Abstract
Activity-dependent changes in the strength of synaptic connections in the hippocampus are central for cognitive processes such as learning and memory storage. In this study, we reveal an activity-dependent presynaptic mechanism that is related to the modulation of synaptic plasticity. In acute mouse hippocampal slices, high-frequency stimulation (HFS) of the mossy fiber (MF)-CA3 pathway induced a strong and transient activation of extracellular-regulated kinase (ERK) in MF giant presynaptic terminals. Remarkably, pharmacological blockade of ERK disclosed a negative role of this kinase in the regulation of a presynaptic form of plasticity at MF-CA3 contacts. This ERK-mediated inhibition of post-tetanic enhancement (PTE) of MF-CA3 synapses was both frequency- and pathway-specific and was observed only with HFS at 50 Hz. Importantly, blockade of ERK was virtually ineffective on PTE of MF-CA3 synapses in mice lacking synapsin I, 1 of the major presynaptic ERK substrates, and triple knockout mice lacking all synapsin isoforms displayed PTE kinetics resembling that of wild-type mice under ERK inhibition. These findings reveal a form of short-term synaptic plasticity that depends on ERK and is finely tuned by the firing frequency of presynaptic neurons. Our results also demonstrate that presynaptic activation of the ERK signaling pathway plays part in the activity-dependent modulation of synaptic vesicle mobilization and transmitter release.
Keywords: MAP kinase, mossy fibers, post-tetanic potentiation
The extracellular-regulated kinase (ERK) signaling pathway plays a crucial role in the regulation of activity-dependent changes in the strength of synaptic transmission in the hippocampus (1, 2). Indeed, multiple studies have shown that altering the normal function of ERK causes severe impairments of both short- and long-term forms of synaptic plasticity at hippocampal synapses (3–6, but see also ref. 7). Remarkably, behavioral analyses of animals with altered ERK signaling have revealed a crucial involvement of this cascade in learning and memory (8–10), supporting the idea that ERK-dependent synaptic plasticity is essential for cognitive processes.
Because potential molecular substrates of ERK are present in distinct neuronal compartments (2), the precise subcellular location in which ERK activation occurs is a major determinant of ERK function. For example, phosphorylated ERK translocates into the nucleus and regulates gene expression in response to synaptic activity by directly or indirectly phosphorylating transcription factors (2, 11, 12). Moreover, there is increasing evidence that ERK phosphorylates target proteins in both dendritic (13–15) and axonal compartments (16, 17). Synapsin I (Syn I), a synaptic vesicle-associated phosphoprotein implicated in the regulation of synaptic strength, is a major ERK substrate in nerve terminals and presents 3 ERK-dependent phosphorylation sites (18, 19). Interestingly, it has been shown that ERK phosphorylation regulates the interaction of Syn I with the actin cytoskeleton, a mechanism that is considered important in the regulation of transmitter release (20). There is strong evidence that ERK activation in axon terminals contributes to regulate the distribution and recycling of synaptic vesicles in both invertebrate and vertebrate neurons (16, 21–23), thus supporting a role for this kinase in the modulation of presynaptic forms of plasticity, possibly via phosphorylation of Syn I (20, 21, 24). However, the physiological conditions under which presynaptic ERK becomes activated and the mechanisms by which it contributes to hippocampal synaptic plasticity are not known.
In the present study, we investigated the spatiotemporal dynamics of ERK activation in mouse hippocampal slices by using immunofluorescence and confocal microscopy. We report the occurrence of a robust phosphorylation of ERK in mossy fiber (MF) axon terminals following high-frequency stimulation (HFS) of MF-CA3 connections. In addition, we show that pharmacological blockade of ERK increases post-tetanic enhancement (PTE) at these synapses, but does not interfere with short-term plasticity elicited at the CA3-CA1 pathway. Finally, we demonstrate that presynaptic action of ERK is achieved through a frequency-dependent mechanism involving Syn I.
Results
Role of ERK in Short-Term Synaptic Plasticity.
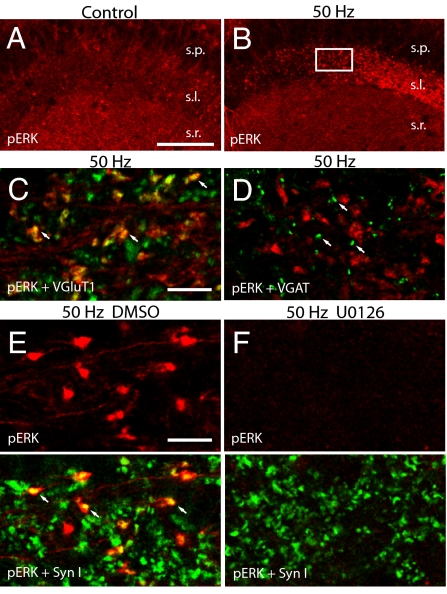
Activity-dependent short-term enhancement of synaptic transmission has been shown to be caused mainly by presynaptic modifications (25). Although it is known that short-term presynaptic plasticity critically depends on rapid elevations of intracellular Ca2+ in presynaptic nerve terminals, the precise molecular mechanisms are still undefined (26, 27). Here we asked whether PTE of MF-CA3 synapses is regulated by local activation of ERK in presynaptic compartments. We initially analyzed the subcellular localization of phospho-ERK (pERK) in acute hippocampal slices by using a monoclonal antibody directed against the dually phosphorylated form of ERK1/2. Confocal analysis of CA3 stratum lucidum, where MF terminals are positioned (28), revealed that very few pERK-positive profiles were detectable in untetanized slices, suggesting that only minor ERK activation is present under baseline stimulation (Fig. 1A). Remarkably, PTE induced by tetanic stimulation (1 s at 50 Hz; see Fig. 2D) caused the appearance of pERK-positive puncta in stratum lucidum (Fig. 1B). The size (>3 μm in diameter) and localization of immunoreactive puncta strongly suggested ERK activation in giant MF terminals (28). In addition, double-immunofluorescence labeling revealed that ERK phosphorylation occurred exclusively in vesicular glutamate transporter 1 (VGluT1)-positive varicosities (Fig. 1C), but not in vesicular GABA transporter (VGAT)-positive axon terminals (Fig. 1D). These results indicate that PTE-induced activation of ERK occurs exclusively in excitatory MF varicosities and exclude the possibility of a concomitant activation of ERK in GABAergic terminals. Notably, ERK activation was selective for MF terminals, and no significant increase of pERK immunoreactivity was observed in CA3 pyramidal neurons (Fig. 1B) or in the cell body of granule cells in the dentate gyrus.
Fig. 1.
ERK activation occurs in excitatory giant terminals after tetanization of the CA3-MF pathway. (A and B) Confocal images show the distribution of immunolabeling for pERK in CA3 fields of a representative control slice that received baseline stimulation (A) and a potentiated slice (B) that was harvested 2 min after tetanization (1 × 50 Hz) of MF-CA3 synapses (s.l., stratum lucidum; s.r., stratum radiatum; s.p., stratum piramidale). (C and D) Representative confocal micrographs showing pERK-positive MF terminals in CA3 stratum lucidum after 50-Hz stimulation. pERK-IR (red) is clearly colocalized (arrows) with VGluT1 (green, C) but not with VGAT (green, D) immunosignal (arrows). (E and F) Confocal micrographs show double labeling for pERK (red) and Syn I (green) in CA3 stratum lucidum of representative tetanized (1 × 50 Hz) slices in the presence of either vehicle (E) or U0126 20 μM (F). Note the colabeled profiles representing ERK activation in Syn I-positive mossy fiber terminals (arrows in E) and the complete absence of pERK labeling after application of U0126 (F). White box in B indicates the location where images C–F were acquired. [Scale bars: 100 μm (A and B); 10 μm (C–F).]
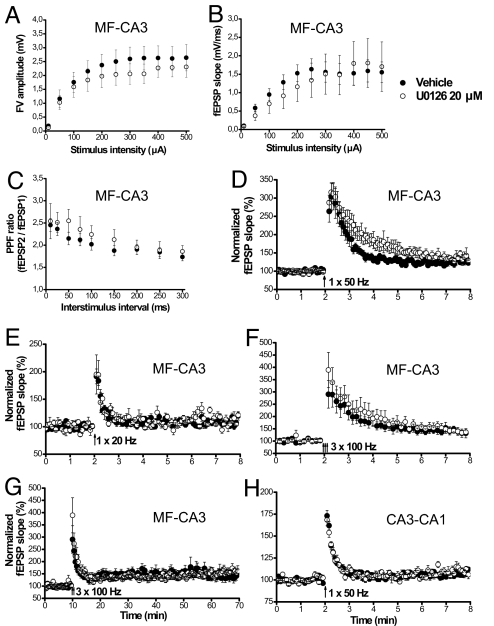
Fig. 2.
ERK modulates short-term presynaptic plasticity in the MF-CA3 pathway at a specific stimulation frequency. (A and B) Basal transmission in MF was unaltered by U0126 application. Input–output curves show that both fiber volley (A) and fEPSP (B) of synaptic responses were intact in the presence of the inhibitor [n = 6 both for DMSO (●) and U0126 (○)]. (C) Paired-pulse facilitation (PPF) was not changed by U0126 (DMSO, n = 8; U0126, n = 7). (D) The magnitude of PTE induced by one 50-Hz burst of 1 s was enhanced in slices treated with U0126. Mean fEPSP slope, during mins 2–3 post-tetanus, and time constant of decay of PTE were significantly increased after the delivery of the tetanus in U0126-treated slices (○, n = 8) compared with slices perfused with vehicle (●, n = 7). (E) PTE induced by a 20-Hz tetanus for 1 s was unmodified by U0126 (vehicle, n = 8; U0126, n = 5). (F) PTE induced by 3 trains of 100 Hz for 1 s did not differ between vehicle treated slices (n = 6) and U0126 treated slices (n = 6). (G) The magnitude of LTP after 100-Hz tetanization was also unchanged in the presence of U0126 (n = 6 for both vehicle and U0126-treated groups). (H) PTP of the Schaffer collateral-CA1 pathway was similar in slices treated with U0126 (n = 8) or with vehicle (n = 9).
Double-labeling experiments revealed that pERK-positive structures were almost invariably labeled also for Syn I (Fig. 1E). Interestingly, these experiments showed that ERK was phosphorylated in only a subset of axon terminals in stratum lucidum, likely belonging to the stimulated MFs (see SI Materials and Methods). A major difference between the 2 immunolabelings was that pERK-immunoreactivity was not exclusively confined to the axon boutons, where synaptic vesicles are clustered, but also invaded to some extent axonal processes (Fig. 1E). Importantly, we found that pERK-immunofluorescence was completely abolished in tetanized slices pretreated with U0126 (20 μM), an inhibitor of the ERK upstream kinase MEK (Fig. 1F).
Application of a single 50-Hz tetanus produced a robust PTE of MF-CA3 synapses that peaked (303.42 ± 38.40%) immediately after the tetanus and decayed progressively to baseline levels following a kinetic that fitted to an exponential decay with a time constant of 36.6 ± 5.08 sec (Fig. 2D, closed circles). To investigate the physiological significance of presynaptic ERK activation, we prevented ERK phosphorylation while inducing PTE at various stimulation frequencies. In the presence of the inhibitor U0126, we found no alterations in either the input-output relationship (Fig. 2 A and B) or the paired-pulse facilitation (Fig. 2C) of the MF-CA3 pathway, indicating that U0126 action did not interfere with basal neurotransmission and a form of presynaptic short term plasticity at these synapses. Intriguingly, when ERK activation was blocked, the lifetime of PTE was markedly prolonged, showing a decay time constant of 86.70 ± 17.94 sec and an increased magnitude which was significant at 1–2 min post-induction (P < 0.05) (Fig. 2D, open circles). In contrast, induction of PTE in the same region by either 20-Hz (Fig. 2E) or 100-Hz (Fig. 2F) stimulation was not affected by application of the ERK inhibitor, indicating that ERK-dependent modulation of synaptic strength is frequency-specific. Moreover, both the expression and maintenance of long-term potentiation (LTP) induced by 100-Hz tetani were normal in slices pretreated with U0126 (154.34 ± 14.45% U0126, 143.20 ± 16.98% vehicle, P > 0.1) (Fig. 2G), indicating that LTP of MF-CA3 synapses does not require ERK activation (see also ref. 7). Interestingly, we also found that ERK-dependent regulation of short-term plasticity was pathway-specific. Indeed, when post-tetanic potentiation (PTP) was induced by 50-Hz stimulation at the CA3-CA1 pathway in the presence of the NMDA receptor antagonist APV (50 μM) to block NMDA receptor-dependent LTP, application of U0126 had no effect on either the temporal dynamics or the magnitude of PTP (173.03 ± 6.18% vehicle, 168.39 ± 6.78% U0126; P > 0.25) (Fig. 2H). Together, these results show that presynaptic ERK activation negatively regulates short-term plasticity of MF-CA3 synapses only at specific stimulation frequencies.
Activity-Dependent Phosphorylation of ERK in Presynaptic Terminals Is Transient and Synapse-Specific.
A simple explanation for the frequency-dependence of ERK function is that ERK activation may not be equally engaged by all stimulation frequencies. To quantitatively assess the temporal dynamics of presynaptic ERK activation, we analyzed the number of pERK-positive structures that coexpressed Syn I immunolabeling (Fig. 3). Tetanic stimulation of MF-CA3 synapses delivered at both 50 (Fig. 3B) and 100 Hz (Fig. 3C) produced a robust presynaptic activation of ERK, that was completely blocked by application of the U0126 inhibitor. In contrast, presynaptic expression of pERK was not modified by a single 20-Hz train delivered for 1 sec (Fig. 3 H–J). Interestingly, quantification of the number of pERK-positive axon terminals showed no significant differences between the 50-Hz and the 100-Hz stimulation protocols (Fig. 3 D and E). Whereas the number of axonal profiles containing pERK immunolabeling was negligible in untetanized control slices, both 50-Hz and 100-Hz HFS caused a rapid increase (4–5-fold) of pERK-positive terminals, that was already significant 30 sec after stimulation (Fig. 3 D and E). For both stimulation frequencies, the number of pERK-positive axon terminals peaked at 2 min post-tetanus, was still significant after 15 min and returned to baseline level after 30 min. Interestingly, the total number of Syn I-IR puncta remained unaltered at any time following either 1 × 50-Hz (Fig. 3F) or 3 × 100-Hz tetanic stimulation (Fig. 3G), suggesting that HFS-dependent activation of ERK was not accompanied by modifications in the number of presynaptic structures. Remarkably, although it has been reported that prolonged stimulation protocols induce the dispersion of Syn I from presynaptic boutons (21, 24, 29), the pattern of Syn I-IR did not change following the brief tetanic stimulations used in the present study. Thus, these data indicate that 100-Hz stimulation activates presynaptic ERK, despite blockade of ERK does not affect PTE or LTP induced by the same stimulation.
Fig. 3.
Time-course of ERK activation in presynaptic terminals. (A–C) Laser confocal images show double-immunofluorescence labeling for pERK and Syn I in CA3 stratum lucidum of representative slices under baseline stimulation (A) or following HFS (B, 2 min after 50-Hz tetanus; C, 5 min after 100-Hz tetanus). Note that both HFS protocols resulted in a noticeable increase in the density of pERK-positive puncta colocalized with Syn I (arrows). (D and E) Bar graphs illustrate the time course of synaptic ERK activation in CA3 stratum lucidum after delivery of either 50-Hz (D) or 100-Hz (E) HFS to the MF-CA3 pathway. Data are expressed as mean number of pERK/Syn I-positive puncta in confocal fields of 3772.42 μm2. With both stimulation frequencies, the densities of pERK-positive terminals was significantly increased in the 0.5–15 min interval with respect to control values (2-way ANOVA, P < 0.0001). However, the pattern of ERK activation was indistinguishable between the 50 Hz and 100 Hz stimulation protocols (2-way ANOVA, P = 0.39). For 50-Hz HFS, data are from 8 (control), 5 (0.5 min), 6 (2 min), 6 (5 min), 5 (10 min), 5 (15 min), and 5 (30 min) slices. For 100-Hz HFS, data are from 9 (control), 4 (0.5 min), 4 (2 min), 6 (5 min), 4 (10 min), 4 (15 min), and 4 (30 min) slices. (F and G) Bar graphs show the mean number of Syn-I positive puncta per field after 50-Hz (F) or 100-Hz (G) HFS (quantified in the same images used for D and E). No statistically significant variations were observed along the time course. (H–J) 20-Hz tetanization of the MF-CA3 pathway does not induce ERK activation in MF terminals. No variation in presynaptic pERK expression (arrows) could be seen in MF terminals of slices that were potentiated and harvested 2 min (I) or 5 min (J) after the onset of HFS (1 sec at 20 Hz) delivered at MF-CA3 synapse, compared with slices that received baseline stimulation (H). **, P < 0.01; #, P > 0.12. (Scale bars, 10 μm.)
To assess whether ERK activation in axon terminals is a common mechanism associated with activity-dependent changes in synaptic strength, we next analyzed the subcellular localization of pERK after HFS of the CA3-CA1 pathway. Although we could not detect any marked change in ERK activation after 50-Hz stimulation (Fig. S1 B and E), we found that pERK-IR was considerably increased in CA1 stratum radiatum 5 min after a 100-Hz stimulation (Fig. S1C). However, in contrast to the presynaptic induction observed in the CA3 area, ERK activation occurred mostly in the cell body and the dendrites of CA1 pyramidal neurons (see also ref. 4). Remarkably, high magnification images revealed that Syn I-positive axon terminals were in close apposition with pERK-positive dendritic profiles, but there was no colocalization of pERK and Syn I within the same neuronal compartments (Fig. S1F).
ERK Modulation of MF-CA3 Short-Term Plasticity Requires Synapsin I.
Syn I is 1 of the major ERK substrates in nerve terminals (18, 19) and the other isoforms, Syn II and Syn III, also display potential consensus sequences for ERK phosphorylation (30, 31). To investigate whether ERK exerts its presynaptic action through phosphorylation of Syn I, we examined both the phosphorylation state of Syn I at sites 4 and 5 during PTE and the effect of ERK blockade on PTE in Syn I-KO and Syn I/II/III-KO mice.
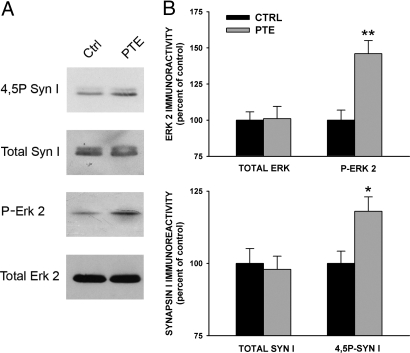
Immunoblotting analysis of ERK and Syn I phosphorylation in hippocampal slices incubated under resting conditions or after HFS (1 × 50 Hz) revealed that the strong increase in ERK phosphorylation observed after tetanic stimulation was accompanied by a smaller, but significant increase in Syn I phosphorylation at the ERK phosphorylation sites 4 and 5 (Fig. 4 A and B). The proportionally smaller increase in Syn I phosphorylation with respect to the extent of ERK activation may be explained by the concomitant activation of calcineurin-dependent dephosphorylation of synapsin (18, 32, 33) and by the fact that the ERK-mediated phosphorylation of Syn I is likely to occur only in a fraction of MF axon terminals (see Fig. 1E).
Fig. 4.
Effects of PTE induction in the CA3 area of the hippocampus on the endogenous phosphorylation of ERK 2 and Syn I. Hippocampal slices incubated under basal conditions (Ctrl) or subjected to HFS (50 Hz) to induce PTE were harvested and homogenized. After homogenization, equal amounts of protein (10 μg protein/sample) were subjected to immunoblotting for the total and phosphorylated forms of ERK2 and Syn I, respectively. A representative immunoblot is reported in A. (B) For each slice (n = 5 per experimental group), the amounts of total and phosphorylated ERK 2 and Syn I were quantified and normalized by an internal standard of brain homogenate. Immunoreactivity levels for both total and phosphorylated proteins detected in HFS-treated slices are expressed in percent of the respective levels in control slices (means ± SEM). *, P < 0.05; **, P < 0.01; 1-way ANOVA vs. respective control.
We then tested whether ERK phosphorylation caused by HFS was affected in Syn I-KO mice. These experiments revealed no difference in the subcellular localization of activated ERK in mutant mice compared with wild-type littermates following 50-Hz tetanic stimulation of MF-CA3 synapses (Fig. 5 A and B). As expected, however, we did not detect any Syn I-IR in slices from Syn I-KO mice (Fig. 5B). To assess whether Syn I-null mutation affects short-term synaptic plasticity in the MF-CA3 pathway, we next analyzed PTE in mutant mice. A single 50-Hz tetanus was able to reliably induce PTE in the CA3 region of Syn I-KO slices (Fig. 5C, closed circles). Notably, the amplitude (264.64 ± 46.26%) and time constant of PTE (41.68 ± 6.09 sec) were not statistically different from those in WT slices, similar to previous findings in the CA1 region (34, 35). Intriguingly, and in sharp contrast with WT animals, blockade of ERK activation by U0126 in slices from Syn I-KO mice did not cause any significant change in either the peak (252.98 ± 23.17%; P > 0.8) or the decay time constant (50.86 ± 5.97, P > 0.2) of PTE. Thus, these data indicate that ERK-dependent modulation of synaptic strength requires the expression of its substrate Syn I in the axon terminals of MFs.
Fig. 5.
ERK modulation of 50 Hz-induced PTE requires Syn I. (A and B) ERK activation in MF terminals in response to a 50-Hz tetanus was normal in Syn I KO mice. ERK activation is visible in MF terminals of Syn I KO mice in response to a 50-Hz tetanus (B). Note that the immunolabeling pattern for pERK in large MF terminals is similar in both genotypes, despite the absence of Syn I immunoreactivity in mutant slices. (Scale bar, 10 μm.) (C) PTE of MF-CA3 synapses induced with a single 50-Hz burst is similar in hippocampal slices from Syn-I KO mice (●, n = 12) and WT mice (gray triangles, data partially replotted from Fig. 2D). The magnitude and kinetics of this PTE were not affected by the application of ERK inhibitor U0126 (○, n = 11). (D) The duration of PTE induced by 50 Hz in synapsin TKO mice (○, n = 10) was found to be significantly increased with respect to WT animals (●, n = 5).
To further characterize short-term plasticity of MF-CA3 synapses and to uncover a possible involvement of the other synapsin isoforms, we conducted 50-Hz potentiation experiments in synapsin triple KO (TKO) mice. Strikingly, we found that TKOs showed an increased PTE (Fig. 5D) with respect to their WT littermates (decay time constant of 33.63 ± 1.35 sec WT, 65.43 ± 8.78 sec TKOs; P < 0.05). Intriguingly, this increase paralleled the increase of PTE observed in the presence of the ERK inhibitor in slices obtained from WT animals (see Fig. 2D) (comparison of time constants: TKOs vs. WT + U0126, P > 0.2). Thus, these results suggest that synapsins exert a redundant role in limiting the potentiation of the synaptic response induced by 50-Hz stimulation of the MFs and that a crucial part of this limiting function is linked to ERK-mediated phosphorylation of Syn I.
Discussion
In the last years, ERK has emerged as a key molecule in the modulation of synaptic plasticity in the hippocampus, as well as in the underlying learning and memory formation (1, 2, 12). Although the involvement of ERK in postsynaptic processes required for synaptic plasticity has been extensively investigated (for review, see ref. 1, 2, 11, 36, 37), the precise role of axonal ERK in activity-dependent modifications of synaptic strength is not fully understood. In this study, we demonstrate that ERK is responsible for the presynaptic modulation of short-term synaptic plasticity selectively in 1 of the major hippocampal pathways. Our results indicate that tetanic stimulation applied to the MF-CA3 synapses causes a transient activation of ERK in presynaptic MF terminals. Moreover, we describe a functional correlation between ERK activation in presynaptic terminals and short-term plasticity, which clearly depends on the stimulation frequency. Finally, we show that ERK-dependent synaptic plasticity requires Syn I, suggesting that ERK contributes to modulate neurotransmitter release at MF-CA3 synapses by regulating synaptic vesicle availability.
Molecular Mechanisms of Presynaptic Short-Term Plasticity in the CA3 Area.
One of the main goals of this study was to clarify whether ERK is activated in presynaptic axonal terminals during synaptic plasticity in the hippocampus. Moreover, we aimed to investigate the functional relationship between ERK activation and presynaptic modulation of transmitter release and to determine the molecular mechanisms associated with this modulation. We show that both PTE- and LTP-inducing stimuli trigger layer-specific ERK activation in the hippocampus. Notably, the activation of ERK in CA3 occurred uniquely in presynaptic structures located in stratum lucidum, likely corresponding to MF terminals, whereas in CA1 region ERK was activated almost exclusively in postsynaptic somato-dendritic profiles. Interestingly, ERK was found to have a role in limiting the expression of PTE generated with one 50-Hz train, because infusion with the specific ERK inhibitor U0126 increased PTE magnitude in the CA3 field.
What are the molecular mechanisms underlying the effects of ERK at MF synapses? One important target of ERK in nerve terminals is Syn I (17, 18), a phosphoprotein that controls the binding of synaptic vesicles to the actin cytoskeleton in response to neuronal activity (30). Using pharmacological inhibition, it has been proposed that ERK may regulate the proportion of vesicles that are available for rapid release by phosphorylating Syn I at specific sites (16, 18). Similarly, there is evidence that ERK controls brain derived neurotrophic factor-induced enhancement of glutamate release from synaptosomes (20). However, these studies are manifold different from our current investigation. For example, in these previous studies either biochemically purified synaptosomal preparations or dissociated neuronal cultures were used. Moreover, the relevance of presynaptic ERK function within the context of synaptic plasticity was not addressed by using electrophysiology. A key finding of the present study is that the ERK-dependent modulation of the magnitude of PTE at MF-CA3 synapses was no longer observed in Syn I-KO mice. Accordingly, we also found that PTE was associated with a significant increase in Syn I phosphorylation at sites that are specific targets of ERK. Together, these data suggest that 1 important role of presynaptic ERK is to modulate short-term increases of glutamate release at MF-CA3 connections through Syn I phosphorylation. Intriguingly, Syn II and III may also be involved in limiting synaptic strengthening, as PTE in Syn TKOs was significantly prolonged, mimicking the effect of ERK inhibition in WT mice. At present, it is not clear whether the limiting effect mediated by Syn II and Syn III also requires ERK-dependent phosphorylation, or whether it occurs independently of this kinase. In addition, it is of note that a possible involvement of Syn II and Syn III was only disclosed in the TKOs, suggesting that under normal conditions Syn I is the predominant isoform underlying the ERK-mediated modulation of PTE. In support of this interpretation, immunoblotting experiments have shown that Syn I is quantitatively predominant compared with both Syn II (38) and Syn III (39).
Remarkably, the action of ERK on short-term plasticity appears to be dynamically modulated by the frequency of MF stimulation. Our results indicate that ERK negatively couples Syn I to the vesicle release machinery only when it is activated by a 50-Hz tetanization. Under these conditions, blocking ERK activation with the MEK inhibitor U0126 would increase vesicle releasing capabilities of the MF terminal and transiently enhance neurotransmitter release. In contrast, PTE induced either with 20- or 100-Hz tetanization was not affected by ERK blockade. Whereas a simple explanation for the 20-Hz stimulation protocol is provided by the result showing that this form of PTE does not activate ERK, the interpretation of why ERK activation is dispensable when 100-Hz tetanization is used to elicit PTE is less straightforward. One possibility is that at 100 Hz, a ceiling effect could exist which may be associated with the concomitant activation of calcineurin and calcineurin-dependent full dephosphorylation of synapsin I at ERK sites (32, 33). Another hypothesis is that simultaneous activation of other protein kinases, for example, cAMP-dependent protein kinase or Ca2+/calmodulin-dependent protein kinase II, could either occlude or overcome ERK action in this form of short-term plasticity (31).
The mechanisms by which the frequency of axonal firing influences ERK function are unclear. However, it has been recently shown (21) that phosphorylation of Syn I at specific ERK sites is regulated over an extended range of stimulation frequencies, a mechanism that may allow nerve cells to control the efficacy of vesicle recycling. Whereas at low-stimulation frequency, the ERK sites of Syn I need to be in the phosphorylated state to confer efficient synaptic vesicle recycling, during HFS the ERK sites must become dephosphorylated to sustain efficient synaptic transmission (21). Together these data indicate that 1 important role of presynaptic ERK activation is to fine tune synaptic strengthening in response to specific firing rates and underscore the importance of the dynamics of ERK-dependent phosphorylation of Syn I in neuronal information processing.
Subcellular Targeting of ERK Activation During Synaptic Plasticity.
Increasing evidences indicate that ERK signaling may be triggered in synaptic structures by direct neuronal activation or behavioral stimuli (6, 16, 17, 40–42). However, the physiological mechanisms determining the subcellular targeting of pERK remain unclear. Our results disclose a pathway-specific regulation of ERK in individual synaptic structures during synaptic plasticity. We found that PTE in the CA3 area induced by a single 50-Hz tetanus and also by 100-Hz trains produced a transient ERK activation that was selectively confined to excitatory presynaptic terminals. Interestingly, the kinetics of ERK activation matched the time course of PTE, occurring immediately (30 s) after the application of the stimulus and decaying to baseline levels after several minutes.
Intriguingly, we found that induction of both 50-Hz PTP and 100-Hz LTP in the CA1 region were not accompanied by presynaptic ERK activation (Fig. S1), although we observed a selective localization of pERK in postsynaptic structures of CA1 pyramidal neurons. These findings are in agreement with earlier studies (4), and indicate that the activity-dependent subcellular targeting of ERK action can be pathway-specific. Moreover, we found that pharmacological ERK blockade had no effects on PTP in the CA1 area, indicating that MF-CA3 and CA3-CA1 pathways do not use identical ERK-dependent molecular mechanisms for the induction of synaptic plasticity. In apparent contrast with our results, a recent work (6) has shown that constitutive presynaptic activation of the Ras-ERK pathway in transgenic mice causes changes in short-term synaptic plasticity in the CA1 area, that involve Syn I. However, a plausible explanation for these discrepancies is that in this mouse model presynaptic ERK activation is long-lasting and produces both structural and functional synaptic modifications that are likely to be responsible for the observed changes in plasticity. Clearly, the effects of chronic ERK induction are different from the rapid physiological actions that we have investigated in our study by acutely blocking ERK only during the induction of synaptic plasticity. Moreover, the 50-Hz stimulation protocol used in the present study to induce PTP in CA1 is substantially different from the protocols used in the previous work (6). Finally, and most importantly, our 50-Hz stimulation protocol failed to elicit detectable levels of presynaptic ERK activation in area CA1, as shown by our immunohistochemical analysis.
Is presynaptic ERK involved in long-lasting forms of synaptic plasticity? Interestingly, when LTP at MF-CA3 synapses was induced with a HFS paradigm (3 × 100 Hz), ERK was also activated in MF presynaptic terminals of granule cells. Quantitation of immunofluorescence showed that HFS produced a robust increase of pERK-positive MF terminals in CA3 stratum lucidum, that lasted for ≈30 min before returning to baseline levels. Our immunocytochemical results are in contrast with earlier findings based on western blot analysis, showing no significant increase of active ERK1/2 levels in CA3 area following MF-CA3 LTP (7). One plausible explanation for this difference lies in the technical approaches that were used in the 2 studies. In fact, western blot analysis on total CA3 tissue might not be as sensitive as quantitative immunofluorescence to detect changes of ERK activation occurring only in a fraction of the MF terminals. Another possibility is that LTP-inducing stimuli may produce lower levels of ERK activation in MF terminals of the rat hippocampal slices (7) compared with mouse. Indeed, substantial differences of ERK involvement in the molecular processes underlying LTP in these 2 species have been reported previously (3, 43). Nonetheless, our results agree with previous data (7) in revealing that ERK, despite being activated by HFS, is not required for the induction and maintenance of MF-CA3 LTP for at least 1 h. Whether later effects of ERK signaling may be involved in the modifications underlying long-lasting plasticity at this pathway remains to be explored.
In conclusion, these data argue in favor of a mechanistic model according to which synaptic ERK is under a tight spatiotemporal regulation by specific patterns of neuronal activity and generates physiological responses that are circuit specific.
Methods
Animals.
All of the experiments performed in this study were conducted in accordance with the European Community Council Directive 86/609/EEC for care and use of experimental animals and were approved by the Animal Care and Use Committee of Turin University. Adult homozygous Syn I KO mice (44), synapsin TKO (45), and C57BL/6J littermates were kept on a 12-h light/dark cycle, and had access to food and water ad libitum.
Electrophysiology.
Extracellular field excitatory postsynaptic potentials (fEPSPs) were recorded in stratum radiatum of CA1 or in stratum lucidum of CA3. When indicated, slices were incubated for 60–120 min before and during tetanic stimulation with ACSF containing U0126 to inhibit ERK activation (20 μM in DMSO, Promega). Data were collected and analyzed on line (10-kHz sampling rate) by using pClamp software (Clampex, Axon Instruments). For details, see SI Materials and Methods.
Immunocytochemistry and Confocal Imaging.
Hippocampal slices were fixed in ice-cold paraformaldehyde [4% in 0.1 M phosphate buffer (PB)] with 1 mM sodium orthovanadate to block endogenous phosphatases. After overnight fixation at 4° C, slices were rinsed, cryoprotected (10, 20, and 30% sucrose) and subsequently cut in 35-μm sections with a cryostat. Free-floating sections were then processed for double immunofluorescence. For quantitative analysis, confocal images were processed with Imaris software (Bitplane), immunolabeled MF terminals were counted manually, and colocalization of signals in overlaid images was confirmed in x, y, and z dimensions. For details, see SI Materials and Methods.
Western Blotting.
Hippocampal slices were frozen in liquid nitrogen 5 min after HFS, extracted in boiling SDS (1% wt/vol plus 1 mM sodium orthovanadate) and subjected to SDS-polyacrylamide gel electrophoresis (SDS/PAGE) and quantitative immunoblotting. For details, see SI Materials and Methods.
Supplementary Material
Acknowledgments.
We thank Drs. P. Greengard (The Rockefeller University, New York), H.T. Kao (Brown University, Providence, RI) and A. Nairn (Yale University, New Haven, CT) for generously providing the mutant synapsin mouse strains as well as anti-synapsin phospho-specific antibodies; Dr. A. Ciccarelli for his valuable help with immunohistochemistry; Dr. M. Messa for his generous help with biochemistry experiments; and Dr. R. Piva for very helpful discussions. This work was supported by Ministero dell'Istruzione, dell'Università e della Ricerca (M.G. and F.O.), Fondo per gli Investimenti della Ricerca di Base (M.S.-P. and F.B.), Regione Piemonte Ricerca Sanitaria Finalizzata (n. 142 to M.G.), Fondazione Pierfranco e Luisa Mariani (R-07-59 to F.B.), Telethon-Italy (GGP05134 to F.B., GGP05236A to M.G.), and Compagnia di San Paolo (M.S.-P. and F.B.). H.V. was supported by a fellowship from Secretaría de Estado de Universidades e Investigación, Ministerio de Educación y Ciencia (EX2006-0294).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0900077106/DCSupplemental.
References
- 1.Grewal SS, York RD, Stork PJ. Extracellular-signal-regulated kinase signaling in neurons. Curr Opin Neurobiol. 1999;9:544–553. doi: 10.1016/S0959-4388(99)00010-0. [DOI] [PubMed] [Google Scholar]
- 2.Thomas GM, Huganir RL. MAPK cascade signalling and synaptic plasticity. Nat Rev Neurosci. 2004;5:173–183. doi: 10.1038/nrn1346. [DOI] [PubMed] [Google Scholar]
- 3.English JD, Sweatt JD. Activation of p42 mitogen-activated protein kinase in hippocampal long term potentiation. J Biol Chem. 1996;271:24329–24332. doi: 10.1074/jbc.271.40.24329. [DOI] [PubMed] [Google Scholar]
- 4.Impey S, et al. Cross talk between ERK and PKA is required for Ca2+ stimulation of CREB-dependent transcription and ERK nuclear translocation. Neuron. 1998;21:869–883. doi: 10.1016/s0896-6273(00)80602-9. [DOI] [PubMed] [Google Scholar]
- 5.Winder DG, et al. ERK plays a regulatory role in induction of LTP by theta frequency stimulation and its modulation by beta-adrenergic receptors. Neuron. 1999;24:715–726. doi: 10.1016/s0896-6273(00)81124-1. [DOI] [PubMed] [Google Scholar]
- 6.Kushner SA, et al. Modulation of presynaptic plasticity and learning by the H-ras/extracellular signal-regulated kinase/synapsin I signaling pathway. J Neurosci. 2005;25:9721–9734. doi: 10.1523/JNEUROSCI.2836-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kanterewicz BI, et al. The extracellular signal-regulated kinase cascade is required for NMDA receptor-independent LTP in area CA1 but not area CA3 of the hippocampus. J Neurosci. 2000;20:3057–3066. doi: 10.1523/JNEUROSCI.20-09-03057.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Atkins CM, Selcher JC, Petraitis JJ, Trzaskos JM, Sweatt JD. The MAPK cascade is required for mammalian associative learning. Nat Neurosci. 1998;1:602–609. doi: 10.1038/2836. [DOI] [PubMed] [Google Scholar]
- 9.Schafe GE, Nadel NV, Sullivan GM, Harris A, LeDoux JE. Memory consolidation for contextual and auditory fear conditioning is dependent on protein synthesis, PKA, and MAP kinase. Learn Mem. 1999;6:97–110. [PMC free article] [PubMed] [Google Scholar]
- 10.Blum S, Moore AN, Adams F, Dash PK. A mitogen-activated protein kinase cascade in the CA1/CA2 subfield of the dorsal hippocampus is essential for long-term spatial memory. J Neurosci. 1999;19:3535–3544. doi: 10.1523/JNEUROSCI.19-09-03535.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thiels E, Klann E. Extracellular signal-regulated kinase, synaptic plasticity, and memory. Rev Neurosci. 2001;12:327–345. doi: 10.1515/revneuro.2001.12.4.327. [DOI] [PubMed] [Google Scholar]
- 12.Adams JP, Sweatt JD. Molecular psychology: Roles for the ERK MAP kinase cascade in memory. Annu Rev Pharmacol Toxicol. 2002;42:135–163. doi: 10.1146/annurev.pharmtox.42.082701.145401. [DOI] [PubMed] [Google Scholar]
- 13.Adams JP, et al. The A-type potassium channel Kv4.2 is a substrate for the mitogen-activated protein kinase ERK. J Neurochem. 2000;75:2277–2287. doi: 10.1046/j.1471-4159.2000.0752277.x. [DOI] [PubMed] [Google Scholar]
- 14.Yuan LL, Adams JP, Swank M, Sweatt JD, Johnston D. Protein kinase modulation of dendritic K+ channels in hippocampus involves a mitogen-activated protein kinase pathway. J Neurosci. 2002;22:4860–4868. doi: 10.1523/JNEUROSCI.22-12-04860.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Banko JL, Hou L, Klann E. NMDA receptor activation results in PKA- and ERK-dependent Mnk1 activation and increased eIF4E phosphorylation in hippocampal area CA1. J Neurochem. 2004;91:462–470. doi: 10.1111/j.1471-4159.2004.02734.x. [DOI] [PubMed] [Google Scholar]
- 16.Schenk U, et al. A novel pathway for presynaptic mitogen-activated kinase activation via AMPA receptors. J Neurosci. 2005;25:1654–1663. doi: 10.1523/JNEUROSCI.3074-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Corvol JC, et al. Depolarization activates ERK and proline-rich tyrosine kinase 2 (PYK2) independently in different cellular compartments in hippocampal slices. J Biol Chem. 2005;280:660–668. doi: 10.1074/jbc.M411312200. [DOI] [PubMed] [Google Scholar]
- 18.Jovanovic JN, et al. Neurotrophins stimulate phosphorylation of synapsin I by MAP kinase and regulate synapsin I-actin interactions. Proc Natl Acad Sci USA. 1996;93:3679–3683. doi: 10.1073/pnas.93.8.3679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Matsubara M, et al. Site-specific phosphorylation of synapsin I by mitogen-activated protein kinase and Cdk5 and its effects on physiological functions. J Biol Chem. 1996;271:21108–21113. doi: 10.1074/jbc.271.35.21108. [DOI] [PubMed] [Google Scholar]
- 20.Jovanovic JN, Czernik AJ, Fienberg AA, Greengard P, Sihra TS. Synapsins as mediators of BDNF-enhanced neurotransmitter release. Nat Neurosci. 2000;3:323–329. doi: 10.1038/73888. [DOI] [PubMed] [Google Scholar]
- 21.Chi P, Greengard P, Ryan TA. Synaptic vesicle mobilization is regulated by distinct synapsin I phosphorylation pathways at different frequencies. Neuron. 2003;38:69–78. doi: 10.1016/s0896-6273(03)00151-x. [DOI] [PubMed] [Google Scholar]
- 22.Humeau Y, et al. Synapsin controls both reserve and releasable synaptic vesicle pools during neuronal activity and short-term plasticity in Aplysia. J Neurosci. 2001;21:4195–4206. doi: 10.1523/JNEUROSCI.21-12-04195.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chin J, Angers A, Cleary LJ, Eskin A, Byrne JH. Transforming growth factor beta1 alters synapsin distribution and modulates synaptic depression in Aplysia. J Neurosci. 2002;22:RC220. doi: 10.1523/JNEUROSCI.22-09-j0004.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chi P, Greengard P, Ryan TA. Synapsin dispersion and reclustering during synaptic activity. Nat Neurosci. 2001;4:1187–1193. doi: 10.1038/nn756. [DOI] [PubMed] [Google Scholar]
- 25.Zucker RS, Regehr WG. Short-term synaptic plasticity. Annu Rev Physiol. 2002;64:355–405. doi: 10.1146/annurev.physiol.64.092501.114547. [DOI] [PubMed] [Google Scholar]
- 26.Brager DH, Cai X, Thompson SM. Activity-dependent activation of presynaptic protein kinase C mediates post-tetanic potentiation. Nat Neurosci. 2003;6:551–552. doi: 10.1038/nn1067. [DOI] [PubMed] [Google Scholar]
- 27.Regehr WG, Delaney KR, Tank DW. The role of presynaptic calcium in short-term enhancement at the hippocampal mossy fiber synapse. J Neurosci. 1994;14:523–537. doi: 10.1523/JNEUROSCI.14-02-00523.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Henze DA, Urban NN, Barrionuevo G. The multifarious hippocampal mossy fiber pathway: A review. Neuroscience. 2000;98:407–427. doi: 10.1016/s0306-4522(00)00146-9. [DOI] [PubMed] [Google Scholar]
- 29.Bonanomi D, et al. Phosphorylation of synapsin I by cAMP-dependent protein kinase controls synaptic vesicle dynamics in developing neurons. J Neurosci. 2005;25:7299–7308. doi: 10.1523/JNEUROSCI.1573-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hilfiker S, et al. Synapsins as regulators of neurotransmitter release. Philos Trans R Soc Lond B. 1999;354:269–279. doi: 10.1098/rstb.1999.0378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fdez E, Hilfiker S. Vesicle pools and synapsins: New insights into old enigmas. Brain Cell Biol. 2006;35:107–115. doi: 10.1007/s11068-007-9013-4. [DOI] [PubMed] [Google Scholar]
- 32.Jovanovic JN, et al. Opposing changes in phosphorylation of specific sites in synapsin I during Ca2+-dependent glutamate release in isolated nerve terminals. J Neurosci. 2001;21:7944–7953. doi: 10.1523/JNEUROSCI.21-20-07944.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yamagata Y, Jovanovic JN, Czernik AJ, Greengard P, Obata K. Bidirectional changes in synapsin I phosphorylation at MAP kinase-dependent sites by acute neuronal excitation in vivo. J Neurochem. 2002;80:835–842. doi: 10.1046/j.0022-3042.2001.00753.x. [DOI] [PubMed] [Google Scholar]
- 34.Rosahl TW, et al. Short-term synaptic plasticity is altered in mice lacking synapsin I. Cell. 1993;75:661–670. doi: 10.1016/0092-8674(93)90487-b. [DOI] [PubMed] [Google Scholar]
- 35.Rosahl TW, et al. Essential functions of synapsins I and II in synaptic vesicle regulation. Nature. 1995;375:488–493. doi: 10.1038/375488a0. [DOI] [PubMed] [Google Scholar]
- 36.Sweatt JD. Mitogen-activated protein kinases in synaptic plasticity and memory. Curr Opin Neurobiol. 2004;14:311–317. doi: 10.1016/j.conb.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 37.Valjent E, Caboche J, Vanhoutte P. Mitogen-activated protein kinase/extracellular signal-regulated kinase induced gene regulation in brain: A molecular substrate for learning and memory? Mol Neurobiol. 2001;23:83–99. doi: 10.1385/MN:23:2-3:083. [DOI] [PubMed] [Google Scholar]
- 38.De Camilli P, Benfenati F, Valtorta F, Greengard P. The synapsins. Annu Rev Cell Biol. 1990;6:433–460. doi: 10.1146/annurev.cb.06.110190.002245. [DOI] [PubMed] [Google Scholar]
- 39.Kao HT, et al. A third member of the synapsin gene family. Proc Natl Acad Sci USA. 1998;95:4667–4672. doi: 10.1073/pnas.95.8.4667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Boggio EM, Putignano E, Sassoe-Pognetto M, Pizzorusso T, Giustetto M. Visual stimulation activates ERK in synaptic and somatic compartments of rat cortical neurons with parallel kinetics. PLoS ONE. 2007;2:e604. doi: 10.1371/journal.pone.0000604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sindreu CB, Scheiner ZS, Storm DR. Ca2+ -stimulated adenylyl cyclases regulate ERK-dependent activation of MSK1 during fear conditioning. Neuron. 2007;53:79–89. doi: 10.1016/j.neuron.2006.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schrick C, et al. N-cadherin regulates cytoskeletally associated IQGAP1/ERK signaling and memory formation. Neuron. 2007;55:786–798. doi: 10.1016/j.neuron.2007.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Selcher JC, et al. A role for ERK MAP kinase in physiologic temporal integration in hippocampal area CA1. Learn Mem. 2003;10:26–39. doi: 10.1101/lm.51103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chin LS, Li L, Ferreira A, Kosik KS, Greengard P. Impairment of axonal development and of synaptogenesis in hippocampal neurons of synapsin I-deficient mice. Proc Natl Acad Sci USA. 1995;92:9230–9234. doi: 10.1073/pnas.92.20.9230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gitler D, et al. Different presynaptic roles of synapsins at excitatory and inhibitory synapses. J Neurosci. 2004;24:11368–11380. doi: 10.1523/JNEUROSCI.3795-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.