Abstract
Because glioma etiology is largely unknown, the inverse association of glioma risk with atopic conditions is promising and deserves close scrutiny. We examined the association between a history of allergies, asthma, and eczema and glioma risk using sibling, friend, and clinic-based controls. This analysis included 388 incident glioma cases and 80 sibling, 191 friend, and 177 clinic-based controls. Each subject’s medical history was assessed via a web-based or telephone survey. Odds ratios (ORs) and their 95% confidence intervals for the associations with allergies, asthma, eczema, and the overall number of these conditions were calculated from conditional (for sibling and friend controls) and unconditional (for clinic-based controls) logistic models. Allergies were consistently inversely associated with the glioma: ORs were 0.53 (95% CI, 0.15–1.84), 0.54 (95% CI, 0.28–1.07), and 0.34 (95% CI, 0.23–0.50) with sibling, friend, and clinic-based controls, respectively. Asthma showed an inverse association only in the comparison with sibling controls (OR=0.43; 95% CI, 0.19–1.00). Eczema showed an inverse association only in the comparison with friend controls (OR=0.42; 95% CI, 0.15–1.18). The overall number of these conditions (ordinal score 0, 1, 2, 3) was inversely associated with glioma: The risk decreased 31–45% with each addition of an atopic condition. These estimates were the most stable when different control groups were considered. Comparing the prevalence of these conditions in the three control groups with published data, we note that clinic-based controls generally better approximate the prevalence data for population-based groups. These controls appear to present a reasonable choice for clinic-centered case-control studies.
Keywords: Glioma, Allergy, Eczema, Asthma, Epidemiology
Introduction
Glioma is one of the most fatal tumors and its etiology is largely unknown (1, 2). Several recent reviews (1, 2) and a meta-analysis (3) indicate that a history of atopic conditions, such as allergies, asthma, and eczema, is inversely associated with the risk of glioma. The biological mechanism behind this association is thought to be elimination of pro-cancerous cells by increased immuno-surveillance due to the hyperactive immune state seen in atopy. Currently, the number of studies that have evaluated the association between glioma and atopy history is less than a dozen (4–14). Thus, this association is far from being classified as established and/or causal. Although very intriguing, the hypothesis that atopic conditions lower the risk of glioma awaits further confirmation.
We examined this hypothesis using the data from our ongoing clinic-based case-control study of glioma being conducted at Duke University in collaboration with the University of Illinois at Chicago (UIC). This study enrolled glioma patients and three groups of controls – siblings, friends, and clinic-based controls – presenting an interesting opportunity for assessing associations with allergies, asthma, and eczema. For example, with the genotypic similarity of siblings, an association detected with sibling controls might speak for a strong environmental component. In contrast, friends at least during some period of their lives may share a similar environment, but not genes. Clinic-based controls present a group that is unrelated to cases and should share neither genes nor environment. Contrasting the results of these analyses gives us an opportunity to further explore the nature of associations between a history of allergies, asthma, and eczema and glioma and to determine the robustness of the relative risk estimates with different control groups.
Methods
The recruitment of the study subjects, along with data collection, was conducted at the Duke University Medical Center (DUMC), in North Carolina, in collaboration with investigators from UIC. Institutional Review Board approvals were obtained from both institutions.
Study Subjects
The study subjects included glioma patients, who made up the cases group, and the participants in the three separate control groups. We included in this analysis all subjects who completed the survey by August 18, 2008. Because the majority of cases (93%) were non-Hispanic whites, we limited our analysis to this ethnic group.
Cases
Glioma cases were identified during the period of August 2003 – April, 2008 with a confirmed new diagnosis (ICDO-3 sites C70.0-C72.9 and C75.1-C75.3) of glioblastoma (ICDO-3 histology codes 9440–9442), astrocytoma (9400–9411 and 9420–9421), or oligodendroglioma (9450–9460). Patients who were 18 or older, English speaking, and residents of the United States were eligible for recruitment. After screening (n=1712), 16 patients were excluded from participation, because they did not have mental capacity to consent (as determined by standard neurological questions that were part of the initial physician assessment); and 1039 were determined as eligible. Seven hundred forty-one patients consented to participate (participation rate=71%). Among those who consented, 429 (41% of eligible) completed the self-report surveys as of August 18, 2008. Thirty six cases were excluded from the analysis as non-White (the rationale explained above), and five cases were excluded because of missing data. As a result, a total of 388 cases were included in the analysis.
Controls
The glioma patients were asked to provide names and contact information for up to two siblings and three friends who might agree to serve as study controls. Eligible sibling and friend controls had to be at least 18 years old, be residents of the United States, and have had no history of brain cancer. Sibling and friend controls were recruited, and consent was obtained by our research staff through telephone or mail contact. Of the 169 siblings and 407 friends eligible to participate as controls, 147 (87%) and 330 (81%), respectively, consented to participate, which represents an overall response rate of 83%. Among those eligible, 108 siblings (61%) and 245 (60%) friends completed the self-report surveys as of August 18, 2008. Three sibling and 12 friend controls were excluded from the analysis as non-White; one sibling and four friend controls were excluded because of missing data. Of the remaining controls, 80 sibling and 191 friend controls had a matching patient who completed the survey (cases that were matched to the remaining controls did not complete the survey and were excluded from the analyses).
The clinic-based control group was selected from the patients seen at the DUMC clinics. They were frequency-matched to cases by age (10-year interval), gender, and race/ethnicity (Caucasian, African-American, Hispanic, Asian, other). For recruitment, we employed two tactics. First, we actively recruited patients from an orthopedic clinic; potential controls were identified from an appointment list and were invited to participate via an introductory letter signed by their doctor. Second, we invited other DUMC patients to participate as controls via flyers placed in DUMC clinics. Approximately 96% of the recruited clinic-based controls were patients of the DUMC orthopedic clinic, whereas 4% were those who responded to the flyers.
Clinic-based controls were recruited in the clinic at the time of their doctor’s appointment. During this meeting, our research staff reviewed the eligibility requirements, and if all were met, the subject was asked to participate in the study. The eligibility criteria for clinic-based controls included the following: (1) age of 18–80, (2) residence within the United States, (3) no history of brain tumors or any cancers, (4) no history of neurodegenerative disease (amyotrophic lateral sclerosis, Creutzfeldt-Jakob disease, Alzheimer’s disease, Parkinson’s disease, vascular dementia, dementia with Lewy bodies, frontotemporal dementia, Huntington’s disease, or Niemann-Pick disease), and (5) fluency in English. Of the 311 eligible controls contacted in clinics, 295 (95%) consented to participate. Among those eligible, 194 (62%) completed the self-report surveys as of August 18, 2008. Seventeen clinic-based controls were excluded form the analysis as non-White; none had missing data; a total of 177 clinic-based controls have been included in the analysis.
Data Collection
Subjects who consented to participate were asked to choose between a web-based or telephone survey. This survey focused on occupational and environmental exposures associated with known or suspected animal neurocarcinogens. In addition, information was collected on demographics, family history, medical history, and history of diagnostic and therapeutic radiation as potential confounding and modifying variables. For the atopy-related questions, the survey asked the participants:
Please check the box next to the condition if any healthcare worker ever told you before two years ago (in bold) that you had the condition. If you were (in bold) diagnosed with the condition, please select the age at which you were diagnosed.
The participant was offered three specific atopy-related conditions from which to choose, allergy, asthma, and eczema. For allergy, a participant was asked to indicate the number of (a) seasonal allergies to pollens, molds, etc. (0, 1, 2, 3+), and (b) allergies to medications (0, 1, 2, 3+), (c) and to specify allergies to foods (check all that apply: wheat, peanuts, soy, tree nuts, eggs, milk, fish/shellfish, other).
Statistical Analysis
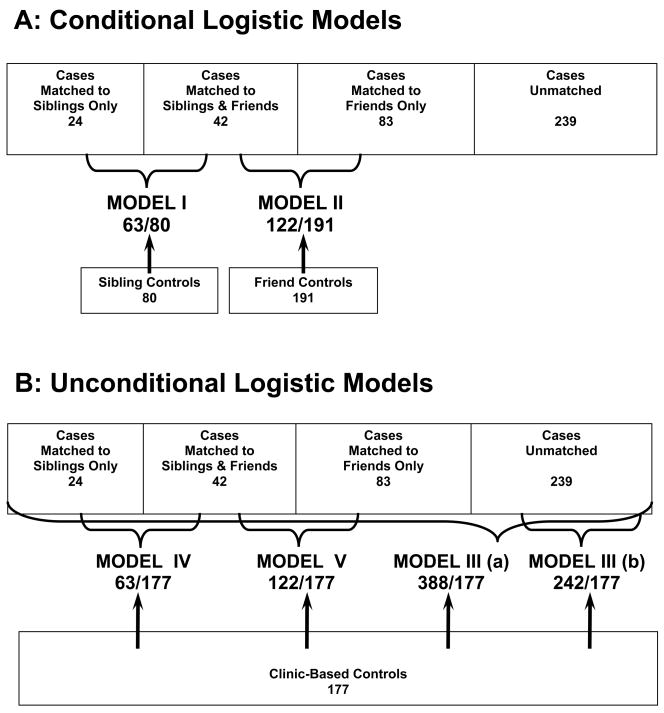
To estimate the association between glioma and history of atopy-related conditions, we calculated age- and gender-adjusted odds ratios (ORs) and their 95% confidence intervals (CIs) from logistic regression models. A total of five models were constructed. The inclusion of cases and controls in the different models is presented in Fig. 1. For cases and matched controls, both siblings and friends, conditional logistic regression was used (Models I and II). For all models, including clinic-based controls, unconditional logistic regression was used (Models III, IV, and V). Model III(a) includes all cases with information available about atopy-related history, including the cases that were matched to siblings and friends in Models I and II (Fig. 1). Model III(b) includes cases that have been matched to neither sibling nor friend controls. To examine whether the OR estimates change when the same group of cases is compared to a different control group, we constructed two additional models: Cases matched to siblings (Model IV) or cases matched to friends (Model V) were compared to clinic-based controls (Fig. 1). We analyzed the associations with three specific conditions (allergy, asthma, and eczema) and with the overall number of reported conditions. For the latter, we created two analytical variables: an ordinal score (0, 1, 2, 3) assuming linear relationships with the risk of glioma and a categorical indicator variable (0, 1, >2) to explore the dose-response pattern and omitting the linearity assumption.
Figure 1.
Inclusion of cases and controls in different models: A – Conditional Logistic Models; B – Unconditional Logistic Models.
Results
Cases (n=388) included in the different models had comparable distributions of age and gender (Table 1). The majority of cases had glioblastoma (63%), with astrocytoma, oligodendroglioma, and mixed glioma presenting much smaller portions (22%, 9%, and 3%, respectively). The histologies presented by single digit number of cases were giant cell glioblastoma (n=3), gliosarcoma (n=3), and gemistocytic astrocytoma (n=2). Among controls, the clinic-based group appeared to have a higher proportion of older people.
Table 1.
Demographic characteristics and prevalence of atopic conditions of cases and controls
| Characteristics* | Cases, N (%) |
Controls, N (%) |
||||||
|---|---|---|---|---|---|---|---|---|
| All (N=388) | Matched with Siblings (N=63) | Matched with Friends (N=122) | Unmatched Cases* (N=239) | Siblings (N=80) | Friends (N=191) | Clinic-based (N=177) | ||
| Age | < 40 | 83 (21) | 18 (29) | 27 (22) | 49(21) | 19 (24) | 38 (20) | 26 (15) |
| 40–49 | 96 (25) | 15 (24) | 35 (29) | 55(23) | 22 (28) | 39 (20) | 20 (11) | |
| 50–59 | 108 (28) | 16 (25) | 37 (30) | 63(26) | 24 (30) | 71 (37) | 46 (26) | |
| 60–69 | 87 (22) | 12 (19) | 22 (18) | 60(25) | 10 (13) | 36 (19) | 51 (29) | |
| ≥ 70 | 14 (4) | 2 (3) | 1 (1) | 12(5) | 5 (6) | 7 (4) | 34 (19) | |
|
| ||||||||
| Gender | Female | 150 (39) | 20 (32) | 51 (42) | 88(37) | 49 (61) | 98 (51) | 106 (60) |
|
| ||||||||
| Atopy-related Conditions | Allergies | 88 (23) | 4 (6) | 22 (18) | 65(27) | 10 (13) | 47 (25) | 88 (50) |
| Asthma | 62 (16) | 16 (25) | 26 (21) | 29(12) | 36 (45) | 51 (27) | 29 (16) | |
| Eczema | 15 (4) | 2 (3) | 5 (4) | 10(4) | 4 (5) | 18 (9) | 10 (6) | |
| At least 1 of the above | 139 (36) | 20 (32) | 43 (35) | 88(37) | 38 (48) | 92 (48) | 96 (54) | |
The numbers of cases matched to siblings, those matched to friend controls, and unmatched cases do not add up to 388, because there is an overlap between cases matched to siblings and friend controls (see Figure 1).
Prevalence of Allergy, Asthma, and Eczema Conditions among Cases and Controls
The reported prevalence of allergies varied vastly between different groups of cases: The difference between sibling-matched cases (6%) and all cases (23%) was almost 4-fold. Among controls, this parameter also varied approximately 4-fold, from 13% in siblings to 50% in clinic-based controls. It is worth noting that the prevalence of allergies among controls was proportionally (approximately twice) higher than in the respective case groups (Table 1). For asthma, the reported prevalence did not vary substantially among the different groups of cases, but varied more than twofold among control groups (Table 1). The reported history of eczema showed the lowest variation among case and control groups. Between control groups, the reported prevalence of at least one atopy-related condition was the parameter showing the least variation (Table 1).
Association between Glioma and the Number of Atopy-Related Conditions
The number of reported conditions was inversely associated with the case-control status. The risk of glioma decreased 31–45% with each addition of a condition (Table 2). When analyzed using categorical variables, the models with sibling controls (Model I) and with clinic-based controls (Model III) were compatible with a linear inverse dose-response, whereas the model with friend controls (Model II) was not.
Table 2.
Association between the history of atopy and case-control status
| History of Atopy | OR (95% CI) |
|||
|---|---|---|---|---|
| Model I | Model II | Model III | ||
| 63 Cases & 80 Matched Sibling Controls matched analysis | 122 Cases & 191 Matched Friend Controls matched analysis | (a) 388 Cases & 177 Clinic-based Controls unmatched analysis | (b) 239 Cases & 177 Clinic-based Controls unmatched analysis | |
| Number of atopic conditions | ||||
| Atopy-related ordinal score (0,1,2,3) | 0.55 (0.30, 1.01)* | 0.69 (0.48, 1.0)* | 0.57 (0.44, 0.75)* | 0.60 (0.44, 0.81)* |
|
| ||||
| Categories: None |
ref. | ref. | ref. | ref. |
| 1 condition |
0.65 (0.28, 1.49) | 0.62 (0.37, 1.04) | 0.64 (0.42, 0.96) | 0.70 (0.45, 1.1) |
| ≥2 conditions | 0.22 (0.04, 1.16) | 0.57 (0.24, 1.33) | 0.28 (0.15, 0.52) | 0.27 (0.13, 0.56) |
|
| ||||
| Specific Atopy-related Conditions | ||||
| Allergies | 0.53 (0.15, 1.84) | 0.54 (0.28, 1.07) | 0.34 (0.23, 0.50) | 0.43 (0.28, 0.66) |
| Asthma | 0.43 (0.19, 1.00) | 0.84 (0.47, 1.50) | 0.96 (0.58, 1.59) | 0.71 (0.39, 1.27) |
| Eczema | 0.74 (0.13, 4.35) | 0.42 (0.15, 1.18) | 0.70 (0.30, 1.64) | 0.79 (0.31, 2.01) |
OR per increase in one atopic condition
Association between Glioma and Specific Conditions
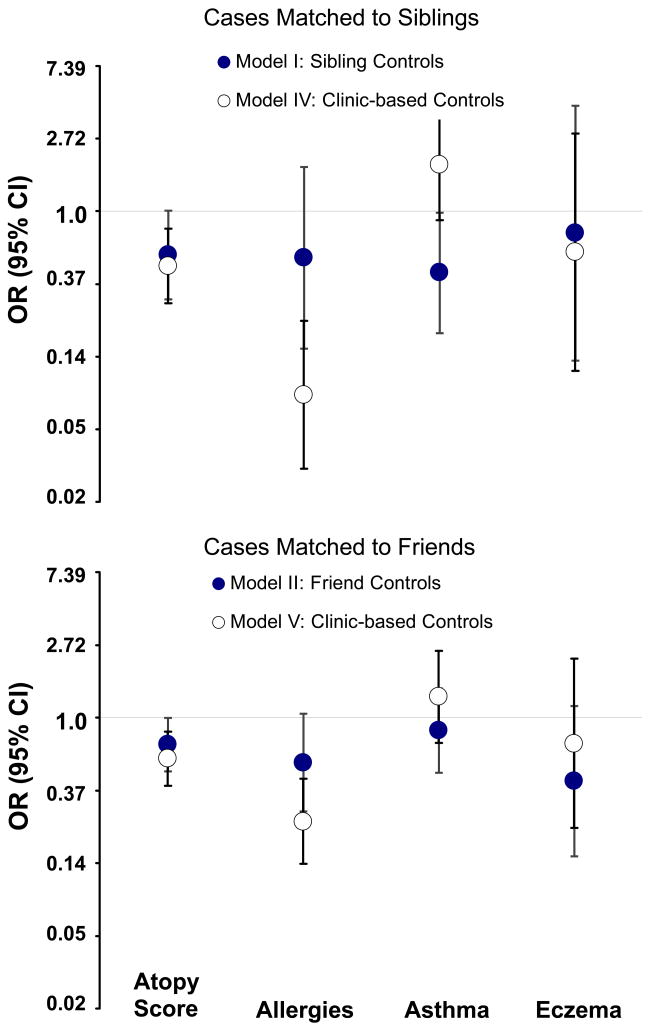
The direction of associations with allergies, asthma, and eczema did not change with different control groups (Table 2). As expected, the smaller sample sizes in models with sibling and friend controls produced wide confidence intervals, although the strength of this association was similar in the two models. In the case of clinic-based controls, the allergy association was stronger and statistically significant. This increase in the association strength cannot be solely attributed to the control group, because the cases involved in the three comparisons are not identical. That is, cases in Models I and II overlap partially and present a portion (38%) of cases in Model III (Fig. 1). To refine the effect of the control group, we kept the case groups intact (i.e., included cases that were matched to siblings in Model IV or to friends in Model V) and changed only the control group to clinic-based (Fig. 1). Whether cases were from sibling- or from friend-matched models, the comparison to clinic-based controls yielded a stronger inverse association with allergies (Fig. 2).
Figure 2.
Effect of control group on the association between glioma and atopic conditions.
Among the observed associations with asthma, only the model with sibling controls (Model I) yielded a statistically significant inverse association (Table 2). When those same cases were compared to the clinic-based controls (Model IV, Fig. 2), the association changed direction (OR=1.90; 95% CI, 0.89–4.07). In the analogous comparison involving cases matched to friends (Model V, Fig. 2), both OR estimates were close to the null value (Fig. 2).
The direction of the eczema association in all models was inverse. However, the estimates for eczema were less stable (i.e., had wide confidence intervals) than those for allergy or asthma probably because the prevalence of eczema was relatively low. Substitution of siblings and friends with clinic-based controls did not change meaningfully either the direction or the strength of the association with eczema.
Discussion
Glioma is a rare cancer and is a classic example of a disease to be studied by using case-control methodology. Except for the twin cohort studies conducted by Schwartzbaum et al. (11), all previous studies on atopy-related conditions and glioma have been case-control. Because the results of such studies depend on the prevalence of atopy-related history among controls, the choice of controls — a well-known Achilles’ heel of case-control design — is important. The control groups in previous studies include friends (6), hospital-based controls (4), and various population-based groups (5, 7–10, 13) (Table 3). According to the existing consensus in the field, population-based groups should produce the most valid results, because they presumably reflect the prevalence of the atopy-related conditions in the base population. Therefore, it is important to know how well the prevalence of these conditions in our control groups approximates those reported for the population-based controls.
Table 3.
Prevalence of atopic conditions in previously published studies of brain tumors and two US cross-sectional studies
| Study | Population (Country) | Prevalence | |||
|---|---|---|---|---|---|
| Allergy | Asthma | Eczema | Any of the Three Conditions | ||
| Brenner et al. (4) | Hospital-based controls (USA) | 38–41% | |||
| Cicuttini et al. (5) | Population-based controls (Australia) | 11% | 8% | ||
| Hochberg et al. (6) | Friend controls | 34% | |||
| Schlehofer et al., 1999 (8) | Population-based controls (International: 6 countries)* | 31% | 7% | 9% | |
| Schlehofer et al., 1992 (9) | Population-based controls (Germany) | 38% | |||
| Schoemaker et al. (10) | Population-based controls (UK) | 23% | 13% | 13% | 62% |
| Schwartzbaum et al. (11) | Twin’s cohorts (Sweden) | 17–31% | 3% | ||
| Wiemels et al. (12) | Population-based controls (USA) | 85% | |||
| Wigertz et al. (13) | Population-based controls (International: 5 countries)† | 46% | 10% | 18% | |
| Arbes et al. (15) | NHANES III‡ (USA) | 54% | |||
| Kilmer et al. (16) | BRFSS§ (USA) | 4.5% | |||
Countries included are Australia, Canada, France, Germany, Sweden, and USA.
Countries included are Denmark, Finland, Norway, Sweden, and UK.
National Health and Nutrition Examination Survey III – population-based cross-sectional study.
Behavioral Risk Factor Surveillance System – population-based cross-sectional study.
The prevalence of allergies in most population-based studies varies within the range of 30–45%, with the lowest and the highest published values of 23% (10) and 85% (12) (Table 3). Although epidemiological studies assess the history of atopy-related conditions mostly by self-report, it is important to note that these estimates are greatly influenced by the specifics of assessment. In this context it is interesting to consider the results of the National Health and Nutrition Examination Survey III (NHANES III). The NHANES III estimated the prevalence of skin test response and found such response to at least 1 of the 10 most common allergens in 54% in the US population (15). The authors believed that their results might underestimate the real prevalence of atopy, because only 10 allergens had been tested. On the other hand, a positive skin test reflects the presence of IgE antibodies after exposure to common environmental allergens, whereas the prevalence of a clinically-defined allergy could be lower. Although it is difficult to evaluate whether these counteracting considerations balance each other, the NHANES III estimate is similar to the prevalence of allergy seen in most epidemiological studies.
In our study, both the sibling controls and friend controls had a lower prevalence of allergies (13% and 25%, respectively), whereas clinic-based controls best approximated allergy prevalence in the general US population (50%). The prevalence of asthma varied between 7% and 13% in different population-based control groups (Table 3). A recent population-based estimate of asthma prevalence in the US is 4.5% (Table 3) (16). In a twin cohort, the prevalence of asthma was even lower, specifically, 3% (Table 3). In our study, all control groups had a higher prevalence of asthma than published population-based estimates (Tables 1 and 3). Among these control groups, siblings had the highest (and the most remote from the plausible range, 45%) and clinic-based controls had the lowest asthma prevalence (16%), with clinic-based controls best approximating the prevalence of asthma previously observed in population-based control groups. The published prevalence estimates for eczema varied from 8% to 18% (Table 3) (5, 8, 10, 13). In our study, the prevalence of eczema among friend controls lies within this range (9%), while the prevalence in clinic-based controls is near the lower lend of the published range (6%). Thus, overall for the three conditions of interest, clinic-based controls appear to best approximate the population prevalence.
When considering the number of atopy-related conditions, at least one is found in approximately 50% of the individuals in all control groups. This estimate is likely to be driven by allergy prevalence and seems plausible in light of the above-described comparisons. Interestingly, despite the wide variations of the specific conditions among the different control groups, the prevalence of this atopy-related measure is similar. Following this similarity, the adjusted ORs associated with at least one condition varied in our study only slightly: ORs were 0.53–0.61. These findings agree with the results from an earlier population-based case-control study (OR=0.63, 95% CI, 0.53–0.76).(10) Overall, the association between glioma and the number of conditions is quite stable in our study and represents the most robust finding (Table 2).
With the three groups of controls, it is tempting to speculate on the role of the genetic and environmental components. The low precision (wide confidence intervals) of the estimates involving sibling and friend controls precludes well-grounded speculations. However, one tendency is apparent. Comparison with clinic-based controls yielded consistently stronger association with allergies as compared to the models with sibling or friend controls (Table 2, Fig. 2). This indicates that overmatching controls by genetic and/or environmental factors in siblings and friends may reduce the magnitude of the association between glioma and allergies.
In the context on this analysis, it is important to explain the rationale for selection of clinic-based controls as opposed to population-based controls. The cases in our study were recruited at the Duke Brain Tumor Center. This population of cases comes only partially (approximately 34%) from North Carolina, but mostly from states adjacent to or close to North Carolina. Because of such disperse distribution of patients’ residences, it was impractical to define base population geographically, which is required for selection of population-based controls. The closest option to population-based controls could be community controls matched by residence. We rejected matching by residence, because recruitment of community-based controls in multiple distant areas is associated with low feasibility, low response rate, and high cost. In this study, we piloted recruitment of three alternative groups of controls with potentially high response rate – siblings, friends, and clinic-based controls. We reasoned that these groups of controls would have similar probability to be seen at the Duke Brain Tumor Center had they been diagnosed with brain tumor – a major requirement to reduce selection bias in clinic-based studies (17, 18). An additional consideration for the selection of clinic-based controls was Berkson’s bias, a possible relationship between exposure of interest (atopy-related conditions) and the reason controls were being seen at the clinic (19). We selected the orthopedic patients as the main source for controls, because we are not aware of an association between orthopedic conditions and glioma or the risk/protective factors for glioma, including atopy-related conditions. The current analysis confirmed that clinic-based controls are most likely the preferred choice for information on medical history. However, because the direction of the association is known and an approximate estimate of the association has been evaluated by the meta-analysis (3), it would be interesting to compare these associations using population-based controls recruited in North Carolina. We invite the researchers with access to such data to participate in this interesting exploration.
A limitation of the current study is the rate of survey completion, ranging from 41% among cases to 60–62% among controls. Taking into account that completion of the survey took from 1.5 to 2.5 hours, the difference in the completion rate between cases and controls can be explained by the low health status of the patients. Importantly, the study excluded mentally incompetent patients and did not have proxy-responders, which potentially may improve the quality of collected information.
In conclusion, we found an inverse association between glioma and the history of atopy-related conditions, the strength of which increased with the number of conditions. With the specific conditions, only allergies convincingly show inverse associations, although the pattern with eczema was similar. Given the similar prevalence of conditions in the clinic-based controls compared to other population-based groups in the literature, we believe that these controls approximate the general population and present a reasonable choice for clinic-centered case-control studies for hypotheses involving a history of medical conditions.
Acknowledgments
This research was funded by NIH SPORE Grant 5P50 CA108786-4
Reference List
- 1.Fisher JL, Schwartzbaum JA, Wrensch M, Wiemels JL. Epidemiology of brain tumors. Neurol Clin. 2007;25:867–90. doi: 10.1016/j.ncl.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 2.Schwartzbaum JA, Fisher JL, Aldape KD, Wrensch M. Epidemiology and molecular pathology of glioma. Nat Clin Pract Neurol. 2006;2:494–503. doi: 10.1038/ncpneuro0289. [DOI] [PubMed] [Google Scholar]
- 3.Linos E, Raine T, Alonso A, Michaud D. Atopy and Risk of Brain Tumors: A Meta-analysis. J Natl Cancer Inst. 2007;99:1544–50. doi: 10.1093/jnci/djm170. [DOI] [PubMed] [Google Scholar]
- 4.Brenner AV, Linet MS, Fine HA, et al. History of allergies and autoimmune diseases and risk of brain tumors in adults. Int J Cancer. 2002;99:252–9. doi: 10.1002/ijc.10320. [DOI] [PubMed] [Google Scholar]
- 5.Cicuttini FM, Hurley SF, Forbes A, et al. Association of adult glioma with medical conditions, family and reproductive history. Int J Cancer. 1997;71:203–7. doi: 10.1002/(sici)1097-0215(19970410)71:2<203::aid-ijc13>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 6.Hochberg F, Toniolo P, Cole P, Salcman M. Nonoccupational risk indicators of glioblastoma in adults. J Neurooncol. 1990;8:55–60. doi: 10.1007/BF00182087. [DOI] [PubMed] [Google Scholar]
- 7.Ryan P, Lee MW, North B, McMichael AJ. Risk factors for tumors of the brain and meninges: results from the Adelaide Adult Brain Tumor Study. Int J Cancer. 1992;51:20–7. doi: 10.1002/ijc.2910510105. [DOI] [PubMed] [Google Scholar]
- 8.Schlehofer B, Blettner M, Preston-Martin S, et al. Role of medical history in brain tumour development. Results from the international adult brain tumour study. Int J Cancer. 1999;82:155–60. doi: 10.1002/(sici)1097-0215(19990719)82:2<155::aid-ijc1>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 9.Schlehofer B, Blettner M, Becker N, Martinsohn C, Wahrendorf J. Medical risk factors and the development of brain tumors. Cancer. 1992;69:2541–7. doi: 10.1002/1097-0142(19920515)69:10<2541::aid-cncr2820691025>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 10.Schoemaker MJ, Swerdlow AJ, Hepworth SJ, et al. History of allergies and risk of glioma in adults. Int J Cancer. 2006;119:2165–72. doi: 10.1002/ijc.22091. [DOI] [PubMed] [Google Scholar]
- 11.Schwartzbaum J, Jonsson F, Ahlbom A, et al. Cohort studies of association between self-reported allergic conditions, immune-related diagnoses and glioma and meningioma risk. Int J Cancer. 2003;106:423–8. doi: 10.1002/ijc.11230. [DOI] [PubMed] [Google Scholar]
- 12.Wiemels JL, Wiencke JK, Sison JD, et al. History of allergies among adults with glioma and controls. Int J Cancer. 2002;98:609–15. doi: 10.1002/ijc.10239. [DOI] [PubMed] [Google Scholar]
- 13.Wigertz A, Lonn S, Schwartzbaum J, et al. Allergic conditions and brain tumor risk. Am J Epidemiol. 2007;166:941–50. doi: 10.1093/aje/kwm203. [DOI] [PubMed] [Google Scholar]
- 14.Schwartzbaum J, Ahlbom A, Malmer B, et al. Polymorphisms Associated with Asthma Are Inversely Related to Glioblastoma Multiforme. Cancer Res. 2005;65:6459–65. doi: 10.1158/0008-5472.CAN-04-3728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arbes SJ, Jr, Gergen PJ, Elliott L, Zeldin DC. Prevalences of positive skin test responses to 10 common allergens in the US population: results from the third National Health and Nutrition Examination Survey. J Allergy Clin Immunol. 2005;116:377–83. doi: 10.1016/j.jaci.2005.05.017. [DOI] [PubMed] [Google Scholar]
- 16.Kilmer G, Roberts H, Hughes E, et al. Surveillance of certain health behaviors and conditions among states and selected local areas--Behavioral Risk Factor Surveillance System (BRFSS), United States, 2006. MMWR Surveill Summ. 2008;57:1–188. [PubMed] [Google Scholar]
- 17.Wacholder S, Chatterjee N, Hartge P. Joint effect of genes and environment distorted by selection biases: implications for hospital-based case-control studies. Cancer Epidemiol Biomarkers Prev. 2002;11:885–9. [PubMed] [Google Scholar]
- 18.Boyd AV. Testing for association of diseases. J Chronic Dis. 1979;32:667–72. doi: 10.1016/0021-9681(79)90097-3. [DOI] [PubMed] [Google Scholar]
- 19.Schwartzbaum J, Ahlbom A, Feychting M. Berkson’s bias reviewed. Eur J Epidemiol. 2003;18:1109–12. doi: 10.1023/b:ejep.0000006552.89605.c8. [DOI] [PubMed] [Google Scholar]