Abstract
Previous studies have shown that the addition of sirolimus to tacrolimus/mini-methotrexate (tac/sir/mtx) as GVHD prophylaxis produces low rates of acute GVHD after reduced intensity (RIC) allogeneic SCT. To assess whether the post transplant methotrexate can be safely eliminated altogether, we conducted a prospective clinical trial testing the combination of tacrolimus and silrolimus (tac/sir) alone as GVHD prophylaxis after RIC SCT from matched related donors. We compare the results with patients who received tac/sir/mtx as GVHD prophylaxis after RIC SCT from matched related donors in a previous prospective study. Patients on both trials received fludarabine 30 mg/m2/d IV and intravenous busulfan 0.8 mg/kg/d IV from day −5 through day −2 as conditioning, followed by transplantation of unmanipulated filgrastim mobilized peripheral blood stem cells. Both cohorts received tacrolimus and sirolimus orally starting on day-3, with doses adjusted to achieve trough serum levels of 5–10 ng/ml and 3–12 ng/ml, respectively. The tac/sir/mtx cohort received additional mini-methotrexate (5 mg/m2 IV) on day +1,3,6. Filgrastim 5 mcg/kg SC QD was started on day +1 until neutrophil engraftment. Twenty nine patients were transplanted on tac/sir, and 46 were transplanted on tac/sir/mtx. The two groups were balanced in age, gender, and disease characteristics. Engraftment was brisk and donor chimerism after transplant was robust in both groups. The cumulative incidence of grade II–IV acute GVHD were similar, 17% vs 11% for the tac/sir and tac/sir/mtx cohorts, respectively (p=0.46). There was also no difference in cumulative incidence of extensive chronic GVHD, treatment related mortality, disease relapse, or survival. The GVHD prophylaxis combination of tacrolimus and sirolimus is well tolerated, and is associated with a low incidence of acute GVHD in matched related donor RIC transplantation. The omission of mini-methotrexate from the tacrolimus and sirolimus GVHD prophylaxis combination does not appear to impact adversely on the development of acute GVHD.
Keywords: GVHD, Sirolimus, Rapamycin, Tacrolimus, Methotrexate, Transplantation
Introduction
While regimen related toxicity is minimized in reduced intensity allogeneic stem cell transplantation, acute graft-versus-host disease (GVHD) remains a significant cause of morbidity and mortality. A variety of immune suppressive drug combinations are used as GVHD prophylaxis in RIC SCT, but an optimal regimen has not been defined. Commonly used combinations include a calcineurin-inhibitor with low dose methotrexate, mycophenolate mofetil, [1, 2] or the addition of in vivo T-cell depletion with alemtuzumab or anti-thymocyte globulin. [3, 4] The latter are associated with low acute GVHD incidence, but loss of graft-versus-malignancy effect can be problematic, and T-cell add-back or donor lymphocyte infusions are often necessary. These issues highlight the fact that in RIC SCT, where the graft-versus malignancy effect is tantamount, an effective GVHD prophylaxis regimen must achieve GVHD control without impairing immune reconstitution and graft-versus malignancy.
Sirolimus, is a macrocyclic lactone immune suppressive agent similar in structure to tacrolimus, is increasingly used as an agent in GVHD prophylaxis regimens after allogeneic stem cell transplantation. Unlike tacrolimus or cyclosporine, sirolimus is an inhibitor of the mammalian target of Rapamycin (mTOR). Sirolimus binds with FKBP12 and the raptor/rictor proteins.[5] This sirolimus-FKBP12-mTOR complex inhibits pathways needed for signal transduction and cell cycle progression, resulting in suppression of T cells and impairment of dendritic cell function. In experimental models, sirolimus does not impede the expansion of CD4+CD25+FoxP3 regulatory T cells, and as such, it is suggested that it would preserve the GVL effect. [6–8]
Several studies have shown that the combination of sirolimus and tacrolimus, with or without low dose methotrexate, is effective in preventing acute GVHD after myeloablative hematopoietic stem cell transplantation. In these studies, which employed high dose cyclophosphamide and total body irradiation (1400cGy) as conditioning, tac/sir/±mtx was associated with grade II–IV acute GVHD rates of 19% for matched related donor, and 28–30% for mismatched related or matched unrelated donor transplantation. [9, 10] The experience with tac/sir combinations as GVHD prophylaxis in reduced intensity conditioning (RIC) transplantation is more limited. We have recently shown that that addition of sirolimus to tacrolimus and low-dose methotrexate (Tac/Sir/Mtx) resulted in a low incidence of grade II–IV acute GVHD after RIC allogeneic transplantation from matched related and unrelated donors in patients with hematologic malignancies [11]. To assess whether the methotrexate can be safely eliminated without compromising GVHD outcomes, we conducted a prospective clinical trial testing the combination of tacrolimus and silrolimus (tac/sir) alone as GVHD prophylaxis after RIC SCT from matched related donors. We report the results of 29 patients transplanted on this trial, and compare them to the 46 patients who received tac/sir/mtx after RIC SCT from matched related donors in a previous reported prospective study.[11] We demonstrate that the combination of sirolimus and tacrolimus alone, omitting the methotrexate, is sufficient for acute GVHD prevention in patients receiving RIC allogeneic transplantation from matched related donors.
Patients and Methods
Patients with hematologic malignancies who have a matched related donor and who were deemed to be appropriate candidates for RIC allogeneic transplantation were eligible for the 2 prospective clinical trials. Enrollment for the tac/sir/mtx trial occurred between September 2002 and December 2004, and enrollment for the tac/sir trial occurred January 2006 and October 2007. Transplantation eligibility requirements included ECOG performance status 0–2, absence of uncontrolled infection at the time of study entry, left ventricular ejection fraction >30%, and normal or near normal parameters of kidney and liver function. All patients were considered to have relative contraindications to myeloablative transplantation. Relative contraindications to myeloablative transplantation included prior autologous stem cell transplantation, age >50 years, significant medical co-morbidity, or organ dysfunction. Low risk disease was defined as acute leukemia in CR1, de-novo early stage myelodysplastic syndrome (RA or RARS), and first chronic phase CML. All others were considered high risk. Both clinical trial protocols were approved by The Human Subjects Protection Committee of Dana-Farber/Harvard Cancer Center. Written informed consent was obtained from all patients prior to transplantation.
All the related donors in these studies were siblings of the patient and were HLA matched at A, B, and DRB1 loci at the allele level. HLA typing was performed with sequence specific oligonucleotide probes (SSOP). All patients received filgrastim mobilized peripheral blood stem cells (PBSC). Donors were mobilized with filgrastim at 10 μg/kg/day for 5 days. Stem cell collection was initiated on the fifth day of filgrastim and continued until a sufficient number of CD34+ cells were obtained. Target cell dose was 5.0 × 106 CD34+ cells/kg. The first day of stem cell infusion was defined as Day 0.
In both studies, the conditioning regimen consisted of fludarabine (30 mg/m2/d IV days −5, −4, −3, −2) and intravenous busulfan (0.8 mg/kg/d days −5, −4, −3, −2). Tacrolimus was administered by mouth at 0.05 mg/kg/day (in 2 daily divided doses) starting on day −3 with a target serum concentration of 5–10 ng/mL. Sirolimus was administered as a 12 mg oral loading dose on day −3, followed by a 4 mg daily dose, adjusted to maintain a target serum concentration of 3–12 ng/mL by HPLC. In the tac/sir/mtx trial, mini-methotrexate (5 mg/m2 IV) was given on day +1,+3,+6. In the tac/sir trial, the mini-methotrexate was omitted. All patients received unmanipulated PBSC. Filgrastim was administered at 5 μg/kg/day beginning on day +1 and was continued until neutrophil engraftment. Patients received oral acyclovir as prophylaxis against herpes virus infections, and sulfamethoxazole/trimethoprim or atovoquone as prophylaxis against Pneumocystis jurivecii. No systemic prophylactic antifungal therapy was given. Patients were monitored for peripheral blood CMV reactivation by DNA hybrid capture during the first 100 days post transplant, and pre-emptive therapy with ganciclovir or valganciclovir was started if CMV DNA over 1.0 pg/mL was detected. Acute GVHD was graded according to the consensus grading scale [12]. Taper of tacrolimus and sirolimus was encouraged after day +64, with the goal to be off immune suppression by approximately 6 months post transplant if there was no GVHD. If a patient developed evidence of recurrent or progressive disease, rapid withdrawal/taper of tacrolimus or sirolimus was allowed at the discretion of the treating physician. No pre-emptive or prophylactic donor lymphocyte infusion was planned as part of either protocol.
Chimerism Analysis
Unfractionated donor chimerism was assessed from bone marrow aspirates and/or peripheral blood at approximately day +30–45, and 3–4 months post transplant. Genotype of donor and recipient were determined using DNA extracted from pre transplant samples. Nine short tandem repeat (STR) loci were typed using the ABI Profiler Plus Kit (Applied Biosystems Inc) and the ABI 310 Genetic Analyzer to resolve alleles. “Informative” alleles that were present only in the donor or recipient were used in the chimerism calculations.
Statistical Analysis
Both clinical trials testing tac/sir and tac/sir/mtx were single arm prospective phase II trials with the primary objective of assessing the rate of grade II–IV acute GVHD. The tac/sir study was a one-stage design with an accrual goal of 30 patients and an alternative hypothesis of 15% grade II–IV acute GVHD rate. An early stopping rule was imposed to stop the study early in the event of excessive grade III–IV acute GVHD. This study reached full accrual; however, one patient’s information was unavailable at the time of analysis and was thus excluded in this report.
Descriptive statistics was provided for patient baseline characteristics. Two-sided Fisher’s exact test was used to compare categorical variables, and two-sided Wilcoxon-Rank-Sum test was used to compare continuous variables.
Cumulative incidence curves for acute GVHD and chronic GVHD were constructed reflecting early death and death or relapse as a competing risk, respectively. In constructing the cumulative incidence curves for acute GVHD, patients who suffer early disease progression/relapse and subsequently develop acute GVHD in the setting of rapid termination of tacrolimus and sirolimus were categorized as having relapsed instead of as having acute GVHD. Cumulative incidence curves for treatment-related death and relapse with or without death were constructed reflecting time to relapse and time to treatment related death as competing risks. The difference between cumulative incidence curves in the presence of a competing risk was tested using the Gray method. [13] Time to relapse and time to treatment-related death were measured from the date of stem cell infusion. Patients who were alive without relapse were censored at the time last seen alive. Overall survival (OS) and progression-free survival (PFS) were calculated using the Kaplan-Meier method. Overall survival was defined as the time from stem cell infusion to death from any cause. Progression-free survival was defined as the time from stem cell infusion to relapse, disease progression or death from any cause. The Log-rank test was used for the comparisons of Kaplan-Meier curves. Prognostic factors for overall survival and progression-free survival were examined in Cox proportional hazard models, whereas acute GVHD, chronic GVHD, relapse, and treatment related death were examined in competing risks regression model.[14]
Results
Patient Characteristics
Characteristics of these 29 patients and the 45 patients on the tac/sir/mtx study are shown in Table 1. The 2 study cohorts were well balanced in terms of age, gender, donor recipient sex mismatch, prior autologous transplant, and disease risk. The median ages for the tac/sir and tac/sir/mtx cohorts were 53 years (range 29 to 64 yrs) and 55 years (range 20–69 yrs), respectively. Approximately 35% of patients in both cohorts had undergone prior myeloablative autologous or allogeneic transplantation. The median follow-up for survivors is 3.26 yrs for the tac/sir/mtx cohort, and 1.84 yrs for the tac/sir cohort.
Table 1.
Patient Characteristics
| Tac/Sir | Tac/Sir/Mtx | ||||
|---|---|---|---|---|---|
| N | % | N | % | p-value | |
| Total | 29 | 100 | 46 | 100 | |
| Age | 0.80 | ||||
| ≥50 | 20 | 69 | 33 | 72 | |
| < 50 | 9 | 31 | 13 | 28 | |
| median (range) | 53 (29–64) | 55 (20–69) | |||
| Sex | 0.25 | ||||
| M | 17 | 59 | 33 | 72 | |
| F | 12 | 41 | 13 | 28 | |
| Patient-Donor Sex Matching | 0.64 | ||||
| MF | 11 | 38 | 18 | 39 | |
| MM | 6 | 21 | 15 | 32 | |
| FF | 7 | 24 | 6 | 13 | |
| FM | 5 | 17 | 7 | 15 | |
| Prior Transplant | 0.98 | ||||
| Yes | 10 | 34 | 16 | 35 | |
| No | 19 | 66 | 30 | 65 | |
| Diagnosis | - | ||||
| AML | 6 | 21 | 14 | 30 | |
| Anemia/Red Cell Disorder | - | - | 1 | 2 | |
| CLL, SLL, PLL | 3 | 10 | 7 | 15 | |
| CML | 3 | 10 | 2 | 4 | |
| Hodgkin Disease | 5 | 17 | 4 | 44 | |
| MDS | 1 | 3 | 8 | 17 | |
| MM/Plasma Cell Disorder | 2 | 7 | 2 | 4 | |
| MPD | - | - | 1 | 2 | |
| NHL | 9 | 31 | 7 | 15 | |
| Risk Status | 0.37 | ||||
| High Risk | 12 | 41 | 11 | 24 | |
| Low Risk* | 17 | 59 | 35 | 76 | |
Low risk defined as acute leukemia in CR1, de-novo MDS-RA or RARS, or first chronic phase CML. All others were considered high risk.
Stem Cell Product and Engraftment
The median number of CD34+ cells infused was 8.0 × 106 cells/kg (range, 2.6–38 × 106) for the tac/sir cohort, and 8.5 × 106 cells/kg (range, 1.8–19 × 106) for the tac/sir/mtx cohort (p=0.73). No cases of primary graft failure were observed in either cohort. In the tac/sir cohort, only 6 of 29 patients (21%) developed neutropenia (absolute neutrophil count <500 cells/μL) or significant thrombocytopenia (platelets <20,000 cells/μL). In contrast, 52% developed neutropenia (p=0.008), and 41% developed thrombocytopenia in the tac/sir/mtx group (p=0.047). Among the patients whose blood counts nadired, there was no difference in time to neutrophil or platelet engraftment between the 2 groups (median time to neutrophil engraftment: 13 vs 13 days (p=0.84) platelet engraftment: 19 vs. 20 days, p=0.85).
Donor derived hematopoiesis was assessed on total nucleated peripheral blood cells between day 25 to 40, and again approximately 100 days after transplantation. In the tac/sir group, median donor chimerism at approximately 1 month post transplant was 94% (range 32–99%), with 71% of all patients achieving ≥90% donor derived hematopoiesis. Median donor chimerism around day +100 for patients with chimerism data available was 95% (range 11–100%), with 78% of patients achieving ≥90% donor derived hematopoiesis. Similarly, median donor chimerism at approximately 30 days post-transplant for the tac/sir/mtx cohort was 92% (range 11–100%), with 61% achieving ≥90% donor derived hematopoiesis. The percentage of patients achieving ≥90% donor chimerism at 1 month (71% vs 61%) in the 2 groups were not statistically different (p =0.44). Because 35% of chimerism data around day +100 was missing for patients in the tac/sir/mtx cohort, median donor chimerism was not calculated for this time point.
Graft-versus-Host Disease
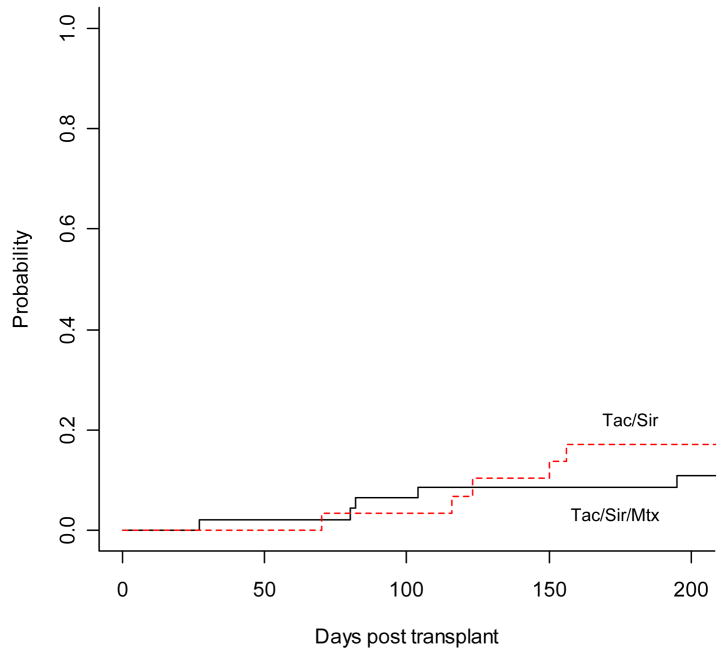
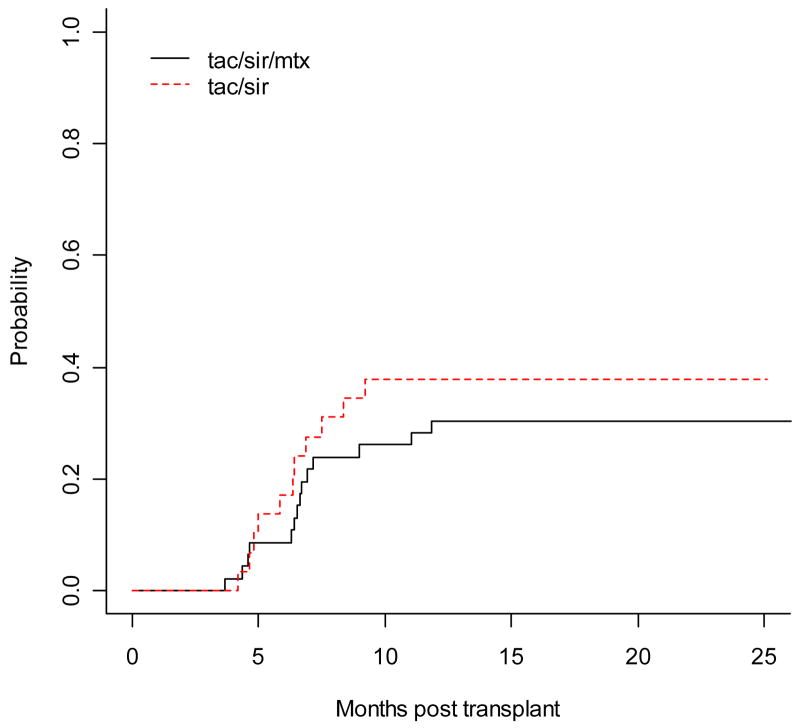
The cumulative incidence of grade II–IV acute GVHD was similar between the tac/sir and tac/sir/mtx cohort, 17.2% vs. 10.9%, respectively, p= 0.46 (Figure 1). There was 1 case of grade IV acute GVHD in the tac/sir/mtx group. There were no acute GVHD related deaths in either cohort. There was a higher cumulative incidence of chronic GVHD in the tac/sir group compared to the tac/sir/mtx group, 74% vs. 43%, respectively, p= 0.02. However, this difference was mainly due to a higher incidence of limited chronic GVHD, as the cumulative incidence of extensive chronic GVHD was similar in the 2 cohorts, 38% for tac/sir and 30% for tac/sir/mtx, p=0.47. (Figure 2) The new NIH consensus scoring definitions for chronic GVHD were not yet used during the times of these 2 studies.
Figure 1.
Cumulative incidence of grade II–IV acute GVHD with early death as a competing risk.
Figure 2.
Cumulative incidence of extensive chronic GVHD with death/relapse a competing risk
Relapse, TRM and Survival
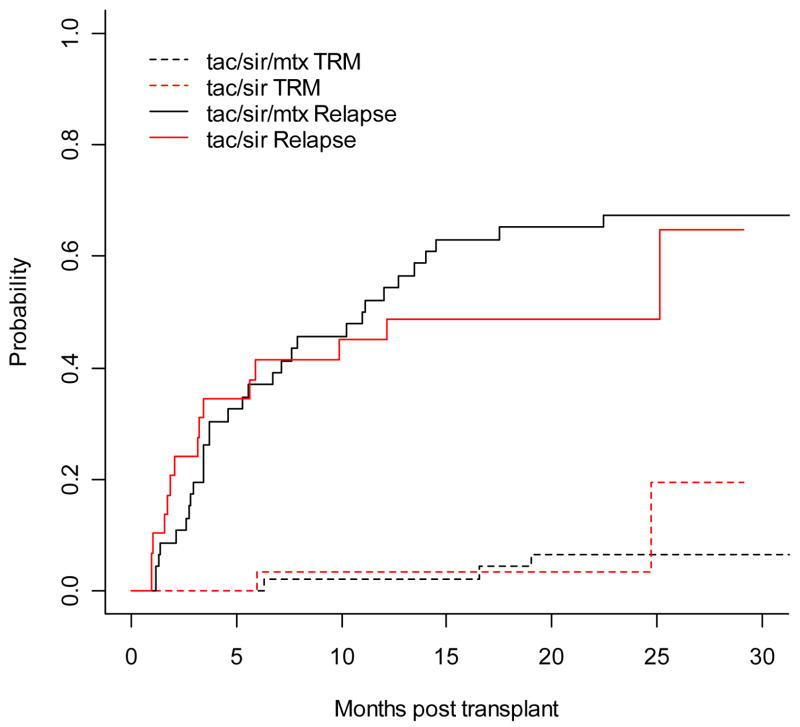
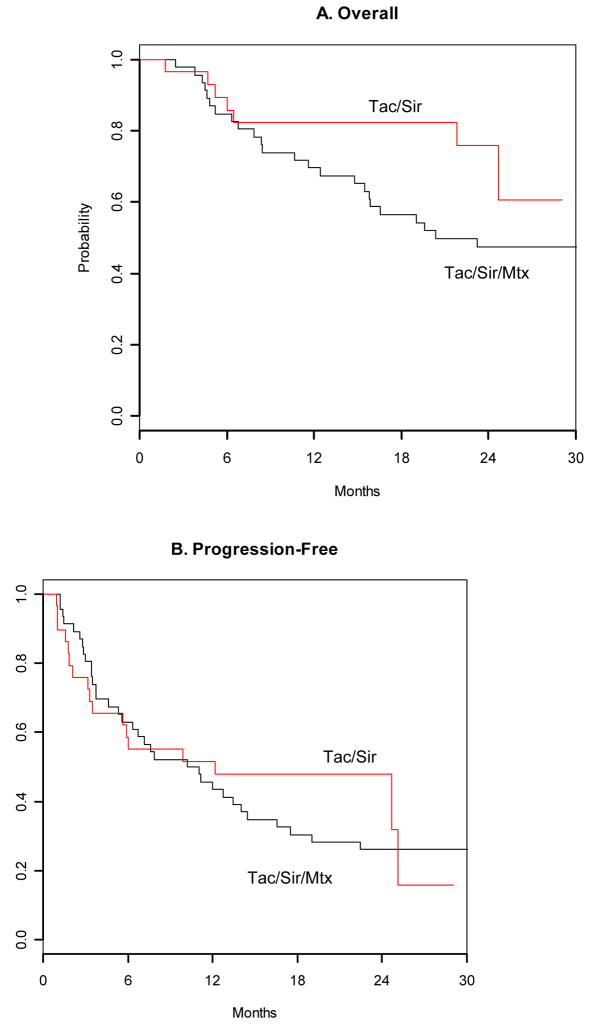
Overall survival at day +100 were 97% for tac/sir, and 98% for tac/sir/mtx. There were no cases of hepatic veno-occlusive disease. Transplant associated thrombotic microangiopathy was observed in 2 patients, one in each cohort. Both cases resolved with discontinuation of tacrolimus. Cumulative incidence of disease relapse and treatment related mortality were also similar for the two cohorts. (Figure 3) With a median follow up time of 1.84 years for the tac/sir cohort, and 3.26 years for the tac/sir/mtx cohort, there was a trend towards better overall survival for the tac/sir group compared to the tac/sir/mtx group (overall survival at 2 years: 76% vs. 47%, respectively, p=0.07, Figure 4a). However, this might be due to the shorter follow-up time in the tac/sir cohort. Progression free survival estimates at 2 years are similar in the 2 groups: 48% for tac/sir and 26% for tac/sir/mtx, P= 0.58, Figure 4b.
Figure 3.
Cumulative incidence of relapse and TRM as competing risks
Figure 4.
Overall and progression free survival
Factors Associated with Outcome and Toxicity
Cox regression analysis was performed to identify factors associated with grade II–IV acute GVHD, extensive chronic GVHD, treatment related mortality, overall survival, and progression free survival. Factors analyzed included age, patient-donor gender mismatch, tac/sir vs. tac/sir/mtx, prior autologous transplant and disease risk at time of transplantation. In competing risk regression models for acute GVHD and extensive chronic GVHD, reflecting early disease progression/relapse as a competing risk, omission of mini-methotrexate, age ≥50, prior transplantation, high disease risk, and donor recipient gender mismatch were not associated with an increased risk for acute or extensive chronic GVHD. Omission of mini-methotrexate was not associated with any difference in relative risk for transplant related mortality, overall survival or progression free survival.
DISCUSSION
We have previously shown that the potent immunosuppressive agent, sirolimus, combined with tacrolimus and low-dose methotrexate results in low rates of grade II–IV acute GVHD for both related and unrelated donor RIC transplantation.[11] In the current study, we demonstrated that the combination of tacrolimus and sirolimus alone, omitting the low dose methotrexate, is similarly effective in the prevention of acute GVHD. After adjusting for relapsed patients who had acute GVHD induced by rapid withdrawal of immune suppression, the cumulative incidence of grade II–IV acute GVHD was 17% for patients receiving tac/sir as GVHD prophylaxis. This result is also concordant with previously published results using this same prophylaxis regimen in myeloablative MRD PBSC transplantation, where the grade II–IV acute GVHD incidence was 10%, and treatment related mortality was minimal.[15] A multi-center randomized trial testing the combination of tacrolimus and sirolimus versus tacrolimus and methotrexate in matched sibling donor myeloablative transplantation is on-going through the BMT Clinical Trials Network.
Considering the equivalence in GVHD control, the elimination post transplant methotrexate from the tac/sir combination may be associated with some potential benefits. The omission of methotrexate may have accounted for the significant reduction in patients from the tac/sir group who actually developed neutropenia (21%) or significant thrombocytopenia (21%), compared to 52% and 41% in the tac/sir/mtx group, respectively. The elimination of methotrexate may provide cost savings and added convenience for patients since it obviates the need for multiple clinic infusion visits during the first week after stem cell infusion.
The incidence of acute GVHD noted in this study compares favorably with other pharmacologic prophylactic strategies of GVHD after RIC transplantation. In RIC related donor transplantation, the incidence of grade II–IV acute GVHD ranges from 16–47% with tacrolimus or cyclosporine and mycophenolate mofetil (MMF)[16, 17], and 12–36% with tacrolimus or cyclosporine and methotrexate.[18] Incorporation of alemtuzumab or antithymocyte globulin as GVHD prophylaxis is associated with a low incidence of acute and chronic GVHD after RIC transplantation. However, these in-vivo T cell depletion approaches are associated with an increased risk of infection and disease relapse, often necessitating the use of donor lymphocyte infusion to restore the GVL effect. [4, 19]
In addition to its immune suppressive properties, sirolimus may have additional benefits in allogeneic transplantation beyond GVHD prevention. Along with other mTOR inhibitors, sirolimus has been shown to have direct anti-cancer activity in several hematologic malignancies. [20] The anti-cancer activity of sirolimus appears to be mediated through inhibition of the mTOR pathway and inhibits immature cells by blockade in G0/G1 phase of the cell cycle. While it is unclear if the doses used to prevent acute GVHD are sufficient to mediate anti-cancer activity, results of a recent retrospective study on lymphoma patients receiving RIC transplant with Bu/Flu conditioning demonstrated that those transplanted using tacrolimus/sirolimus based GVHD prophylaxis enjoy superior disease free survival than those receiving non-sirolimus containing GVHD prophylaxis. [21] Preliminary results incorporating sirolimus into a RIC regimen in a group of patients with high risk leukemia also suggested improved disease control after transplant.[22] Larger series will be needed to assess the impact of sirolimus on disease control. Interestingly, there has also been recent data suggesting that the combination of mTOR inhibitors with methotrexate can provide a synergistic effect against acute lymphoblastic leukemia in cell lines and animal models.[23]
As true in many single institution non-randomized phase II studies, our data are limited by the fact that the study populations for the 2 trials are small, and there is insufficient power to detect small differences in GVHD outcomes. Furthermore, the tac/sir/mtx trial was conducted a few years earlier than the tac/sir trial. This could have impacted the transplant related mortality comparisons because supportive care and antifungal therapy have improved in recent years, although this difference should not influence the development of GVHD. Definitive confirmation of acute GVHD rates with tac/sir vs. tac/sir/mtx will require a prospective randomized phase III trial.
In summary, our study demonstrates that the combination of tacrolimus and sirolimus alone is associated with good acute GVHD control and little toxicity after RIC transplantation from matched sibling donors. The use of low dose intravenous busulfan with sirolimus in this RIC setting does not appear to increase the risk of hepatic VOD or TMA. While the incidence of chronic GVHD remains high, long-term survival outcomes are encouraging, suggesting that the graft-versus-malignancy effect is preserved. Sirolimus containing GVHD prophylaxis regimens are worthy of further investigation in reduced intensity transplantation.
Acknowledgments
Supported in part by the Ted and Eileen Pasquarello Research Fund and NIH grant P01 HL070149-02.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Giralt S, et al. Melphalan and purine analog-containing preparative regimens: reduced-intensity conditioning for patients with hematologic malignancies undergoing allogeneic progenitor cell transplantation. Blood. 2001;97(3):631–7. doi: 10.1182/blood.v97.3.631. [DOI] [PubMed] [Google Scholar]
- 2.McSweeney P, et al. Outpatient PBSC allografts using immunosuppression with low-dose TBI before and cyclosporine (CSP) and mycophenolate mofetil (MMF) after transplant. Blood. 1998;92(Suppl 1):519a. [Google Scholar]
- 3.Kottaridis PD, et al. In vivo CAMPATH-1H prevents graft-versus-host disease following nonmyeloablative stem cell transplantation. Blood. 2000;96(7):2419–25. [PubMed] [Google Scholar]
- 4.Mohty M, et al. Graft-versus-host disease following allogeneic transplantation from HLA-identical sibling with antithymocyte globulin-based reduced-intensity preparative regimen. Blood. 2003;102(2):470–6. doi: 10.1182/blood-2002-12-3629. [DOI] [PubMed] [Google Scholar]
- 5.Sarbassov DD, et al. Rictor, a novel binding partner of mTOR, defines a rapamycin-insensitive and raptor-independent pathway that regulates the cytoskeleton. Curr Biol. 2004;14(14):1296–302. doi: 10.1016/j.cub.2004.06.054. [DOI] [PubMed] [Google Scholar]
- 6.Baan CC, et al. Differential effect of calcineurin inhibitors, anti-CD25 antibodies and rapamycin on the induction of FOXP3 in human T cells. Transplantation. 2005;80(1):110–7. doi: 10.1097/01.tp.0000164142.98167.4b. [DOI] [PubMed] [Google Scholar]
- 7.Zeiser R, et al. Differential impact of mammalian target of rapamycin inhibition on CD4+CD25+Foxp3+ regulatory T cells compared with conventional CD4+ T cells. Blood. 2008;111(1):453–62. doi: 10.1182/blood-2007-06-094482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Battaglia M, Stabilini A, Roncarolo MG. Rapamycin selectively expands CD4+CD25+FoxP3+ regulatory T cells. Blood. 2005;105(12):4743–8. doi: 10.1182/blood-2004-10-3932. [DOI] [PubMed] [Google Scholar]
- 9.Antin JH, et al. Sirolimus, tacrolimus, and low-dose methotrexate for graft-versus-host disease prophylaxis in mismatched related donor or unrelated donor transplantation. Blood. 2003 doi: 10.1182/blood-2003-02-0489. [DOI] [PubMed] [Google Scholar]
- 10.Cutler C, et al. Extended follow-up of methotrexate-free immunosuppression using sirolimus and tacrolimus in related and unrelated donor peripheral blood stem cell transplantation. Blood. 2006 doi: 10.1182/blood-2006-09-046219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alyea EP, et al. Sirolimus, tacrolimus, and low-dose methotrexate as graft-versus-host disease prophylaxis in related and unrelated donor reduced-intensity conditioning allogeneic peripheral blood stem cell transplantation. Biol Blood Marrow Transplant. 2008;14(8):920–6. doi: 10.1016/j.bbmt.2008.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Przepiorka D, et al. Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant. 1994;15(6):825–8. [PubMed] [Google Scholar]
- 13.Gray R. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Annals of Statistics. 1988;16:1141–1154. [Google Scholar]
- 14.Fine J, Gray R. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 15.Cutler C, et al. Sirolimus and tacrolimus without methotrexate as graft-versus-host disease prophylaxis after matched related donor peripheral blood stem cell transplantation. Biol Blood Marrow Transplant. 2004;10(5):328–36. doi: 10.1016/j.bbmt.2003.12.305. [DOI] [PubMed] [Google Scholar]
- 16.Nieto Y, et al. Tacrolimus and mycophenolate mofetil after nonmyeloablative matched-sibling donor allogeneic stem-cell transplantations conditioned with fludarabine and low-dose total body irradiation. Biol Blood Marrow Transplant. 2006;12(2):217–25. doi: 10.1016/j.bbmt.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 17.McSweeney PA, et al. Hematopoietic cell transplantation in older patients with hematologic malignancies: replacing high-dose cytotoxic therapy with graft-versus-tumor effects. Blood. 2001;97(11):3390–400. doi: 10.1182/blood.v97.11.3390. [DOI] [PubMed] [Google Scholar]
- 18.Couriel DR, et al. Acute and chronic graft-versus-host disease after ablative and nonmyeloablative conditioning for allogeneic hematopoietic transplantation. Biol Blood Marrow Transplant. 2004;10(3):178–85. doi: 10.1016/j.bbmt.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 19.Perez-Simon JA, et al. Nonmyeloablative transplantation with or without alemtuzumab: comparison between 2 prospective studies in patients with lymphoproliferative disorders. Blood. 2002;100(9):3121–7. doi: 10.1182/blood-2002-03-0701. [DOI] [PubMed] [Google Scholar]
- 20.Witzig TE, Kaufmann SH. Inhibition of the phosphatidylinositol 3-kinase/mammalian target of rapamycin pathway in hematologic malignancies. Curr Treat Options Oncol. 2006;7(4):285–94. doi: 10.1007/s11864-006-0038-1. [DOI] [PubMed] [Google Scholar]
- 21.Armand P, et al. Improved Survival in Lymphoma Patients Receiving Sirolimus for Graft-Versus-Host Disease Prophylaxis After Allogeneic Hematopoietic Stem-Cell Transplantation With Reduced-Intensity Conditioning. J Clin Oncol. 2008 doi: 10.1200/JCO.2008.17.7279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Claxton DF, Ehmann C, Rybka W. Control of advanced and refractory acute myelogenous leukaemia with sirolimus-based non-myeloablative allogeneic stem cell transplantation. Br J Haematol. 2005;130(2):256–64. doi: 10.1111/j.1365-2141.2005.05600.x. [DOI] [PubMed] [Google Scholar]
- 23.Teachey DT, et al. mTOR inhibitors are synergistic with methotrexate: an effective combination to treat acute lymphoblastic leukemia. Blood. 2008;112(5):2020–3. doi: 10.1182/blood-2008-02-137141. [DOI] [PMC free article] [PubMed] [Google Scholar]