Abstract
Host anti-donor effector T cells represent a major barrier to the successful engraftment of allogeneic donor hematopoietic progenitor and stem cells. Here, administration of a complex of IL-2 and anti-IL 2 antibody (IAC) significantly enhanced donor chimerism early as well as long-term engraftment following reduced intensity conditioning and allogeneic MHC-matched hematopoietic cell transplant (HCT). Timing of administration of this complex was crucial: administration of IAC post-HCT more efficiently facilitated marrow engraftment than pre-HCT treatment. Donor chimerism persisted to >6 months post-HCT. Importantly, this approach clearly suppressed the emergence of host anti-donor CD8 T cells 2 – 3 weeks post-HCT as assessed by tetramer staining. Following in vivo reactivation of IAC-treated and control recipients at >5 months post-HCT with donor antigen, only PBS-treated control marrow allograft recipients responded with tetramer-binding CD8 cells. In total, the present findings support the notion that the transient activation and expansion of host Tregs in situ post-HCT can be explored as a new approach to regulate host alloreactivity post-transplant. Interestingly, direct stimulation of recipient Treg cells in reduced intensity conditioned recipients obviated a requirement for exogenous Treg cell transfusion in this model and may represent a viable alternative to, and/or complement the adaptive transfer of Treg populations in clinical HCT.
Introduction
The regulatory functions of CD4+ CD25+ FoxP3+ cells (Tregs) in maintaining peripheral tolerance1,2 to self antigens has led to studies investigating their use in inhibiting alloreactive T cell responses following allogeneic marrow transplantation.3–5 Naturally occurring Tregs can induce transplantation tolerance in mice.4–6 A significant reduction of lethal graft-versus-host disease (GVHD) has been observed following injection of freshly isolated or ex vivo activated Tregs.5,7,8 Tregs have been reported to act in an antigen-specific manner9–13 and are known to prevent CD8 T cell-mediated allograft rejection.10 Notably, most existing experimental models of allogeneic hematopoietic cell transplantation (HCT) utilizing Tregs have employed the collection, purification and transfer of exogenous populations of donor CD4+ CD25+ Treg cells.12,14,15 Recent studies from our group have reported the presence and persistence of host CD4+CD25+FoxP3+ regulatory cells following reduced intensity conditioning (RIC) and T cell depleted HCT.16 Boyman and colleagues demonstrated that mouse IL-2 complexed with different anti-IL2 mAbs could selectively activate or inhibit certain lymphoid populations.17 Interestingly, one of these complexes (IL-2/JES6-1A12 mAb) efficiently expanded CD4+ CD25+Tregs. To address the application of these cells in facilitating allogeneic hematopoietic and progenitor cell engraftment following HCT, IL-2 complexed with anti-IL 2 mAb17 was administered to RIC recipients following MHC-matched allogeneic HCT. Strikingly, this protocol induced transient activation and expansion of host Tregs accompanied by rapid and significant early chimerism followed by stable, long term donor cell engraftment. Notably, a post-HCT regimen utilizing two injections of IAC was sufficient to inhibit the emergence of host T cells reactive to donor minor histocompatibility transplant antigens.18,19 The present results demonstrate that the in situ activation of host Tregs early following allogeneic MHC-matched, minor transplantation antigen (MiHA) mismatched HCT can regulate host versus graft (HVG) responses to support hematopoietic progenitor cell engraftment. These findings raise the possibility for considering clinical approaches to manipulate endogenous Tregs in situ to facilitate engraftment of marrow allografts and the induction of tolerance.
2. Materials and methods
Mice
All mice were used at 8 to 10 weeks of age. C57BL/6 (B6, H-2b) and 129P3/J (H-2b) were purchased from The Jackson Laboratory. BALB. B (H-2b) mice are bred and maintained at the UM Animal Facility. All experimental procedures were performed in compliance with the UM Division of Veterinary Resources guidelines.
Bone marrow transplant and IAC administration
B6 mice were non-ablatively conditioned (5.5 Gy TBI). Donor bone marrow cells from sex-matched BALB. B or 129P3/J were obtained from femurs and tibia. A single cell suspension of the bone marrow cells was prepared by flushing with a 21-gauge needle and the cells filtered through a 100μ nylon mesh. Donor marrow cells thus obtained were then depleted of T cells via complement mediated lysis using a mixture of T-cell specific antibodies (H0-13-4 hybridoma supernatant, mouse anti-Thy1.2 IgM, ATCC, Rockville, MD; GK1.5, rat IgG2b ascites, mouse anti-CD4, generously provided by Dr. Bruce Blazar, University of Minnesota) and rabbit complement (Cedarlane Laboratories, Burlington, NC). The marrow cells were incubated at 37°C for 45 minutes, washed twice in RPMI and resuspended for HCT. Marrow T-cell depletion was routinely >99%. Recipient mice were transplanted (day 0) with T cell-depleted bone marrow (TCD-BM, 4 × 106) by tail vein (iv) injection.
IAC was prepared using recombinant murine (rm) IL-2 with the anti-IL2 monoclonal antibody (clone JES6-1A12), both reagents purchased from e-Bioscience (San Diego, CA). The reagents were incubated at a molar ratio of 2 to 1 (1μg IL-2 and 5μg Ab) for 15 minutes at RT as previously described.17 The resulting IAC was injected into each mouse intraperitoneally in 500μl PBS immediately after preparation. IAC administration was performed prior to, (days −5 and −3) or following (days +3 and +5) allogeneic HCT.
Flow cytometric analyses
The following antibodies were used for flow cytometry: FITC or cychrome-conjugated anti-B220, biotinylated anti Ly9.1, PE-conjugated streptavidin, FITC labeled anti-CD4 and CD8, FITC-conjugated Ki-67 (all antibodies were purchased from BD Pharmingen, San Diego, CA) and FITC-conjugated FoxP3 (e-Bioscience). Blood was collected from recipient mice by retro-orbital bleeding. In some experiments, complete blood counts (CBC) were performed using a Hemavet 950 Cell Counter (Drew Scientific, Waterbury, CT) to compute absolute numbers (cell numbers/100μl blood) of circulating FoxP3+ CD4+ CD25+ T cells. Red cells were lysed using ammonium chloride lysis buffer (ACK). Lysed cells were washed twice in staining buffer and stained with the appropriate antibody or tetramer reagents. The tetramer H60 (Kb/LTFNYRNL, NIH Tetramer Facility) was used to stain cells as described with minor modification.18 Briefly, recipient blood collected in heparinized tubes, lysed with ACK buffer and incubated at RT for 15 minutes together with cychrome anti-CD8 mAb. The cells were analyzed with the LSR-1 cytometer (Becton Dickinson, San Jose, CA). We sought to determine if IAC-treated hosts are tolerant to donor antigens and whether or not re-encounter with donor antigens post-HCT would induce host anti-donor CD8 T cells detectable by H60 tetramer staining. In some experiments previously transplanted B6 mice which were subsequently chimeric or non-chimeric were injected ip with 2 × 107 BALB. B splenocytes to compare responses to donor antigen in these 2 groups. Analysis of circulating host anti-donor CD8 cells was then performed using H60 tetramer staining between 1 and 3 weeks following donor splenocyte injection.
Cell Culture
In some experiments, splenocytes were prepared from splenic fragments obtained by partial splenectomy of IAC-treated or control recipients of BALB. B T cell depleted bone marrow. Briefly, a laparotomy was performed under general anesthesia using 2% Avertin. A small fragment of the spleen was excised and hemostasis achieved by thermocautery. The abdominal muscle incision was closed with 6-0 silk. A single cell suspension was prepared from these splenic fragments and cultured in RPMI 1640 medium supplemented with 10% FBS, penicillin/streptomycin, L-glutamine and 2-ME (Gibco, Grand Island NY). Cells (2.5 × 105/well) were cultured in quadruplicate in 96 well flat bottom plates in the presence of 1μg/ml LPS (Sigma, St. Louis, MO) or anti-CD3 mAb (Clone 145-2C-11, 20% v/v culture supernatant). Following 3 days of culture, cells were pulsed with tritiated thymidine for 6 hours, harvested and cpm obtained using a Filtermate Harvester (Perkin Elmer, Turku, Finland).
Treg cell enrichment
Treg cell enrichment was performed as described14,16 with some modifications. Briefly, pooled lymph node and spleen single cell suspensions from IAC or PBS-treated mice were first incubated with an anti-CD8 cell antibody (H02.2 hybridoma supernatant), washed and “panned” on Petri dishes coated with goat anti-mouse IgG/IgM (Chemicon International/Millipore, Billerica, MA) to respectively deplete CD8 and B cells. Cells were then coated with PE-conjugated anti-CD25 mAb (PC61, BD Pharmingen) followed by anti-PE magnetic beads for positive selection of CD4+ CD25+ cells on a magnetic column (Miltenyi Biotech, Auburn, CA). This procedure routinely yielded CD4+CD25+ cells with >95% purity (these cells comprised >99% of total CD4 cells).
Statistical analysis
The statistical significance of outcome parameters (frequencies, numbers, MFI values) between experimental and control groups was evaluated by a standard 2-tailed, unpaired Student t-test (95% confidence) using Graphpad Prism 4 software.
Results
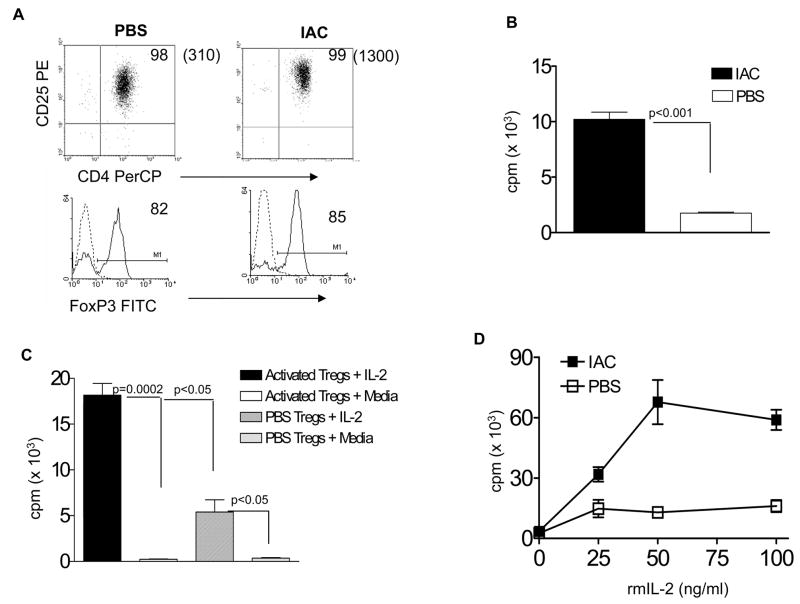
We initially investigated the capacity of IAC treatment to induce the proliferation of CD4+ CD25+ cells in normal mice based upon the observations reported by Boyman et al.17 Highly enriched CD4+ CD25+ Tregs (>95% enriched) were prepared from 129P3/J mice two days following the second IAC injection or from PBS control (Fig 1A) mice were 3H-TdR pulsed for 4hr and examined for incorporation. These populations expressed comparable levels of FoxP3. Tregs from IAC-treated mice exhibited significantly higher levels of 3H-TdR incorporation compared to unmanipulated Tregs (Fig. 1B). These observations are consistent with the activation and proliferation of Tregs following IAC treatment but do not exclude a contribution by FoxP3−cells to this proliferation. Since IAC treatment utilizing JES6-1A12 mAb preferentially expands Tregs bearing high affinity IL-2 receptors17, we next hypothesized that following their exposure to IAC, the selective expansion/activation of these cells would enhance their response to IL-2. In an independent experiment performed, one day following a single IAC treatment or PBS, purified CD4+ CD25+ Tregs from 129P3/J mice were cultured in the presence of rmIL-2 and assessed for 3HTdR incorporation. Tregs from IAC-treated mice incorporated significantly higher levels of tritiated thymidine compared to those obtained from PBS-treated controls (Fig. 1C). These findings illustrate the capacity of IL-2 to expand Tregs in vitro following a single in vivo IAC administration. To further examine the enhanced responsiveness of Treg cells from IAC treated mice to IL-2 induced proliferation, an experiment examined varying doses of rmIL-2 (Fig. 1D). Results indicated that at each dose of cytokine examined, Tregs from IAC treated mice exhibited enhanced proliferation.
Fig. 1. IAC administration induces in vivo activation of Tregs from normal mice and enhances their ability to proliferate in response to rmIL-2 ex-vivo.
(A) Tregs were purified (>95% pure) from spleens and LN of normal 129P3/J mice 2 days after the second infusion with IAC or PBS (n = 2 mice/group) and analyzed for CD25 (dot plots) and FoxP3 expression (histograms), the latter on gated CD4+CD25+ cells. Dot plots: numbers in parentheses represent mean fluorescence intensity (MFI). (B) Two days after the second IAC injection, 5 × 104 purified Tregs from the experiment in Fig. 1A were plated in quadruplicate and pulsed with 3H-TdR for 4hrs. (C) In an independent experiment Tregs from IAC mice exhibit enhanced responsiveness to IL-2 ex vivo. Normal 129P3/J mice were administered a single injection of IAC or PBS (n=2 mice/group). Tregs were purified (>95% pure) one day later, the cells cultured in the presence of 100ng rmIL-2 for 72 hr and pulsed with tritiated thymidine for 4 hr. (D) Tregs from IAC-treated mice exhibit enhanced sensitivity to rmIL-2 ex vivo. Purified Tregs were obtained from 129P3/J mice treated with IAC or PBS as described in Fig. 1C. These Tregs were then cultured (4 × 105/well) in quadruplicate in the presence of the indicated concentrations of rmIL-2. After 72 hrs of culture, the cells were pulsed with 3H-TdR for 6hrs.
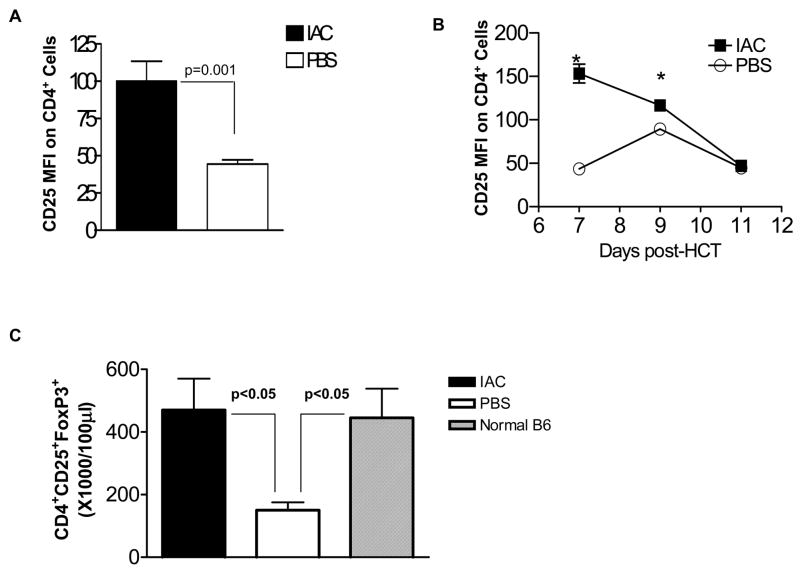
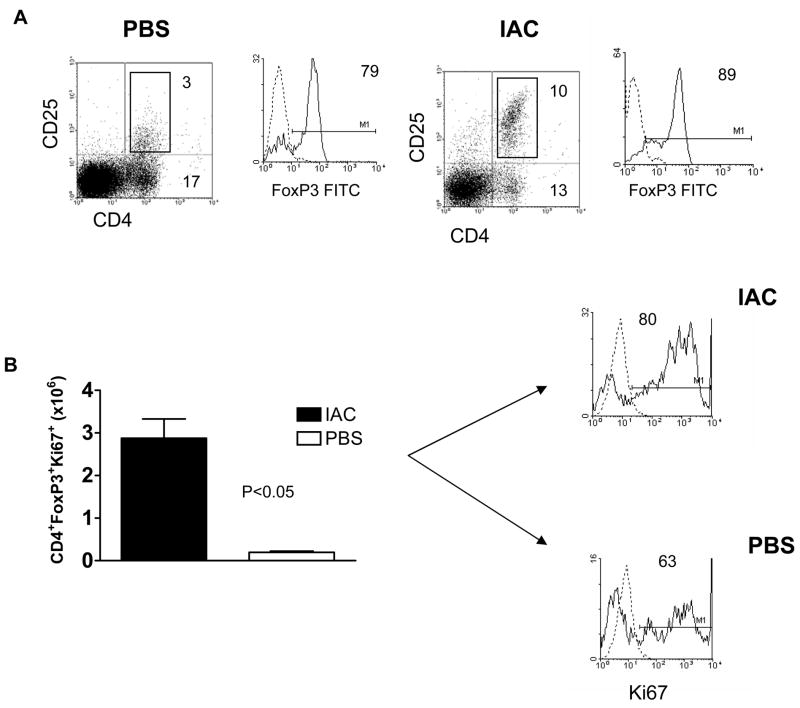
Based on our and others previous findings demonstrating recipient Treg cell presence in B6 recipients following HCT16,20, we next investigated the capacity of IAC to stimulate Treg cells in mice following reduced intensity conditioning and HCT. We administered IAC at days 3 and 5 post-HCT. Notably, a significant upregulation of surface CD25 expression was also observed (Fig. 2A) consistent with the in situ activation of these cells following IAC treatment. To examine the kinetics of CD25 upregulation, MFI was analyzed at 7, 9 and 11 days post-HCT (Fig. 2B). Results indicated the transient nature of in situ Treg CD25 upregulation. Analysis of circulating Tregs 1 week post-HCT, i.e. 2 days following the second IAC injection, clearly demonstrated a significant increase in the numbers of circulating host CD4+ CD25+ FoxP3+Tregs in treated recipients versus levels in PBS-treated control mice (Fig. 2C). Notably, the numbers of Treg cells in IAC treated, conditioned and transplanted recipients were comparable to the circulating numbers in normal, unconditioned B6 mice (Fig. 2C). Tregs in IAC-treated hosts constituted >40% of total peripheral blood CD4+ cells, representing a >2-fold percentage increase of circulating Tregs over control mice (data not shown). These observations regarding activation in recipient PBL were recapitulated in the spleen one week post-HCT (Fig. 3A, IAC-treated). Furthermore, the intracellular expression of Ki-67 was enhanced in splenic FoxP3+ Tregs of IAC-treated recipients vs. PBS control mice (Fig. 3B, IAC-treated) indicating that host Tregs exposed to IAC entered the cell cycle and were actively proliferating in this compartment. To determine if more frequent or earlier IAC injection could enhance these observations, recipients were injected at days 1, 3, 5 and 6 post-HCT (data not shown). No significant differences in the % of CD25+ CD4 T cells or the levels of CD25 expression were observed. Thus all subsequent experiments involving IAC post-HCT utilized the two injection (i.e. days +3 and +5) regimen.
Fig. 2. IAC induces transient upregulation of CD25 and expansion of host Tregs following MHC-matched HCT.
(A) B6 (H-2b) mice were non-ablatively conditioned (5.5Gy TBI) and 24 hours later transplanted with 4 × 106 129P3/J B6 (H-2b) T-cell depleted bone marrow (Day 0). Three and 5 days post-HCT, recipient mice were administered IAC ip. At one week post-HCT, circulating PBL were analyzed for CD25 expression on CD4+ cells, represented by MFI (results represent MFI values ± SEM; n = 3 mice/group, 3 independent experiments). (B) In an independent experiment, B6 mice were conditioned and transplanted with 129P3/J as described in Fig. 1A. IAC or PBS was infused at days +3 and +5. At the indicated intervals post-HCT, PBL were analyzed for CD25 MFI on CD4+ cells (*p < 0.05; n = 3 mice/group). Data presented as MFI ± SEM. Data points without error bars indicate SEM values within symbols. IAC induced rapid and transient activation of host Treg cells. (C) B6 mice conditioned with 5.5 Gy TBI and transplanted with 4 × 106 BALB. B TCD-BM were infused with IAC or PBS at days +3 and +5. Recipient mice or normal B6 PBL were then analyzed for circulating CD4+ CD25+ FoxP3+ cells (gated on mononuclear cells) at 1 week post-HCT. Results represent mean cell counts/100μl ± SEM (n = 3 pools of 2 mice/group; normal B6 control mice: n = 4 mice/group, individual data points analyzed). IAC infusion resulted in marked expansion of circulating host Treg populations post-HCT.
Fig. 3. In situ activation of host Tregs induces upregulation of CD25 and Ki-67 expression in the spleen.
B6 mice were conditioned with 5.5 Gy TBI and one day later transplanted with 4 × 106 BALB. B TCD-BM. A) One day after the second IAC injection, splenocytes from PBS (left) or IAC (right) infused recipients were analyzed for CD25 (dot plot) and FoxP3 expression (histograms). The dotted curve in the histogram represents isotype control staining. Results are from a representative mouse in each group (n = 3–4/group) from one of two independent experiments performed. B) Splenocytes from these PBS or IAC treated mice were analyzed for intracellular expression of Ki-67. Tregs expressing FoxP3 were analyzed on gated mononuclear cells (mean ± SEM). Numbers on histograms represent % Ki-67+ cells gated on CD4+ FoxP3+ T cells. Histogram markers represent intersect between Ki-67 (solid) and isotype control (dotted) mAb staining.
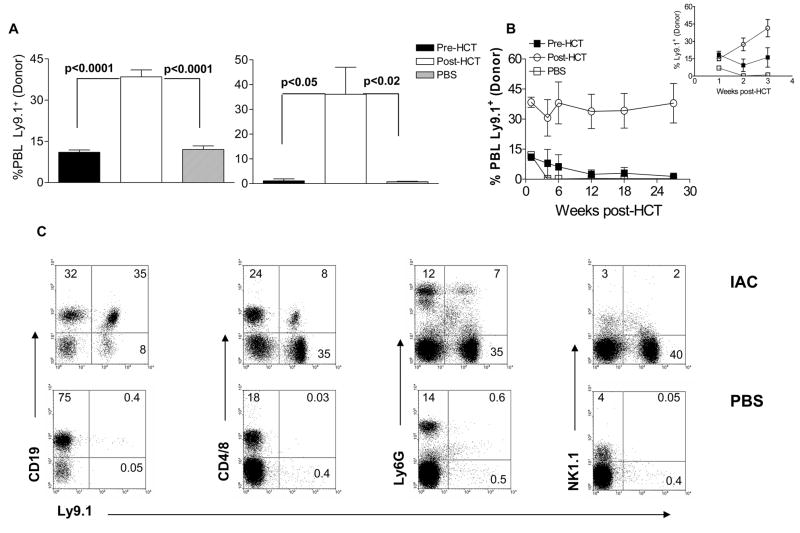
One potential consequence of the increased number of host Treg cells after RIC and HCT could be regulation of host anti-donor responses which inhibit engraftment. Peripheral blood was therefore analyzed for the presence of circulating donor (Ly9.1+, CD19, CD4/CD8 and granulocyte) cells in MHC-matched MiHA mismatched recipients following RIC and TCD-HCT (5.5 Gy TBI) in BALB. B or 129P3/J → B6 HCT models.19 As early as 1 week post-BALB. B (or 129P3/J data not shown) transplant into B6 recipients, a dramatic and significant increase in the percent peripheral lymphoid donor cells was present in recipients treated with IAC after HCT (Days +3, +5) versus recipients treated with IAC pre-HCT (Days −5, −3) or in PBS-treated controls (Fig. 4A). In recipients treated with IAC post-HCT, the levels of peripheral donor chimerism underwent a rapid increase over the next 3 weeks and persisted for >6 months post-HCT (Fig. 4B and inset). As noted above, we again observed no significant differences at this time in the levels of donor chimerism between mice receiving 2 vs. 4 IAC injections during the first week post-HCT (data not shown). During the later intervals (6 months) post-HCT, significant levels of myeloid (Ly6G+) as well lymphoid cells (CD19+ and CD4/CD8+, NK1.1+) were readily detectable in IAC-treated recipients, consistent with stable multi-lineage reconstitution in recipients with donor cells (Fig. 4C).
Fig. 4. Administration of IAC post-HCT enhances early and stable long-term peripheral donor (Ly9.1+) chimerism.
B6 mice were sublethally irradiated (5.5 Gy TBI) and injected with IAC pre-HCT (days −5 and −3) or post-HCT (days +3 and +5). Recipient mice received 4 × 106 BALB. B TCD-BM (day 0). Peripheral donor cell (Ly9.1+) frequency was assessed by staining recipient PBL with Ly9.1 at 1 week (A, left) and 27 weeks (A, right) post-HCT. No lethality was observed in any group in this experiment. (B) Kinetics of donor chimerism in the BALB. B → B6 experiment described in 4A following RIC, HCT and pre-HCT or post-HCT IAC administration. Inset represents the rapid increase in donor chimerism levels in IAC infused recipients during the initial 3 weeks post-HCT. (C) Representative dot plots of recipient PBL stained for Ly9.1, CD19, CD4/CD8 and NK1.1 gated on mononuclear cells and Ly6G gated on total donor cells in IAC-treated (top panels) or PBS-treated (bottom panels) control hosts. Data represent dot plots from individual B6 animals 21 weeks post-HCT with BALB. B TCD-BM as described above. Experiments presented above reflect 4 independent experiments (n = 3 – 7 mice/group; results = % donor cells ± SEM). The results demonstrate the presence of multi-lineage donor chimerism at >5 months post-transplant.
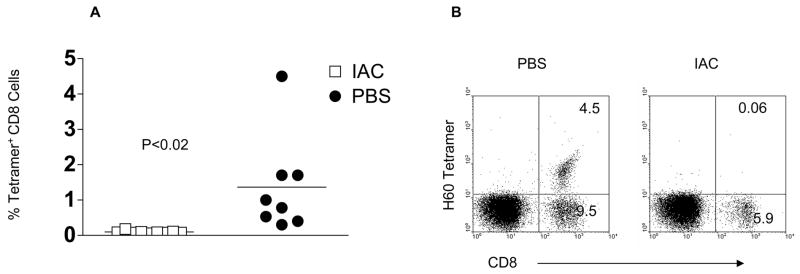
These observations were consistent with the hypothesis of host Treg suppression of HVG. To address this issue, BALB. B TCD-BM cells were administered to RIC B6 recipients followed by IAC at days +3 and +5. The H60 tetramer detects TCRs on B6 host CD8 cells that are reactive to the MiHA immunodominant H60 peptide expressed in donor BALB. B and 129P3/J hematopoietic lymphoid cells.18,19,21 The presence of these CD8 tetramer+ cells becomes readily detectable at 2 – 3 weeks post-HCT in unmanipulated, RIC B6 recipients of BALB. B or 129P3/J TCD-BM (Fig. 5).19 In contrast, there was a consistent and statistically significant reduction (p < 0.02) in the frequency of these CD8+ T cells in recipients treated with IAC post-HCT compared to both recipients treated with IAC pre-HCT (data not shown) and to control, PBS-treated B6 recipients (Fig. 5A). These observations indicate that the response of donor-specific host CD8 cells following encounter with donor MiHA was suppressed in recipients treated with IAC post-HCT as illustrated by their failure to expand early post-HCT. Notably, a consistent observation was that in recipients with detectable levels of tetramer binding CD8 T cells at 2 – 3 weeks post-HCT, there was an absence of peripheral donor chimerism.
Figure 5. Administration of IAC post-HCT suppresses the emergence and early expansion of host anti-donor CD8 T cells.
PBL from the indicated IAC treatment groups (n=9 for IAC; n = 8 for PBS) were stained with the H60 tetramer 2 weeks post-HCT. The frequency of circulating tetramer-binding cells is expressed as tetramer+ CD8 cells as % of gated mononuclear cells (A) *p<0.02 (IAC-treated vs. PBS-treated control recipients). B) Representative dot plots for H60 staining obtained from IAC treated and PBS-treated B6 recipients of BALB. B bone marrow.
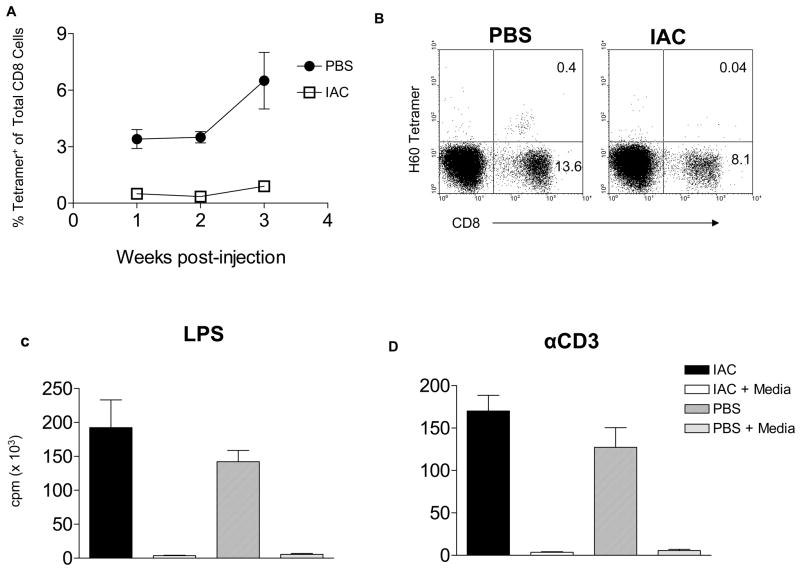
Following the initial response of host CD8 T cells to donor antigens in the marrow allograft detected by H60 tetramer staining, the frequency of these cells decline to barely detectable levels (data not shown and18). However, upon re-activation with BALB. B antigens, host anti-donor CD8 cell populations are induced to expand and the elevated levels following subsequent re-activation are sustained for long periods.18 We next hypothesized that chimeric, IAC-treated B6 recipients of donor marrow allograft would be tolerant to donor antigens and therefore unresponsive to challenge with donor spleen cells. In contrast, PBS-treated B6 mice that eliminated donor allografts should respond to donor antigens upon reactivation with donor splenocytes. 2 × 107 BALB. B splenocytes were administered to IAC-treated or PBS-treated B6 recipients of BALB. B bone marrow 27 weeks post-HCT and the frequency of circulating host tetramer+ CD8 cells analyzed in these mice (Fig. 6). One to 3 weeks following BALB. B splenocyte challenge, the frequency of circulating host tetramer+ CD8 T cells was found to be markedly elevated in PBS-treated B6 control recipients but barely detectable in the chimeric recipients (Fig. 6A, B). This in vivo response by PBS-treated recipients indicates the immunological capacity of these animals to respond to donor antigen challenge. However, the lack of the anti-H60 CD8 T cell response by chimeric, IAC-treated mice could be a result of either a specific or a more global unresponsive state. To distinguish between these possibilities, partial splenectomies were performed in IAC and control recipients ~7 months post-HCT and splenocytes were cultured in the presence of LPS or anti-CD3 mAb. Responses to LPS (Fig. 6C) and anti-CD3 mAb stimulation (Fig. 6D) were comparable (p>0.05) regardless of whether the animals had or had not engrafted with donor hematopoietic cells. These findings demonstrate that although engrafted animals fail to respond to donor transplantation antigens (i.e. H60) they are not globally immunodeficient and thus support the notion that tolerance to donor MiHA is present in these IAC treated recipients.
Figure 6. Chimeric, IAC-treated B6 recipients of BALB. B marrow are tolerant to donor antigens 21 weeks post-HCT.
B6 mice treated with IAC or PBS (n = 3/group) were injected with 2 × 107 BALB. B splenocytes at 27 weeks post-HCT. (A) One to 3 weeks later, PBL were analyzed for tetramer-binding CD8 cells. Results are presented as the average % tetramer positive cells of total CD8 cells from 3 individual mice. (B) Representative dot plot of H60 tetramer (PE) CD8 staining (cychrome) cells from each group is shown. (C, D) Suppression of host anti-donor responses in chimeric, IAC-treated recipients of BALB. B marrow is not associated with inhibition of host responses to LPS and anti-CD3 activation. Post-HCT IAC treated or PBS-treated mice were partially splenectomized at ~7 months post-HCT and splenocytes cultured in quadruplicate in the presence of LPS (C) or anti-CD3 (D) for 72 hrs, and then pulsed with 3HTdR for 6hrs.
Discussion
The present studies demonstrate for the first time that the administration of IL-2 and anti-IL2 mAb complex post-HCT was highly efficient in the facilitation of hematopoietic cell engraftment following MHC-matched allogeneic HCT in RIC recipients. The protocol employed consistently induced significant expansion and activation of circulating host Tregs as assessed by the elevated numbers and frequency of these cells, their up-regulation of CD25, thymidine incorporation and Ki-67 expression following IAC administration. Together with the finding that following IAC infusion, expansion of host anti-donor alloantigen reactive CD8 T cells was not observed, we hypothesize that stimulation of host Tregs inhibits HVG to support donor hematopoietic cell engraftment.
A number of laboratories have reported the ability of co-transplanted donor Treg cell populations to modulate GVHD and facilitate engraftment.13,14,22 The rationale underlying such studies was the supposition that activated Treg cells can inhibit alloreactive T cell responses which complicate such transplants. Treg cells of host origin have been less well explored, however, un-manipulated Tregs of host origin do not appear to readily enhance chimerism following allogeneic HCT.6,23 Recent studies by Boyman and colleagues reported the capacity of IL-2/anti-IL2 mAb conjugates to expand CD4+CD25+ T cells in situ in normal mice17. We therefore sought to determine whether it is possible to expand host Treg cells in HCT recipients using IAC injection as a strategy to regulate HVG alloreactive T cell responses in order to facilitate hematopoietic engraftment. Observations in reduced intensity conditioned animals demonstrated that host Treg cells present could in fact be efficiently stimulated by one or two IAC injections evidenced by enhanced CD25 expression as well proliferative activity by these cells (Figs. 1–3). Thus, similar to Tregs in normal mice (Fig. 1), these findings show that residual host Treg cells present following RIC and transplant16,20 could be activated and expanded in situ by systemic administration of IAC. The efficacy of the IAC protocol employed here was evidenced by the finding that the numbers of circulating Tregs in complex infused HCT B6 recipients early post-transplant were indistinguishable from Treg numbers in normal, unconditioned, non-transplanted B6 mice.
We then examined the consequence of these IAC activated host Tregs on hematopoietic outcome following transplant of T cell depleted, MHC-matched MiHA mismatched marrow. Our studies clearly illustrate consistent and dramatic enhancement of donor chimerism in reduced intensity conditioned hosts following MHC-matched allogeneic HCT and IAC treatment post-HCT. Notably, the early enhanced levels of circulating donor hematopoietic cells in such recipients persisted long term as evidenced by donor chimerism >6 months post-HCT. These observations demonstrate that hematopoietic donor lymphoid and myeloid progenitor cell engraftment took place following treatment with IAC post-HCT. Notably in these studies, the timing of IAC administration was found to be crucial in enhancing initial donor chimerism and prolonging allogeneic marrow engraftment post-HCT. We propose that early (1 – 2 weeks) post-HCT, the activation of host Tregs following IAC administration enhances their capacity to suppress host anti-donor CD8 T cell responses that mediate resistance to engraftment in this MHC-matched HCT model. The consistent absence of detectable peripheral H60 tetramer binding CD8 cells 2 – 3 weeks post-HCT in IAC-treated marrow allograft recipients indicated that there was significant inhibition of the initial activation and expansion of these host effector CD8 T cells. These findings are consistent with the notion that the suppression of host anti-donor CD8 T cells is a key mechanism underlying the enhancement of donor chimerism. Ex vivo, Tregs have been shown to efficiently regulate CD8 T cell responses by suppressing proliferation and IFNγ production induced by antigen-or polyclonal stimulation.24
A recent study reported that the adoptive transfer of ex vivo activated Tregs of host origin, induced antigen-specific tolerance to donor marrow in MHC-mismatched HCT.13 The findings reported here suggest that the strategy of 2 injections of IAC post-HCT may be sufficient to induce tolerance to donor antigens in the MHC-matched, MiHA mismatched model. As mentioned above, the inhibition of the expansion of host anti-donor reactive CD8 T cells was apparent within several weeks post-transplant using this regimen. Importantly, assessment for these anti-donor alloreactive T cells months later in transplanted mice indicated that although they were readily induced by introducing donor alloantigen bearing cells in non-IAC, i.e. non-chimeric recipients (that had previously rejected the donor marrow allograft), our attempts to elicit their “re-emergence” in IAC treated chimeric recipients were unsuccessful (Fig. 6). The capacity of both chimeric and non-chimeric recipients to respond equivalently to anti-CD3 and LPS stimulation ruled out global immunodeficiency in chimeric animals and further supports the notion that tolerance to donor alloantigen is present in these post-transplant IAC treated recipients. We conclude that treatment with IAC post-HCT resulted in efficient host reconstitution with donor derived allogeneic hematopoietic cells and speculate that central tolerance contributes to the pattern of responses we observed.
Together, the findings in our study plausibly demonstrate for the first time that a brief, timely in vivo IAC regimen in reduced intensity conditioned recipients of MHC-matched marrow allografts leads to enhanced and stable, long term donor chimerism. Suppression of host anti-donor responses in IAC-treated mice early post-HCT is consistent with regulation, i.e. inhibition of the barrier to engraftment shortly after transplant as evidenced by the failure of host anti-donor CD8 T cells to emerge during the first 4 weeks post-HCT. Thus, the strategy employing IAC in our pre-clinical MHC-matched allogeneic HCT may support the use of this treatment for enhancing marrow progenitor engraftment and tolerance induction in selected clinical MiHA-mismatched allogeneic marrow recipients. Notably, following their in vivo activation by IAC, Tregs were rendered more sensitive to IL-2 driven ex-vivo proliferation and expansion (Fig. 1). These findings suggest that in vivo, a single IAC treatment followed by IL-2 may be sufficient to achieve equivalent levels of chimerism as observed by the multiple IAC treatments used here. IL-2 has been examined in T cell replete experimental BMT and has been effectively used in the clinic for therapy.25–27 Recent studies have reported that such cytokine therapy can result in increased numbers of Treg cells in clinical and experimental disorders.28–32 Treg expansion induced by IAC could therefore be considered to increase the numbers of Tregs targeted by IL-2 in the settings of autoimmune disease and transplantation to further their activation and expansion. This strategy would minimize the use of cytokine-antibody complex, thus reducing the risk of immune complex disease.
Acknowledgments
We gratefully acknowledge the NIH Tetramer Facility, Emory, GA for providing the H60 tetramer. This work is supported by NIH grants CA120776-01 and AI46689-5A1 (to RL) and the University of Miami Scientific Awards Committee (AS).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Shevach EM, DiPaolo RA, Andersson J, et al. The lifestyle of naturally occurring CD4+ CD25+ Foxp3+ regulatory T cells. Immunol Rev. 2006;212:60–73. doi: 10.1111/j.0105-2896.2006.00415.x. [DOI] [PubMed] [Google Scholar]
- 2.Sakaguchi S, Ono M, Setoguchi R, et al. Foxp3+ CD25+ CD4+ natural regulatory T cells in dominant self-tolerance and autoimmune disease. Immunol Rev. 2006;212:8–27. doi: 10.1111/j.0105-2896.2006.00427.x. [DOI] [PubMed] [Google Scholar]
- 3.Jiang S, Lechler RI, He XS, et al. Regulatory T cells and transplantation tolerance. Hum Immunol. 2006;67:765–76. doi: 10.1016/j.humimm.2006.07.013. [DOI] [PubMed] [Google Scholar]
- 4.Wood KJ, Sakaguchi S. Regulatory T cells in transplantation tolerance. Nat Rev Immunol. 2003;3:199–210. doi: 10.1038/nri1027. [DOI] [PubMed] [Google Scholar]
- 5.Nguyen VH, Zeiser R, Negrin RS. Role of naturally arising regulatory T cells in hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2006;12:995–1009. doi: 10.1016/j.bbmt.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 6.Joffre O, van Meerwijk JP. CD4(+)CD25(+) regulatory T lymphocytes in bone marrow transplantation. Semin Immunol. 2006;18:128–35. doi: 10.1016/j.smim.2006.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Taylor PA, Lees CJ, Blazar BR. The infusion of ex vivo activated and expanded CD4(+)CD25(+) immune regulatory cells inhibits graft-versus-host disease lethality. Blood. 2002;99:3493–9. doi: 10.1182/blood.v99.10.3493. [DOI] [PubMed] [Google Scholar]
- 8.Hoffmann P, Ermann J, Edinger M, et al. Donor-type CD4(+)CD25(+) regulatory T cells suppress lethal acute graft-versus-host disease after allogeneic bone marrow transplantation. Journal of Experimental Medicine. 2002;196:389–99. doi: 10.1084/jem.20020399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cohen JL, Trenado A, Vasey D, et al. CD4(+)CD25(+) immunoregulatory T Cells: new therapeutics for graft-versus-host disease. J Exp Med. 2002;196:401–6. doi: 10.1084/jem.20020090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kingsley CI, Karim M, Bushell AR, et al. CD25+CD4+ Regulatory T Cells Prevent Graft Rejection: CTLA-4- and IL-10-Dependent Immunoregulation of Alloresponses. J Immunol. 2002;168:1080–6. doi: 10.4049/jimmunol.168.3.1080. [DOI] [PubMed] [Google Scholar]
- 11.van Maurik A, Herber M, Wood KJ, et al. Cutting edge: CD4+CD25+ alloantigen-specific immunoregulatory cells that can prevent CD8+ T cell-mediated graft rejection: implications for anti-CD154 immunotherapy. J Immunol. 2002;169:5401–4. doi: 10.4049/jimmunol.169.10.5401. [DOI] [PubMed] [Google Scholar]
- 12.Joffre O, Gorsse N, Romagnoli P, et al. Induction of antigen-specific tolerance to bone marrow allografts with CD4+CD25+ T lymphocytes. Blood. 2004;103:4216–21. doi: 10.1182/blood-2004-01-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Joffre O, Santolaria T, Calise D, et al. Prevention of acute and chronic allograft rejection with CD4+CD25+Foxp3+ regulatory T lymphocytes. Nat Med. 2008;14:88–92. doi: 10.1038/nm1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hanash AM, Levy RB. Donor CD4+CD25+ T cells promote engraftment and tolerance following MHC-mismatched hematopoietic cell transplantation. Blood. 2005;105:1828–36. doi: 10.1182/blood-2004-08-3213. [DOI] [PubMed] [Google Scholar]
- 15.Nguyen VH, Shashidhar S, Chang DS, et al. The impact of regulatory T cells on T-cell immunity following hematopoietic cell transplantation. Blood. 2008;111:945–53. doi: 10.1182/blood-2007-07-103895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bayer AL, Jones M, Chirinos J, et al. Host CD4+CD25+ T cells can expand and comprise a major component of the Treg compartment after experimental HCT. Blood. 2009;113:733–43. doi: 10.1182/blood-2008-08-173179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boyman O, Kovar M, Rubinstein MP, et al. Selective stimulation of T cell subsets with antibody-cytokine immune complexes. Science. 2006;311:1924–7. doi: 10.1126/science.1122927. [DOI] [PubMed] [Google Scholar]
- 18.Choi EY, Yoshimura Y, Christianson GJ, et al. Quantitative analysis of the immune response to mouse non-MHC transplantation antigens in vivo: The H60 histocompatibility antigen dominates over all others. Journal of Immunology. 2001;166:4370–9. doi: 10.4049/jimmunol.166.7.4370. [DOI] [PubMed] [Google Scholar]
- 19.Shatry A, Roopenian DC, Levy RB. A single donor MiHA epitope can elicit a host CD8 T memory population that mediates resistance to engraftment of marrow allografts. Biol Blood Marrow Transplant. 2007;13:293–8. doi: 10.1016/j.bbmt.2006.12.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Anderson BE, McNiff JM, Matte C, et al. Recipient CD4+ T cells that survive irradiation regulate chronic graft-versus-host disease. Blood. 2004;104:1565–73. doi: 10.1182/blood-2004-01-0328. [DOI] [PubMed] [Google Scholar]
- 21.Zimmerman Z, Shatry A, Deyev V, et al. Effector cells derived from host CD8 memory T cells mediate rapid resistance against MiHA-mismatched allogeneic marrow grafts without participation of perforin, FasL and the simultaneous inhibition of three TNF family effector pathways. Biol Blood Marrow Transplant. 2005;11:576–86. doi: 10.1016/j.bbmt.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 22.Taylor PA, Panoskaltsis-Mortari A, Swedin JM, et al. L-Selectin(hi) but not the L-selectin(lo) CD4(+)25(+) T-regulatory cells are potent inhibitors of GVHD and BM graft rejection. Blood. 2004;104:3804–12. doi: 10.1182/blood-2004-05-1850. [DOI] [PubMed] [Google Scholar]
- 23.Steiner D, Brunicki N, Bachar-Lustig E, et al. Overcoming T cell-mediated rejection of bone marrow allografts by T-regulatory cells: synergism with veto cells and rapamycin. Exp Hematol. 2006;34:802–8. doi: 10.1016/j.exphem.2006.02.017. [DOI] [PubMed] [Google Scholar]
- 24.Piccirillo CA, Shevach EM. Cutting edge: control of CD8+ T cell activation by CD4+CD25+ immunoregulatory cells. J Immunol. 2001;167:1137–40. doi: 10.4049/jimmunol.167.3.1137. [DOI] [PubMed] [Google Scholar]
- 25.Rosenberg SA, Lotze MT, Yang JC, et al. Prospective randomized trial of high-dose interleukin-2 alone or in conjunction with lymphokine-activated killer cells for the treatment of patients with advanced cancer. J Natl Cancer Inst. 1993;85:622–32. doi: 10.1093/jnci/85.8.622. [DOI] [PubMed] [Google Scholar]
- 26.Wei S, Kryczek I, Edwards RP, et al. Interleukin-2 administration alters the CD4+FOXP3+ T-cell pool and tumor trafficking in patients with ovarian carcinoma. Cancer Res. 2007;67:7487–94. doi: 10.1158/0008-5472.CAN-07-0565. [DOI] [PubMed] [Google Scholar]
- 27.Murphy WJ, Bennett M, Kumar V, et al. Donor-type activated natural killer cells promote marrow engraftment and B cell development during allogeneic bone marrow transplantation. J Immunol. 1992;148:2953–60. [PubMed] [Google Scholar]
- 28.Ahmadzadeh M, Rosenberg SA. IL-2 administration increases CD4+ CD25(hi) Foxp3+ regulatory T cells in cancer patients. Blood. 2006;107:2409–14. doi: 10.1182/blood-2005-06-2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang H, Chua KS, Guimond M, et al. Lymphopenia and interleukin-2 therapy alter homeostasis of CD4+CD25+ regulatory T cells. Nat Med. 2005;11:1238–43. doi: 10.1038/nm1312. [DOI] [PubMed] [Google Scholar]
- 30.Tang Q, Adams JY, Penaranda C, et al. Central role of defective interleukin-2 production in the triggering of islet autoimmune destruction. Immunity. 2008;28:687–97. doi: 10.1016/j.immuni.2008.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zorn E, Nelson EA, Mohseni M, et al. IL-2 regulates FOXP3 expression in human CD4+CD25+ regulatory T cells through a STAT-dependent mechanism and induces the expansion of these cells in vivo. Blood. 2006;108:1571–9. doi: 10.1182/blood-2006-02-004747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zorn E, Mohseni M, Kim H, et al. Combined CD4+ donor lymphocyte infusion and low-dose recombinant IL-2 expand FOXP3+ regulatory T cells following allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2009;15:382–8. doi: 10.1016/j.bbmt.2008.12.494. [DOI] [PMC free article] [PubMed] [Google Scholar]