Abstract
Serotonin synthesis in mammals is initiated by 2 distinct tryptophan hydroxylases (TPH), TPH1 and TPH2. By genetically ablating TPH2, we created mice (Tph2−/−) that lack serotonin in the central nervous system. Surprisingly, these mice can be born and survive until adulthood. However, depletion of serotonin signaling in the brain leads to growth retardation and 50% lethality in the first 4 weeks of postnatal life. Telemetric monitoring revealed more extended daytime sleep, suppressed respiration, altered body temperature control, and decreased blood pressure (BP) and heart rate (HR) during nighttime in Tph2−/− mice. Moreover, Tph2−/− females, despite being fertile and producing milk, exhibit impaired maternal care leading to poor survival of their pups. These data confirm that the majority of central serotonin is generated by TPH2. TPH2-derived serotonin is involved in the regulation of behavior and autonomic pathways but is not essential for adult life.
Keywords: growth retardation, maternal care, respiration, serotonin, sleep
Serotonin (5-hydroxytryptamine, 5-HT) is an extracellular signaling molecule with a multitude of functions in the central nervous system (CNS) and in the periphery. 5-HT effects are conveyed by at least 13 receptors classified in 7 families, 5-HT1 to 5-HT7. Serotonin synthesis from tryptophan is initiated by the enzyme tryptophan hydroxylase (TPH) generating 5-hydroxytryptophan followed by aromatic amino acid decarboxylase (AADC), which produces 5-HT. We have recently discovered that 2 TPH isoenzymes exist in all vertebrates, TPH1 and TPH2, encoded by 2 distinct genes (1, 2). Tph1 is mainly expressed in the gut, generating serotonin that is distributed into the whole body by thrombocytes, and in the pineal gland, where the resulting 5-HT is metabolized to melatonin. The Tph1-deficient mice generated by us (2) and others (3, 4) revealed that 95% of peripheral 5-HT is produced by TPH1. They also revealed that 5-HT in platelets and other peripheral cells is involved in such diverse processes as thrombosis (5), liver regeneration (6), hepatitis (7), colon cancer (8), mammary gland plasticity (9), pulmonary hypertension (10), and bone formation (11). TPH2, on the other hand, is responsible for the synthesis of serotonin in the raphé nuclei of the brainstem, from where all central serotonergic projections originate (12). Accordingly, polymorphisms and functional mutations in the human and mouse genes for this enzyme have been linked to neurological and behavioral abnormalities (13–16).
In this study, we generated mice lacking TPH2 by gene targeting and analyzed the physiological consequences resulting from a lack of brain serotonin.
Results and Discussion
Generation and Basic Characteristics of Tph2-Deficient Mice.
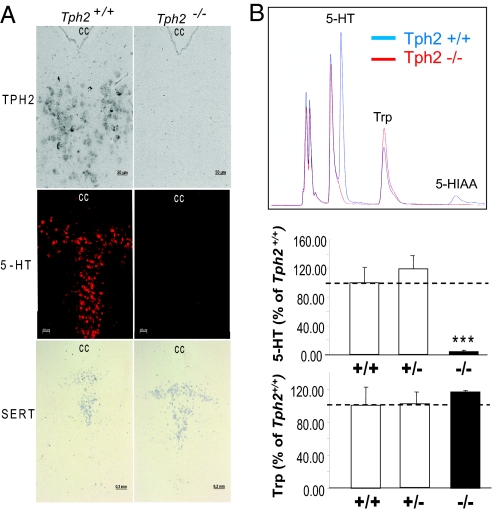
Tph2-deficient (Tph2−/−) mice were generated by deleting the coding sequence in exons 1 and 2 (supporting information (SI) Fig. S1 A and B). In the resulting Tph2−/− mice, no Tph2 mRNA could be found by RT-PCR (Fig. S1C) and in situ hybridization in the brain (Fig. 1A). Immunohistochemistry did not detect serotonin in sections of the raphé nuclei (Fig. 1A). By high-performance liquid chromatography (HPLC) quantification we measured <1–4% of residual levels of the monoamine compared to control animals and no detectable amounts of its metabolite 5-HIAA in the whole brain, in raphé nuclei, and in other brain areas such a striatum, hippocampus, cortex, and hypothalamus of Tph2−/− mice while the concentration of the precursor tryptophan was unchanged (Fig. 1B, Table 1, Table S1). TPH1 may be responsible for this minute residual 5-HT generation, because it is expressed in the brain at a level of about 4% compared to TPH2 (17). However, TPH1 is obviously not able to compensate for the loss of TPH2, confirming that TPH2 is the only relevant enzyme for 5-HT synthesis in the CNS. There was no difference compared to control mice in the brain 5-HT concentrations of heterozygous mice (Tph2+/−) (Fig. 1B, Table S1). Only 5-HIAA levels were slightly but significantly reduced in some brain regions (Table S1). These data show that 50% of a Tph2 gene dose is enough to maintain normal brain serotonin levels, partially the result of a decreased metabolism of the monoamine. Furthermore, there was no difference in serotonin levels in any other organ or in the blood of Tph2−/− mice, confirming that peripheral serotonin is not depending on TPH2 (Table S2). The bowel is an exception because expression of both isoforms has been described there, TPH1 in enterochromaffin cells (2) and TPH2 in enteric neurons (18). Because TPH2-derived serotonin is only a minor portion of total intestinal serotonin, the 5-HT concentration in the duodenum remains unchanged in Tph2−/− mice (Table S2).
Fig. 1.
Serotonin system in the brain of Tph2-deficient mice. (A) Detection of serotonin (Middle panel) by immunofluorescence and of Tph2 and serotonin transporter (SLC6A4, SERT) transcripts by in situ hybridization (Upper and Lower panels, respectively) in the dorsal raphé (DR) of Tph2−/− mice. CC, central canal. (B) Detection of serotonin (5-HT), its degradation product 5-hydroxyindoleacetic acid (5-HIAA), and the serotonin precursor tryptophan (Trp) in the DR by HPLC (representative HPLC-chromatogram, Upper panel and its quantification, Middle and Lower panels). ***, P < 0.001 Tph2−/− vs. Tph2+/+ and Tph2+/−, Student's t test.
Table 1.
Monoamines and their metabolites, GABA, and glutamate levels in the brains of Tph2-deficient mice
| 5-HT (pg) | 5-HIAA (pg) | NA (pg) | Glutamate (pmol) | GABA (pmol) | DA (pg) | DOPAC (pg) | HVA (pg) | |
|---|---|---|---|---|---|---|---|---|
| Control | 710.1 ± 56.9 | 147.4 ± 13.2 | 419.6 ± 17.2 | 10.2 ± 0.5 | 2.93 ± 0.09 | 1116.4 ± 32.8 | 119.6 ± 4.2 | 147.3 ± 12.3 |
| Tph2−/− | 28.4 ± 3.6*** | ND | 395.7 ± 23.9 | 10.1 ± 0.6 | 2.80 ± 0.04 | 1139.4 ± 27.6 | 121.1 ± 12.7 | 156.4 ± 10.5 |
Serotonin (5-HT), 5-hydroxyindoleacetic acid (5-HIAA), norephinephrine (NA), Glutamate, γ-aminobutyric acid (GABA), dopamine (DA), dihydroxyphenylacetic acid (DOPAC), and homovanillic acid (HVA) levels were measured in whole brain lysates per milligram of wet tissue of Tph2−/− (n = 4) and control (n = 6) mice (mixed background). Data are presented as mean ± SEM; ND, not detectable. ***, P < 0.001 vs. control group, 1-way ANOVA, followed by Tukey's post hoc test.
Serotonergic neurons were still present at their normal location and pattern in the raphé nuclei of Tph2−/− mice as detected by in situ hybridization for serotonin transporter (SLC6A4) mRNA (Fig. 1A). Consistent with the findings in other Tph2−/− mice (4, 19) and Pet-1-deficient mice, which lack most serotonergic neurons (20), we did not detect major alterations in brain structures using magnetic resonance imaging (Movies S1 and S2) but detailed morphological studies are still pending. Thus, neither the development of serotonergic neurons nor whole brain morphogenesis depends on TPH2-derived serotonin. However, we cannot exclude that during embryogenesis maternal or TPH1-derived circulating 5-HT reaches the brain because of the lack of a blood–brain barrier at early stages of development.
We next studied the effects of the near complete lack of serotonin on other neurotransmitters such as dopamine, γ-aminobutyric acid, norepinephrine, and glutamate in the brain of adult Tph2−/− mice. To our surprise the level of no other transmitter was altered in comparison to control animals (Table 1). Moreover, gene expression profiling in FVB/N-F4 Tph2-deficient mice using Affymetrix Mouse genome arrays also did not reveal any marked change (>1.8-fold) in mRNA abundances in the whole brains of Tph2−/− animals (data not shown). Thus, we could not detect any compensatory mechanisms in the CNS of mice drastically deficient in serotonin. Nevertheless, these studies need to be repeated with defined brain structures to detect possible local alterations in gene expression or neurotransmitter levels.
Survival and Growth.
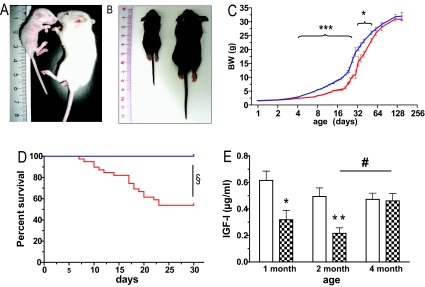
Tph2−/− mice were born at normal Mendelian ratios and at the day of birth were undistinguishable from control littermates. However, already 3 days later Tph2−/− mice were visibly smaller, had softer skin, and appeared weaker in comparison to control littermates and this difference was aggravated in the following 3 weeks (Fig. 2A, B, and C). Moreover, Tph2−/− mice on a mixed genetic background showed considerable lethality (27%) in the first 4 weeks of life. Backcross to FVB/N background improved the survival rate (88.6%), whereas after backcross to the C57BL/6 strain the lethality got more pronounced and about half of the Tph2−/− pups were lost (Fig. 2D, 52.6% survival). Because of the growth retardation it was not possible to wean Tph2−/− mice at 3 weeks of age but only 2 weeks later. After weaning they started a catch-up growth and reached near normal size at 4 months of age (Fig. 2C), when also no increased mortality could be observed anymore at least until 1.5 years of age on the mixed genetic background.
Fig. 2.
Growth retardation and postnatal lethality in Tph2-deficient mice. Representative photographs of 15-day-old FVB/N-Tph2-deficient (A) and 21-day-old C57BL/6-Tph2-deficient (B) mice. On both panels: Left, Tph2−/− mouse; Right, Tph2+/+ mouse from the same litter. Body weight (BW) development (mixed genetic background) (C) and survival (C57BL/6 genetic background) (D) of Tph2-deficient animals. Red line, Tph2−/−; blue line, control mice. (E) IGF1 concentration in the serum of the Tph2-deficient animals (FVB/N-F4 genetic background) at different ages. Filled bars, Tph2−/−; open bars, control mice. *, P < 0.05; **, P < 0.01; and ***, P < 0.001 (Student's t test); and §, P < 0.0001 (Logrank test) Tph2−/− vs. age-matched control mice; #, P < 0.05 (Student's t test) 4-month-old vs. 2-month-old Tph2−/− mice.
We do not yet know the reasons for the lack of weight gain in early postnatal life. Newborn Tph2−/− mice drink milk and show normal digestion. All trials to quantify food intake or to force feed failed, because any handling of the pups worsened the phenotype. Pups raised in litters with mixed genotypes showed the same phenotype as pups raised in pure Tph2−/− litters excluding competition with control littermates as cause for the effect. The growth retardation also did not depend on the genotype of the mother because it appeared in pups born and raised by Tph2−/− and Tph2+/− dams. Tph2−/− pups vocalize equally as controls when separated from their mothers excluding a deficit in this behavior as cause of the growth retardation (Fig. S2). Serum levels of insulin-like growth factor I (IGF1) were markedly lower in Tph2−/− mice until 2 months of age but afterward reached the levels of control animals, when the animals had attained normal size (Fig. 2E). Although these reduced IGF1 concentrations are probably causing the postnatal growth retardation in Tph2−/− mice, they could easily be secondary to undernutrition (21). Thus, further studies are required to clarify whether behavioral or sensory deficits causing malnutrition or a primary impairment of the growth hormone/IGF1 axis by the lack of stimulatory serotonin actions (22) lead to the impaired thriving of these mice.
Fertility and Maternal Care.
The number of pups per litter was normal even when both parents lacked Tph2 (6.0 ± 1.5 in Tph2−/− vs. 5.9 ± 0.4 in controls; mixed genetic background). Thus, neither male nor female fertility was affected by the lack of brain serotonin. However, a high percentage of the dams did not take care of their pups. Of 10 litters born by Tph2−/− mothers bred with Tph2+/− fathers (FVB/N-F4 genetic background), only 4 survived for more than several days, compared to 8 litters of 9 born from couples of Tph2+/− mothers and Tph2−/− fathers. The same phenotype was observed when wild-type fathers were used for breeding and, thus, did not depend on the genotype of the offspring (Table 2). At the day of delivery (day 0) Tph2−/− mothers showed normal milk production, were feeding pups (the stomach of newborns was visibly filled with milk a few hours after delivery), organizing and keeping a nest. However, in the following days pups of Tph2−/− were neglected and even often cannibalized by the mother in an aggressive manner. Consequently, most of the pups of Tph2−/− mothers were dead on day 2 or 3 after birth. The same results were obtained when we performed cross-fostering experiments. Tph2−/− dams ate 50% of the pups (30% of whole litters) born by Tph2+/+ mothers, while Tph2+/+ mothers only cannibalized 5.6% of the pups (zero whole litter) from Tph2−/− mothers. This alteration in maternal instincts in Tph2−/− mothers was confirmed by the pups retrieval test: on day 1, 8 of 9 Tph2−/− dams were not able to collect their scattered pups within 30 min, whereas it took on average 3.9 ± 0.7 min for the control dams (n = 6) to achieve this (Movies S3 and S4). However, there was no significant difference between females of each genotype in the time it took to find a hidden cookie (42.3 ± 15.5 sec in Tph2−/− vs. 29.7 ± 9.3 sec in control mothers), indicating that maternal neglect is not caused by disturbed olfaction in Tph2−/− mice. These data confirm a recent study showing that mice lacking most serotonergic neurons exhibit a drastic impairment in maternal care (23). It has been shown that maternal neglect can go along with aggressiveness in mice (24) and, indeed, we observed a more pronounced aggressive behavior of female and male Tph2-deficient mice compared to controls. Even females housed with Tph2−/− females were often wounded by fighting that never happens in control animals of the same genetic background. These observations are consistent with the hypothesis that increased aggression is associated with states of low serotonergic system activity (25).
Table 2.
Maternal neglect in Tph2-deficient mice
| Mother genotype | Number of dams | Litter size | Number of pups born | Number of pups survived until day 5 | % survival | Number of dead litters on day 5 |
|---|---|---|---|---|---|---|
| Control | n = 23 | 10.13 ± 0.45 | 233 | 228 | 97.9% | 0/23 |
| Tph2−/− | n = 29 | 9.28 ± 0.48 | 269 | 149 | 55.4% | 11/29 |
Tph2−− and control females (mixed background) were mated with NMRI (wild type) males and separated into single cages when visibly pregnant. The number of pups was counted on the day of birth (day 0) and at day 5. Litter size is given as average ± SEM.
Body Temperature.
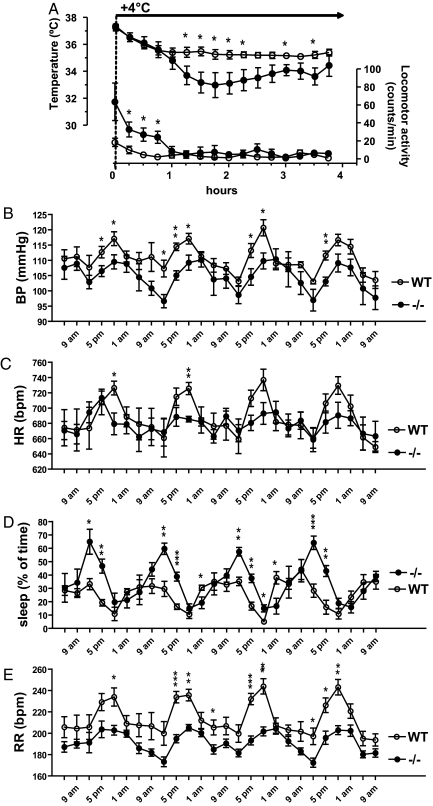
Because central serotonin is known to be an important modulator of autonomic functions (26), we evaluated blood pressure (BP), heart rate (HR), respiration rate (RR), and the control of sleep and body temperature by telemetry. Basic body temperature was normal in Tph2−/− mice (36.60 ± 0.04 °C vs. 36.61 ± 0.22 °C in controls). However, after a challenge by a cold (4 °C) environment, the observed drop in body temperature was more pronounced in serotonin-deficient mice than in control animals (Fig. 3A). At the same time, Tph2−/− mice exhibited hyperactivity in the first hour at 4 °C probably compensating by skeletal muscle movement for a stronger loss in body temperature (Fig. 3A). When after 1 hour the animals reduced their locomotion to the levels of control animals, their temperature started to fall more rapidly than in the controls. This is in accordance with data recently obtained in mice lacking most of the serotonergic neurons (27). In this study, the authors show that shivering and nonshivering thermogenesis is impaired by the perturbation of the brain serotonin system, confirming an important function of central 5-HT in thermoregulation.
Fig. 3.
Telemetric analysis of physiological parameters in Tph2-deficient mice. (A) Changes in the body temperature and locomotor activity after a challenge by a cold (4 °C) environment. Filled circles, Tph2−/− (n = 7); open circles, control mice (n = 8) (4-month-old female mice, FVB/N F4 genetic background). Circadian variations in sleep (B), mean arterial blood pressure (BP) (C), heart rate (HR) in beats per minute (bpm) (D), and respiratory rate (RR) in breaths per minute (Bpm) (E) in Tph2-deficient 3-month-old female mice (FVB/N F4 genetic background). Bold line indicates nighttime. Filled circles, Tph2−/− (n = 5), open circles, control mice (n = 5). *, P < 0.05; **, P < 0.01; and ***, P < 0.001 (Student's t test) Tph2−/− vs. control mice at the same time point.
Sleep.
Telemetric recordings revealed marked disturbances in sleep of Tph2−/− mice. During the daytime, these animals sleep more frequently (Fig. 3B, Fig. S3A) and for longer periods of time than their control littermates (3.96 ± 0.78 30-min inactivity periods from 7 a.m. to 7 p.m. in Tph2−/− vs. 1.04 ± 0.29 in control mice, P < 0.01). In the night, sleeping periods were rarer in both strains and did not significantly differ in length and frequency. There is a long-lasting debate on the role of serotonin in the regulation of sleep and wakefulness (28). Both sleep promotion and induction of arousal by 5-HT have been reported, mostly depending on the origin of the serotonergic neurons analyzed. Our animal model strongly supports sleep promotion and a suppression of arousal as the net effect of a lack of serotonin in the CNS.
Cardiovascular Parameters.
The same animals were also analyzed for the circadian variation of cardiovascular parameters. Thereby, significant decreases in blood pressure and heart rate could be detected mainly in the late afternoon and in the night in Tph2−/− mice (Fig. 3 C and D, Fig. S3 B and C). In the CNS, serotonin is of major importance for cardiovascular control (29). Considerable evidence supports a role of 5-HT1A receptors in the brainstem, in particular in centers involved in sympathetic regulation, which get strong serotonergic innervation from the dorsal group of raphé nuclei, B1 to B4. When these 5-HT1A receptors are activated, a fall in blood pressure is observed accompanied by a decrease in sympathetic nerve activity. Concurrently, central administration of 5-HT1A agonists potentiates the vagal outflow to the heart (30). However, when these agonists are applied to forebrain nuclei, sympathoexcitation and hypertension is induced. These areas get their serotonergic input from the more rostral raphé nuclei, B5 to B9. To further complicate the issue, 5-HT2, 5-HT3, and 5-HT7 receptors have been shown to participate in central serotonergic regulation of the sympathetic and parasympathetic tone (29, 30). Despite that the role of single 5-HT receptors in specific brain areas remains to be clarified in future experiments, our data clearly show that a ubiquitous lack of serotonin decreases blood pressure and heart rate at least at nighttime probably by inhibition of the sympathetic and stimulation of the parasympathetic nervous system.
Respiration.
Further analysis of the telemetric data revealed that respiration rate was significantly reduced in Tph2−/− mice (185.5 ± 9.7 min−1 in Tph2−/− vs. 231.5 ± 6.8 min−1 in WT, P < 0.05) but with normal circadian variations (Fig. 3E, Fig. S3D). These data were confirmed by whole body plethysmography (data not shown). Such dampening of respiration has been described in other pharmacologic (31) and genetic (27, 32) models of partial serotonin deficiency in the CNS. However, there were also contradictory studies postulating that serotonin inhibits breathing (reviewed in ref. 33). Our data strongly support the concept of a tonic excitatory input of serotonergic fibers on respiratory neurons.
Conclusions.
In conclusion, the lack of serotonin in the brain of Tph2−/− mice confirms that TPH2 is the major enzyme responsible for serotonin synthesis in the brain and that circulating 5-HT cannot enter the brain after birth under normal conditions. The lack of central serotonin in these mice leads to impaired early postnatal growth and altered autonomic control of sleep, breathing, thermoregulation, heart rate, and blood pressure. Furthermore, it promotes aggressive behavior and maternal neglect. However strikingly, besides these relatively mild alterations, animals lacking most serotonin in the brain are viable, morphologically normal, and fertile. Thus, TPH2-derived serotonin is involved in the regulation of behavior and autonomic pathways but is not essential for adult life. Recently, mice lacking both TPH isoforms were generated and shown to be viable (ref. 4 and N.A., and M.B., unpublished work). Using these animals, it should be possible to clarify the functional importance of the serotonin system as a whole.
Materials and Methods
Generation of Tph2-Knockout Mice.
A Tph2+/− embryonic stem (ES) cell line was created by homologous recombination of an “expression-selection cassette” described below into the Tph2 locus of the ES cell line E14Tg2A (BayGenomics) leading to the deletion of a 5.2-kb-long region, which contains the coding part of exon 1 (Ex1) and Ex2, and, thus, disabling the transcription and translation of the entire Tph2 gene (Fig. S1A).
First, 1- and 6-kb-long Tph2-homology arms were amplified by PCR from mouse genomic DNA. The pIRES-EGFP vector backbone (Clontech) was used to introduce the 5′ Tph2 homology arm together with neoR and dsRed2 (dsRedN1 vector, Clontech) genes creating a Tph2-neoR-IVS-IRES-dsRed2-pA expression cassette. An additional SV40-EM7-Bsd cassette (kindly provided by F. Stewart, Technical University, Dresden, Germany) was inserted by homologous recombination in bacteria using Red/ET recombination kit (Genebridges) downstream of the dsRed2 to enable the selection in ES cells. The resulting expression-selection cassette was then flanked by the 3′ Tph2 homology arm and used for homologous Red/ET recombineering with the Tph2-containing BAC RP23–226H2 to elongate the Tph2-homology arms. After the successful integration, Tph2-BAC was shaved according to the Red/ET protocol to a 24.6-kb-long plasmid with 14.7-kb 5′ and 3.8-kb 3′ homology regions. The targeting construct was then linearized with I-Sce I and electroporated into ES cells (Bio-Rad Gene Pulser, 800 mV, 3 μF) and clones were picked after 8 days of blasticidin selection. Correct genomic integration of the expression-selection cassette introduced an additional SpeI restriction site, which was used for restriction mapping and Southern blot analysis (Fig. S1B). The 298-bp Southern probe used to detect homologous recombination (Fig. S1A) was amplified by PCR with primers TPH2 South 5 (5′ CAG GAA GCG CTG GAT CTC C) and TPH2 South 3 (5′ CCA AGC ACG TTT ATG ACT CAG) and labeled with radioactive CTP using Prime-It RmT Random Primer labeling kit (Stratagene).
Tph2+/− ES cells were injected into C57BL/6 blastocysts and transferred into pseudopregnant females. After transmission of the knockout allele from chimera to F1 generation, Tph2−/− mice were obtained from heterozygous breeding and the line was further maintained on the mixed background by breeding +/− with +/− animals. To obtain such mice on a pure genetic background, we bred F1 (129/OlaHsd/C57BL/6 background) heterozygous Tph2-deficient animals to the inbred FVB/N and C57BL/6 mouse line (Charles River) for 7 and 6 generations, respectively.
Genotyping of animals was performed using PCR with primer TPH34 (5′AGC TGA GGC AGA CAG AAA GG), TPH54 (5′ CCA AAG AGC TAC TCG ACC TAC G), and Neo3 (5′ CTG CGC TGA CAG CCG GAA CAC). The absence of Tph2-transcripts in the brain of Tph2-deficient animals was confirmed by RT-PCR with primer pairs spanning Ex1–Ex6 (TPH2Ex1.5: 5′ GAT TCT GCT GTG CCA GAA GAT C; TPH2Ex6.3: 5′ GCA AGC ATG AGT CGG GTA GAG) and Ex10–Ex12 (TPH2 Ex10.5: 5′ CCA TCG GAG AAT TGA AGCA; TPH2 Ex12.3: 5′ GTC CTG CAC CAC ATT CTCA).
Animals.
Mice were maintained in IVC cages (Tecniplast Deutschland) under standardized conditions with an artificial 12-h dark–light cycle, with free access to standard chow (0.25% sodium; SSNIFF Spezialitäten) and drinking water ad libitum. Local German authorities approved the studies with standards corresponding to those prescribed by the American Physiological Society.
Telemetry experiments, gene expression analysis, brain MRI, immunohistochemistry, HPLC, and behavior studies were performed in adult (12–24 weeks old) Tph2-deficient mice, using +/+ and +/− littermates as controls. The genetic background of the animals (mixed, FVB/N-F4, FVB/N-F7, and C57BL/6-F6) used in each experiment is indicated in the text and in the figure legends. However, unless otherwise stated, similar results were obtained with all analyzed backgrounds.
To collect organs for RT-PCR and gene expression analysis, animals were killed by cervical dislocation, and tissues were isolated and immediately snap frozen in liquid nitrogen. For the HPLC analysis, animals were first anesthetized by i.p. ketamine (100 mg/kg) and xylazine (10 mg/kg) injection and perfused with 1× PBS-heparin (5,000 IU/L) to wash out blood. To isolate brain areas, brains were rapidly removed, immediately frozen on dry ice, and stored at −80 °C until use. Various brain areas including frontal cortex, hippocampus, hypothalamus, and striatum, were dissected from the frozen brains on a cold plate (−10 °C).
For in situ hybridization and immunocytochemistry, animals were transcardially perfused first with 1× PBS-heparin and then with buffered 4% paraformaldehyde (PFA). Brains were removed and postfixed in the same buffered 4% PFA overnight at 4 °C.
Biochemical Analyses.
For the evaluation of IGF1 levels, blood was taken periorbitally and 25 μL of serum were used for the IGF1 measurement using a commercially available EIA kit (DSL-10-29000, Diagnostic Systems Laboratories).
For the determination of monoamines and their metabolites, GABA, and glutamate, frozen tissues were homogenized in lysis buffer containing 10 μM ascorbic acid and 2.4% perchloric acid, centrifuged for 30 min at 20,000 g, and the supernatant was used for the HPLC measurement. The brain tissue levels of serotonin, 5-hydroxyindoleacetic acid, 3,4-dihydroxyphenylacetic acid and homovanillic acid, were analyzed as described previously using HPLC with electrochemical detection (HPLC-ECD) (34). Dopamine and noradrenaline were measured by HPLC-ECD technique with electrochemical detection after extraction to alumina, according to Felice et al. with minor modifications (35, 36). For determination of γ-aminobutyric acid (GABA) and glutamate tissue levels, amino acids were precolumn derivatized with o-phthalaldehyde/2-mercaptoethanol using a refrigerated autoinjector and then separated on a HPLC column (ProntoSil C18 ace-EPS, 50 mm × 3 mm i.d.) at a flow rate of 0.6 mL/min and a column temperature of 40 °C. The mobile phase was 50 mM sodium acetate pH 5.7 in a linear gradient from 5% to 21% acetonitrile. Derivatized amino acids were detected by their fluorescence at 450 nm after excitation at 330 nm (37).
Immunocytochemistry and in Situ Hybridization.
PFA-fixed brains were incubated in 30% sucrose solution and cryosectioned at 20 μm. The sections were dried after mounting onto SuperFrost Plus (Menzel) slides and directly used for immunohistochemistry, as previously described (38) using rabbit polyclonal anti-5HT primary antibodies (1:1,000; Immunostar) and Cy3-conjugated anti-rabbit IgG secondary antibody (1:500; Jackson ImmunoResearch). Fluorescence images were collected using Axioplan2 imaging microscope and a Sensicam 12BIT camera (Zeiss).
For the in situ hybridization, PFA-fixed brains were embedded in paraffin and sectioned at 8 μm. The sections were deparaffinized, rehydrated, and treated with proteinase K (Roche). Hybridization was performed as previously described (39, 40) using digoxigenin-UTP (Qiagen)-labeled mouse Tph2 and Sert antisense-RNA probes. Pictures were taken with Axioplan2 Imaging microscope/Axiophoto camera (Zeiss).
Telemetry.
The telemetric techniques are described in detail elsewhere (41). Briefly, PhysioTel PA-C20 pressure transmitters (DSI) were implanted into the femoral artery and recordings of BP, HR, and locomotor activity were started 10 days after the surgery. RR was calculated from the telemetric blood pressure data using the RespiRATE module of the Dataquest A.R.T software (DSI).
The sleeping time was calculated from the locomotor activity data. An animal was considered “sleeping” when it was displaying 0 activity during at least 5 min. The percentage of sleeping over a period was calculated as relation between the number of “5-min sleeping episodes” and all 5-min intervals during this period. More than 30 min of immobility was considered an “extended sleeping period.” Data for the day/nighttime BP, HR, RR, and sleeping time were averaged from 4 or 5 consecutive days.
To perform the body temperature measurements, PhysioTel TA-F20 transmitters (DSI) were implanted into the peritoneal cavity of 4-month-old female mice (FVB/N-F4 genetic background) and 10 days after surgery temperatures were recorded. After 2 days of baseline recordings at room temperature (22 °C), animals were subjected to a cold room (4 °C) for 4 h and then returned back to ambient temperature.
Behavioral Studies.
For the evaluation of maternal care females of different genotypes were mated for 1 week with wild-type males of the NMRI mouse strain (which are considered to be good breeders). Several days before delivery, females were separated to single cages and were observed every morning for the presence of newborns. The day when pups were born was called day 0. The behavior of mothers and pups survival were recorded daily from day 0 until day 5. For the cross-fostering test, pups from Tph2−/− dams were transferred to control mothers and vice versa on day 1 after birth just after the lactation was started. Pups-retrieval test was performed in the home cage, covered with a transparent Plexiglas lid under normal light conditions. Mice were given a 30-min habituation time and thereafter the nest was destroyed and pups were scattered in the cage. Mothers were given 30 min for the construction of a new nest and huddling of the pups. Maternal behavior was monitored with a video camera and analyzed with a suitable software (Biobserve, version 2.2.0.91). “Hidden cookie” tests were conducted to check for gross malfunction of the main olfactory system in Tph2−/− females. After overnight food deprivation (≈24 h), the time to find a small piece of a food pellet, buried in the fresh sawdust, was recorded for each mouse.
Statistics.
Results are expressed as mean ± SEM. Tests of significance (PRISM, GraphPad) were conducted by unpaired Student's t test and 1-way ANOVA, followed by Tukey's post hoc test. The survival of animals was analyzed using the Kaplan/Meier method followed by a Logrank test.
SI.
Further details may be found in SI Materials and Methods.
Supplementary Material
Acknowledgments.
We acknowledge the help of Herbert Schulz in the analysis of the Affymetrix data and the excellent technical assistance of Susanne Wollenzin, Andrea Müller, Sabine Grüger, Reika Langanki, Manfred Strohmann, and Carolin Gärtner. This study was funded by the European Framework Program 6 (FunGenES Integrated Project) and D.K. and V.M. were supported by fellowships of the German Academic Exchange Service (DAAD).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0810793106/DCSupplemental.
References
- 1.Walther DJ, Bader M. A unique central tryptophan hydroxylase isoform. Biochem Pharmacol. 2003;66:1673–1680. doi: 10.1016/s0006-2952(03)00556-2. [DOI] [PubMed] [Google Scholar]
- 2.Walther DJ, et al. Synthesis of serotonin by a second tryptophan hydroxylase isoform. Science. 2003;299:76. doi: 10.1126/science.1078197. [DOI] [PubMed] [Google Scholar]
- 3.Cote F, et al. Disruption of the nonneuronal tph1 gene demonstrates the importance of peripheral serotonin in cardiac function. Proc Natl Acad Sci USA. 2003;100:13525–13530. doi: 10.1073/pnas.2233056100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Savelieva KV, et al. Genetic disruption of both tryptophan hydroxylase genes dramatically reduces serotonin and affects behavior in models sensitive to antidepressants. PLoS ONE. 2008;3:e3301. doi: 10.1371/journal.pone.0003301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Walther DJ, et al. Serotonylation of small GTPases is a signal transduction pathway that triggers platelet α-granule release. Cell. 2003;115:851–862. doi: 10.1016/s0092-8674(03)01014-6. [DOI] [PubMed] [Google Scholar]
- 6.Lesurtel M, et al. Platelet-derived serotonin mediates liver regeneration. Science. 2006;312:104–107. doi: 10.1126/science.1123842. [DOI] [PubMed] [Google Scholar]
- 7.Lang PA, et al. Aggravation of viral hepatitis by platelet-derived serotonin. Nat Med. 2008;14:756–761. doi: 10.1038/nm1780. [DOI] [PubMed] [Google Scholar]
- 8.Nocito A, et al. Serotonin regulates macrophage-mediated angiogenesis in a mouse model of colon cancer allografts. Cancer Res. 2008;68:5152–5158. doi: 10.1158/0008-5472.CAN-08-0202. [DOI] [PubMed] [Google Scholar]
- 9.Matsuda M, et al. Serotonin regulates mammary gland development via a novel autocrine-paracrine loop. Dev Cell. 2004;6:193–203. doi: 10.1016/s1534-5807(04)00022-x. [DOI] [PubMed] [Google Scholar]
- 10.Morecroft I, et al. Effect of tryptophan hydroxylase 1 deficiency on the development of hypoxia-induced pulmonary hypertension. Hypertension. 2007;49:232–236. doi: 10.1161/01.HYP.0000252210.58849.78. [DOI] [PubMed] [Google Scholar]
- 11.Yadav VK, et al. Lrp5 controls bone formation by inhibiting serotonin synthesis in the duodenum. Cell. 2008;135:825–837. doi: 10.1016/j.cell.2008.09.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dahlström A, Fuxe K. Evidence for the existence of monoamine-containing neurons in the central nervous system. I. Demonstration of monoamines in the cell bodies of brain stem neurons. Acta Physiol Scand Suppl. 1964;232:1–55. [PubMed] [Google Scholar]
- 13.Zhang X, Beaulieu JM, Sotnikova TD, Gainetdinov RR, Caron MG. Tryptophan hydroxylase-2 controls brain serotonin synthesis. Science. 2004;305:217. doi: 10.1126/science.1097540. [DOI] [PubMed] [Google Scholar]
- 14.Harvey M, et al. Support for the involvement of TPH2 gene in affective disorders. Mol Psychiatry. 2004;9:980–981. doi: 10.1038/sj.mp.4001557. [DOI] [PubMed] [Google Scholar]
- 15.Zhang X, et al. Loss-of-function mutation in tryptophan hydroxylase-2 identified in unipolar major depression. Neuron. 2005;45:11–16. doi: 10.1016/j.neuron.2004.12.014. [DOI] [PubMed] [Google Scholar]
- 16.Beaulieu JM, et al. Role of GSK3 beta in behavioral abnormalities induced by serotonin deficiency. Proc Natl Acad Sci USA. 2008;105:1333–1338. doi: 10.1073/pnas.0711496105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abumaria N, Ribic A, Anacker C, Fuchs E, Flugge G. Stress upregulates TPH1 but not TPH2 mRNA in the rat dorsal raphe nucleus: Identification of two TPH2 mRNA splice variants. Cell Mol Neurobiol. 2008;28:331–342. doi: 10.1007/s10571-007-9259-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gershon MD. Enteric serotonergic neurones finally! J Physiol. 2009;587:507. doi: 10.1113/jphysiol.2008.167676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gutknecht L, et al. Deficiency of brain 5-HT synthesis but serotonergic neuron formation in Tph2 knockout mice. J Neural Transm. 2008;115:1127–1132. doi: 10.1007/s00702-008-0096-6. [DOI] [PubMed] [Google Scholar]
- 20.Hendricks TJ, et al. Pet-1 ETS gene plays a critical role in 5-HT neuron development and is required for normal anxiety-like and aggressive behavior. Neuron. 2003;37:233–247. doi: 10.1016/s0896-6273(02)01167-4. [DOI] [PubMed] [Google Scholar]
- 21.Thissen JP, Ketelslegers JM, Underwood LE. Nutritional regulation of the insulin-like growth factors. Endocr Rev. 1994;15:80–101. doi: 10.1210/edrv-15-1-80. [DOI] [PubMed] [Google Scholar]
- 22.Papageorgiou A, Denef C. Stimulation of growth hormone release by 5-hydroxytryptamine (5-HT) in cultured rat anterior pituitary cell aggregates: Evidence for mediation by 5-HT2B, 5-HT7, 5-HT1B, and ketanserin-sensitive receptors. Endocrinology. 2007;148:4509–4522. doi: 10.1210/en.2007-0034. [DOI] [PubMed] [Google Scholar]
- 23.Lerch-Haner JK, Frierson D, Crawford LK, Beck SG, Deneris ES. Serotonergic transcriptional programming determines maternal behavior and offspring survival. Nat Neurosci. 2008;11:1001–1003. doi: 10.1038/nn.2176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Takayanagi Y, et al. Pervasive social deficits, but normal parturition, in oxytocin receptor-deficient mice. Proc Natl Acad Sci USA. 2005;102:16096–16101. doi: 10.1073/pnas.0505312102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Popova NK. From genes to aggressive behavior: The role of serotonergic system. BioEssays. 2006;28:495–503. doi: 10.1002/bies.20412. [DOI] [PubMed] [Google Scholar]
- 26.Jacobs BL, Martin-Cora FJ, Fornal CA. Activity of medullary serotonergic neurons in freely moving animals. Brain Res Brain Res Rev. 2002;40:45–52. doi: 10.1016/s0165-0173(02)00187-x. [DOI] [PubMed] [Google Scholar]
- 27.Hodges MR, et al. Defects in breathing and thermoregulation in mice with near-complete absence of central serotonin neurons. J Neurosci. 2008;28:2495–2505. doi: 10.1523/JNEUROSCI.4729-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saper CB, Chou TC, Scammell TE. The sleep switch: Hypothalamic control of sleep and wakefulness. Trends Neurosci. 2001;24:726–731. doi: 10.1016/s0166-2236(00)02002-6. [DOI] [PubMed] [Google Scholar]
- 29.Ramage AG. Central cardiovascular regulation and 5-hydroxytryptamine receptors. Brain Res Bull. 2001;56:425–439. doi: 10.1016/s0361-9230(01)00612-8. [DOI] [PubMed] [Google Scholar]
- 30.Jordan D. Vagal control of the heart: Central serotonergic (5-HT) mechanisms. Exp Physiol. 2005;90:175–181. doi: 10.1113/expphysiol.2004.029058. [DOI] [PubMed] [Google Scholar]
- 31.Mueller RA, Towle AC, Breese GR. Supersensitivity to the respiratory stimulatory effect of TRH in 5,7-dihydroxytryptamine-treated rats. Brain Res. 1984;298:370–373. doi: 10.1016/0006-8993(84)91440-9. [DOI] [PubMed] [Google Scholar]
- 32.Erickson JT, Shafer G, Rossetti MD, Wilson CG, Deneris ES. Arrest of 5HT neuron differentiation delays respiratory maturation and impairs neonatal homeostatic responses to environmental challenges. Respir Physiol Neurobiol. 2007;159:85–101. doi: 10.1016/j.resp.2007.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Richerson GB. Serotonergic neurons as carbon dioxide sensors that maintain pH homeostasis. Nat Rev Neurosci. 2004;5:449–461. doi: 10.1038/nrn1409. [DOI] [PubMed] [Google Scholar]
- 34.Sperk G. Simultaneous determination of serotonin, 5-hydroxindoleacetic acid, 3,4-dihydroxyphenylacetic acid and homovanillic acid by high performance liquid chromatography with electrochemical detection. J Neurochem. 1982;38:840–843. doi: 10.1111/j.1471-4159.1982.tb08708.x. [DOI] [PubMed] [Google Scholar]
- 35.Felice LJ, Felice JD, Kissinger PT. Determination of catecholamines in rat brain parts by reverse-phase ion-pair liquid chromatography. J Neurochem. 1978;31:1461–1465. doi: 10.1111/j.1471-4159.1978.tb06573.x. [DOI] [PubMed] [Google Scholar]
- 36.Sperk G, Berger M, Hortnagl H, Hornykiewicz O. Kainic acid-induced changes of serotonin and dopamine metabolism in the striatum and substantia nigra of the rat. Eur J Pharmacol. 1981;74:279–286. doi: 10.1016/0014-2999(81)90046-7. [DOI] [PubMed] [Google Scholar]
- 37.Piepponen TP, Skujins A. Rapid and sensitive step gradient assays of glutamate, glycine, taurine and gamma-aminobutyric acid by high-performance liquid chromatography-fluorescence detection with o-phthalaldehyde-mercaptoethanol derivatization with an emphasis on microdialysis samples. J Chromatogr B Biomed Sci Appl. 2001;757:277–283. doi: 10.1016/s0378-4347(01)00156-6. [DOI] [PubMed] [Google Scholar]
- 38.Muller DN, et al. NF-kappaB inhibition ameliorates angiotensin II-induced inflammatory damage in rats. Hypertension. 2000;35:193–201. doi: 10.1161/01.hyp.35.1.193. [DOI] [PubMed] [Google Scholar]
- 39.Dony C, Gruss P. Specific expression of the Hox 1.3 homeo box gene in murine embryonic structures originating from or induced by the mesoderm. EMBO J. 1987;6:2965–2975. doi: 10.1002/j.1460-2075.1987.tb02602.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schmidt-Ullrich R, et al. NF-kappaB transmits Eda A1/EdaR signalling to activate Shh and cyclin D1 expression, and controls post-initiation hair placode down growth. Development. 2006;133:1045–1057. doi: 10.1242/dev.02278. [DOI] [PubMed] [Google Scholar]
- 41.Xu P, et al. Endothelial dysfunction and elevated blood pressure in Mas gene-deleted mice. Hypertension. 2008;51:574–580. doi: 10.1161/HYPERTENSIONAHA.107.102764. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.