Abstract
The hypothesized role of rapid eye movement (REM) sleep, which is rich in dreams, in the formation of new associations, has remained anecdotal. We examined the role of REM on creative problem solving, with the Remote Associates Test (RAT). Using a nap paradigm, we manipulated various conditions of prior exposure to elements of a creative problem. Compared with quiet rest and non-REM sleep, REM enhanced the formation of associative networks and the integration of unassociated information. Furthermore, these REM sleep benefits were not the result of an improved memory for the primed items. This study shows that compared with quiet rest and non-REM sleep, REM enhances the integration of unassociated information for creative problem solving, a process, we hypothesize, that is facilitated by cholinergic and noradrenergic neuromodulation during REM sleep.
Keywords: human, implicit, memory, remote-associates, sleep
The night before Easter Sunday of that year I awoke, turned on the light, and jotted down a few notes on a tiny slip of paper. Then I fell asleep again. It occurred to me at 6 o'clock in the morning that during the night I had written down something most important, but I was unable to decipher the scrawl. The next night, at 3 o'clock, the idea returned. It was the design of an experiment to determine whether or not the hypothesis of chemical transmission that I had uttered 17 years ago was correct. I got up immediately, went to the laboratory, and performed a single experiment on a frog's heart according to the nocturnal design.
Otto Loewi, 1938 German, Nobel laureate for his work on the chemical transmission of nerve impulses.
Creativity has been defined as “the forming of associative elements into new combinations which either meet specified requirements or are in some way useful” (1). It has been further proposed that creative problem solving is reached in four successive phases: first, intense but unsuccessful confrontations with the elements of the problem; second, a decision to put the problem aside; third, a dormant period with no further conscious work on the problem, e.g., incubation; and finally, a “flash of insight” in which the solution suddenly enters consciousness while the individual is dreaming or engaged in idle thought (2–4). Evidence for the role of these phases in creative problem solving (e.g., a dormant period or incubation), however, is inconsistent (5–7). Yet, it has been long hypothesized that creative problem solving is enhanced by states of mind, such as sleep or quiet reflection, which foster insights. Furthermore, several famous anecdotes attribute creative revelations to dreaming in particular, ranging from musical compositions to insightful advances in scientific discovery (8).
Evidence for the role of sleep in creative problem solving has been suggested by prior research, but the most critical questions about this effect remain unanswered. First, sleep appears to enhance creative and associative memory processing compared with wake, but the underlying mechanisms, such as sleep stages, have not been explored (9–12). The seminal article by Wagner and colleagues (12) suggested that sleep might facilitate “cognitive flexibility” and lead to increased occurrences of insight; however, information about the operative sleep stage was not provided. Second, no study has demonstrated that REM [a potentially more facilitative state of mind than non-REM (NREM)] enhances creativity more than wake, NREM, or simply the passage of time (i.e., incubation) (13, 14). Circadian confounds in the timing of testing periods may be a possible reason for the lack of difference between the REM group and wake controls. Last, although these studies suggest that exposure to the elements of a problem before sleep is necessary for insights to occur, they do not successfully distinguish between improved memory and enhanced creative processing as the cause of better performance on these associative tasks (15). In conclusion, prior studies suggest that sleep, particularly REM, may enhance the formation of associative networks and the integration of unassociated information, but no study to date has shown REM to enhance creative processing directly more than any other sleep or wake state. The present study (i) directly compared REM, NREM, and wake controls while using a nap paradigm to control for circadian effects, and (ii) probed contributions of both memory and associative processing in creative problem solving.
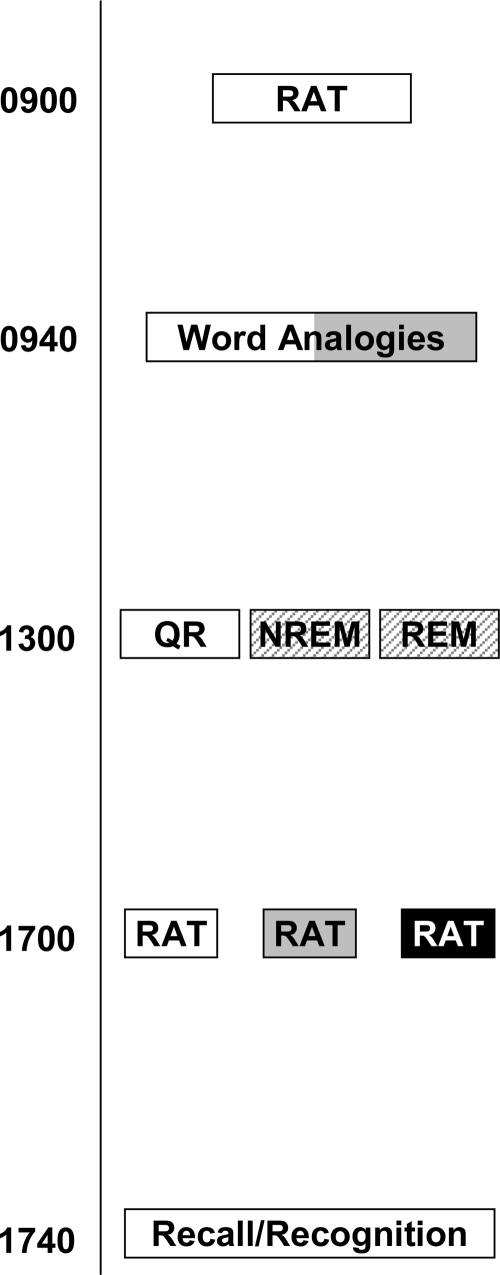
We compared incubation and sleep on three forms of prior exposure to the elements of a creative problem (repeated exposure, no exposure, or priming) on the Remote Associates Test (RAT) (Fig. 1). Using a nap paradigm allowed us specifically to compare sleep with or without REM with incubation. In the creativity task (RAT), subjects are required to produce a word that is associated with three test words that are seemingly unrelated to each other (1). The exposure conditions were designed to access three different methods for creative problem solving. First, the repeated-exposure condition examined the role of incubation on creative problem solving. Second, the priming condition examined whether stimulation of information nodes by an unrelated source can increase solutions to creative problems. Last, the no-exposure condition examined whether general creative problem solving can be enhanced with repetition of the same type of task.
Fig. 1.
Experimental design. Subjects were administered the creative problem solving test in the morning and then a word analogy priming task. After an intervening polysomnographically recorded sleep or quiet rest period, subjects were tested on the three prior exposure conditions: repeated exposure (white box), primed exposure (gray box), or no exposure (black box). Memory tests for the analogy solutions followed.
We hypothesized that: (i) incubation alone would increase creative associations in the repeated condition; (ii) sleep, specifically REM, is required for associating information primed in an unrelated task to the solutions for a creativity task; and (iii) creative problem solving requires prior exposure to problem-related information, such that no benefit would be seen for items in the no-exposure condition. To reduce interference effects that occur during normal waking, a quiet rest group with EEG monitoring was used instead of uncontrolled wake or sleep deprivation groups.
Results
Incubation-Dependent Improvements in Performance Are Independent of Sleep.
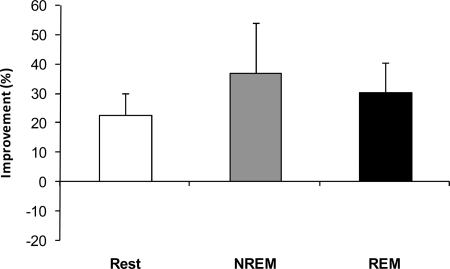
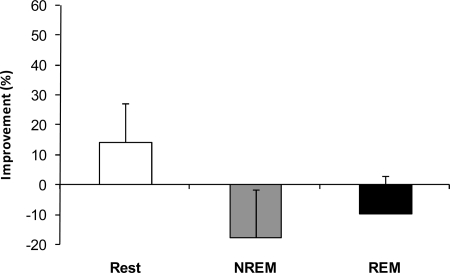
Subjects were first tested on the RAT at 9 AM and retested at 5 PM. To compare sleep and the passage of time, subjects were administered the same RAT in both the morning and afternoon sessions. No differences were found between groups (P = 0.67, 1-way ANOVA) (Fig. 2), and post hoc analysis showed that all three groups, NREM sleep, REM sleep, and quiet rest, improved similarly on the repeated items compared with the morning baseline performance [confidence interval (CI) 95%, 6.5–38.3%; CI 95%, 10.6–50.2%; and CI 95%, 2.4–71.0% for the REM, NREM, and rest groups, respectively]. These results indicate that passage of time (i.e., incubation) was sufficient to increase creative problem solving. This is consistent with the theory of incubation in which solutions to problems will emerge spontaneously after not actively working on the problem for some time (i.e., allowing the problem to incubate) (16).
Fig. 2.
Incubation-dependent improvements in performance are independent of sleep. NREM sleep, REM sleep, and quiet rest similarly improved performance on the repeat items compared with the morning baseline performance (CI 95%, 6.5–38.3%; CI 95%, 10.6–50.2%; and CI 95%, 2.4–71.0% for the REM, NREM, and rest groups, respectively).
REM Sleep, Not Incubation nor NREM Sleep, Improved Solutions After Priming.
We tested whether priming of associative networks would improve creative problem solving with the primed items and whether REM sleep would enhance this effect compared with NREM sleep or quiet rest. After the morning RAT, subjects completed a set of analogies (e.g., CHIPS: SALTY; CANDY: S___) in which half of the answers (e.g., SWEET) were also the answers to the afternoon RAT items (e.g., HEART, SIXTEEN, COOKIES; answer: SWEET).
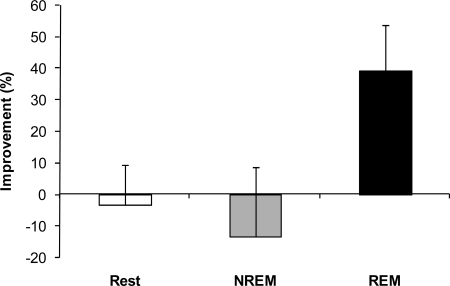
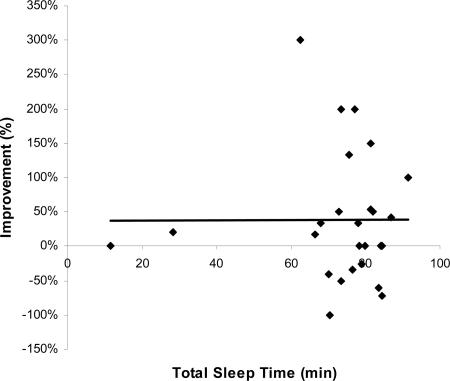
In contrast to the incubation results, subjects that had REM sleep displayed a significant improvement above NREM sleep and quiet rest groups (Fig. 3; P = 0.04, 1-way ANOVA and post hoc analysis). Strikingly, although the quiet rest and NREM sleep groups received the same priming, they displayed no improvement on the primed RAT items, whereas the REM sleep group improved by almost 40% above the morning performance. Although naps with REM typically had longer total sleep time (TST) (see Table 1), surprisingly, TST was not correlated with performance on the primed RAT items (r = .104, P = 0.53, Fig. 4). We also separated subjects by their TST with a median split of the data and compared low TST with high TST on the priming results, which was nonsignificant (t = 1.4, P = 0.17). Thus, it is unlikely that the REM results were the result of TST differences in the nap. Consistent with previous studies reporting a benefit of sleep on cognition (17–20), we find that it was not the quantity (i.e., TST), but the quality (i.e., specific sleep stages) that led to improved performance. These results suggest that REM enhanced the formation of associative networks and the integration of unassociated information, compared with quiet rest and NREM sleep.
Fig. 3.
REM sleep facilitates the use of prior information for creative problem solving. Subjects who had REM sleep displayed a significant improvement above NREM and quiet rest groups (P = 0.047, 1-way ANOVA and post hoc analysis). Strikingly, although the quiet rest and NREM nap groups received the same priming, they displayed no improvement on the primed RAT items, whereas the REM group improved by almost 40% above the morning performance.
Table 1.
Sleep statistics comparing REM and NREM naps
| Sleep type | NREM group | REM group |
|---|---|---|
| Total sleep time* | 50.2 (±4.2) | 72.9 (±2.9) |
| Stage 1, NS | 5.4 (±1.4) | 3.9 (±1.0) |
| Stage 2, NS | 24.6 (±3.0) | 31.4 (±2.0) |
| SWS, NS | 20.0 (±4.2) | 23.3 (±2.8) |
| REM* | 0.2 (±2.3) | 14.3 (±1.6) |
Results are shown as mean (±SEM).
*, P < 0.001; NS, not significant.
Fig. 4.
Amount of sleep does not contribute to creative problem solving. TST and improvement on primed RAT items were not significantly correlated (r = 0.104, P = 0.53, trend line shown).
Creative Problem Solving Requires Prior Exposure.
Because daytime sleep has been shown to increase alertness and improve a range of cognitive functions (perceptual, verbal, and motor learning; declarative and implicit memory) (18, 21–24), we tested whether sleep or quiet rest might enhance general creativity on new RAT items. Baseline assessments were measured on the morning RAT. In the PM session, subjects were tested on new RAT items. Surprisingly, no group (NREM, REM, quiet rest) differences were found on the new RAT items (P = 0.261 1-way ANOVA) (Fig. 5), and no improvement in PM performance above baseline was observed in the three groups (CI 95%, −13.0 to 41.2%; CI 95%, −16.0 to 35.4%; and CI 95%, −15.4 to 51.0% for the REM, NREM, and rest groups, respectively). Although daytime sleep has been shown to improve performance on some cognitive tasks and to increase alertness and restore homeostatic drive, neither NREM nor REM sleep improved general creative problem solving in the absence of prior exposure (e.g., priming).
Fig. 5.
REM sleep does not improve creative problem solving without prior exposure. No group differences were found on the new RAT items (P = 0.261 1-way ANOVA), and PM performance was not different from baseline in the any of the three groups (CI 95%, −13.0 to 41.2%; CI 95%, −35.4 to 16.0%; and CI 95%, −51.0 to 15.4% for the REM, NREM, and rest groups, respectively). Although daytime sleep has been shown to improve a range of cognitive functions (perceptual, verbal, and motor learning; declarative and implicit memory) and to increase alertness and restore homeostatic drive, neither NREM nor REM sleep improves general creative problem solving in the absence of prior exposure (e.g., priming).
REM Improvements in Creative Problem Solving Are Not Caused by Improved Memory.
Previous studies have shown that sleep facilitates the retention of declarative memories (25–27). We, therefore, examined whether enhancement on the RAT items after priming and REM sleep was caused by memory of the answers in the priming task. To address memory, recognition and cued recall were assessed for answers to the morning analogies during the afternoon session. The process dissociation procedure (28) was also used to investigate how sleep may act on implicit and explicit memory processes.
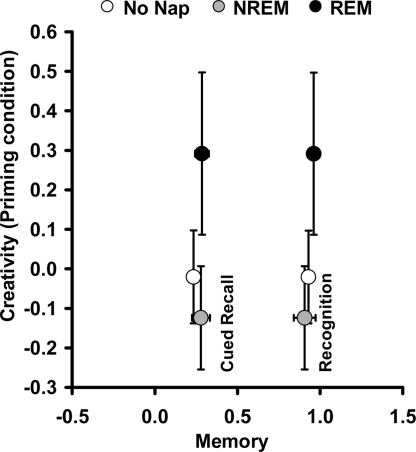
Surprisingly, yet consistent with the incubation findings, no difference was observed among the three groups (NREM, REM, and quiet rest) for any of the memory measures, including recognition (P = 0.283, 1-way ANOVA), cued recall (P = 0.353, 1-way ANOVA), explicit (P = 0.435, 1-way ANOVA), and implicit (P = 0.229, 1-way ANOVA). Interestingly, all three groups (rest, NREM naps, REM naps) had >90% correct on the recognition test, suggesting that all groups formed memories of the answers from the morning analogies, i.e., priming. NREM sleep, REM sleep, and quiet rest groups were indistinguishable on the memory measures [recognition (P = 0.28, 1-way ANOVA), cued recall (P = 0.35, 1-way ANOVA), explicit (P = 0.43, 1-way ANOVA), and implicit (P = 0.22, 1-way ANOVA) memory]. Performance on these memory measures was not correlated with performance on the primed RAT items (Fig. 6). Importantly, although all groups had similar memory for primed answers, only subjects with REM sleep promoted generalization of the analogy answers to new and useful solutions on an unrelated creative problem-solving task.
Fig. 6.
REM sleep improvements in creativity are dissociated from memory. Creative problem solving performance in the priming condition (y axis) and memory tasks (cued recall and recognition) are shown. The three groups perform equally well on all memory tasks, although REM sleep improvements in creativity are only seen in performance for primed unrelated items.
Discussion
Here, we report that REM sleep can improve creative problem solving. We found that: (i) the passage of time (i.e., incubation period) improves problem solving for previously exposed items, and this was independent of the sleep condition; (ii) sleep enhanced creative problem solving for items that were primed before sleep, but this was only true for naps that included REM sleep; (iii) REM sleep improvements in creative problem solving are not the result of selective improvements in memory; and (iv) general problem-solving abilities were not improved in wake or sleep conditions. These findings have important implications for how sleep, specifically REM sleep, might foster the formation of associative networks.
A longstanding, critical issue in sleep and cognition research is whether improvements in behavioral performance are the result of sleep-specific enhancement or reduction of interference. Experiences during waking have been shown to interfere with memory consolidation (29). Thus, performance benefits observed after sleep may be the result of lack of interference. For example, a recent study found that a quiet wake interval provided benefits for auditory tone sequence learning similar to those from a sleep interval, and both were better than the active wake interval (30). Our methods control for interference effects by comparing sleep periods with quiet rest periods. Subjects were relaxed on a recliner, with polysomnographic monitoring, and they listened to instrumental music of their choice for a time interval equal to the naps. By controlling for verbal input, we can be confident that performance across sleep and wake groups was not caused by a difference in verbal interference during the nap, but specifically by processes occurring during incubation.
Current models for why we sleep posit an important role of sleep in memory consolidation (31–34). While controlling for circadian effects, the present study found that sleep specifically enhanced the associative network for primed solutions but did not improve memory consolidation. Although other studies have reported that sleep enhances explicit verbal memory (22, 35), our memory measures were not comparable with these recall tasks because exposure to the verbal material was primed and not explicitly presented. Furthermore, not only were NREM sleep, REM sleep, and quiet rest groups indistinguishable on the memory measures, performance on these memory measures was not correlated with performance on the primed RAT items. This indicates that the improvement on the primed RAT was not a consequence of the REM sleep group simply remembering the primed words better than the other groups. These results are consistent with the previous finding that memory strength of previously encountered insight problems is not directly related to the solution acquisition to those problems (36).
The results support the hypothesis that the brain is subconsciously spreading activation of previously activated nodes. Prior literature suggests that during a “dormant period” between two active encounters with a problem, the memory trace of a target item, and the progression of this target through other relevant stored information generate spreading activation through a network (16). For example, by priming the solution SWEET before sleep, the SWEET node is activated, and during subsequent REM sleep, the associative nodes (in this case HEART, SIXTEEN, COOKIE) are more likely to be activated and increased above threshold. Therefore, when the three words that were previously unrelated (HEART, SIXTEEN, COOKIE) are seen, there will be an increased probability of the node SWEET being chosen as the solution. We propose that the most optimal dormant period occurs during REM sleep, which provides the most spreading of activation.
One possible mechanism for the spreading-activation model involves cholinergic and noradrenergic neuromodulation that occurs specifically during REM sleep. During wake, higher levels of norepinephrine and acetylcholine inhibit recurrent connections in the neocortex. During REM sleep, however, high levels of acetylcholine in the hippocampus suppress feedback from hippocampus to the neocortex, whereas lower levels of acetylcholine and norepinephrine in the neocortex could facilitate the spread of activity within neocortical areas without strong hippocampal influence (37). This is supported by behavioral evidence from amnesiacs that activation of this associative network during sleep is independent of the medial temporal lobe structures and may reflect reactivation of remote memories that are less dependent on the hippocampus (38). In this theoretical framework, REM sleep would allow neocortical structures to reorganize associative hierarchies, in which information from the hippocampus would be reinterpreted in relation to previous semantic representations or nodes.
We propose that REM sleep is important for assimilating new information into past experience to create a richer network of associations for future use. Fluid interpretation is a hallmark of a creative mind, from idle word play to the abstraction of shapes that led to the solving of neurochemical transmission or the structure of the benzene ring. These findings on the role of REM sleep in creative problem solving underscore the Nobel Laureate Friedrich A. Kekule's recommendation: “Let us learn to dream.”
Experimental Procedures
General procedures are outlined in this section. Deviations from this procedure are described in each section.
Subjects.
A total of 77 native English speakers between the ages of 18 and 35 with no personal history of neurological, psychological, or other chronic illness gave informed consent to participate in the experiment, which was approved by the Institutional Review Board of the University of California at San Diego. Subjects were required to sleep an average of 6 h per night for the 5 days leading up to the experimental day and at least 6.5 h the night before the test day. Subjects filled out sleep diaries and wore actigraphs 5–7 days before testing as subjective and objective measures of sleep–wake activity. Subjects were restricted from consuming caffeine and alcohol 24 h before and during the experiment day.
Materials
RAT is a paper-and-pencil task adapted from Mednick (1). Each RAT item contains a triplet of words presented horizontally along with a blank space. Each item requires the subject to combine or relate the three words drawn from mutually remote associative clusters (e.g., COOKIES, SIXTEEN, HEART: _____). The subject is required to find a fourth word that could serve as an associative link between these three words. The answer to this item is SWEET (cookies are sweet, sweet sixteen, sweetheart). The three test words HEART, SIXTEEN, COOKIE are associated with the solution SWEET by formation of a compound (sweetheart), by a syntactic association (sweet sixteen), and by a semantic relationship (cookies are sweet). Thus, reaching a solution requires “creative thought” because the first, most highly probable associate to each of the items is often not correct, so the solver must think of more distantly related information to connect the three words. Performance on the RAT correlates reliably with other established insight problems (39).*
Subjects were read the instructions aloud and given four practice items to ensure understanding of the task. The score was calculated as the proportion of items answered correctly. Subjects completed two versions of the RAT, one in the morning and one in the afternoon. The two versions were counterbalanced across sessions. In the morning session, subjects completed the AM RAT, and AM scores were used as baseline.
Analogies.
Analogies (e.g., FAST:SLOW as HARD:E___), were administered in the AM session. The first letter of each answer was given. Half of the analogy answers served as primes for the answers to the RAT administered during the PM session. There was no time limit for completing the analogies. The mean log HAL word frequency (40) for all words used in the analogies was 1.58 (SD = 0.73). The mean word length was 5.36 letters (SD = 1.75).
General Method.
All subjects were tested on the RAT twice in 1 day (Fig. 1). At 0900, subjects were administered the RAT followed by the analogies. Subjects returned at 1300, at which time they were randomly assigned to a nap or a quiet rest group. Subjects in the nap group took a polysomographically recorded (PSG) nap (90 min of sleep maximum or up to 2 h in bed), whereas those in the rest condition listened to instrumental music with PSG monitoring for 90 min. At 1630, subjects returned for testing of the afternoon RAT.
Experiment 1.
RAT.
Subjects completed 30 RAT items. They were given 40 min to complete all of their responses.
Analogies.
Two versions with 30 analogies each were administered in the AM session. Fifteen analogies in the AM had the same answers as 15 items in the PM RAT.
Study procedure.
In the morning session, subjects completed the AM RAT and then filled in responses to 30 analogies. In the PM RAT, 15 of the items were primed during the AM analogies. The other 15 PM RAT items were unprimed. A total of 25 subjects, REM (n = 10), NREM (n = 6), and rest (n = 9), participated in experiment 1.
Experiment 2.
RAT-S.
Two shortened versions of the RAT were used, each with 15 items. These versions were created by dividing Version A of the RAT into odd and even items. The two versions were counterbalanced across sessions. Subjects were given 20 min to complete all of their responses. The morning RAT-S was used as a baseline performance measure. In the afternoon session, two RAT-S forms were administered. One form was the same form as the morning (i.e., uncued RAT-S), and the other form had the 15 answers cued from the morning analogies (i.e., cued RAT-S).
Analogies.
Subjects completed 75 analogies. Fifteen correct answers were primes for the afternoon cued-RAT-S. The remaining 60 analogy answers were used in the afternoon session to test for memory retention with three distinct methods. Twenty correct answers were tested on a recognition test, 20 correct answers were tested on an inclusion test, and 20 correct answers were tested on an exclusion test (see below for test description).
Inclusion and exclusion tests.
A modified version of the process-dissociation procedure (15) was used to dissociate between explicit and implicit processes for verbal memory. For the inclusion test, subjects were given 20 stem completions and asked to complete them with words they recalled from the answers to the morning analogies. If they could not recall the word, they were asked to fill in the stem with the first word that came to mind. For the exclusion test, subjects were also given 20 stem completions and asked to complete with words that were NOT answers to the morning analogies. If they could not recall the word, they were to fill in the stem with the first word that came to mind. The 40 words were counterbalanced across the inclusion–exclusion test and condition. Explicit memory was calculated as inclusion − exclusion. Implicit was computed as exclusion/[1−(inclusion − exclusion)] (28).
Study procedure.
At 0900, subjects were administered the RAT-S, followed by the analogies. Subjects returned at 1300, at which time they were randomly assigned to a nap or a quiet rest group. At 1700, subjects returned to execute the cued RAT-S, noncued RAT-S, recognition, inclusion and exclusion tests. A total of 52 subjects, REM (n = 18), NREM (n = 6), and rest (n = 28) participated in experiment 2.
Statistical Analyses.
Three tests of percentage improvement on RAT performance (repeat exposure, primed exposure, no exposure) were examined with a 2-tailed, 1-way ANOVA by using three levels of the variable Group (REM, NREM, quiet rest). Percentage improvement was calculated as (number of correct responses on afternoon RAT − number of correct responses on morning baseline RAT)/(number of correct responses on morning baseline RAT). Percentage improvement was computed for each individual and then averaged across subjects for each group. CI values were set at 95%, with a 2-tailed probability to examine whether percentage improvement on the afternoon RATs differed from 0 (i.e., no improvement). To examine the effect of specific sleep stages on creative problem solving, we categorized naps by the presence or absence of REM sleep as indicated by PSG. This segregated the data into naps with REM, NREM, and quiet rest groups. Performance for new and repeat exposure was calculated from experiments 1 and 2, respectively. There were no differences between experiments 1 and 2 for performance in primed-exposure RAT items [P = 0.26, 2-way ANOVA (Experiment × Group)], so percentage improvement for each subject was collapsed across experiments 1 and 2.
Acknowledgments.
We thank Drs. John T. Wixted, Harold Pashler, William A. Alaynick, David E. Huber, and Sean P. A. Drummond for their insightful comments. This work was supported by National Institutes of Health Grant K01 MH080992-01.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Dallob PI, Dominowski RL (April, 1993) Erroneous Solutions to Verbal Insight Problems: Effects of Highlighting Critical Material. Paper presented at the 73rd Annual Meeting of the Western Psychological Association, Portland, OR.
References
- 1.Mednick SA. The associative basis of the creative process. Psychol Rev. 1962;69:220–232. doi: 10.1037/h0048850. [DOI] [PubMed] [Google Scholar]
- 2.Wallas G. The Art of Thought. New York: Harcourt Brace; 1962. [Google Scholar]
- 3.Koestler A. The Act of Creation. New York: Dell; 1964. [Google Scholar]
- 4.Patrick C. Creative thought in artists. J Psychol. 1937;4:35–73. [Google Scholar]
- 5.Olton RM. Experimental studies of incubation: Searching for the elusive. J Creative Behav. 1979;13:9–22. [Google Scholar]
- 6.Olton RM, Johnson DM. Mechanisms of incubation in creative problem solving. Am J Psychol. 1976;89:617–630. [Google Scholar]
- 7.Vul E, Pashler H. Incubation benefits only after people have been misdirected. Mem Cognit. 2007;35:701–710. doi: 10.3758/bf03193308. [DOI] [PubMed] [Google Scholar]
- 8.Stickgold R, Walker M. To sleep, perchance to gain creative insight? Trends Cognit Sci. 2004;8:191–192. doi: 10.1016/j.tics.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 9.Gomez RL, Bootzin RR, Nadel L. Naps promote abstraction in language-learning infants. Psychol Sci. 2006;17:670–674. doi: 10.1111/j.1467-9280.2006.01764.x. [DOI] [PubMed] [Google Scholar]
- 10.Dumay N, Gaskell MG. Sleep-associated changes in the mental representation of spoken words. Psychol Sci. 2007;18:35–39. doi: 10.1111/j.1467-9280.2007.01845.x. [DOI] [PubMed] [Google Scholar]
- 11.Ellenbogen JM, et al. Human relational memory requires time and sleep. Proc Natl Acad Sci USA. 2007;104:7723–7728. doi: 10.1073/pnas.0700094104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wagner U, et al. Sleep inspires insight. Nature. 2004;427:352–355. doi: 10.1038/nature02223. [DOI] [PubMed] [Google Scholar]
- 13.Walker MP, et al. Cognitive flexibility across the sleep–wake cycle: REM-sleep enhancement of anagram problem solving. Brain Res Cognit Brain Res. 2002;14:317–324. doi: 10.1016/s0926-6410(02)00134-9. [DOI] [PubMed] [Google Scholar]
- 14.Stickgold R, et al. Sleep-induced changes in associative memory. J Cognit Neurosci. 1999;11:182–193. doi: 10.1162/089892999563319. [DOI] [PubMed] [Google Scholar]
- 15.Scrima L. Isolated REM sleep facilitates recall of complex associative information. Psychophysiology. 1982;19:252–259. doi: 10.1111/j.1469-8986.1982.tb02556.x. [DOI] [PubMed] [Google Scholar]
- 16.Yaniv I, Meyer DE. Activation and metacognition of inaccessible stored information: Potential bases for incubation effects in problem solving. J Exp Psychol Learn Mem Cognit. 1987;13:187–205. doi: 10.1037//0278-7393.13.2.187. [DOI] [PubMed] [Google Scholar]
- 17.Mednick S, Nakayama K, Stickgold R. Sleep-dependent learning: A nap is as good as a night. Nat Neurosci. 2003;6:697–698. doi: 10.1038/nn1078. [DOI] [PubMed] [Google Scholar]
- 18.Nishida M, Walker MP. Daytime naps, motor memory consolidation and regionally specific sleep spindles. PLoS ONE. 2007;2:e341. doi: 10.1371/journal.pone.0000341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nishida M, Pearsall J, Buckner RL, Walker MP. REM sleep, prefrontal theta, and the consolidation of human emotional memory. Cereb Cortex. 2008;19:1158–1166. doi: 10.1093/cercor/bhn155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Walker MP, et al. Practice with sleep makes perfect: Sleep-dependent motor skill learning. Neuron. 2002;35:205–211. doi: 10.1016/s0896-6273(02)00746-8. [DOI] [PubMed] [Google Scholar]
- 21.Mednick SC, Arman AC, Boynton GM. The time course and specificity of perceptual deterioration. Proc Natl Acad Sci USA. 2005;102:3881–3885. doi: 10.1073/pnas.0407866102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mednick SC, et al. Comparing the benefits of caffeine, naps and placebo on verbal, motor and perceptual memory. Behav Brain Res. 2008;193:79–86. doi: 10.1016/j.bbr.2008.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mednick SC, et al. The restorative effect of naps on perceptual deterioration. Nat Neurosci. 2002;5:677–681. doi: 10.1038/nn864. [DOI] [PubMed] [Google Scholar]
- 24.Monk TH, et al. Effects of afternoon “siesta” naps on sleep, alertness, performance, and circadian rhythms in the elderly. Sleep. 2001;24:680–687. doi: 10.1093/sleep/24.6.680. [DOI] [PubMed] [Google Scholar]
- 25.Ellenbogen JM, et al. Interfering with theories of sleep and memory: sleep, declarative memory, and associative interference. Curr Biol. 2006;16:1290–1294. doi: 10.1016/j.cub.2006.05.024. [DOI] [PubMed] [Google Scholar]
- 26.Gais S, Born J. Declarative memory consolidation: Mechanisms acting during human sleep. Learn Mem. 2004;11:679–685. doi: 10.1101/lm.80504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marshall L, Born J. The contribution of sleep to hippocampus-dependent memory consolidation. Trends Cognit Sci. 2007;11:442–450. doi: 10.1016/j.tics.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 28.Jacoby LL. A process dissociation framework: Separating automatic from intentional uses of memory. J Mem Language. 1991;30:513–541. [Google Scholar]
- 29.Wixted JT. The psychology and neuroscience of forgetting. Annu Rev Psychol. 2004;55:235–269. doi: 10.1146/annurev.psych.55.090902.141555. [DOI] [PubMed] [Google Scholar]
- 30.Gottselig JM, et al. Sleep and rest facilitate auditory learning. Neuroscience. 2004;127:557–561. doi: 10.1016/j.neuroscience.2004.05.053. [DOI] [PubMed] [Google Scholar]
- 31.Maquet P. The role of sleep in learning and memory. Science. 2001;294:1048–1052. doi: 10.1126/science.1062856. [DOI] [PubMed] [Google Scholar]
- 32.Sejnowski TJ, Destexhe A. Why do we sleep? Brain Res. 2000;886:208–223. doi: 10.1016/s0006-8993(00)03007-9. [DOI] [PubMed] [Google Scholar]
- 33.Stickgold R. Sleep-dependent memory consolidation. Nature. 2005;437:1272–1278. doi: 10.1038/nature04286. [DOI] [PubMed] [Google Scholar]
- 34.Walker MP, Stickgold R. Sleep, memory, and plasticity. Annu Rev Psychol. 2006;57:139–166. doi: 10.1146/annurev.psych.56.091103.070307. [DOI] [PubMed] [Google Scholar]
- 35.Tucker MA, et al. A daytime nap containing solely non-REM sleep enhances declarative but not procedural memory. Neurobiol Learn Mem. 2006;86:241–247. doi: 10.1016/j.nlm.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 36.Dougal S, Schooler JW. Discovery misattribution: When solving is confused with remembering. J Exp Psychol Gen. 2007;136:577–592. doi: 10.1037/0096-3445.136.4.577. [DOI] [PubMed] [Google Scholar]
- 37.Hasselmo ME. Neuromodulation: Acetylcholine and memory consolidation. Trends Cognit Sci. 1999;3:351–359. doi: 10.1016/s1364-6613(99)01365-0. [DOI] [PubMed] [Google Scholar]
- 38.Stickgold R, et al. Replaying the game: hypnagogic images in normals and amnesics. Science. 2000;290:350–353. doi: 10.1126/science.290.5490.350. [DOI] [PubMed] [Google Scholar]
- 39.Schooler JW, Melcher J. The ineffability of insight. In: Smith SM, Ward TB, Finke RA, editors. The Creative Cognition Approach. Cambridge, MA: MIT Press; 1995. pp. 249–268. [Google Scholar]
- 40.Balota DA, et al. The English Lexicon Project. Behav Res Methods. 2007;39:445–459. doi: 10.3758/bf03193014. [DOI] [PubMed] [Google Scholar]