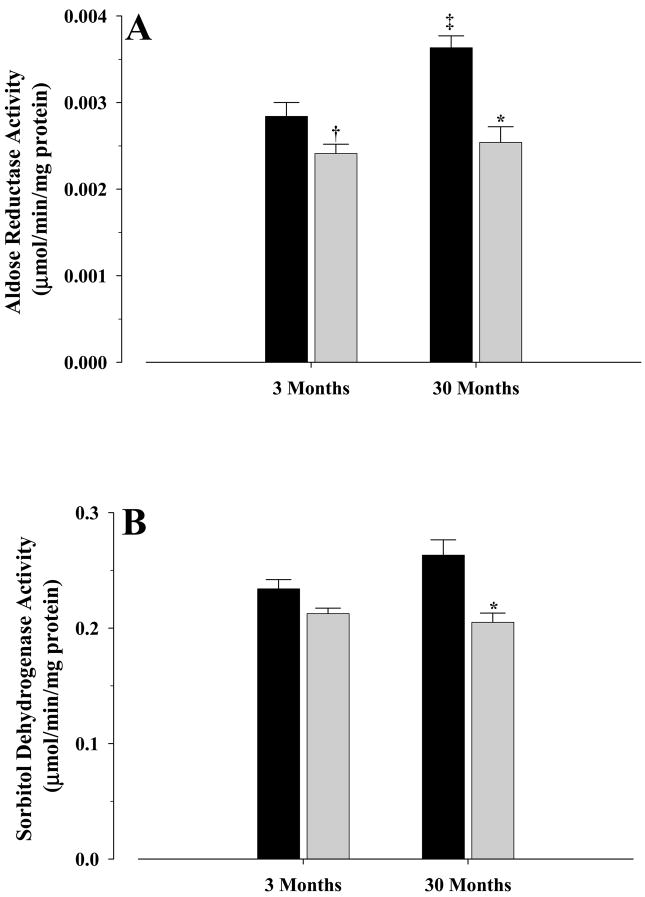
The activities of the sorbitol pathway enzymes were measured in both young and old mice, fed either control or CR diets (Fig. 1). In the case of AR (Fig. 1A), a 24% (P < 0.005) and 15% (P < 0.05) decrease in activity in old and young CR mice, respectively, was observed when compared with their corresponding controls. Old controls showed 28% higher activity when compared with young controls (P < 0.004), while no differences were observed between young and old CR mice.
Figure 1. Activities of aldose reductase (A) and sorbitol dehydrogenase (B) in livers of young and old mice.
Activities were measured as described in the text and were mean±S.E.M of at least five independent experiments and expressed as μmol/min/mg protein. Controls, solid bars; CR, gray bars. Significance levels for (A): * P < 0.005 old CR vs old control; † P < 0.05 young CR vs young control; ‡ P < 0.004 old control vs young control. Significance levels for (B): * P < 0.004 old CR vs old control.
In the case of SDH (Fig. 1B), a 22% decrease in activity (P < 0.004) was observed in old CR mice when compared with old controls, while a trend (P = 0.06) towards a decrease (9%) in activity of young CR was observed when compared with young controls. No significant differences were observed between young and old CR mice, however, a trend (P = 0.09) towards an increase in old controls (12.5%) was observed when compared with young controls.
Sorbitol pathway metabolite concentrations
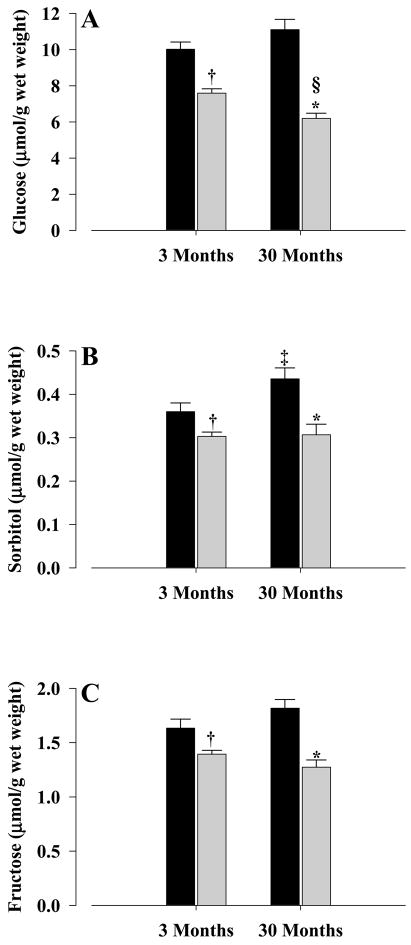
The concentrations of the sorbitol pathway metabolites were determined. The levels of glucose (Fig. 2A) were significantly decreased in young (P < 0.001) and old (P < 0.001) CR mice, when compared with controls. Sorbitol levels (Fig. 2B) were also decreased significantly in both young (P < 0.03) and old (P < 0.004) CR mice when compared with their corresponding controls. This pattern was also observed for fructose (Fig. 2C), with both young and old CR mice showing significantly decreased levels (P < 0.02 and P < 0.001, respectively).
Figure 2. Polyol pathway metabolite concentrations.
Glucose (A), sorbitol (B), and fructose were determined from the livers of young and old mice. All concentrations were mean±S.E.M of at least five independent experiments and expressed as μmol/g wet weight. Controls, solid bars; CR, gray bars. Significance levels for (A): * P < 0.001 old CR vs old control; † P < 0.001 young CR vs young control; § P < 0.004 old CR vs young CR. Significance levels for (B): * P < 0.003 old CR vs old control; † P < 0.03 young CR vs young control; ‡ P < 0.04 old control vs young control. Significance levels for (C): * P < 0.001 old CR vs old control; † P < 0.02 young CR vs young control.
When age-related comparisons were made, old CR mice showed decreased glucose levels (P < 0.004) when compared with young CR, but no differences were observed between old and young controls (Fig. 2A). In the case of sorbitol (Fig. 2B), no age-related differences were observed between old and young CR, however, old controls showed significantly higher (P < 0.04) levels when compared with young controls. As for fructose (Fig. 2C), no age related differences were observed between young and old controls or young and old CR mice.
Other metabolite concentrations
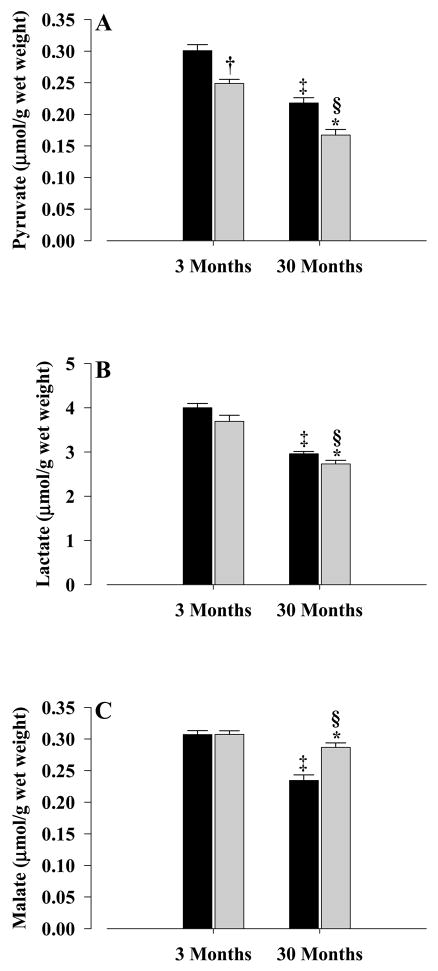
The concentrations of three other hepatic metabolites were also measured. The levels of pyruvate (Fig. 3A) were decreased significantly in young (P < 0.001) and old (P < 0.001) CR mice. Lactate levels (Fig. 3B) were also decreased significantly (P < 0.04) in old CR mice when compared with controls, however, no differences between young control and CR mice were observed. Malate levels (Fig. 3C) showed increased levels (P < 0.001) in old CR when compared with old controls but no differences between young controls and CR mice were observed.
Figure 3. Hepatic metabolite concentrations.
Pyruvate (A), lacate (B) and malate (C) were determined from the livers of young and old mice. All concentrations were mean±S.E.M of at least five independent experiments and expressed as μmol/g wet weight. Controls, solid bars; CR, gray bars. Significance levels for (A): * P < 0.001 old CR vs old control; † P < 0.001 young CR vs young control; § P < 0.001 old CR vs young CR; ‡ P < 0.001 old control vs young control. Significance levels for (B): * P < 0.04 old CR vs old control; § P < 0.001 old CR vs young CR; ‡ P < 0.001 old control vs young control. Significance levels for (C): * P < 0.001 old CR vs old control; § P < 0.05 old CR vs young CR; ‡ P < 0.001 old control vs young control.
When age-related comparisons were made, pyruvate levels (Fig. 3A) in old CR mice were significantly lower (P < 0.001) than those of young CR, and old controls were lower that young controls (P < 0.001). This pattern was also true for lactate (Fig. 3B), where old control and CR levels were significantly lower (P < 0.001) than the corresponding levels in young animals. A similar pattern was also observed for malate (Fig. 3C), with old controls showing lower levels than young controls (P < 0.001), as do old CR when compared with young CR (P < 0.05).
Redox state
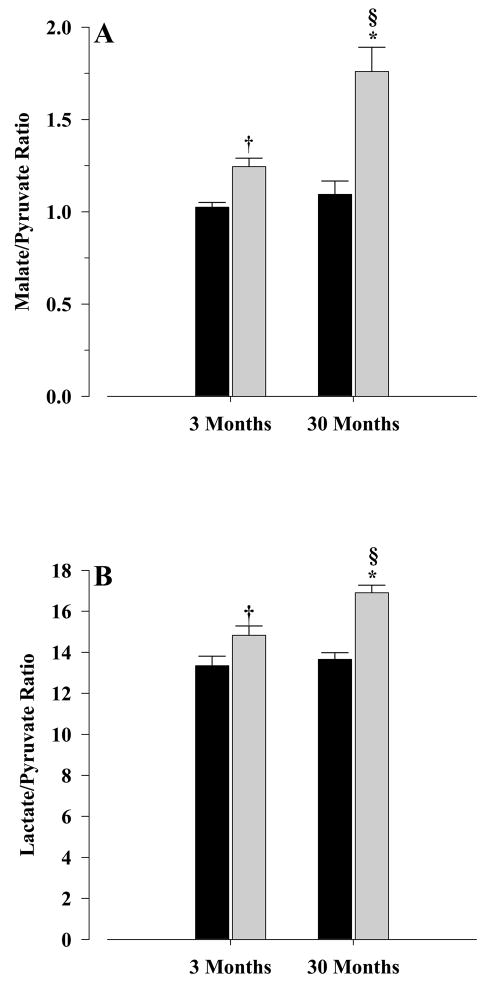
Using the values obtained from the above metabolite section, the Malate/Pyruvate and Lactate/Pyruvate ratios were calculated (Fig. 4) to give an indication of the NADPH/NADP and NADH/NAD redox couples, respectively. The results showed that Malate/Pyruvate ratio (Fig. 4A) increased significantly in both young and old CR mice (P < 0.001 for both) when compared with their corresponding controls. Similar pattern was also observed for Lactate/Pyruvate ratio (Fig. 4B), with significant increases in old and young CR mice (P < 0.001 and P < 0.04, respectively) when compared with their corresponding controls.
Figure 4. Metabolite ratios.
Malate/Pyruvate (A) and Lactate/Pyruvate (B) ratios were determined for young and old, control and CR animals. All ratios were mean±S.E.M of at least six independent measurements. Significance levels for (A): * P < 0.001 old CR vs old control; † P < 0.001 young CR vs young control; § P < 0.005 old CR vs young CR. Significance levels for (B): * P < 0.001 old CR vs old control; † P < 0.04 young CR vs young control; § P < 0.004 old CR vs young CR.
To see if these ratios were influenced by age, the Malate/Pyruvate ratio was significantly higher (P < 0.005) in old CR mice when compared with young CR (Fig. 4A), while no differences were observed between ratios of old and young control mice. The same pattern was also observed for the Lactate/Pyruvate ratio (Fig. 4B), with the ratio in old CR mice being significantly higher (P < 0.004) than in the young CR, while no differences were observed between the ratios from young and old controls.
DISCUSSION
The sorbitol (polyol) pathway (Fig. 5) under normal physiological conditions plays a minor role in glucose metabolism. However, under conditions of elevated glucose levels that result in saturation of the glycolytic pathway at the hexokinase step (Kinoshita et al. 1963), the excess glucose is diverted to the polyol pathway to be metabolized to sorbitol and then to fructose (Gabbay 1973). Our results show higher AR activity in young and old controls, when compared to young and old CR (Fig. 1A), indicating that more glucose is entering the polyol pathway. This is supported by the fact that glucose levels were also high in young and old controls, when compared to young and old CR (Fig. 2A), therefore, allowing the excess to be diverted to the polyol pathway. These events were, however, counteracted by CR, with significantly reduced glucose levels (Fig. 2A) resulting in decreased AR activity (Fig. 1A). The glucose results from this study are similar to our previously reported hepatic glucose levels (Hagopian et al. 2003a). The availability of glucose plays a major role in the activities of both the glycolytic and polyol pathways. This is evident from the effects of CR whereby decreased glycolysis under CR conditions is observed (Dhahbi et al.1999; Hagopian et al. 2003a), which includes a dramatic decrease in glucokinase activity (Hagopian et al. 2003a), as well as decreased polyol pathway activity, as reported here. Therefore, it is possible to conclude that the higher activities of both AR and SDH of the controls observed in this study (Fig. 1 A and B, respectively) were due to the levels of glucose that were high enough to result in the diversion of the excess amounts to the polyol pathway. On the other hand, CR resulted not only in decreased glucose levels but also in low glucokinase activity (Hagopian et al. 2003a), and these decreased levels of glucose may have contributed to the decreased polyol pathway enzyme activities, possibly due to the lack of glucokinase saturation, which could allow the available glucose under CR conditions to be metabolized via glycolysis.
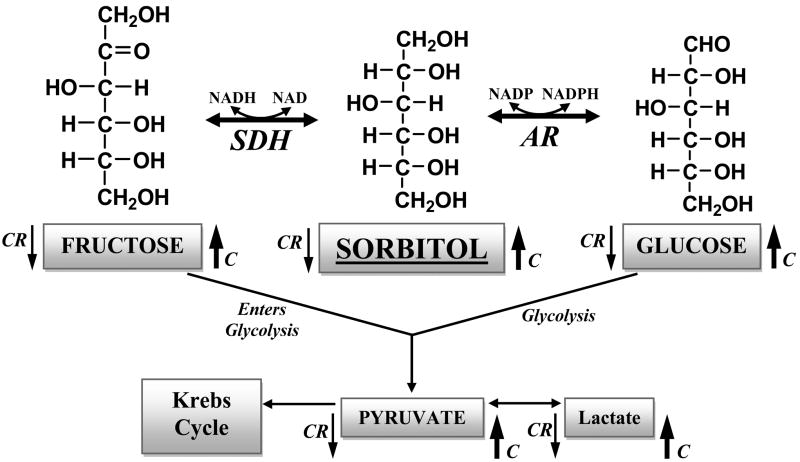
Figure 5.
The sorbitol (polyol) metabolism pathway and its connection to other pathways. The two enzymes assayed were aldose reductase (AR) and sorbitol dehydrogenase (SDH). The metabolites measured were glucose, sorbitol, fructose, as well as lacatate and pyruvate. Arrows next to the metabolites indicate either higher (up arrow) or lower (down arrow) metabolite level under control (C) or caloric restriction (CR) conditions, respectively.
Decreased glucose levels could lead to decreased sorbitol levels by decreasing AR activity, and this in turn can decrease SDH activity (Fig. 1B), due to decreased levels of sorbitol (Fig. 2B). Decreased SDH activity and sorbitol levels could also lead to significantly decreased fructose levels in both old and young CR mice when compared with their corresponding controls (Fig. 2C). The fructose results in this study are in agreement with our previous findings (Hagopian et al.2005a). These results indicate that by decreasing glucose availability by CR, the enzymes and metabolites of the polyol pathway are also decreased.
It is also interesting to note the effects of age on the polyol pathway enzyme activities and metabolite levels. Increased polyol pathway enzyme activities with age were observed in control mice (Fig. 1A and B) which could correlate well with age-related pathophysiological conditions such as various diabetic complications (neuropathy, retinopathy, nephropathy) (Chung et al. 2003; El-Kabbani et al. 2004). This increase was significant for AR (Fig. 1A) while a trend towards an increase for SDH was observed (Fig. 1B). However, the results also indicate that CR counteracted these age-related increases by lowering the activities of both AR and SDH (Fig. 1A and 1B). Both enzymes from old CR mice showed activities that were similar or lower than the activities in young control or CR mice (Fig. 1A and B). Our results are in agreement with previous findings in rat liver (Ugochukwa and Figgers 2006) where AR activity decreased under CR conditions. In that same study (Ugochukwa and Figgers 2006) no differences were observed in SDH activity and this agrees with our findings of no significant differences in SDH activity in young animals. In our study, decreases in SDH activity occurred only with long-term CR (28 months). Also, the previous study (Ugochukwa and Figgers 2006) did not investigate the influence of age on the activities. However, the lack of age-related SDH activity differences reported in our study between young and old controls was similar to a previous report from rat liver (Danh et al. 1985). We did not observe any SDH activity differences between young and old CR (Fig. 1B), and this was not investigated in the previous study (Danh et al. 1985). It has also been reported that pathological conditions, such as hyperglycemia, high osmotic pressure and cancer, induce AR gene expression (Fujii et al. 1999), therefore, it is quite possible that CR could have the opposite effect and that decreased AR activity under CR conditions could also be the result of decreased gene expression.
Increased glucose metabolism through the polyol pathway, due to increased AR activity, can influence the NADPH/NADP ratio. To determine the redox state, the levels of malate and pyruvate were measured and the Malate/Pyruvate ratio was taken as an indicator of the NADPH/NADP ratio. The inter-conversion of these two metabolites is catalyzed by malic enzyme and has been used for the determination of the NADPH/NADP ratio (Krebs and Veech 1969; Veech et al. 1969). The pyruvate and malate levels determined here (Fig. 3A and C) were in agreement with our previous findings (Hagopian et al. 2003a; Hagopian et al. 2004) and clearly show that CR leads to a significantly higher Malate/Pyruvate ratio in both young and old mice, indicating a higher NADPH/NADP ratio (Fig. 4A). This higher ratio in CR mice indicates higher NADPH levels at least partially due to decreased utilization by AR. It has been suggested previously that tissue damages under pathological conditions related to high glucose levels could be in part due to increased NADPH consumption by AR during glucose reduction (Sheetz and King 2002). It is possible, therefore, to assume that CR has resulted in sparing NADPH utilization by AR and contributing to the overall provision of NADPH for other reductive systems. One such system is the glutathione reductase/glutathione peroxidase system, which is responsible for glutathione regeneration and detoxification of ROS and hydroperoxides, whereby a significant strain on the NADPH pool by other pathways could decrease the cell’s ability to defend against oxidative stress (Engerman et al.1994; Mullineaux and Creissen 1997; Srivastava et al. 2005).
Also measured was the concentration of lactate (Fig. 3B), and the results were in agreement with our previous findings (Hagopian et al. 2003a). The values of lactate and pyruvate were then used to determine the Lactate/Pyruvate ratio, which in turn indicates the NADH/NAD ratio, and our results were in agreement with our previous finding (Hagopian et al. 2003a), showing increased Lactate/Pyruvate ratio in young and old CR animals, when compared with their corresponding controls. The increased NADH/NAD and NADPH/NADP ratios indicate a more reduced state in CR animals. This increased reduced state is important since an oxidized state could increase pro-oxidant molecules like ROS, therefore, contributing to the aging process (Sohal and Weindruch 1996;Weindruch and Sohal 1997). Moreover, the NADH/NAD and NADPH/NADP ratios are of critical importance for the behavior of reducible and oxidizable substrates and the direction of reversible reaction such as glycolysis and gluconeogenesis (Krebs 1967).
Our results indicate that under CR conditions, sorbitol pathway enzymes AR and SDH showed decreased activities, and the sorbitol pathway metabolites glucose, sorbitol and fructose decreased as well. These decreases correlate with increased NADPH/NADP and NADH/NAD ratios, indicating a shift from pro-oxidizing to a more reduced state under CR conditions. It is possible that these changes may be beneficial in terms of allowing more reducing equivalents to be available for various other pathways, such glutathione regeneration. Moreover, decreased sorbitol levels could help in decreasing the harmful role this polyol plays in various diabetic complications.
Acknowledgments
The work was supported by the National Institutes of Health grants PO1 AG11915 and RO1 AG028125.
List of Abbreviations
- CR
caloric restriction
- SDH
sorbitol dehydrogenase
- AR
aldose reducatse
- ROS
reactive oxygen species
References
- Balendiran GK, Rajkumar B. Fibrates inhibit aldose reductase activity in the forward and reverse reactions. Biochem Pharmacol. 2005;70:1653–1663. doi: 10.1016/j.bcp.2005.06.029. [DOI] [PubMed] [Google Scholar]
- Beutler H-O. D-Sorbitol. In: Bergmeyer HU, editor. Methods of Enzymatic Analysis. VI. Verlag-Chemie GmbH; Weinheim: 1984. pp. 356–360. [Google Scholar]
- Chung SSM, Ho ECM, Lam KSL, Chung SK. Contribution of polyol pathway to diabetes-induced oxidative stress. J Am Soc Nephrol. 2003;14:S233–S236. doi: 10.1097/01.asn.0000077408.15865.06. [DOI] [PubMed] [Google Scholar]
- Danh HC, Benedetti MS, Dostert P. Age-related changes in sorbitol dehydrogenase activity of rat brain, liver, kidney and eye. J Pharm Pharmacol. 1985;37:910–912. doi: 10.1111/j.2042-7158.1985.tb05000.x. [DOI] [PubMed] [Google Scholar]
- Dhahbi JM, Mote PL, Wingo J, Tillman JB, Walford RL, Spindler SR. Calories and aging alter gene expression for gluconeogenic, glycolytic, and nitrogen-metabolizing enzymes. Am J Physiol. 1999;277:E352–E360. doi: 10.1152/ajpendo.1999.277.2.E352. [DOI] [PubMed] [Google Scholar]
- El-Kabbani O, Darmanin C, Chung RP-T. Sorbitol dehydrogenase: Structure, function and ligand design. Curr Med Chem. 2004;11:465–476. doi: 10.2174/0929867043455927. [DOI] [PubMed] [Google Scholar]
- Engerman R, Kern TS, Larson ME. Nerve conduction and aldose reductase inhibition during 5 years of diabetes or galactosaemia in dogs. Diabetologia. 1994;37:141–144. doi: 10.1007/s001250050084. [DOI] [PubMed] [Google Scholar]
- Fujii J, Takahashi M, Hamaoka R, Kawasaki Y, Miyazawa N, Taniguchi N. Physiological relevance of aldehyde reductase and aldose reductase gene expression. Adv Exp Med Biol. 1999;463:419–426. doi: 10.1007/978-1-4615-4735-8_52. [DOI] [PubMed] [Google Scholar]
- Gabbay KH. The sorbitol pathway and the complications of diabetes. New Engl J Med. 1973;288:831–836. doi: 10.1056/NEJM197304192881609. [DOI] [PubMed] [Google Scholar]
- Gonzalez RG, Barnett P, Aguayo J, Cheng H-M, Chylack LT. Direct measurement of polyolpathway activity in the ocular lens. Diabetes. 1984;33:196–199. doi: 10.2337/diab.33.2.196. [DOI] [PubMed] [Google Scholar]
- Gaynes BI, Watkins JB., III Comparison of glucose, sorbitol and fructose accumulation in lens and liver of diabetic and insulin-treated rats and mice. Comp Biochem Physiol. 1989;92B:685–690. doi: 10.1016/0305-0491(89)90250-2. [DOI] [PubMed] [Google Scholar]
- Hagopian K, Ramsey JJ, Weindruch R. Influence of age and caloric restriction on liver glycolytic enzyme activities and metabolic concentrations in mice. Exp Gerontol. 2003b;38:253–266. doi: 10.1016/s0531-5565(02)00203-6. [DOI] [PubMed] [Google Scholar]
- Hagopian K, Ramsey JJ, Weindruch R. Caloric restriction increases gluconeogenic and transaminase enzyme activities in mouse liver. Exp Gerontol. 2003b;38:267–278. doi: 10.1016/s0531-5565(02)00202-4. [DOI] [PubMed] [Google Scholar]
- Hagopian K, Ramsey JJ, Weindruch R. Krebs cycle enzymes from livers of old mice are differentially regulated by caloric restriction. Exp Gerontol. 2004;39:1145–1154. doi: 10.1016/j.exger.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Hagopian K, Ramsey JJ, Weindruch R. Fructose metabolizing enzymes form mouse liver: influence of age and caloric restriction. Biochim Biophys Acta. 2005a;1721:37–43. doi: 10.1016/j.bbagen.2004.10.001. [DOI] [PubMed] [Google Scholar]
- Hagopian K, Ramsey JJ, Weindruch R. Serine utilization in mouse liver: influence of caloric restriction and aging. FEBS Lett. 2005b;579:2009–2013. doi: 10.1016/j.febslet.2005.02.062. [DOI] [PubMed] [Google Scholar]
- Hagopian K, Ramsey JJ, Weindruch R. Enzymes of glycerol and glyceraldehydes metabolism in mouse liver: effects of caloric restriction and age on activities. Biosci Rep. 2008;28:107–115. doi: 10.1042/BSR20080015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagopian K, Butt J, Munday MR. Regulation of fatty acid synthesis in lactating rat mammary gland in the fed to starved transition: asynchronous control of pyruvate dehydrogenase, phosphofructokinase and acetyl-CoA carboxylase. Comp Biochem Physiol. 1991;100B:527–534. doi: 10.1016/0305-0491(91)90215-y. [DOI] [PubMed] [Google Scholar]
- Hoshi A, Takahashi M, Fujii J, Myint T, Kaneto H, Suzuki K, Yamasaki Y, Kamada T, Taniguchi N. Glycation and inactivation of sorbitol dehydrogenase in normal and diabetic rats. Biochem J. 1996;318:119–123. doi: 10.1042/bj3180119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwata N, Inazu N, Satoh T. The purification and properties of aldose reductase from rat ovary. Arch Biochem Biophys. 1990;282:70–77. doi: 10.1016/0003-9861(90)90088-g. [DOI] [PubMed] [Google Scholar]
- Jay D, Hitomi H, Griendling KK. Oxidative stress and diabetic cardiovascular complications. Free Rad Biol Med. 2006;40:183–192. doi: 10.1016/j.freeradbiomed.2005.06.018. [DOI] [PubMed] [Google Scholar]
- Jeffery J, Jörnvall H. Sorbitol dehydrogenase. Adv Enzymol Relat Areas Mol Biol. 1988;61:47–106. doi: 10.1002/9780470123072.ch2. [DOI] [PubMed] [Google Scholar]
- Kador PF, Robinson WJ, Kinoshita JH. The pharmacology of aldose reducatse inhibitors. Ann Rev Pharmacol Toxicol. 1985;25:691–714. doi: 10.1146/annurev.pa.25.040185.003355. [DOI] [PubMed] [Google Scholar]
- Kinoshita JH, Futterman S, Satoh K, Merola LO. Factors affecting the formation of sugar alcohols in ocular lens. Biochim Biophys Acta. 1963;74:340–350. doi: 10.1016/0006-3002(63)91377-5. [DOI] [PubMed] [Google Scholar]
- Krebs HA. The redox state of nicotinamide adenine dinucleotide in the cytoplasm and mitochondria of rat liver. Adv Enz Reg. 1967;5:409–437. doi: 10.1016/0065-2571(67)90029-5. [DOI] [PubMed] [Google Scholar]
- Krebs HA, Veech RL. Pyridine nucleotide interrelations. In: Papa S, Tager JM, Quagliariello E, Slater EC, editors. The Energy Level and Metabolic Control in Mitochondria. Adriatica Editrice; Bari: 1969. pp. 329–379. [Google Scholar]
- Lee S-M, Schade SZ, Doughty CC. Aldose reductase, NADPH and NADP+ in normal, galactose-fed and diabetic rat lens. Biochim Biophys Acta. 1985;841:247–253. doi: 10.1016/0304-4165(85)90065-0. [DOI] [PubMed] [Google Scholar]
- Mullineaux PM, Creissen GP. Glutathione reductase: regulation and role in oxidative stress. In: Scandalios JG, editor. Oxidative Stress and the Molecular Biology of Antioxidant Defenses. Cold Spring Harbor Laboratory Press; Plainville, NY: 1997. pp. 667–713. [Google Scholar]
- Pugh TD, Klopp RG, Weindruch R. Controlling caloric consumption: protocols for rodents and rhesus monkeys. Neurobiol Aging. 1999;20:157–165. doi: 10.1016/s0197-4580(99)00043-3. [DOI] [PubMed] [Google Scholar]
- Sheetz MJ, King GL. Molecular understanding of hyperglycemia’s adverse effects for diabetic complications. JAMA. 2002;288:2579–2588. doi: 10.1001/jama.288.20.2579. [DOI] [PubMed] [Google Scholar]
- Sohal RS, Weindruch R. Oxidative stress, caloric restriction, and aging. Science. 1996;273:59–63. doi: 10.1126/science.273.5271.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava SK, Ramana KV, Bhatnagar A. Role of aldose reductase and oxidative damage in diabetes and the consequent potential for therapeutic options. Endocr Rev. 2005;26:380–392. doi: 10.1210/er.2004-0028. [DOI] [PubMed] [Google Scholar]
- Tilton R, Chang K, Nyengaard JR, Enden MV, Ido Y, Williamson JR. Inhibition of sorbitol dehydrogenase. Effects of vascular and neuronal dysfunction in streptozotocin-induced diabetic rats. Diabetes. 1995;44:234–242. doi: 10.2337/diab.44.2.234. [DOI] [PubMed] [Google Scholar]
- Ugochukwa NH, Figgers CL. Modultaion of the flux patterns in carbohydrate metabolism in the livers of streptozoticin-induced diabetic rats by dietary caloric restriction. Pharmacol Res. 2006;54:172–180. doi: 10.1016/j.phrs.2006.04.004. [DOI] [PubMed] [Google Scholar]
- Veech RL, Eggleston LV, Krebs HA. The redox state of free nicotinamide-adenine dinucleotide phosphate in the cytoplasm of rat liver. Biochem J. 1969;115:609–619. doi: 10.1042/bj1150609a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weindruch R, Sohal RS. Caloric intake and aging. New Eng J Med. 1997;337:986–994. doi: 10.1056/NEJM199710023371407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weindruch R, Walford RL. The retardation of aging and disease by dietary restriction. Charles C. Thomas Publisher; Springfield: 1988. [Google Scholar]