Abstract
Dengue is a mosquito-borne viral disease that is a major public health problem worldwide. In 2004, the Pediatric Dengue Cohort Study was established in Managua, Nicaragua, to study the natural history and transmission of dengue in children. Here, the authors describe the study design, methods, and results from 2004 to 2008. Initially, 3,721 children 2–9 years of age were recruited through door-to-door visits. Each year, new children aged 2 years are enrolled in the study to maintain the age structure. Children are provided with medical care through the study, and data from each medical visit are recorded on systematic study forms. All participants presenting with suspected dengue or undifferentiated fever are tested for dengue by virologic, serologic, and molecular biologic assays. Yearly blood samples are collected to detect inapparent dengue virus infections. Numerous information and communications technologies are used to manage study data, track samples, and maintain quality control, including personal data assistants, barcodes, global information systems, and fingerprint scans. Close collaboration with the Nicaraguan Ministry of Health and use of almost entirely local staff are essential components for success. This study is providing critical data on the epidemiology and transmission of dengue in the Americas needed for future vaccine trials.
Keywords: Central America, cohort studies, dengue, information systems, methods, Nicaragua
Dengue virus (DENV) causes the most prevalent mosquito-borne viral illness in humans, with an estimated 50 million cases occurring annually in tropical and subtropical countries (1). Four serotypes of the virus (DENV-1, DENV-2, DENV-3, DENV-4) are transmitted by the domestic, daytime-biting mosquitoes Aedes aegypti and Ae. albopictus. From 50% to 90% of DENV infections can be inapparent (2–4). Of those that develop symptoms, most cases present as undifferentiated fever or classic dengue fever, a debilitating but self-limiting acute febrile illness characterized by headache, retroorbital pain, arthralgia, myalgia, rash, and mild hemorrhagic manifestations. A subset of dengue cases progress to the potentially lethal dengue hemorrhagic fever/dengue shock syndrome, characterized by vascular leakage leading to shock, low platelet counts, and hemorrhagic manifestations (5). Infection by 1 DENV serotype provides lifelong immunity against that serotype but confers only partial and transient protection against subsequent infections with other DENV serotypes (6). Substantial epidemiologic evidence indicates that sequential infection with different DENV serotypes increases the risk of dengue hemorrhagic fever/dengue shock syndrome (7).
Currently, the only way to control dengue is by targeting its mosquito vector through insecticides and elimination of potential breeding sites. Treatment for dengue is limited to supportive care. However, a number of vaccines are under development (8, 9), with several in phase 2 trials. Phase 3 trials are planned over the next few years to test the efficacy of these vaccines in children. Prior to these trials, further epidemiologic information is needed about the incidence and burden of dengue worldwide to design trials with sufficient statistical power while minimizing any potential risks. Large-scale prospective cohort studies of dengue can provide these data and prepare for vaccine trials, and these are needed in both Asia and the Americas, the 2 regions with greatest dengue activity (1, 10).
The design, logistics, and execution of prospective large-scale cohort studies are challenging in any setting, but they can be especially difficult in a developing country where infrastructure and services may be unreliable or nonexistent. These challenges include the following: 1) lack of street names and addresses, 2) low prevalence of telephone ownership by the families of many participants, 3) no reliable postal system, 4) a proportion of illiterate participants, and 5) irregular electrical and water services.
A pediatric cohort study following approximately 3,800 children was conducted in Managua, Nicaragua, from 2004 to the present. The objective of the Nicaraguan Pediatric Dengue Cohort Study is to study the natural history of dengue, document its incidence and seroprevalence, characterize the symptoms and disease spectrum, collect biologic samples for immunology and vaccine safety research, and prepare for a potential phase 2/3 vaccine trial. This paper describes the methods used to establish and conduct the Nicaraguan Pediatric Dengue Cohort Study and extend it to study influenza and other respiratory diseases, as well as the information technologies used to overcome logistic challenges and ensure the high quality of the study.
MATERIALS AND METHODS
Study site and organization
This study is conducted at the Health Center Sócrates Flores Vivas (HCSFV) in District II of Managua, the capital of Nicaragua. District II is adjacent to Lake Managua, and the Health Center serves a population of approximately 60,047. The primary health-care facility is the HCSFV; study children requiring additional medical attention are transferred to the study hospital, the National Pediatric Reference Hospital, Hospital Infantil Manuel de Jesús Rivera (HIMJR). Clinical laboratory tests are performed in the HCSFV, while all virologic and serologic tests are performed at the National Virology Laboratory of the Centro Nacional de Diagnóstico y Referencia. All study facilities are part of the Nicaraguan Ministry of Health, and the national directors of epidemiology, the reference laboratories, and the health province of Managua sit on the Executive Committee of the Pediatric Dengue Cohort Study. A formal agreement (“convenio”) supporting the study is signed by the Minister of Health and the principal investigator of the study. Ninety-five percent of study personnel are Nicaraguan, and all decisions are made by consensus by a technical committee composed of Pediatric Dengue Cohort Study site directors, informatics and quality control directors, the study coordinator, and the principal investigator. This study was approved by the institutional review boards at the University of California, Berkeley, the International Vaccine Initiative, and the Nicaraguan Ministry of Health (Centro Nacional de Diagnóstico y Referencia and HIMJR).
Recruitment
To recruit cohort participants, in August and September of 2004, study teams performed house-to-house visits in neighborhoods served by the HCSFV and invited all eligible children to participate. Eligibility criteria included the following: 1) age between 2 and 9 years, 2) residence in the study area, 3) no plan to leave the study area during the following 3 years, 4) willingness to attend the study clinic for all medical needs, 5) no immune-compromising conditions, such as current chemotherapy treatment or human immunodeficiency virus positivity, 6) informed parental consent, and 7) participant assent for children over 5 years of age. Children were eligible to remain in the study until the age of 12 years or until they moved from the study area. To maintain the age structure of the cohort, we recruited children aged 2 years between July and September of each year. The initial informed consent applied to the first 3 years of the study; in 2007, study participants aged 11 years or younger and their families were given the opportunity to continue for an additional 3 years, and a second informed consent was performed. In the fourth year of the study, additional children aged 3–9 years were enrolled to even out the age distribution.
Enrollment and baseline visit
At enrollment, demographic data are collected, a global positioning system (GPS) point is taken of the child's house, and children are scheduled for a baseline study visit at the HCSFV, which consists of collection of a clinical history, additional demographic data, and a blood sample. The child is issued a study identification card with photograph, study code, and barcode, and a fingerprint scan is performed to aid in participant identification. Families are instructed to bring the child to the HCSFV at the first sign of fever or illness. Participants are provided with free medical care and laboratory testing through the study. Study physicians and an ambulance are available 24 hours/day, 365 days/year. Participants requiring hospitalization or secondary-level care are transferred to the study hospital, HIMJR, by study staff. Data from all medical appointments are collected systematically on standardized study forms at both the HCSFV and the HIMJR.
Case surveillance
Upon presentation to the HCSFV, participants identify themselves at the reception desk. They are then directed to the study area, where a study nurse measures their height, weight, and tympanic temperature and records the information by using a standardized medical visit form. Study physicians perform a medical examination and record data systematically on the history of the illness and current symptoms, consisting of approximately 80 variables including blood pressure, cardiac and respiratory rates, a second tympanic temperature measurement, lower and upper respiratory symptoms, gastrointestinal symptoms, indicators of dehydration, urinary tract symptoms, musculoskeletal pain, rashes and other skin abnormalities, hemorrhagic manifestations, and nutritional status. Data are recorded on any medical tests ordered and treatments prescribed.
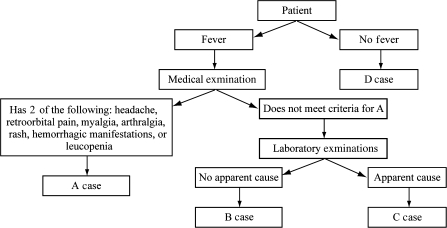
The physician then classifies the patient in 1 of 4 categories: A, B, C, or D (Figure 1). Patients classified as category A meet the World Health Organization's classification of suspected dengue, namely, an acute febrile illness with 2 or more of the following symptoms or signs: headache, retroorbital pain, myalgia, arthralgia, rash, hemorrhagic manifestations, or leucopenia. Patients classified as category B display undifferentiated acute febrile illness or febrile illness without an evident cause. Patients classified as category C are febrile cases with a defined focus, such as pneumonia or dysentery. Category D patients are those with afebrile illnesses, chronic diseases, and accidents.
Figure 1.
Flowchart of case classification during initial medical consults of children in the Pediatric Dengue Cohort Study, Managua, Nicaragua, 2004–2008.
Category A patients.
Category A patients are treated as suspected dengue cases. An acute blood sample is drawn at the first visit, and an examination is performed. A complete blood count (CBC) and reverse transcriptase-polymerase chain reaction (RT-PCR) and virus isolation for DENV are performed immediately. The child is scheduled for follow-up visits each day until complete recovery from the illness. If the child misses an appointment, study staff visit the child's home to provide continuing medical care. A convalescent blood sample is collected 14–30 days after the onset of symptoms for dengue serology tests.
Category B patients.
Category B patients are treated as possible dengue cases and are handled similarly to category A cases, except that RT-PCR and virus isolation for DENV are performed retrospectively on the acute sample only if serologic assays on paired acute- and convalescent-phase samples indicate a current DENV infection.
Category C and category D patients.
Category C (febrile) and category D (afebrile) patients receive medical care and tests as indicated by their symptoms. In December 2005 and January 2006 and then from June 2006 to the present, a convenience sample of acute blood specimens from about 40% of category C patients was collected and stored for future dengue testing.
Field visits
An annual field visit is conducted to assess and encourage participant participation. Study teams attempt to visit each child at his/her home, which is located by using GPS data. If the team cannot interview a parent or guardian at the first visit, 2 additional attempts are made. Study teams composed of 2 field staff are preassigned a set of participants to visit, maps of the location of each team's set are printed, and the GPS points and children's information are downloaded into hand-held GPS devices. Throughout the day, data are transferred from the field staff's personal data assistants (PDAs) to a supervisor's PDA to prevent data loss and to allow for multiple updates of the study database throughout the day. This allows for real-time monitoring, enabling rapid assessment of progress, efficient reassignment of participant visits when necessary, and effective planning of the next day's visits. Questions in the study participation survey elicit information about attendance to the HCSFV for all illnesses and use of medical providers outside of the cohort. Periodic field visits are also conducted to collect convalescent samples, provide follow-up medical care to children who miss appointments, and check on the status of participants who have not had contact with the study in the prior 6-month period.
Yearly sample collection
Each July or August, an annual blood sample is collected to examine the incidence of subclinical DENV infection. The annual sampling takes place in 2 phases. In the first phase, participants present at the HCSFV, and the annual sample is collected at a temporary specimen collection center in the HCSFV auditorium. Briefly, children are identified by identification card or fingerprint scanning at the identification and registration station, where barcode labels for all documents and sample tubes are automatically printed. Children continue to a nurses’ station where a nurse uses a barcode scanner-enabled PDA for automatic retrieval of data and paperless data entry of interview and specimen information. The nurse draws a blood sample for CBC and dengue serologic tests. The CBC results are returned to the parent or guardian within 2 days, and if any abnormalities are noted (e.g., anemia), appropriate medical follow-up is scheduled. When they pick up the CBC results, participants are provided with a study gift of school supplies worth approximately US $1.00. Test results indicating whether the child has been infected with DENV during the previous year are also returned. In the second phase, field teams visit the homes of all participants who did not present to the HCSFV during the first phase. As in field visits, households are located using GPS devices. Each household is visited at least 3 times in an attempt to locate the participant. After 3 unsuccessful attempts to locate the participant or confirmation that the child no longer lives in the study area, study staff record the child as lost to follow-up.
Electronic sample tracking
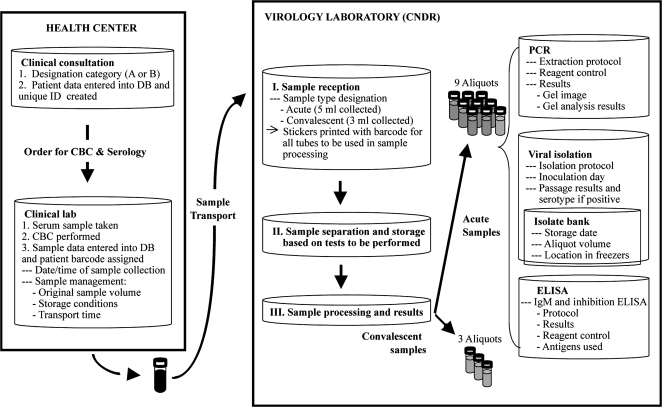
In order to ensure the quality of samples and laboratory results, a sample-tracking system was developed to handle the thousands of blood samples processed each year (refer to Figure 2 for an example of category A samples). All samples collected in the study are labeled with 2-dimensional barcodes, which are appropriately sized for collection, microcentrifuge, and cryopreservation tubes. This minimizes input error and allows real-time sample tracking from collection in the HCSFV or in the field to processing and storage in the National Virology Laboratory. Barcode labels for sample collection tubes are automatically printed during the year upon a child’s presentation with illness at the clinical laboratory in the HCSFV following the scanning of a study identification card or child's fingerprint and selection of the medical tests to be performed. When samples are collected in the field, barcode labels are preprinted prior to the visit. At every step, from collection of the sample to transport to reception at the Virology Laboratory, the tubes are scanned, and an automatic time and date stamp is recorded. Once received at the Virology Laboratory, each sample is scanned, at which time barcode labels are automatically printed for sample processing and storage of all aliquots. The sample-tracking system is used to automatically record the box, rack, and freezer in which a sample is placed for storage. This system also enables monitoring of sample volume in each aliquot, as the volume used in every assay is automatically subtracted from the initial volume. Additionally, the barcoding system ensures participant confidentiality, as tubes are not labeled with the participant's name, and it alerts the medical staff if consent has not been obtained, such as for DNA or influenza, thereby preventing protocol violations.
Figure 2.
Flowchart of sample and information flow for probable dengue cases in the Laboratory Information Management System of the Pediatric Dengue Cohort Study, Managua, Nicaragua, 2004–2008. CBC, complete blood count; CNDR, Centro Nacional de Diagnóstico y Referencia; DB, database; ELISA, enzyme-linked immunosorbent assay; ID, identification; IgM, immunoglobulin M; PCR, polymerase chain reaction.
Data collection and management
To handle the large amounts of data collected through the Pediatric Dengue Cohort Study, information technology staff developed a series of interrelated databases. To ensure data quality, databases were designed with value-restricted fields wherever possible. As many data as possible, such as study code, date, and time, are entered automatically through barcode scanning. Additionally, many laboratory results are electronically transferred to the study databases. For example, CBC results are concurrently printed for inclusion in the patient's chart and automatically transferred to the study database. This system reduces data entry errors and omissions. All data that are manually entered undergo double data entry. Data from field visits, the annual sample, and clinical histories are collected directly into PDAs, whose databases are designed with skip patterns to advance the survey to the appropriate question based on the respondent's prior answers and with questions to verify responses. Use of PDAs in data collection ensures that no fields are skipped, as is possible with paper-based questionnaires, thereby minimizing missing data. A series of quality control queries are run daily on all databases to detect and allow for real-time correction of any errors that occur. Additionally, use of PDAs with barcode scanners allows for access to study databases and the sample tracking system during electrical power outages. During power outages, essential study computers, such as those used for barcode printing and data entry, are maintained by using backup batteries or a small power generator. A description of the components of the data management and sample tracking system is located in Appendix Table 1.
Extension of the cohort
In June 2007, the cohort study was extended to include investigation of influenza and other viral respiratory diseases. These were chosen because of the large burden of respiratory diseases in children in developing countries, lack of detailed epidemiologic data about these diseases, and similarities in study design required to investigate viral respiratory diseases and dengue. Initially, the focus was on influenza, since numerous commonalities exist that make the cohort ideal for studying both dengue and influenza. For instance, both are acute febrile diseases, early symptoms are similar, and cases must present soon after symptom onset to enable detection and isolation of viruses. Participants in the dengue cohort were presented with the opportunity to participate in the Nicaraguan Influenza Cohort Study, and informed consent procedures were performed. As the dengue cohort was already established and the infrastructure was in place, adding other diseases did not require extensive resources or effort. In fact, the only major additions needed to establish the Nicaraguan Influenza Cohort Study were the collection of a respiratory specimen (nasal and oropharyngeal swabs) and laboratory testing for influenza. Subsequently, testing for other respiratory viruses, including respiratory syncytial virus, parainfluenza viruses 1–3, human metapneumovirus, adenoviruses, and rhinoviruses, was initiated by using the respiratory samples.
Laboratory assays
An acute case is considered positive for dengue if 1) dengue virus (DENV) is isolated in C6/36 Ae. albopictus cells (11) and serotyped by RT-PCR (12), 2) DENV RNA is detected by RT-PCR (12, 13) after extraction from serum samples by using the QIAamp Viral RNA Mini Kit (Qiagen, Valencia, California), 3) seroconversion is demonstrated by using acute and convalescent paired sera by a DENV-specific immunoglobulin M capture enzyme-linked immunosorbent assay (14), and/or 4) antibody titer by inhibition enzyme-linked immunosorbent assay (2, 15) demonstrates a 4-fold or greater increase in titer in paired acute and convalescent sera as calculated by using the Reed-Muench method (16). Those whose paired annual samples demonstrate seroconversion or an increase in DENV-specific antibody titer during the year, but who have not had an identifiable febrile episode associated with acute DENV infection, are considered “inapparent DENV infections.”
RESULTS
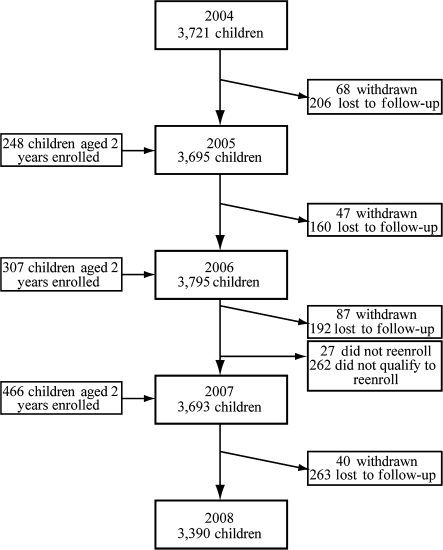
In the first year of the dengue cohort study, 3,721 children were enrolled during an intensive 5-week period that began on August 30, 2004. In subsequent years, all new participants were enrolled during a recruitment period in July or August that typically spanned 4–6 weeks. During the first 4 years of the study (August 2004–July 2008), a total of 4,742 children participated, with yearly participation ranging from 3,693 to 3,795 children (Figure 3). The geographic distribution of participants is shown in Figure 4, and their baseline characteristics by year are shown in Table 1. Over the course of the study, 821 (17.3%) children were lost to follow-up, 245 (5.2%) children were withdrawn from the study, and 289 (6.1%) children were not enrolled again at the end of the first 3 years (Figure 3). Yearly loss to follow-up ranged from 4.3% to 7.1%. The majority of children lost to follow-up (n = 533, 64.9%) had moved and could not be located. Children were withdrawn from the study by study personnel if they moved from the study area, reached the age of 12 years, or failed to follow study procedures, or if their parents requested withdrawal. Of the 289 children who were not enrolled again in the study, 262 (90.7%) had reached the age of 12 years and did not qualify to continue participation.
Figure 3.
Flowchart of participants in the Pediatric Dengue Cohort Study, Managua, Nicaragua, 2004–2008.
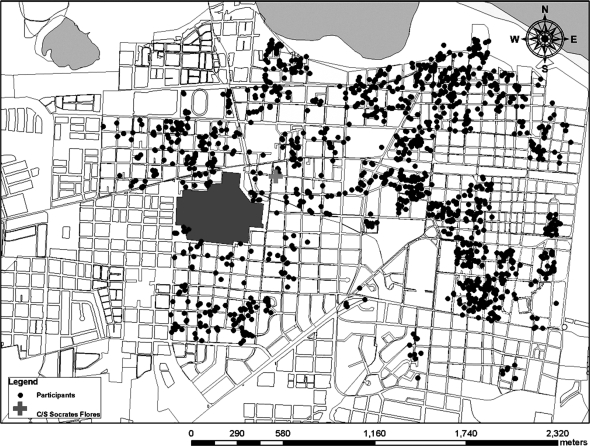
Figure 4.
Map of study area and distribution of cohort participants in the Pediatric Dengue Cohort Study, Managua, Nicaragua, 2004–2008. C/S Socrates Flores, Centro de Salud Sócrates Flores Vivas.
Table 1.
Characteristics of Cohort Participants at the Beginning of Each Study Year in the Pediatric Dengue Cohort Study, Managua, Nicaragua, 2004–2008
| Year 1 (N = 3,721) |
Year 2 (N = 3,695) |
Year 3 (N = 3,795) |
Year 4 (N = 3,693) |
|||||
| No. | % | No. | % | No. | % | No. | % | |
| Sex | ||||||||
| Female | 1,827 | 49.1 | 1,820 | 49.3 | 1,870 | 49.3 | 1,829 | 49.5 |
| Male | 1,894 | 50.9 | 1,875 | 50.7 | 1,925 | 50.7 | 1,864 | 50.5 |
| Age, years | ||||||||
| 2 | 461 | 12.4 | 251 | 6.8 | 300 | 7.9 | 255 | 6.9 |
| 3 | 500 | 13.4 | 419 | 11.3 | 247 | 6.5 | 364 | 9.9 |
| 4 | 543 | 14.6 | 464 | 12.6 | 399 | 10.5 | 307 | 8.3 |
| 5 | 476 | 12.8 | 501 | 13.6 | 432 | 11.4 | 376 | 10.2 |
| 6 | 453 | 12.2 | 436 | 11.8 | 467 | 12.3 | 401 | 10.9 |
| 7 | 477 | 12.8 | 428 | 11.6 | 423 | 11.1 | 436 | 11.8 |
| 8 | 430 | 11.6 | 435 | 11.8 | 407 | 10.7 | 394 | 10.7 |
| 9 | 357 | 9.6 | 399 | 10.8 | 404 | 10.6 | 370 | 10.0 |
| 10 | 24 | 0.6 | 340 | 9.2 | 368 | 9.7 | 370 | 10.0 |
| 11 | NA | 22 | 0.6 | 326 | 8.6 | 338 | 9.2 | |
| 12 | NA | NA | 22 | 0.6 | 82 | 2.2 | ||
Abbreviation: NA, not applicable.
Participants attended 51,609 appointments at the study clinic (Table 2). A vast majority (91.9%) of participants have attended HCSFV for medical attention for 1 or more illnesses. The median number of visits per child was 8 (range, 0–123 visits). Ninety-four percent of all febrile illnesses (categories A, B, and C) and 97% of possible dengue cases (categories A and B) presented within the first 3 days of illness. There were 1,600 suspected dengue cases, of which 1,504 (94%) provided a convalescent sample.
Table 2.
Medical Consults of Participants by Year in the Pediatric Dengue Cohort Study, Managua, Nicaragua, 2004–2008
| Year 1 (N = 11,396) |
Year 2 (N = 12,851) |
Year 3 (N = 13,755) |
Year 4 (N = 13,607) |
|||||
| No. | % | No. | % | No. | % | No. | % | |
| Type | ||||||||
| Primary | 8,463 | 74.3 | 9,148 | 71.2 | 10,512 | 76.4 | 10,127 | 74.4 |
| Follow-up | 2,933 | 25.7 | 3,703 | 28.8 | 3,243 | 23.6 | 3,480 | 25.6 |
| Case classification | ||||||||
| Category A | 756 | 6.6 | 1,451 | 11.3 | 772 | 5.6 | 763 | 5.6 |
| Category B | 424 | 3.7 | 1,182 | 9.2 | 979 | 7.1 | 846 | 6.2 |
| Category C | 4,854 | 42.6 | 4,235 | 32.9 | 5,532 | 40.2 | 5,181 | 38.1 |
| Category D | 5,362 | 47.1 | 5,983 | 46.6 | 6,472 | 47.1 | 6,817 | 50.1 |
In a satisfaction survey of 3,121 (85%) of the cohort participants, 2,990 (96%) rated the medical attention received through the study as excellent or very good. Of the 3,251 children who qualified for reenrollment in the study at the end of the first 3-year period, only 27 (0.8%) chose not to reenroll. In yearly participation surveys, 1.7%–2.5% (average, 2.1%) of participants reported having attended a health-care provider outside of the study, and 0.8%–6.1% (average, 2.8%) reported having had an illness and not attending any medical provider (Table 3). The most common reasons for not seeking medical care included brief duration of illness (<12 hours) and relief of symptoms upon self-medication with over-the-counter pain or cold medications.
Table 3.
Results of Participation Survey by Year in the Pediatric Dengue Cohort Study, Managua, Nicaragua, 2004–2008
| Year 1 (N = 3,721) |
Year 2 (N = 3,695) |
Year 3 (N = 3,795) |
Year 4 (N = 3,693) |
|||||
| No. | % | No. | % | No. | % | No. | % | |
| Participated in survey | 2,937 | 78.9 | 3,121 | 84.5 | 3,196 | 84.2 | 3,351 | 90.7 |
| Had a fever and consulted other health-care provider | 72 | 2.5 | 62 | 2.0 | 72 | 2.3 | 56 | 1.7 |
| Had a fever and did not consult a medical provider | 49 | 1.7 | 78 | 2.5 | 195 | 6.1 | 28 | 0.8 |
Uptake of the Nicaragua Influenza Cohort Study was high, with 4,132 of 4,178 (99%) eligible subjects choosing to participate. After the addition of the influenza study, no decrease in participation or in the quality of the dengue study was observed. In fact, more participants presented to the HCSFV earlier after symptom onset when compared with the 3 prior years, with the mean days post-symptom onset at presentation of 1.77 days in 2007–2008 compared with 2.13 days in 2004–2007 (P = 0.03).
DISCUSSION
In this paper, we describe the study design and methods used to establish and conduct the Pediatric Dengue Cohort Study. This study is the first large-scale prospective cohort study in the Americas to determine the incidence rate of dengue and DENV infection over multiple years. It has also allowed the establishment of infrastructure necessary for high-quality clinical studies and trials, including informatics and quality control systems and experienced Nicaraguan personnel. Expansion of the study to include influenza and viral respiratory diseases was possible with minimal additional cost and has provided key epidemiologic and virologic data about these diseases in Nicaragua, as well as increased benefit to study participants.
One unique aspect of the study is the extensive, low-cost, customized informatics system that was developed to ensure study quality using inexpensive software (17). Implementation of information technologies in the Pediatric Dengue Cohort Study has permitted greater efficiency and quality of data collection, allowing investigators to overcome many of the challenges of performing research in a resource-limited setting. Specifically, use of barcodes and PDAs provided real-time access to study data, allowing constant monitoring to ensure data quality and completeness. GPS data greatly facilitated daily planning and coordination of field visits, thereby improving efficiency and decreasing cost. Barcodes and fingerprint scans dramatically reduced the amount of time required to locate and retrieve medical charts, thus reducing waiting time and improving patient flow (18).
Participation was high throughout the 4 years of the study, as evidenced by the large number of medical visits, the high percentage (94%) of children that attended a convalescent visit, and low loss to follow-up. Compliance was also very high, as demonstrated by the fact that 94% of children with fever attended the HCSFV within the first 3 days of illness, and few children reported having sought medical care outside of the HCSFV. The high participation rates can be attributed to overall satisfaction with the services provided by the cohort study, supported by the extremely high acceptance rate (99%) of continuing participation in the study.
The addition of influenza and other viral respiratory diseases was widely accepted by cohort participants, as evidenced by the 99% acceptance rate of enrolling in the influenza study, and did not negatively impact the dengue study. These additions enhanced the benefits to both participants and the Nicaraguan people with minimal extra burden on the participants. Incorporation of these additional studies by the established cohort was efficient and cost-effective, requiring a fraction of the resources that a separate cohort would require, and it has yielded critical information about the burden and seasonality of viral respiratory diseases in tropical developing countries and allowed establishment of essential laboratory diagnostic capacity for these diseases in the Ministry of Health (19) (A. Gordon, A. Balmaseda, E. Harris, unpublished data).
Close collaboration with the Ministry of Health was vital to the success of the project (20). By working within the Ministry of Health and its facilities in a sustainable manner and not setting up a separate study clinic and laboratory, the study not only benefitted participants and study personnel but also improved the public health infrastructure substantially, bringing physical renovations and basic amenities to the study health center and hospital. Likewise, improvements to the National Virology Laboratory indirectly benefited the Nicaraguan population. In addition, use of almost entirely local staff and coinvestigators increased the acceptability of the project while also training Nicaraguan scientists and public health professionals.
In summary, local “ownership” and the use of information technologies were vital to the implementation, conduct, and high quality of the Pediatric Dengue Cohort Study. The study design allowed for the addition of detailed surveillance for other diseases, such as influenza, in an efficient and cost-effective manner. Importantly, close collaboration with the Nicaraguan Ministry of Health and a shared decision-making process, together with almost entirely Nicaraguan study personnel, have ensured local benefit and a lasting impact of the study. Altogether, this has resulted in international recognition of Nicaragua as a Center of Excellence for dengue research and epidemiology. Finally, this study is providing key information, including incidence data that will be presented in future reports, needed for the design and conduct of dengue vaccine trials in the Americas.
Acknowledgments
Author affiliations: Centro de Salud Sócrates Flores Vivas, Barrio Monseñor Lezcano, Managua, Nicaragua (Guillerimina Kuan); Division of Epidemiology, School of Public Health, University of California, Berkeley, Berkeley, California (Aubree Gordon); Sustainable Sciences Institute, c/o Centro de Salud Sócrates Flores Vivas, Barrio Monseñor Lezcano, Managua, Nicaragua (William Avilés, Douglas Elizondo, Samantha N. Hammond, Oscar Ortega); Departamento de Virología, Centro Nacional de Diagnóstico y Referencia, Ministerio de Salud, Complejo de Salud Dra. Concepcion Palacios, Primero de Mayo, Managua, Nicaragua (Angel Balmaseda, Andrea Nuñez); and Division of Infectious Diseases and Vaccinology, School of Public Health, University of California, Berkeley, Berkeley, California (Josefina Coloma, Eva Harris).
This work was supported by the Pediatric Dengue Vaccine Initiative (grant VE-1).
The authors thank the phenomenal study personnel at the Centro de Salud Sócrates Flores Vivas, Sustainable Sciences Institute, Centro Nacional de Diagnóstico y Referencia, and Hospital Infantil Manuel de Jesús Rivera, in particular, Magaly Amador, Sonia Arguello, José Ramon Cisneros, Carolina Flores, Nicole Fitzpatrick, Juan Carlos Matute, Berman Moraga, Juan Carlos Mercado, Mirtha Monterrey, Zoila Orozco, Sergio Ojeda, Leonel Perez, Miguel Reyes, Crisanta Rocha, Carlos Romero, Saira Saborio, Leyla Saenz, Nery Sanchez, Sheyla Silva, Yolanda Tellez, and Maria José Vargas. The authors are grateful for the longstanding support from the Ministry of Health and especially Drs. Alcides Gonzalez and Juan José Amador. Finally, they would like to thank Rain Mocello for her editorial assistance.
Conflict of interest: none declared.
Glossary
Abbreviations
- CBC
complete blood count
- DENV
dengue virus
- GPS
global positioning system
- HCSFV
Health Center Sócrates Flores Vivas
- HIMJR
Hospital Infantil Manuel de Jesús Rivera
- PDA
personal data assistant
- RT-PCR
reverse transcriptase-polymerase chain reaction
APPENDIX
Appendix Table 1.
Components of the Customized, Low-Cost Informatics System Used in the Pediatric Dengue Cohort Study, Managua, Nicaragua, 2004–2008
| Function | Components |
| Data entry and management | Hardware Computers MS9520 Voyager hand-held scanners (Metrologic, West Deptford, New Jersey) for scanning barcodes Opticon LG2 2D (Opticon, Inc., Orangeburg, New York) for scanning barcodes with automatic time stamp and mobile tracking |
| Software Microsoft Access (Microsoft Corporation, Seattle, Washington) as the primary database management system EpiInfo (Centers for Disease Control and Prevention, Atlanta, Georgia) for double data-entry check | |
| Identification of participants | Hardware U.are.U. 4000 fingerprint scanner (Digital Personna, Redwood City, California) |
| Software Verifinger 4.2 SDK (Neurotechnologija, Vilnius, Lithuania) for fingerprint scanning Print Studio Professional 2.0 software (Jolly Technologies, San Carlos, California) for printing identification cards with barcodes | |
| Localization of participants’ houses | Hardware Garmin GPS 60 and Garmin e-Trex hand-held GPS devices (Garmin, Olathe, Kansas) |
| Software Mapsource (Garmin, Olathe, Kansas) for downloading points from GPS devices ArcView (ESRI, Redlands, California) for analyzing geographic information to search for and locate children in their home and for planning of field activities | |
| Mobile data access and storage | Hardware SPT 1550 with integrated barcode scanner (Symbol, Oakland, California) Palm Tungston (Palm, Sunnyvale, California) with Plug-N-Scan barcode kit (Portable Technology Solutions, LLC, Calverton, New York) Rechargeable batteries and chargers |
| Software Palm OS 4.1 or higher (Palm, Sunnyvale, California) Pendragon Forms 5.0 (Pendragon Software Corporation, Libertyville, Illinois) for PDA databases and user interface of PDA databases Hand-held user licenses | |
| Barcode printing | Hardware TLP 2844 thermal transfer printer, 5095 universal resin ribbons, Polypro 1000 labels for labeling paperwork and blood collection tubes, and Cryocool 3000 labels for labeling Eppendorf tubes and cryovials (Zebra, Vernon Hills, Illinois) |
| Software Print Studio Professional 2.0 software (Jolly Technologies, San Carlos, California) for barcode printing using Microsoft Access databases Visual Basic 6.0 (Microsoft Corporation, Seattle, Washington) for creating tables used to encode study information into barcodes | |
| Automatic entry of laboratory results | Hardware Machine-specific cables for connecting laboratory equipment to computers |
| Software Visual Basic 6.0 (Microsoft Corporation, Seattle, Washington) to manage the PC serial port connector |
Abbreviations: GPS, global positioning system; PC, personal computer; PDA, personal data assistant.
References
- 1.Gibbons RV, Vaughn DW. Dengue: an escalating problem. BMJ. 2002;324(7353):1563–1566. doi: 10.1136/bmj.324.7353.1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Balmaseda A, Hammond SN, Tellez Y, et al. High seroprevalence of antibodies against dengue virus in a prospective study of schoolchildren in Managua, Nicaragua. Trop Med Int Health. 2006;11(6):935–942. doi: 10.1111/j.1365-3156.2006.01641.x. [DOI] [PubMed] [Google Scholar]
- 3.Endy TP, Chunsuttiwat S, Nisalak A, et al. Epidemiology of inapparent and symptomatic acute dengue virus infection: a prospective study of primary school children in Kamphaeng Phet, Thailand. Am J Epidemiol. 2002;156(1):40–51. doi: 10.1093/aje/kwf005. [DOI] [PubMed] [Google Scholar]
- 4.Burke DS, Nisalak A, Johnson DE, et al. A prospective study of dengue infections in Bangkok. Am J Trop Med Hyg. 1988;38(1):172–180. doi: 10.4269/ajtmh.1988.38.172. [DOI] [PubMed] [Google Scholar]
- 5.World Health Organization. Dengue Haemorrhagic Fever: Diagnosis, Treatment, Prevention, and Control. Geneva, Switzerland: World Health Organization; 1997. [Google Scholar]
- 6.Sabin AB. The dengue group of viruses and its family relationships. Bacteriol Rev. 1950;14(3):225–232. [PubMed] [Google Scholar]
- 7.Halstead SB. Epidemiology of dengue and dengue hemorrhagic fever. In: Gubler DJ, Kuno G, editors. Dengue and Dengue Hemorrhagic Fever. New York, NY: CAB International; 1997. [Google Scholar]
- 8.Hombach J. Vaccines against dengue: a review of current candidate vaccines at advanced development stages. Rev Panam Salud Publica. 2007;21(4):254–260. doi: 10.1590/s1020-49892007000300011. [DOI] [PubMed] [Google Scholar]
- 9.Whitehead SS, Blaney JE, Durbin AP, et al. Prospects for a dengue virus vaccine. Nat Rev Microbiol. 2007;5(7):518–528. doi: 10.1038/nrmicro1690. [DOI] [PubMed] [Google Scholar]
- 10.Guzmán MG, Kourí G. Dengue: an update. Lancet Infect Dis. 2002;2(1):33–42. doi: 10.1016/s1473-3099(01)00171-2. [DOI] [PubMed] [Google Scholar]
- 11.Balmaseda A, Sandoval E, Pérez L, et al. Application of molecular typing techniques in the 1998 dengue epidemic in Nicaragua. Am J Trop Med Hyg. 1999;61(6):893–897. doi: 10.4269/ajtmh.1999.61.893. [DOI] [PubMed] [Google Scholar]
- 12.Lanciotti RS, Calisher CH, Gubler DJ, et al. Rapid detection and typing of dengue viruses from clinical samples by using reverse transcriptase-polymerase chain reaction. J Clin Microbiol. 1992;30(3):545–551. doi: 10.1128/jcm.30.3.545-551.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Seah CL, Chow VT, Tan HC, et al. Rapid, single-step RT-PCR typing of dengue viruses using five NS3 gene primers. J Virol Methods. 1995;51(2–3):193–200. doi: 10.1016/0166-0934(94)00104-o. [DOI] [PubMed] [Google Scholar]
- 14.Balmaseda A, Guzmán MG, Hammond S, et al. Diagnosis of dengue virus infection by detection of specific immunoglobulin M (IgM) and IgA antibodies in serum and saliva. Clin Diagn Lab Immunol. 2003;10(2):317–322. doi: 10.1128/CDLI.10.2.317-322.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fernández RJ, Vázquez S. Serological diagnosis of dengue by an ELISA inhibition method (EIM) Mem Inst Oswaldo Cruz. 1990;85(3):347–351. doi: 10.1590/s0074-02761990000300012. [DOI] [PubMed] [Google Scholar]
- 16.Reed LJ, Muench H. A simple method of estimating fifty percent endpoints. Am J Hyg. 1938;27(3):493–497. [Google Scholar]
- 17.Avilés W, Ortega O, Kuan G, et al. Integration of information technologies in clinical studies in Nicaragua. PLoS Med. 2007;4(10):1578–1583. doi: 10.1371/journal.pmed.0040291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Avilés W, Ortega O, Kuan G, et al. Quantitative assessment of the benefits of specific information technologies applied to clinical studies in developing countries. Am J Trop Med Hyg. 2008;78(2):311–315. [PubMed] [Google Scholar]
- 19.Gordon A, Ortega O, Kuan G, et al. Prevalence and seasonality of influenza-like illness in children, Nicaragua, 2005–2007. Emerg Infect Dis. 2009;15(3):408–414. doi: 10.3201/eid1503.080238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Coloma J, Harris E. Sustainable transfer of biotechnology to developing countries: fighting poverty by bringing scientific tools to developing-country partners. Ann N Y Acad Sci. 2008;1136:358–368. doi: 10.1196/annals.1425.014. [DOI] [PubMed] [Google Scholar]