Abstract
The family of ionotropic glutamate receptors includes 2 subunits, delta1 and delta2, the physiological relevance of which remains poorly understood. Both are nonfunctional in heterologous expression systems, although the isolated, crystallized ligand binding domain (LBD) of delta2 is capable of binding D-serine. To investigate these seemingly contradictory observations we tested whether delta receptors can be ligand gated at all. We used a strategy that replaced the native LBD of delta2 by a proven glutamate-binding LBD. Test transplantations between α-amino-3-hydroxy-5-methylisoxazole propionate (AMPA) and kainate receptors (GluR1 and GluR6, respectively) showed that this approach can produce functional chimeras even if only one part of the bipartite LBD is swapped. Upon outfitting delta2 with the LBD of GluR6, the chimera formed glutamate-gated ion channels with low Ca2+ permeability and unique rectification properties. Ligand-induced conformational changes can thus gate delta2, suggesting that the LBD of this receptor works fundamentally differently from that of other ionotropic glutamate receptors.
Keywords: orphan receptor, AMPA receptor, kainate receptor, chimeric receptors, ligand binding domain
Excitatory neurotransmission in the vertebrate central nervous system is mainly mediated by ionotropic glutamate receptors (iGluRs). Molecular cloning identified 18 mammalian iGluR subunits, of which only 16 sort into the traditional pharmacological subfamilies of (AMPA), kainate (KA), and N-methyl-D-aspartate (NMDA) receptors (1). The 2 remaining subunits have escaped electrophysiological characterization: because they are nonresponsive to glutamate (Glu) in heterologous expression systems, they were termed “orphan” receptors, “glutamate-like” receptors, “nonionotropic” receptors, or, most commonly, delta receptors (2).
Mice deficient in the delta2 subunit display ataxia. More precisely, parallel fiber synapses of cerebellar Purkinje cells, the site of specific and abundant expression of delta2, exhibit reduced long-term depression (LTD) and synapse formation with presynaptic granule cells (3–6). Recent work suggests that delta2 supports LTD induction via metabotropic signaling through its C terminus (7–10) and that synapse formation is supported via the subunit's N-terminal domain (11, 12). As previous domain transplantations indicated defunct delta receptor ion pores (13), the notion formed that the receptors might not be ion channels (9).
Evidence to the contrary, that delta receptors can form functional ion channels stems from lurcher mice: the delta2 gene of lurcher mice carries a point mutation that renders delta2 channels constitutively open (14). Electrophysiological analysis of this delta2-lurcher subunit in heterologous cells has so far been the only possible way to deduce information on delta2 channel properties (15–17). Likewise, the delta2-lurcher mutant served as a tool to characterize the action of the recently identified modulators D-serine and Ca2+. Although both D-serine and Ca2+ bind and modulate the LBD of delta2, both molecules fail to activate wild-type delta2 (18, 19).
Thus, it is still unclear whether delta receptors serve as ion channels in vivo and, moreover, whether they share the intrinsic capability of iGluRs to translate ligand binding into ion pore opening. Such gating capability, however, is a prerequisite for ion channel function and cannot be investigated using the delta2-lurcher mutant, given its grossly abnormal gating. Therefore, we sought to obtain a ligand-responsive delta2 receptor by exchanging its LBD for that of another iGluR subunit. First, we tested LBD transplantation between well-characterized AMPA and kainate receptor subunits and show that functional transplantation is possible. We then exchanged the LBD of delta2 for that of a kainate receptor, demonstrating that a functionally proven LBD is indeed capable of gating delta2. This allowed us to characterize rectification and Ca2+ permeability of the delta2 pore using the chimera as a tool that is not compromised by abnormal gating characteristics.
Results
LBD Transplantation Between GluR1 and GluR6.
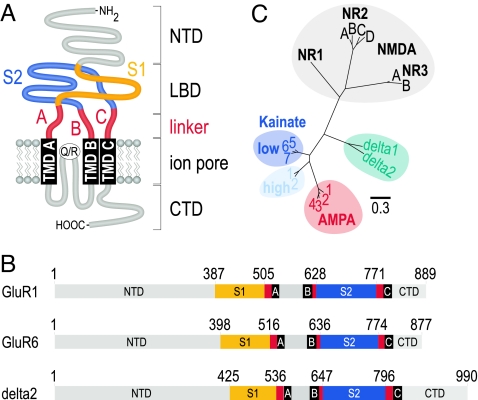
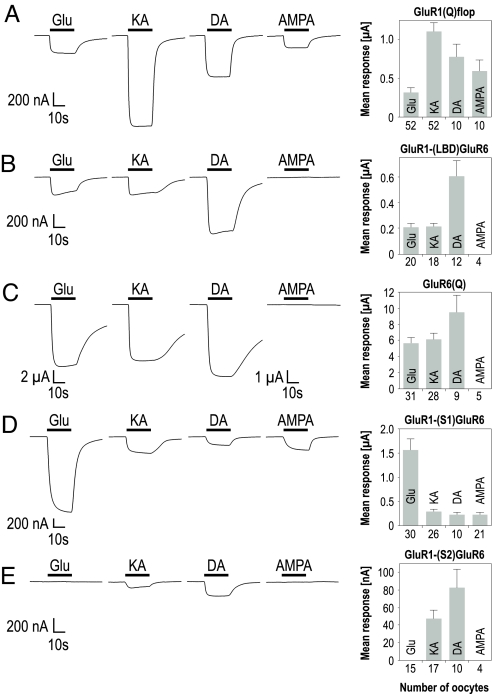
The LBD of iGluRs is formed from 2 discontinuous stretches of sequence, S1 and S2, which are separated by 2 short linkers leading to the transmembrane domains (TMDs) and the ion pore (Fig. 1A). We first set out to show that swapping of the LBD with the chosen domain borders (Fig. 1B) is possible between closely related subunits without loss of function. For that purpose, we performed transplantations between the AMPA receptor GluR1 and the KA receptor GluR6. We constructed the following chimeras: GluR1-(S1)GluR6, GluR1-(S2)GluR6, and GluR1-(LBD)GluR6. All were expressed in Xenopus oocytes and screened for agonist-induced current responses. Interestingly, all 3 chimeras yielded functional homomeric ion channels with distinct agonist profiles (Fig. 2, Table S1). In theory, exchanging the LBD of an AMPA receptor for that of a kainate receptor would be expected to transform the agonist profile of the resulting chimera from “AMPA-like” to “kainate-like.” To detect such a switch in the agonist profile, we recorded not only responses to Glu and KA but also to AMPA and domoate (DA). AMPA does not activate GluR6 receptors (20), and in contrast to GluR1, GluR6 is most sensitive to DA (21). In addition, KA receptor desensitization is potently inhibited by treatment with the lectin Concanavalin A (ConA), while the effect on GluR1 is only moderate (22). We find that after ConA treatment of oocytes expressing the GluR1-(LBD)GluR6 chimera, responses to Glu, KA, and DA but not AMPA were detectable (Fig. 2B). This agonist profile exactly resembles that of wild-type GluR6 receptors (Fig. 2C). Although current amplitudes of GluR1-(LBD)GluR6 were reduced to 5–6% of GluR6-mediated responses, they amounted to approximately 80% of GluR1-mediated responses, which is the ion pore-contributing subunit.
Fig. 1.
Topology, relatedness, and definition of iGluR LBDs. (A) Schematic topology of an iGluR showing the following domains: S1 (orange) and S2 (dark blue), which together form the LBD; the TMDs A, B, and C (black); the three linkers A, B, and C (red) that connect the LBD with the TMDs. The N-terminal domain (NTD), the pore region, and the C-terminal domain (CTD) are shown in gray. (B) Unrooted tree of the LBD sequences of mammalian iGluR subunits: the alignment included 284 aa each from 18 sequences, comprising the S1 and S2 domains. (C) Definition of domain borders for the 3 subunits GluR1, GluR6, and delta2. Shown is a linear representation of the 3 subunits drawn to scale. Numbering starts with the first amino acid of the mature protein. Signal peptide lengths were as follows: GluR1 (18 aa), GluR6 (31 aa), and delta2 (17 aa).
Fig. 2.
LBD swapping between GluR1 and GluR6 transplants the agonist profile. Representative current responses elicited by 300 μM glutamate (Glu), 150 μM kainate (KA), 10 μM domoate (DA), and 10 μM AMPA in oocytes expressing GluR1 wild type (A), GluR1-(LBD)GluR6 (B), GluR6 wild type (C), GluR1-(S1)GluR6 (D), and GluR1-(S2)GluR6 (E). The horizontal black bars indicate the duration of agonist application. To the Right, the respective mean amplitudes are shown, with each bar representing the mean agonist-induced responses (± SEM) of 4–52 oocytes. The responses of GluR1-(S2)GluR6, GluR1-(LBD)GluR6, and GluR6 were recorded after treating the oocytes for 10 min with 10 μM ConA.
The GluR1-(S1)GluR6 chimera was responsive to all 4 agonists before and after ConA treatment (Fig. 2D). Unlike for GluR1 wild type, glutamate-induced steady-state current responses were higher than KA-induced responses for this chimera. In the low time resolution of the Xenopus oocyte expression system, which cannot resolve peak currents of quickly desensitizing iGluRs, higher current amplitudes in response to the full agonist Glu as compared to the partial agonist KA are indicative of reduced desensitization. The swapping of S1 encompassed the replacement of leucine 479 in GluR1 by tyrosine, a mutation which prevents desensitization in AMPA receptors (23–25). Therefore, the agonist profile of GluR1 with the S1 domain of GluR6 suggested reduced desensitization. Unless GluR1-(S2)GluR6-expressing oocytes were treated with ConA, they were unresponsive to all 4 agonists. After ConA treatment, small responses could be recorded to KA or DA, but never to Glu or AMPA application (Fig. 2E). The unresponsiveness to AMPA in wild-type GluR6 receptors is governed by the amino acid at position 721 (20). The corresponding position in GluR1 is changed from threonine to aspartate in the process of exchanging the S2 domain for that of GluR6, and thus likely accounts for the insensitivity of the GluR1-(S2)GluR6 chimera to AMPA.
Because the LBD exchange might have altered agonist potencies, we next recorded dose–response curves (Fig. S1). We found that the mismatch introduced by transplanting just one part of the LBD reduced agonist potency—most profoundly, when S2 was derived from GluR6. Transplantation of the complete LBD, however, results in agonist potencies comparable to those of the LBD's wild-type parent subunit. Hence, LBD transplantation with GluR6 as the LBD donor produced functional chimeras with predictable changes in agonist profile.
The GluR6-Derived LBD Gates delta2 Channels.
What happens when the delta2 receptor is provided with a functional LBD? To find out, we constructed LBD chimeras with the acceptor delta2 and the donor GluR6: delta2-(S1)GluR6, delta2-(S2)GluR6, and delta2-(LBD)GluR6. To maximize our chances of obtaining functional chimeric receptors we adhered to the following rules. First, as for the GluR1/GluR6 exchange, we based the border selection for the transplanted S1 and S2 regions on available crystal structures (Fig. 1B). Second, we identified the degree of relatedness on the level of the LBD sequences (Fig. 1C). An amino acid distance analysis using only the S1 and S2 regions revealed that, in terms of amino acid sequence, the delta receptor LBDs are most closely related to the LBDs of low-affinity KA receptors. We chose the KA receptor GluR6, because GluR6 is AMPA-insensitive and thus provides an easy assay to verify its agonist profile.
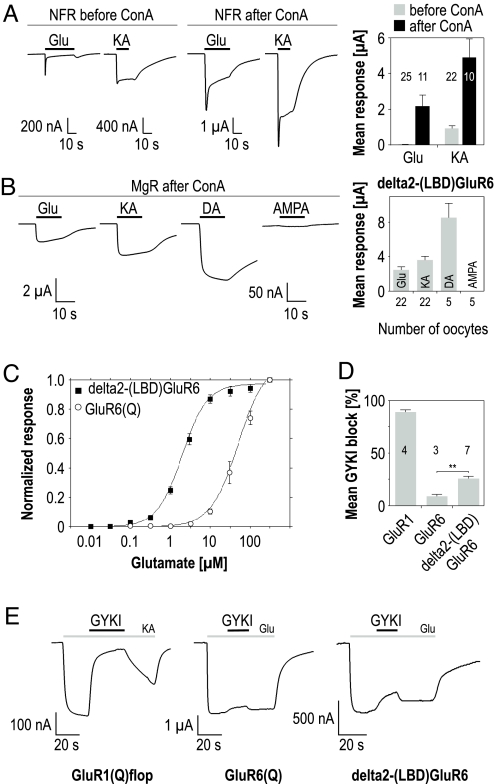
When expressed in Xenopus oocytes, no current responses of delta2-(S1)GluR6 or delta2-(S2)GluR6 could be detected by application of Glu or KA, neither before nor after ConA treatment (n = 6–7, data not shown). Intriguingly, however, the delta2-(LBD)GluR6 chimera formed perfectly functional ion channels. Large delta2-(LBD)GluR6-mediated current responses to Glu and KA were recorded in Ca2+-containing normal frog Ringer's solution (NFR) even before ConA treatment (Fig. 3A). Glu evoked initial peak currents that rapidly declined to a low steady-state response (12 ± 7 nA, n = 25), and KA evoked initial peak responses that declined to a steady-state response of 937 ± 132 nA (n = 22). ConA treatment increased both the peak responses and the steady-state responses to Glu (2181 ± 608 nA, n = 11), and KA (4912 ± 1044 nA, n = 10).
Fig. 3.
A GluR6-derived LBD gates delta2 channels. (A) Typical current responses recorded upon application of Glu and KA before and after ConA treatment in NFR. The respective mean current responses (numbers of oocytes are indicated in the diagram) are shown in a bar diagram to the Right. (B) Typical current responses elicited by 300 μM Glu, 150 μM KA, 10 μM DA, and 10 μM AMPA from oocytes expressing delta2-(LBD)GluR6. To the Right, the respective mean agonist-induced responses (± SEM) of 5–22 oocytes are shown. The responses were recorded in at least 2 independent experiments in Ca2+-free MgR after treating the oocytes with ConA. (C) Dose–response curves for delta2-(LBD)GluR6 (black squares) in comparison to GluR6 wild type (white circles) recorded for Glu; each curve averaged from 5 oocytes. (D) Mean block (± SEM) of agonist-induced current responses by 300 μM GYKI 52466, and respective current traces in (E). Note that GYKI does block delta2-(LBD)GluR6 channels to 26 ± 2%.
The initial peak response in NFR of delta2-(LBD)GluR6-expressing oocytes can reflect 2 phenomena. First, any Ca2+ influx through the delta2 chimera would activate Ca2+-gated chloride channels endogenous to the oocyte. Such outwardly directed chloride currents superimpose iGluR-mediated currents and are known to be responsible for the initial peak responses when recording from Ca2+-permeable channels. Second, a peak response can arise from desensitization that is slow enough to be recordable in the oocyte system (τ > 1–2 s). To test which of the 2 phenomena causes the initial peak currents, we recorded in Ca2+-free Magnesium Ringer's solution (MgR) to avoid any potential Ca2+ influx. Because we detected a smaller but persistent glutamate-evoked peak response of delta2-(LBD)GluR6-expressing oocytes even in Ca2+-free MgR (154 ± 26 nA, n = 7), we conclude that the remaining peak response reflects slow desensitization of the chimera. Consistent with this notion is that ConA treatment—presumably blocking desensitization of delta2-(LBD)GluR6—completely abolished the peak response in MgR (Fig. 3B).
Applying Glu, KA, DA, and AMPA after ConA treatment (Fig. 3B) confirmed that the LBD transplantation transferred the agonist profile of GluR6 to the otherwise nonresponsive delta2 subunit. Interestingly, Glu potency of delta2-(LBD)GluR6 (2.2 ± 0.2 μM, n = 5) was increased roughly 25-fold when compared to GluR6 (Fig. 3C). This is a striking example that agonist potency can be decisively influenced by factors outside the LBD of iGluRs.
The Glutamate-Gated delta2 Chimera Shows Distinct Properties.
The functional delta2-(LBD)GluR6 chimera provided us with the desired tool to examine agonist-evoked currents carried by the delta2 pore. Compared to previous work with the delta2-lurcher mutant (15, 17), the delta2 LBD chimera generated here greatly facilitated the characterization of those delta2 properties that are not determined by the LBD. Thus, we used it to examine noncompetitive antagonism by GYKI 52466 (GYKI) and 1-naphthylacetyl spermine (NASP), and rectification and Ca2+ permeability.
GYKI specifically inhibits AMPA receptors by binding to the highly subtype-specific linker region of iGluRs (26). Because this linker region is delta2-derived in the LBD chimera, we used it to test whether GYKI does act on delta2 (Fig. 3 D and E). In our hands, 300 μM GYKI blocked KA-induced responses of GluR1 by 88 ± 2% (n = 4). By contrast, GluR6-mediated currents were only reduced by 9 ± 2% (n = 3). To our surprise, 300 μM GYKI blocked glutamate-induced currents of delta2-(LBD)GluR6 by 26 ± 2% (n = 7). Unfortunately, higher concentrations of GYKI lowered the pH, which likely inhibits delta2-(LBD)GluR6 currents as delta2-lurcher was reported to be inhibited by protons (27). Hence, we used the concentration range below 300 μM to determine IC50 values for GYKI. Curve fits predict an IC50 value of 894 ± 187 μM (n = 7) for delta2-(LBD)GluR6, whereas GYKI completely blocks GluR1-mediated currents with an IC50 of 56 ± 4 μM (n = 3). The GYKI effect on the chimera is considerably smaller than for AMPA receptors, but significantly different from the GYKI-insensitive KA receptors.
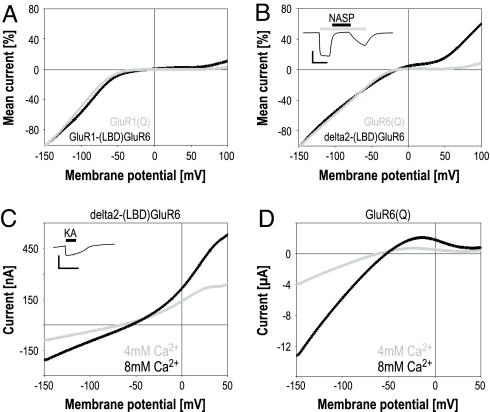
To examine rectification, we next recorded current–voltage relationships. As a control, we first compared GluR1-(LBD)GluR6 to GluR1(Q) (Fig. 4A). The pore region of this LBD chimera is GluR1-derived and hence identical to the one of GluR1(Q). As can be expected from this fact, both current–voltage relationships were inwardly rectifying and did not differ significantly. Wollmuth et al. (17) had suggested that delta2 channel properties are similar to AMPA receptors, a notion that they based on the analysis of the constitutively open delta2-lurcher channels. Furthermore, as the delta2 receptor features a Q at the position that corresponds to the Q/R editing site of AMPA and kainate receptors, the chimera might be expected to show similar rectification properties. Yet, the current–voltage relationships for delta2-(LBD)GluR6 differed substantially from typical inwardly rectifying relationships of AMPA and KA receptor Q variants (Fig. 4B). Agonist-activated delta2-(LBD)GluR6 clearly shows outward current at positive membrane potentials.
Fig. 4.
Ca2+ permeability and rectification of delta2 channels. (A) Current–voltage relationships of wild-type GluR1 and GluR1-(LBD)GluR6 in response to 150 μM KA. To allow direct comparison of the current–voltage relationships, the maximal inward current at −150 mV was set to 100%. The depicted relations represent the mean of 4–6 oocytes. Note that the transplantation of the GluR6 LBD has no significant influence on the current–voltage relationship of GluR1 channels. (B) Same as in A, but for GluR6 wild type in comparison to delta2-(LBD)GluR6, in response to 150 μM KA. Note that the delta2 channels feature a biphasic current–voltage relationship with less pronounced rectification and significantly higher outward current at positive membrane potentials. Traces were normalized for comparison as in A and represent the mean of 4–5 oocytes. Despite the reduced rectification, currents recorded at −70 mV are readily and completely blocked by extracellulary applied NASP (10 μM) (Insert; scale bar: 400 nA/20 s) (C+D) Ca2+ permeability of delta2-(LBD)GluR6 chimeras was estimated by recording current–voltage relationships in 4 mM CaR and 8 mM CaR in response to 150 μM KA. The Ca2+-to-monovalent cation permeability ratio was determined from the resulting reversal potentials. For comparison, the respective current–voltage relationships are shown for GluR6 wild type (D) and delta2-(LBD)GluR6 (C). The Insert in C shows a typical current trace of delta2-(LBD)GluR6 recorded in 8 mM CaR at −70 mV in response to 150 μM KA. (Scale bar: 50 nA/20 s.)
In addition to the rectification properties, the susceptibility to open channel blockers such as NASP is dependent on the amino acid at the Q/R editing site in AMPA and KA receptors. Because intracellular spermines cause inward rectification by blocking the Q variant pores at positive membrane potentials from the intracellular side, both NASP block and inward rectification are thought to be causally dependent on the editing status of the receptor. As we observed clear outward currents at positive membrane potentials for delta2-(LBD)GluR6, the delta2 pore's affinity for intracellular spermines appears to be lower. Thus, we next examined the chimera's sensitivity to extracellularly applied NASP. In agreement with all previous reports that analyzed delta2-lurcher receptors, delta2-(LBD)GluR6 was susceptible to extracellularly applied NASP (Fig. 4B, Insert). KA-induced currents after ConA treatment were blocked by 93 ± 6% (n = 6) using 10 μM NASP.
We next used delta2-(LBD)GluR6 to estimate the Ca2+-to-monovalent cation permeability ratio. For all permeability measurements, oocytes were preinjected with EGTA to prevent the activation of endogenous Ca2+-gated chloride channels by Ca2+ influx. The KA-evoked responses of delta2-(LBD)GluR6 in 8 mM Ca2+ Ringer's solution (CaR), in which the sole permeable extracellular cation was Ca2+, represent qualitative proof that the delta2 channel is Ca2+-permeable (Fig. 4C, Insert). To quantitatively determine the permeability ratio of Ca2+-to-monovalent cations of delta2 pores, reversal potentials were determined under varying concentrations of extracellular Ca2+. For comparison, the respective current–voltage relationships were also recorded for GluR6(Q) and GluR1(Q). Although for GluR1 and GluR6 current–voltage relationships in CaR were strongly inwardly rectifying, relationships of delta2-(LBD)GluR6 in CaR were considerably different in showing no rectification and large outward current components (Fig. 4 C vs. D, GluR1 data not shown).
Changing concentration of an ion species only shifts the reversal potential if the channel that mediates the recorded current is permeable to that particular ion. For all 3 examined receptors, a shift in the reversal potential to more positive values was observed upon doubling the extracellular Ca2+ concentration (Table S2). From the measured reversal potentials, Ca2+-to-monovalent cation permeability ratios were calculated as described before (28, 29). In agreement with previously reported results, the value we obtained for the AMPA receptor Q variant, 3.1, was higher than the result for the KA receptor (1.3). For delta2-(LBD)GluR6, we measured a permeability ratio of 0.87, indicating that the chimera is less Ca2+-permeable than AMPA or KA receptors.
The delta2-Derived LBD Does Not Convert GluR6 Channels to Low-Affinity D-Serine Receptors.
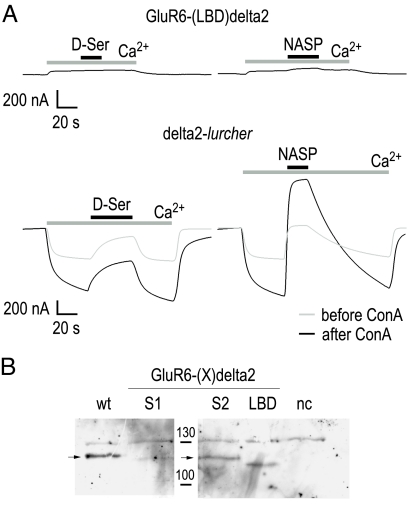
Because the delta2 pore can be gated by the LBD from GluR6, we asked whether the LBD of delta2 is capable of gating a GluR6 channel. For that purpose, we constructed the reverse chimera GluR6-(LBD)delta2 and examined its functionality in oocytes. We screened for current responses to D-serine (1 mM) and for constitutive activity using NASP (10 μM). Because recent reports indicate a role for Ca2+ in the regulation of the delta2 LBD (19), we also assessed the effect of switching from Ca2+-free to Ca2+-containing NFR. We used the delta2-lurcher mutant as a positive control, as wild-type delta2 receptors are nonresponsive (Fig. 5A).
Fig. 5.
The delta2 LBD does not render GluR6 channels responsive to D-serine. (A) The chimera GluR6-(LBD)delta2 is not responsive to 1 mM D-serine, before or after treatment with ConA. The delta2-lurcher point mutant, delta2-(A637T), was used as positive control and responded as reported (18) to both D-serine (1 mM) and NASP (10 μM) applications. (B) Western blot analysis of surface protein fractions (15–20 oocytes) was performed to check for membrane protein expression of the indicated nonfunctional LBD chimeras. The size of the marker bands is given in kDa. Note that all chimeras are expressed; wt, wild-type GluR6(Q); nc, uninjected oocytes. The slight reduction in molecular weight for S1, S2, and LBD constructs compared to wild-type GluR6 reflects the deletion of glycosylation sites (one each in S1 and S2) upon domain transplantation.
We did not observe any currents under any of the conditions examined. Applying neither Ca2+ nor D-serine had any effect on GluR6-(LBD)delta2. Likewise, the test for constitutive activity came up negative, as channel blockers did not alter holding currents (Fig. 5A). Treatment of the GluR6-(LBD)delta2-expressing oocytes with ConA did not change these results. This lack of responsiveness was not the result of a lack of surface expression of the chimeric receptor protein, which we controlled for by Western blot analysis (Fig. 5B). In the same experiment, applying Ca2+ increased spontaneous activity of delta2-lurcher channels, although application of D-serine or NASP reduced spontaneous currents (Fig. 5A). Interestingly, ConA treatment had a mildly potentiating effect on delta2-lurcher-mediated currents. This latter finding fits our observation that delta2-(LBD)GluR6-mediated currents can also be potentiated by ConA treatment and suggests that wild-type delta2 channels are susceptible to ConA modulation.
Discussion
The common membrane topology and modular design of iGluR subunits originally inspired the domain transplantation technique. It has been successfully applied to all major receptor domains (30–34). Here, we extend this approach to the LBD of the nonfunctional delta2 subunit, providing evidence that delta2 subunits can form functional ion channels that are able to sense the conformational status of an integral LBD, i.e., that can be gated by ligands. Although passive ion flux has repeatedly been shown using delta2-lurcher channels (14, 15, 17), “normal” agonist-induced gating behavior has never been demonstrated before for any of the 2 delta subunits.
One caveat of the domain transplantation technique is that LBD transplantation might produce an artificial receptor the characteristics of which are unique to the chimera and not a reflection of the functional properties of the domain-contributing receptors. To better gauge the impact of LBD exchange on gating, we therefore performed control transplantations between GluR1 and GluR6. The GluR1 receptor that carried the complete LBD of GluR6 not only formed functional receptors in Xenopus oocytes, but also showed a “GluR6-like” agonist profile without grossly altered agonist potencies. At the same time, channel properties of the LBD-receiving GluR1 subunit, such as the degree of inward rectification, were not significantly different between chimera and GluR1 wild type. Interestingly, preservation of function was possible even when only S1 or only S2 was exchanged, but the resulting chimeras showed strongly altered agonist potencies and desensitization behavior. The fact that function is preserved in the single S1 or S2 GluR1/GluR6 chimeras, and that these chimeras' properties can be explained using existing structural models for wild-type receptors (see Results), argues against a gross distortion of structure.
Presuming that overall structure and principal gating mechanisms remain essentially intact upon LBD transplantation, we have shown that glutamate-induced domain closure of the GluR6 LBD in a delta2-(LBD)GluR6 chimera translates into opening of the attached delta2 channel. Although the delta2 LBD was recently crystallized in complex with D-serine, and D-serine induced closure of the 2 lobes of the LBD in the crystal structure, D-serine did not elicit a response from wild-type delta2 channels (18). In view of our finding that application of D-serine to the delta2 LBD was incapable of gating an attached GluR6 channel, the main functional difference between delta subunits and other iGluRs seems to reside within the LBD. Structural features unique to delta2 might be present within its LBD that prevent the successful triggering of current responses in heterologous systems. By replacing the delta2 LBD by that of GluR6, this inhibitory feature might be removed and thus successful gating is enabled; conversely, by replacing the GluR6 LBD for that of delta2, we introduce that inhibitory feature and thus block successful gating. iGluRs are believed to alternate between 3 main states (open, resting, and desensitized) via an unstable transition state in which the channel is closed but the agonist bound and locked within the closed binding cavity (35, 36). Our data suggest that delta2 receptors are capable of that part of the normal gating process that proceeds from the transition state toward channel opening, although LBD-intrinsic interactions that govern the step from the transition state toward the desensitized (35) or resting state (37) seem to work differently in delta2.
We reexamined delta2 channel properties, exploiting the much more normal gating properties our chimera has in comparison to the spontaneously gating and thus constitutively open delta2-lurcher channels that others had investigated previously (15, 17).
We find that the chimera can be partially blocked by GYKI—an AMPA receptor-specific noncompetitive antagonist. The identified binding site for GYKI in linker A and linker C is completely different in delta2, offering no obvious explanation for the chimera's GYKI sensitivity. GYKI is known to bind to different gating states with different affinity, exhibiting the lowest affinity toward the open state of AMPA receptors (26). The GYKI sensitivity of the delta2 chimera might therefore reflect a similarity in the overall gating state between the chimera and AMPA receptors on the level of the linkers.
The rectification properties and Ca2+ permeability ratios we determined are in reasonable agreement with values published for delta2-lurcher (15, 17). Also, the current–voltage relationship of delta2 shows a shape similar to that obtained for delta2-lurcher. However, in contrast to Wollmuth et al. (17), we conclude that rectification at positive membrane potentials is significantly less for delta2 than for GluR1(Q) variants, revealing a unique and distinguishing property of delta2 rather than the postulated similarity to AMPA receptors.
Furthermore, Ca2+ permeability ratios indicate that delta2 constitutes the least Ca2+-permeable iGluR channel with a glutamine at the Q/R site. The value for the Ca2+-to-monovalent cation permeability we obtained (0.86) falls into the same range as the one derived for delta2-lurcher in HEK293 cells (0.44) (15). This finding supports the notion that Ca2+ influx through delta2 channels may not be the decisive signal for the induction of LTD at parallel fiber synapses (10). Thus, the physiological role of Ca2+ influx via delta2 channels is probably not comparable to that of Ca2+-permeable AMPA receptors.
Our findings favor the notion that the LBD of delta2 receptors does communicate a signal to the channel pore. Given that the pores of delta receptors are conserved at decisive positions, and given our results that the ion channel is apparently responsive to signals from the LBD, it seems more than peculiar why it has not been possible to show ion channel function for delta2 wild-type channels. Every part of the delta2 subunit that is not directly involved in ion channel function, chiefly the N-terminal domain and the C terminus, has been implicated in supporting in vivo functions that had been identified from the phenotype of germ line delta2 knockout mice. Thus, the N terminus was reported to support synapse formation (11, 12) while the C terminus was implicated in LTD induction (38). As basal synaptic transmission appears to be normal in delta2 knockout mice, no physiological evidence could yet be obtained that delta2 serves as an ion channel (39). However, such a function might have gone undetected in the delta2 knockout mouse because of compensatory effects. Because we find that a GluR6 LBD can successfully engage the delta2 pore, it might be worthwhile to investigate basal synaptic transmission in conditional delta2 knockout mice.
Given the discovery of TARPs as auxiliary AMPA receptor subunits (40), and the recent reports on further new AMPA and KA receptor-associated proteins that alter channel function (41, 42), delta receptor function might be controlled by yet uncharacterized proteins. Such proteins might be required to make the delta receptors responsive to agonists, in essence rendering them switchable receptors that depend on a yet unidentified “on” signal to activate their ion channel function.
Our results suggest that the key difference to other iGluRs lies within the LBD of delta receptors. Future studies focusing on this part of the receptor might uncover a unique mechanism necessary to trigger gating.
Methods
Molecular Biology.
We defined the respective native S1 and S2 sequences as follows (Fig. 1C): S1 domain (118 aa in GluR1, Q387–Q505; 118 aa in GluR6, S398–N516; 111 aa in delta2, V425–A536); S2 domain (143 aa in GluR1, P628–G771; 138 aa in GluR6, P636–E774; 149 aa in delta2, S647–D796) (see Fig. 1C). The GluR1 flop splice variant was used throughout.
Heterologous Expression and Western Blotting.
Experiments were performed using standard procedures for Xenopus laevis oocytes as described before (31). For details, please refer to the specific information given in the supportive information (SI Text).
Supplementary Material
Acknowledgments.
We thank Björn Peters for expert oocyte preparation, and Kasper B. Hansen, Alexander Jackson, and Aaron Milstein for comments and critical reading of this manuscript. S.M.S. was supported by a fellowship of the International Graduate School of Neuroscience (IGSN), Bochum, Germany. C.S. was supported by the Ruhr University Research School, Bochum, Germany.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0900329106/DCSupplemental.
References
- 1.Hollmann M, Heinemann S. Cloned glutamate receptors. Annu Rev Neurosci. 1994;17:31–108. doi: 10.1146/annurev.ne.17.030194.000335. [DOI] [PubMed] [Google Scholar]
- 2.Schmid SM, Hollmann M. To gate or not to gate: Are the delta subunits in the glutamate receptor family functional ion channels? Mol Neurobiol. 2008;37:126–141. doi: 10.1007/s12035-008-8025-0. [DOI] [PubMed] [Google Scholar]
- 3.Hirano T, Kasono K, Araki K, Mishina M. Suppression of LTD in cultured Purkinje cells deficient in the glutamate receptor δ2 subunit. Neuroreport. 1995;6:524–526. doi: 10.1097/00001756-199502000-00029. [DOI] [PubMed] [Google Scholar]
- 4.Kashiwabuchi N, et al. Impairment of motor coordination, Purkinje cell synapse form, and cerebellar long-term depression in GluRδ2 mutant mice. Cell. 1995;81:245–252. doi: 10.1016/0092-8674(95)90334-8. [DOI] [PubMed] [Google Scholar]
- 5.Jeromin A, Huganir RL, Linden DJ. Suppression of the glutamate receptor δ2 subunit produces a specific impairment in cerebellar long-term depression. J Neurophysiol. 1996;76:3578–3583. doi: 10.1152/jn.1996.76.5.3578. [DOI] [PubMed] [Google Scholar]
- 6.Hirano T, Kasono K, Araki K, Shinozuka K, Mishina M. Involvement of the glutamate receptor δ2 subunit in the long-term depression of glutamate responsiveness in cultured rat Purkinje cells. Neurosci Lett. 1994;182:172–176. doi: 10.1016/0304-3940(94)90790-0. [DOI] [PubMed] [Google Scholar]
- 7.Hirai H, et al. Rescue of abnormal phenotypes of the δ2 glutamate receptor-null mice by mutant δ2 transgenes. EMBO Rep. 2005;6:90–95. doi: 10.1038/sj.embor.7400312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yuzaki M. Transgenic rescue for characterizing orphan receptors: A review of δ2 glutamate receptor. Transgenic Res. 2005;14:117–121. doi: 10.1007/s11248-005-2685-6. [DOI] [PubMed] [Google Scholar]
- 9.Kakegawa W, Kohda K, Yuzaki M. The δ2 ‘ionotropic’ glutamate receptor functions as a non-ionotropic receptor to control cerebellar synaptic plasticity. J Physiol. 2007;584:89–96. doi: 10.1113/jphysiol.2007.141291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kakegawa W, et al. Ca2+ permeability of the channel pore is not esssential for the δ2 glutamate receptor to regulate synaptic plasticity and motor coordination. J Physiol. 2007;579:729–735. doi: 10.1113/jphysiol.2006.127100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kuroyanagi T, Yokoyama M, Hirano T. Postsynaptic glutamate receptor delta family contributes to presynaptic terminal differentiation and establishment of synaptic transmission. Proc Natl Acad Sci USA. 2009;106:4912–4916. doi: 10.1073/pnas.0900892106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Uemura T, Mishina M. The amino-terminal domain of glutamate receptor delta2 triggers presynaptic differentiation. Biochem Biophys Res Commun. 2008;377:1315–1319. doi: 10.1016/j.bbrc.2008.10.170. [DOI] [PubMed] [Google Scholar]
- 13.Villmann C, Strutz N, Morth T, Hollmann M. Investigation by ion channel domain transplantation of rat glutamate receptor subunits, orphan receptors and putative NMDA receptor subunit. Eur J Neurosci. 1999;11:1765–1778. doi: 10.1046/j.1460-9568.1999.00594.x. [DOI] [PubMed] [Google Scholar]
- 14.Zuo J, et al. Neurodegeneration in Lurcher mice caused by mutation in delta2 glutamate receptor gene. Nature. 1997;388:769–773. doi: 10.1038/42009. [DOI] [PubMed] [Google Scholar]
- 15.Kohda K, Wang Y, Yuzaki M. Mutation of a glutamate receptor motif reveals its role in gating and δ2 receptor channel properties. Nat Neurosci. 2000;3:315–322. doi: 10.1038/73877. [DOI] [PubMed] [Google Scholar]
- 16.Williams K, Dattilo M, Sabado TN, Kashiwagi K, Igarashi K. Pharmacology of delta2 glutamate receptors: Effects of pentamidine and protons. J Pharmacol Exp Ther. 2003;305:740–748. doi: 10.1124/jpet.102.045799. [DOI] [PubMed] [Google Scholar]
- 17.Wollmuth LP, et al. The lurcher mutation identifies δ2 as an AMPA/kainate receptor-like channel that is potentiated by Ca2+ J Neurosci. 2000;20:5973–5980. doi: 10.1523/JNEUROSCI.20-16-05973.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Naur P, et al. Ionotropic glutamate-like receptor δ2 binds D-serine and glycine. Proc Natl Acad Sci USA. 2007;104:14116–14121. doi: 10.1073/pnas.0703718104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hansen KB, et al. Modulation of the dimer interface at ionotropic glutamate-like receptor delta2 by D-serine and extracellular calcium. J Neurosci. 2009;29:907–917. doi: 10.1523/JNEUROSCI.4081-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Swanson GT, Gereau R, IV, Green T, Heinemann SF. Identification of amino acid residues that control functional behavior in GluR5 and GluR6 kainate receptors. Neuron. 1997;19:913–926. doi: 10.1016/s0896-6273(00)80972-1. [DOI] [PubMed] [Google Scholar]
- 21.Sommer B, et al. A glutamate receptor channel with high affinity for domoate and kainate. EMBO J. 1992;11:1651–1656. doi: 10.1002/j.1460-2075.1992.tb05211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Everts I, Villmann C, Hollmann M. N-glycosylation is not a prerequisite for glutamate receptor function but is essential for lectin modulation. Mol Pharmacol. 1997;52:861–873. doi: 10.1124/mol.52.5.861. [DOI] [PubMed] [Google Scholar]
- 23.Stern-Bach Y, Russo S, Neuman M, Rosenmund C. A point mutation in the glutamate binding site blocks desensitization of AMPA receptors. Neuron. 1998;21:907–918. doi: 10.1016/s0896-6273(00)80605-4. [DOI] [PubMed] [Google Scholar]
- 24.Armstrong N, Gouaux E. Mechanism for activation and antagonism of an AMPA-sensitive glutamate receptor: Crystal structure of the GluR2 ligand binding core. Neuron. 2000;28:165–181. doi: 10.1016/s0896-6273(00)00094-5. [DOI] [PubMed] [Google Scholar]
- 25.Jin R, et al. Mechanism of positive allosteric modulators acting on AMPA receptors. J Neurosci. 2005;25:9027–9036. doi: 10.1523/JNEUROSCI.2567-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Balannik V, Menniti FS, Paternain AV, Lerma J, Stern-Bach Y. Molecular mechanism of AMPA receptor noncompetitive antagonism. Neuron. 2005;48:279–288. doi: 10.1016/j.neuron.2005.09.024. [DOI] [PubMed] [Google Scholar]
- 27.Williams K. Pharmacology of delta 2 glutamate receptors: Effects of pentamidine and protons. J Pharmacol Exp Ther. 2003;305:740–748. doi: 10.1124/jpet.102.045799. [DOI] [PubMed] [Google Scholar]
- 28.Egebjerg J, Heinemann SF. Ca2+ permeability of unedited and edited versions of the kainate selective glutamate receptor GluR6. Proc Natl Acad Sci USA. 1993;90:755–759. doi: 10.1073/pnas.90.2.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Soto F, et al. P2X4: An ATP-activated ionotropic receptor cloned from rat brain. Proc Natl Acad Sci USA. 1996;93:3684–3688. doi: 10.1073/pnas.93.8.3684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morimoto-Tomita M, et al. Autoinactivation of neuronal AMPA receptors via glutamate-regulated TARP interaction. Neuron. 2009;61:101–102. doi: 10.1016/j.neuron.2008.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Villmann C, Strutz N, Morth T, Hollmann M. Investigation by ion channel domain transplantation of rat glutamate receptor subunits, orphan receptors and a putative NMDA receptor subunit. Eur J Neurosci. 1999;11:1765–1778. doi: 10.1046/j.1460-9568.1999.00594.x. [DOI] [PubMed] [Google Scholar]
- 32.Schmid SM, Korber C, Herrmann S, Werner M, Hollmann M. A domain linking the AMPA receptor agonist binding site to the ion pore controls gating and causes lurcher properties when mutated. J Neurosci. 2007;27:12230–12241. doi: 10.1523/JNEUROSCI.3175-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leuschner WD, Hoch W. Subtype-specific assembly of alpha-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid receptor subunits is mediated by their n-terminal domains. J Biol Chem. 1999;274:16907–16916. doi: 10.1074/jbc.274.24.16907. [DOI] [PubMed] [Google Scholar]
- 34.Panicker S, Brown K, Nicoll RA. Synaptic AMPA receptor subunit trafficking is independent of the C terminus in the GluR2-lacking mouse. Proc Natl Acad Sci USA. 2008;105:1032–1037. doi: 10.1073/pnas.0711313105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sun Y, et al. Mechanism of glutamate receptor desensitization. Nature. 2002;417:245–253. doi: 10.1038/417245a. [DOI] [PubMed] [Google Scholar]
- 36.Armstrong N, Jasti J, Beich-Frandsen M, Gouaux E. Measurement of conformational changes accompanying desensitization in an ionotropic glutamate receptor. Cell. 2006;127:85–97. doi: 10.1016/j.cell.2006.08.037. [DOI] [PubMed] [Google Scholar]
- 37.Weston MC. Interdomain interactions in AMPA and kainate receptors regulate affinity for glutamate. J Neurosci. 2006;26:7650–7658. doi: 10.1523/JNEUROSCI.1519-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kakegawa W, Kohda K, Yuzaki M. The 2 ‘ionotropic’ glutamate receptor functions as a non-ionotropic receptor to control cerebellar synaptic plasticity. J Physiol. 2007;584:89–96. doi: 10.1113/jphysiol.2007.141291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kashiwabuchi N, et al. Impairment of motor coordination, Purkinje cell synapse formation, and cerebellar long-term depression in GluR delta 2 mutant mice. Cell. 1995;81:245–252. doi: 10.1016/0092-8674(95)90334-8. [DOI] [PubMed] [Google Scholar]
- 40.Milstein AD, Nicoll RA. Regulation of AMPA receptor gating and pharmacology by TARP auxiliary subunits. Trends Pharmacol Sci. 2008;29:333–339. doi: 10.1016/j.tips.2008.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schwenk J, et al. Functional proteomics identify cornichon proteins as auxiliary subunits of AMPA receptors. Science. 2009;323:1313–1319. doi: 10.1126/science.1167852. [DOI] [PubMed] [Google Scholar]
- 42.Zhang W, et al. A transmembrane accessory subunit that modulates kainate-type glutamate receptors. Neuron. 2009;61:385–396. doi: 10.1016/j.neuron.2008.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.