Abstract
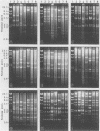
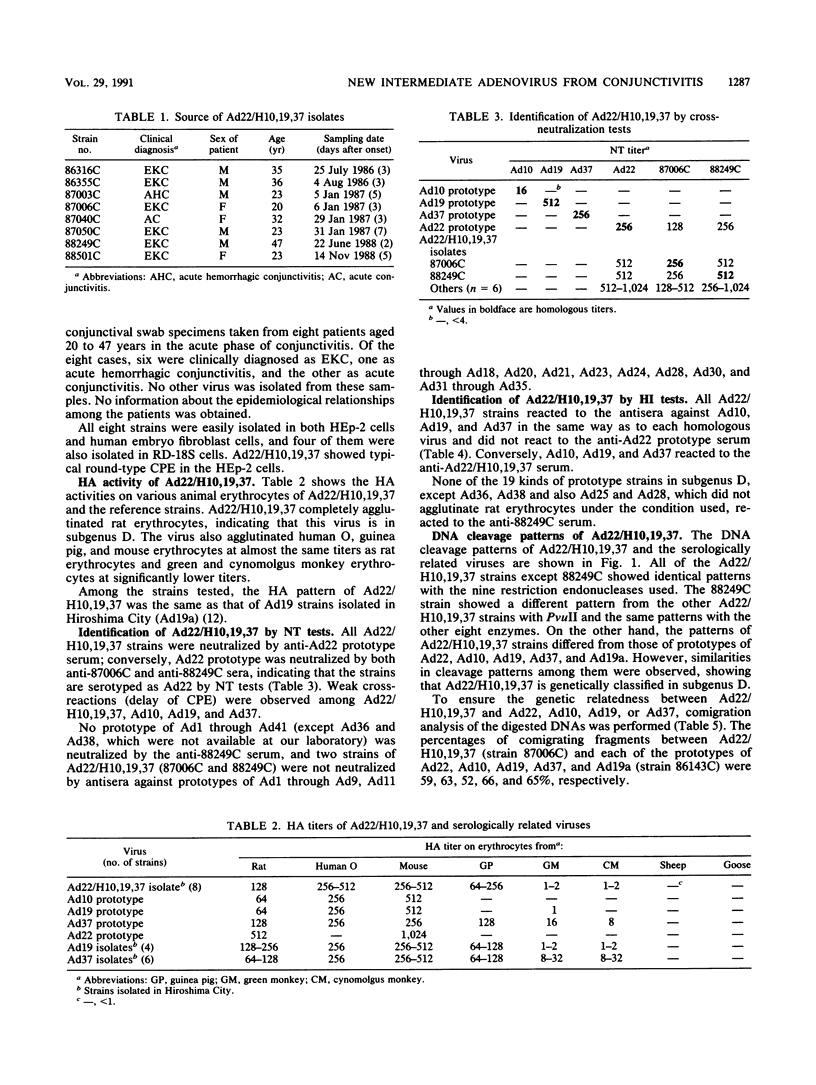
Eight strains of a new intermediate adenovirus were isolated in the course of an investigation of conjunctivitis in Hiroshima City, Japan. The strain was first isolated in July 1986. All eight strains were isolated from conjunctival swab samples from patients with conjunctivitis, mainly epidemic keratoconjunctivitis. The virus was typed as adenovirus type 22 in neutralization tests and was related closely to types 10, 19, and 37 in hemagglutination inhibition tests. The DNA cleavage patterns of the eight strains with nine restriction endonucleases were the same with one exception but different from those of the above serologically related species. We conclude that the intermediate adenovirus is a new etiological agent of conjunctivitis, mainly epidemic keratoconjunctivitis.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adrian T., Bastian B., Wagner V. Restriction site mapping of adenovirus types 9 and 15 and genome types of intermediate adenovirus 15/H9. Intervirology. 1989;30(3):169–176. doi: 10.1159/000150089. [DOI] [PubMed] [Google Scholar]
- Brown D. T., Burlingham B. T. Penetration of host cell membranes by adenovirus 2. J Virol. 1973 Aug;12(2):386–396. doi: 10.1128/jvi.12.2.386-396.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsberg H. S., Lundholm-Beauchamp U., Horswood R. L., Pernis B., Wold W. S., Chanock R. M., Prince G. A. Role of early region 3 (E3) in pathogenesis of adenovirus disease. Proc Natl Acad Sci U S A. 1989 May;86(10):3823–3827. doi: 10.1073/pnas.86.10.3823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hierholzer J. C., Wigand R., Anderson L. J., Adrian T., Gold J. W. Adenoviruses from patients with AIDS: a plethora of serotypes and a description of five new serotypes of subgenus D (types 43-47). J Infect Dis. 1988 Oct;158(4):804–813. doi: 10.1093/infdis/158.4.804. [DOI] [PubMed] [Google Scholar]
- Hierholzer J. C., Wigand R., de Jong J. C. Evaluation of human adenoviruses 38, 39, 40, and 41 as new serotypes. Intervirology. 1988;29(1):1–10. doi: 10.1159/000150023. [DOI] [PubMed] [Google Scholar]
- Irving L., Kennett M., Lewis F., Birch C., Donaldson A. Adenovirus eye infections in an Australian city, 1972-9. J Hyg (Lond) 1981 Feb;86(1):95–103. doi: 10.1017/s0022172400068789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp M. C., Hierholzer J. C., Cabradilla C. P., Obijeski J. F. The changing etiology of epidemic keratoconjunctivitis: antigenic and restriction enzyme analyses of adenovirus types 19 and 37 isolated over a 10-year period. J Infect Dis. 1983 Jul;148(1):24–33. doi: 10.1093/infdis/148.1.24. [DOI] [PubMed] [Google Scholar]
- Newland J. C., Cooney M. K. Characteristics of an adenovirus type 19 conjunctivitis isolate and evidence for a subgroup associated with epidemic conjunctivitis. Infect Immun. 1978 Jul;21(1):303–309. doi: 10.1128/iai.21.1.303-309.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noda M., Otagaki Y., Ikeda Y., Matsuishi T., Ogino T. Genome types of adenovirus types 19 and 37 isolated from patients with conjunctivitis in Hiroshima City. J Med Virol. 1988 Sep;26(1):15–22. doi: 10.1002/jmv.1890260104. [DOI] [PubMed] [Google Scholar]
- Norrby E. The structural and functional diversity of Adenovirus capsid components. J Gen Virol. 1969 Sep;5(2):221–236. doi: 10.1099/0022-1317-5-2-221. [DOI] [PubMed] [Google Scholar]
- Sambrook J., Sleigh M., Engler J. A., Broker T. R. The evolution of the adenoviral genome. Ann N Y Acad Sci. 1980;354:426–452. doi: 10.1111/j.1749-6632.1980.tb27983.x. [DOI] [PubMed] [Google Scholar]
- Wadell G. Molecular epidemiology of human adenoviruses. Curr Top Microbiol Immunol. 1984;110:191–220. doi: 10.1007/978-3-642-46494-2_7. [DOI] [PubMed] [Google Scholar]
- Wadell G., de Jong J. C. Restriction endonucleases in identification of a genome type of adenovirus 19 associated with keratoconjunctivitis. Infect Immun. 1980 Feb;27(2):292–296. doi: 10.1128/iai.27.2.292-296.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigand R., Adrian T., Bricout F. A new human adenovirus of subgenus D: candidate adenovirus type 42. Arch Virol. 1987;94(3-4):283–286. doi: 10.1007/BF01310720. [DOI] [PubMed] [Google Scholar]
- Wigand R., Adrian T. Intermediate adenovirus strains of subgenus D occur in extensive variety. Med Microbiol Immunol. 1989;178(1):37–44. doi: 10.1007/BF00202290. [DOI] [PubMed] [Google Scholar]
- Wigand R., Keller D., Werling I. Immunological relationship among human adenoviruses of subgenus D. Arch Virol. 1982;72(3):199–209. doi: 10.1007/BF01348965. [DOI] [PubMed] [Google Scholar]
- de Jong J. C., Wigand R., Wadell G., Keller D., Muzerie C. J., Wermenbol A. G., Schaap G. J. Adenovirus 37: identification and characterization of a medically important new adenovirus type of subgroup D. J Med Virol. 1981;7(2):105–118. doi: 10.1002/jmv.1890070204. [DOI] [PubMed] [Google Scholar]