Abstract
Invasive nontyphoidal Salmonella (NTS), including Salmonella typhimurium (STm), are major yet poorly-recognized killers of infants in sub-Saharan Africa. Death in these children is usually associated with bacteremia, commonly in the absence of gastrointestinal symptoms. Evidence from humans and animal studies suggest that severe infection and bacteremia occur when specific Ab is lacking. Understanding how Ab responses to Salmonella are regulated will help develop vaccines against these devastating infections. STm induces atypical Ab responses characterized by prominent, accelerated, extrafollicular T-independent (TI) Ab against a range of surface antigens. These responses develop without concomitant germinal centers, which only appear as infection resolves. Here, we show STm rapidly induces a population of TI B220+CD5− B1b cells during infection and TI Ab from B1b cells targets the outer membrane protein (Omp) porins OmpC, OmpD and OmpF but not flagellin. When porins are used as immunogens they can ablate bacteremia and provide equivalent protection against STm as killed bacterial vaccine and this is wholly B cell-dependent. Furthermore Ab from porin-immunized chimeras, that have B1b cells, is sufficient to impair infection. Infecting with porin-deficient bacteria identifies OmpD, a protein absent from Salmonella Typhi, as a key target of Ab in these infections. This work broadens the recognized repertoire of TI protein antigens and highlights the importance of Ab from different B cell subsets in controlling STm infection. OmpD is a strong candidate vaccine target and may, in part, explain the lack of cross-protection between Salmonella Typhi and STm infections.
Keywords: B cells, vaccines
Infections caused by nontyphoidal strains of Salmonella (NTS; primarily the serovars Typhimurium and Enteritidis) have a staggering impact on human health in infants and HIV+ adults, particularly in sub-Saharan Africa. In HIV− infants, the burden of NTS is greatest between 6 mo and 3 years and gastrointestinal symptoms are apparent in <50% of cases indicating NTS disease in Africa may be different to that in the West. Bacteremia is a strong correlate of disease severity and mortality in bacteremic children who reach clinic can be nearly 25%. Thus, restricting bacteremia may be pivotal to control these infections (1, 2).
The requirements for effective immunity to Salmonella depend on the stage of infection. Effective clearance of primary infection requires persistent Th1 T cell responses, whereas at this stage Ab limits bacteremia (3, 4). It is significant that although individuals who lack components of the IL-12 or IL-23 signaling pathways are more susceptible to invasive NTS disease they can still generate serum anti-Salmonella IgG (5). Furthermore, these children do not die from NTS infection, are frequently without bacteremia, and do not commonly appear with chronic NTS infection within their first 2 years of life (6).
The peak risk of disseminated NTS infection of between 6 and 24 mo supports a role for Ab against NTS because this coincides with loss of maternal Ab but before active humoral immunity is acquired (7). Because vaccination of adults with isolated capsular polysaccharide (a wholly TI antigen), induces protective serum IgG against typhoid but poor mucosal responses (8), it implies that serum Ab is sufficient to protect against typhoid and so may protect against NTS too.
We recently reported that STm induces an atypical Ab response with rapid and massive extrafollicular plasma cell responses decoupled from parallel induction of germinal centers (4). The induction of this response is not T cell mediated although switching to the Th1 isotype IgG2a is T-dependent. Our studies identified STm Omp as early targets of switched Ab in this response. These contain many proteins including the related porins OmpC, OmpD, and OmpF, which are of a similar Mr (≈40 kDa). OmpR regulates OmpC and OmpF expression but not OmpD (9). Salmonella Typhi (ST) lack OmpD although it is found in all other Salmonella serovars examined (10). Purified porins from ST induce an atypically long-lived Ab response, which contrasts to the gradually declining responses frequently observed after immunization with alum-precipitated proteins (11).
Here, we show that porins and live STm induce B1b cells in a TI manner, whereas flagellin does not. A single immunization with porins from STm, but not ST, could moderate infection with virulent and attenuated STm. Ab to OmpD was important because porin immunization did moderate infection with bacteria lacking OmpC and OmpF but not bacteria lacking OmpD. Therefore, protein antigens from STm can induce TI antibodies via B1b cells and OmpD may be a candidate for a subunit vaccine against systemic NTS infection. We suggest that Ab-mediated vaccines, although unlikely to result in sterilizing immunity, can restrict the devastating impact of NTS infections.
Results
Salmonella Induce a Rapid TI Plasma Cell Response Against Selective Antigens.
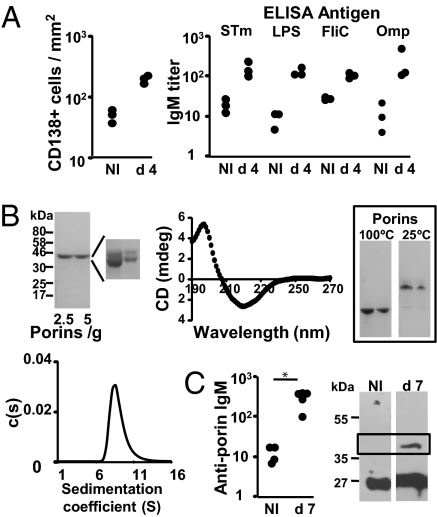
Previous studies show that Salmonella induces a rapid and extensive Ab response (4). Major targets of the early, switched response are Omp but not LPS or the flagellar protein, FliC. The rapid induction of this response suggested a TI element. To dissect this further, we infected mice lacking all T cells (TCRβδ-deficient mice) with attenuated STm. Infection resulted in a large increase in CD138+ plasmacytoid cells by day 4 paralleled by a rapid induction of STm-specific serum IgM (Fig. 1A). Detailed analysis revealed a strong induction of Ab to LPS and Omp, but a less marked response to FliC (Fig. 1A). We focused on the TI response to Omp by examining the response to a highly pure porin preparation from STm containing OmpC and OmpD and some OmpF (Fig. 1B and Materials and Methods). Analyses (Fig. 1B) showed that purified porins, predominantly formed β-strands, were heat-modifiable consistent with folded porins, and were oligomeric with oligomers typically 120–240 kDa thus suggesting a trimeric or hexameric configuration. Therefore, the porins adopt a natively folded oligomeric state consistent with their normal conformation in the outer membrane. ELISA and immunoblotting of sera from immunized T cell-deficient mice indicated that porins induced antigen-specific IgM (Fig. 1C). In contrast anti-porin Ab was only a minor component of the natural Ab pool, because nonimmune sera (at 1:50) reacted with nonporin but not porin proteins. Thus, IgM Ab was induced specifically to STm porins in the absence of T cell help.
Fig. 1.
Porins from STm induce a T-independent Ab response. (A) T cell-deficient TCRβδ−/− mice were infected i.p. with 5 × 105 attenuated STm for 4 days, and splenic CD138+ plasmacytoid cells per square millimeter (Left) were assessed by immunohistology. Anti-STm, anti-LPS, anti-flagellin (FliC), and anti-total Omp serum IgM was assessed by ELISA (Right). Data are representative of 2 experiments. (B) Analysis of porin purity and structure. Electrophoretic separation of porins showing their purity (Left) reveals 2 major species OmpC and OmpD with trace amounts of OmpF (see Materials and Methods). (Center) A spectrum of porins, using CD [millidegrees (mdeg)], indicating they are β-stranded. The boxed gel (Right) shows the electrophoretic pattern of porins under denaturing (100 °C) or seminative (25 °C) conditions. (Lower Left) s distributions (c(s)) for porins after AUC, indicating that the porins had a peak coefficient indicative of oligomeric structures with Mr of 120–240 kDa. (C) ELISA assessment of porin-specific IgM from nonimmunized and 7-day (d) porin-immunized TCRβδ−/− mice. Immunoblot of IgM binding to Omp from naïve and porin-immunized mice. Porins separate to the boxed area. Binding of sera to other proteins (such as 27 kDa) reflects the presence of natural Ab and is independent of porin immunization. ELISA data representative of 3 experiments; immunoblots of 5 naïve and immune sera. The Mann–Whitney test was applied to compare the statistical significance between groups linked by bars. NI, noninfected. *, P < 0.05.
Porin-Induced Impairment of STm Infection Is Mediated Through B Cells.
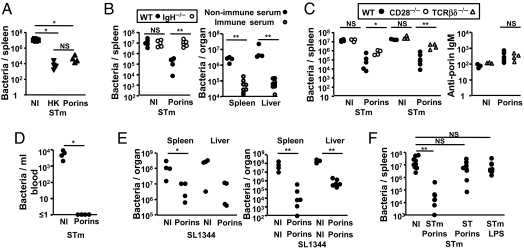
It was then assessed whether Ab to purified porins could impair STm infection. WT mice were immunized with porins or heat-killed bacteria [HK, an established vaccine (8)] and challenged 35 days later. Both immunogens conferred comparable benefit, reducing splenic bacterial burdens by 2–3 logs (Fig. 2A). To assess the role of B cells WT mice and B cell-deficient mice (IgH−/− mice) were immunized with porins and infected after 35 days. Immunized B cell-deficient mice had similar bacterial numbers as nonimmunized mice (Fig. 2B). In contrast, opsonization of bacteria with complement-inactivated sera from porin-immunized T cell-deficient mice reduced infection in these mice. Because the effects of porin immunization were B cell-mediated and TI we assessed whether switched Ab conferred additional benefit over IgM. To test this WT, CD28-deficient and T cell-deficient mice were immunized with porins and infected 35 days later. Although porin-specific IgM was induced in all groups after infection (Fig. 2C), porin-specific IgG was largely undetectable in T cell-deficient mice. Bacterial burdens were 10-fold greater in CD28-deficient animals and 40-fold greater in T cell-deficient mice. Because porin immunization was only effective in the presence of B cells and all groups of naïve animals had similar bacterial numbers 5 days after infection these data suggest that ≥90% of the benefit was IgG mediated (Fig. 2C). Because bacteremia is a marker of severity the blood bacterial burden of nonimmunized and porin immunized animals was assessed. On day 5 of infection in nonporin-primed mice the median bacteremia was 6.0 × 103 bacteria/mL whereas after immunization no bacteria were detected in blood (Fig. 2D). Thus, porin immunization is effective at eliminating bacteremia.
Fig. 2.
Immunization with porins impairs STm infection in a B cell-dependent manner. (A) Nonimmunized (NI), porin-immunized or HK STm immunized WT mice were infected with 5 × 106 STm cells for 5 days and splenic bacterial numbers enumerated. (B) NI or porin immunized WT (filled circles) or B cell-deficient (open circles) (IgH−/−) mice were infected with 5 × 106 STm cells for 5 days and spleen and liver bacterial numbers enumerated (Left) (representative of 3 repeats). B cell-deficient mice were infected with STm opsonized with sera from NI (filled circles) or porin-immunized (open circles) mice (Right) (representative of 2 repeats). (C) NI or porin-immunized WT, CD28−/− or TCRβδ−/− mice were infected with 5 × 106 STm for 5 days and splenic bacterial numbers enumerated (Left) (representative of 3 and 2 experiments). (Right) Serum IgM titers from 5-day STm-infected NI and porin-immunized WT and TCRβδ−/− mice (representative of 2 experiments). (D) Blood bacterial counts from NI and porin-immunized WT mice infected with 5 × 106 STm for 5 days (representative of 2 repeats). (E) Spleen and liver bacterial counts from WT mice immunized once (Left) or twice (Right) with porins and infected with 3 × 103 STm strain SL1344 cells for 4 days (representative of 2 experiments). (F) Splenic bacterial counts from NI WT mice or WT mice immunized with STm porins, ST porins or LPS and infected with 5 × 106 STm for 5 days (representative of 3 experiments). The Mann–Whitney test was applied to compare the statistical significance between groups linked by bars. *, P < 0.05; **, P < 0.01; NS, not significant.
We next assessed how anti-porin Ab moderates infection to virulent STm strain SL1344. A single porin-immunization reduced splenic and liver bacterial numbers 4 days after infection by 20- and 40-fold respectively; a second porin immunization reduced this by 1,700- and 400-fold respectively (Fig. 2E). In these experiments, the reduction in bacterial numbers after porin immunization may prolong survival but would not prevent death. Last, we examined whether LPS from STm or porins from ST could provide similar benefits. Mice were immunized with highly pure STm LPS or with ST porins and infected 35 days later. Neither pure LPS nor ST porins conferred any benefit (Fig. 2F). This was surprising considering the high degree of identity between OmpC and OmpF from these 2 serovars. This suggests responses to the third STm porin, OmpD, may be important during NTS infection.
Ab to OmpD Is Sufficient to Impair STm Infection.
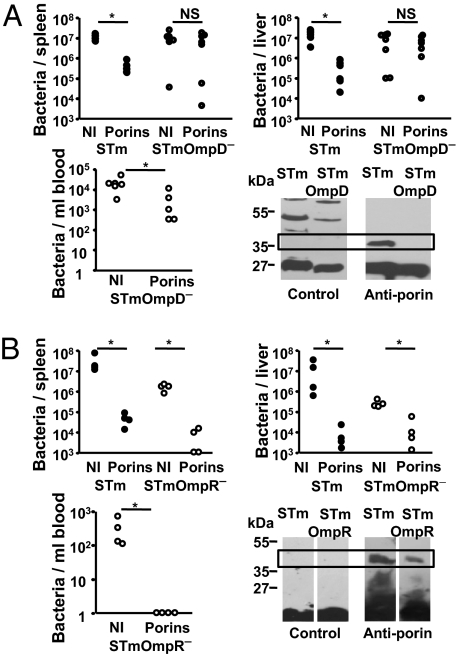
It was then tested whether Ab to OmpD could account for the differing results observed after immunization with STm or ST porins. Mice were immunized with porins and infected with either STm or STm lacking OmpD. Bacterial numbers were similar in the spleen and livers of naïve and immunized mice after infection with OmpD-deficient STm but immunization offered significant protection against bacteraemia (Fig. 3A). This impaired protection correlated with IgM Ab from porin-immunized mice failing to bind to the porin fraction from OmpD-deficient STm (Fig. 3A). In contrast after porin immunization and infection with OmpR-deficient bacteria (lacking OmpC and OmpF but not OmpD) bacterial numbers were reduced, bacteremia was not measurable and binding of IgM Ab from porin-immunized mice to the porin fraction from OmpR-deficient STm was evident (Fig. 3B). This indicates that OmpD is an important target of Ab to STm and Ab to OmpD alone is sufficient to impair STm infection.
Fig. 3.
OmpD is an important target of the humoral immune response to STm. (A) Nonimmunized (NI) or porin-immunized WT mice were infected with 5 × 106 STm (closed circles) or 5 × 106 STm lacking OmpD (STmOmpD− strain RAK126; open circles) for 5 days and splenic, liver and blood bacterial numbers enumerated (representative of 3 experiments for spleen and liver). Immunoblot analysis of IgM from NI or porin-immunized TCRβδ−/− mice against cell walls from STm or STm lacking OmpD. The boxed region shows the approximate Mr of the porins OmpC and F. (B) NI or porin-immunized WT mice were infected with 5 × 106 STm 5 × 106 STm cells (filled circles) or 5 × 106 STm cells lacking OmpR (STmOmpR− strain RAK83) (open circles) for 5 days and splenic, liver, and blood bacterial numbers enumerated (representative of 2 experiments). Immunoblot analysis of IgM from NI or porin-immunized TCRβδ−/− mice against cell walls from STm or STm lacking OmpR. The boxed region shows the approximate Mr of the porin OmpD. The Mann–Whitney test was applied to compare the statistical significance between groups linked by bars. *, P < 0.05; NS, not significant; d, day.
Porins and STm Induce a Population of Peritoneal B Cells with a B1b Phenotype.
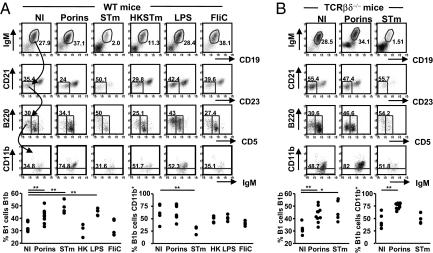
The results above show the effectiveness of porin immunization depends on B cells and these responses are induced in the absence of T cells. Thus, we assessed whether TI B1 cells were recruited against porins. WT mice were immunized and peritoneal responses assessed 4 days later, using 7 color flow cytometry. CD19+ and IgM+ lymphocytes were gated for low/negative CD21 and CD23 expression and these examined for CD5 and B220 expression. Finally, CD11b expression on the B220+CD5− population was assessed. Porin immunized mice, compared with nonimmunized mice, showed a significant induction of B cells with characteristics of B1b cells, being CD19+ and IgM+, B220int but CD5− with low CD21 and CD23 expression (Fig. 4A). B1b cells were also significantly induced by live STm and purified LPS but not HK STm, FliC, or OVA (Fig. 4A, OVA, and data not shown). CD11b expression was variable between the groups. CD11b− B1b cells have been reported previously, at similar proportions to those described here, and have the potential to become CD11b+ after transfer into lymphopenic recipients (12). The T-independence of this B1b induction was shown by giving porins or STm to TCRβδ-deficient mice (Fig. 4B). These experiments showed a significant induction of B1b cells but also confirmed the differential expression of CD11b on B1b cells induced by porins or STm. Thus, STm and porins induce a peritoneal population of TI B1b B cells.
Fig. 4.
Porins induce B1b cells. (A) Peritoneal cells from nonimmunized (NI) WT mice and WT mice immunized with the described antigens for 4 days were stained using 7 color FACS. IgM+CD19+ cells were gated from the lymphocyte population. Cells with low CD21 and CD23 were gated from the IgM+CD19+ population, and B1b cells were IgM+CD19+CD21loCD23lo cells that were CD5−B220+. The final FACS gate shows the proportion of B1b cells that express CD11b. Graphs show the proportion of B1 cells that are B1b and the proportion of B1b cells that are CD11b+. Data representative of ≥2 repeat experiments. (B) B1b cells from TCRβδ−/− mice before and after immunization with porins and STm for 4 days were identified as in A. Graphs show the proportion of B1 cells that are B1b and the proportion of B1b cells that are CD11b+. Numbers refer to the percentages of cells in the gated boxes and are representative of 2 experiments. The Mann–Whitney test was applied to compare the statistical significance between NI and immunized groups linked by bars. *, P < 0.05; **, P < 0.01. Approximately 5 × 103 events are shown in each CD21/CD23 gate. EU, endotoxin units.
Anti-Porin Ab from B1b B Cells Is Sufficient to Impair Infection.
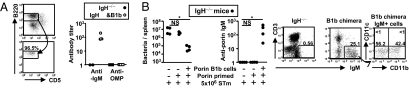
To test whether Ab produced by B1b cells responding to porins could limit STm infection chimeric mice were generated that have B1b cells but lack B1a cells and have few B2 cells (13). Peritoneal B220+CD5− cells were sorted from 4 day porin-immunized WT mice and 2 × 105 sorted cells were transferred into B cell-deficient mice (Fig. 5A). This population does include B2 follicular cells but these cells do not expand after transfer into lymphopenic mice (14) and thus only contribute a small proportion of the final B cell population after B1b cell expansion. Nonimmunized B1b chimeras could produce natural IgM antibody but this did not react to porins (Fig. 5A). Forty-eight hours after cell transfer B1b recipients and B cell-deficient mice that did not receive cells were immunized with porins. After 20 days the success of immunization was confirmed by measuring anti-porin IgM from the immunized chimeras. The next day mice were infected with 5 × 105 STm and bacterial burdens and serum Ab assessed 5 days later. Only mice that had peritoneal B cells and anti-porin Ab could impair STm infection (Fig. 5B). Porin-immunized B cell-deficient mice that did not receive B1b cells had bacterial numbers equivalent to nonimmunized mice. After infection peritoneal B cells in B1b chimeras were IgM+, and approximately half were also CD11b+ (Fig. 5B). Therefore, Ab from B1b cells is sufficient to limit infection.
Fig. 5.
Ab derived from B1b cells against porins is sufficient to impair STm infection. (A) (Upper) FACS plot showing the B220+CD5− cells (boxed) sorted for transfer into B cell-deficient IgH−/− mice. (Lower) Plot showing that the purity of the sorted cells was >95% and were CD5−. Graph shows serum IgM Ab titers from B1b chimeras 20 days after transfer in the absence of porin immunization. (B) Splenic bacterial burden (Left) and IgM titer (Right) of B cell-deficient mice that have either only been infected or been infected after porin immunization or have been infected after B1b transfer and porin immunization. FACS plots show the absence of B cells in the peritoneal cavity of B cell-deficient mice (Left) but their presence in B1b chimeras (Center). (Right) Plot showing CD11b expression on chimera B cells. The Mann–Whitney test was applied to compare the statistical significance between groups linked by bars. *, P < 0.05. NS, not significant.
Discussion
This work has 3 major findings: First, Ab to OmpD can reduce STm infection. Second, porins and viable STm but not FliC, induce a TI B1b cell response. Finally porin-induced Ab from these cells impairs STm infection. There has been controversy about the role of LPS in responses to porins, in part because LPS may associate with some porin trimers (e.g., OmpF). In the current study LPS was unlikely to be a potential confounder because purified porins had low levels of LPS and immunization with highly pure STm LPS did not moderate infection (Fig. 2F). Furthermore, infecting porin-immunized mice with LPS-sufficient, OmpD-deficient STm resulted in a diminution in Ab binding to the cell wall and a failure to reduce bacterial infection.
OmpD is a trimeric porin with homology to OmpF and OmpC (9, 15) whose expression is enhanced by anaerobiosis and suppressed by low pH, whereas OmpF and OmpC expression is sensitive to changes in osmolarity (16). OmpD has an unclear role in infection whereas loss of OmpR is attenuating (17, 18). Studies have identified a lack of cross-reactivity between surface binding mAb to these 3 porins (19). Indeed, for OmpD many antibodies only recognize the protein in trimeric but not monomeric form (19) suggesting exposed regions are under selective immune pressure. Multiple studies have assessed the potential of porins as vaccines, often with conflicting results, but few have specifically examined OmpD, presumably because of its absence in ST (e.g., ref. 20). Some studies that failed to show benefit from porins involved passive transfer of anti-porin antibodies (e.g. ref. 21), which may not have sufficiently covered the porin epitope repertoire. The current study shows that the natural response to OmpD in STm is sufficient to achieve marked reductions in bacterial numbers, although its effects may be potentiated by other factors, such as natural antibody. The importance of antibody to OmpD does not preclude roles for antibody to OmpF or OmpC, because other reasons may account for its minor role here, such as the amount of OmpF and OmpC in the preparations, or their expression in STm at different growth stages.
The failure to achieve sterile immunity after porin immunization may not be an impediment to reducing disease; for in humans multiple lines of evidence suggest sterilizing immunity is not essential to achieve clinical benefit. In infants in sub-Saharan Africa systemic STm infection can lead to fatal bacteremia (2). The peak incidence of this, between 6 and 24 months, coincides with the loss of passively acquired maternal IgG and before protection from active immunity. Nearly all children have Ab by 3 years, indicating previous exposure, indeed Ab correlates with decreased risk of bacteremia (7). Similarly, some patients with defective IL-12/23 signaling lacking a clinical history of Salmonella infection have anti-Salmonella IgG (5). This suggests IL-12/23-independent mechanisms may exist for coping with NTS infections. Therefore, the sterilizing immunity necessary to protect against infection with virulent STm SL1344 in susceptible strains of mice may overstate the degree of immunity required to protect against NTS or typhoid in humans. Indeed in vaccinated humans the protection vaccines confer can be overwhelmed if the infecting dose is sufficiently high (22). Thus, although it is unlikely Ab can confer sterilizing immunity it can diminish bacterial numbers at the time of infection and the extracellular dissemination of infection that results in bacteremia. Therefore, even modest reductions in infecting NTS numbers may result in substantial clinical benefits.
TI Ab to protein antigens has been described for the glycoprotein of vesicular stomatitis virus (23) and complement factor H-binding protein from the bacterial pathogen Borrelia (24). For the latter, self-renewing B1b cells play important roles (25) as they do in pneumococcal infections (26). This suggests B1b cells may play significant roles in a range of infections and reflects our ever increasing understanding of the diversity of B cell responses to pathogens (27). In the current study all benefits from porin immunization were B cell mediated and Ab from B1b cells was sufficient to impair infection (Figs. 2B and 5). The induction of B1b cells to porins and STm may also explain, at least in part, why the early nonswitched extrafollicular plasma cell response occurs similarly in WT and T cell-deficient/impaired mice whereas functional T cell responses are required for extrafollicular responses against model protein antigens (28). The massive extent and rapid induction of the extrafollicular Ab response to STm suggests antigen availability is not limiting (4) and the B1b cell repertoire may be broader than originally thought.
Responses to typhoid Vi antigen may involve B1b cells and so immunization with STm porins or Vi antigen may act similarly in their respective infections. However, Ab to purified Vi antigen in humans is wholly TI and induces poor mucosal Ab (8) but porins can induce good T-dependent responses (29). It has long been appreciated that infants and the elderly have impaired responses to TI-2 antigens, such as capsular polysaccharide. It is tempting to speculate, as others have, that deficiencies in B1b cells in children are responsible for susceptibility to encapsulated bacteria (30). Interestingly, whereas responses to TI-2 antigens involve 2 sets of B cells, B1b cells, and marginal zone (MZ) B cells, mice deficient in the tyrosine kinase pyk2 lack MZ but not B1b B cells yet induce potent IgM and IgG responses to the TI-2 antigen TNP-Ficoll (31). In addition, RAG-1 mice reconstituted with as few as 105 B1b cells and lacking other B cell types mount strong and persistent Ab responses to NP-Ficoll (13). This suggests that responses to TI antigens are likely to be of sufficient evolutionary importance that multiple cell types are involved.
Materials and Methods
Mice, Bacterial Strains, Immunogens, and Biophysical Assessments.
Animal studies had ethical and Home Office approval. WT and gene-deficient mice are reported elsewhere except for TCRβδ−/− mice, which were from Jax (4). Mice were age (6–12 weeks) and sex-matched. STm SL1344 is a virulent strain and SL3261 is an AroA− attenuated strain (described in ref. 4). OmpD− STm strain RAK126 was made by transferring the Tn10::tetr insertion in ompD of STm strain BRD455 (17, 18) by P22 generalized transduction into SL3261. The OmpR− STm strain RAK83 was constructed using red recombinase (32). Briefly, aph, encoding kanamycin resistance, was amplified using primers (5′GGATCGTCTGCTGACCCGTGAATCTTTCCATCTCATGGGTGTAGGCTGGAGCTGCTTC3′ and 5′GTCTGAATATAACGCGGATGTGCCGGATCTTCTTCCACATTCCGGGGATCCGTCGACC3′) that contained sequence flanking the deletion target and electrotransformed into STm SL3261 expressing λ-red recombinase. The ΔompR::aph was transferred to STm SL3261 by P22 transduction. The genotype of strains was confirmed by PCR and PAGE.
Total Omp preparations were made by 2% (vol/vol) Triton X-100 extraction (4). Purified porins from ST (strain ATCC 9993) and STm (strain ATCC 14028) were generated (29) by repeated extraction with SDS and separation by FPLC on a Sephacryl S-200 column before dialysis against PBS/0.1% (wt/vol) SDS. Limulus amebocyte lysate assay showed LPS contamination at 0.06 EU/480 μg porins. Protein identity was confirmed by trypsin digest and mass spectrometry. TLRgrade STm LPS was purchased (Axxora ALX-581-011-L002). Flagellin was made as described in ref. 4. OVA, Imject grade, was from Thermo Scientific.
For sedimentation velocity and CD measurements protein was dialyzed against PBS, 0.1% SDS, pH 7.4, at 20 °C. Far UV CD measurements were performed on a Jasco J-715 spectropolarimeter with a 1-mm path length cell, 2-nm bandwidth, 1-nm increments, 100 nm/min scanning speed, 2-s response time, and continuous scanning mode. 20 scans were averaged and the spectrum corrected for buffer contribution. Data were interpreted using standard methods described in ref. 33.
Analytical ultracentrifugation (AUC) sedimentation velocity was performed in a Beckman Coulter proteome XL-I protein characterization system. Protein and buffer reference were centrifuged at 20,000 rpm in a Ti-50 rotor and analyzed using Sedfit (34).
Immunization and Infection of Mice and Opsonization Experiments.
Mice were immunized i.p. with 20 μg of proteins or LPS in PBS for 35 days unless stated otherwise. HK bacteria (107 per mouse) were heated for 1 h at 65 °C. Bacteria were from cultures with an OD600 of >1.0. Mice were infected i.p. with attenuated bacteria at 5 × 105 or 5 × 106 per animal as described in the text. Mice were infected i.p. with 3 × 103 WT SL1344 bacteria. Bacterial numbers were determined by direct culturing (4). Experiments with mice and opsonized bacteria were performed as described previously except sera were from naïve or immunized TCRβδ−/− mice (4). A single serum was used per mouse and all sera were heat-inactivated at 56 °C for 0.5 h. Bacteria (2.5 × 106/mL) and sera (1:100) were mixed for 0.5 h before infection and bacterial viability and lack of agglutination confirmed.
Immunohistology, ELISA, and Immunoblotting.
Immunohistology was performed on frozen sections as described in ref. 4. CD138+ cells were detected using rat anti-mouse CD138 (BD), biotinylated sheep anti-rat and streptavidinABComplex alkaline phosphatase (AP) (DakoCytomation) (4). Cells per square millimeter were calculated using a point-counting technique (35). ELISA was performed as previously described to detect Ab to STm, LPS, FliC, and OMP (4). Antigen was used at 5 μg/mL and primary antibodies and AP-linked goat anti-mouse isotype antibodies used to identify isotypes. To detect serum IgM posttransfer, wells were coated with Rat anti-mouse IgM and bound IgM detected as above. For immunoblotting, proteins were transferred onto PVDF membrane and endogenous HRP eliminated using SG substrate (Vector Laboratories). Sera were added overnight (1:50 for naïve and 1:100 −500 for immunized sera) and signal detected with HRP-conjugated goat anti-mouse IgM and Supersignal Chemiluminescense (Thermo Scientific).
FACS Analysis and Cell Sorting.
Cells were stained with one or more of these anti-mouse mAb: IgMFITC (eB121–15F9), IgMPE-Cy7 (11/41), CD19APC-Cy7 (1D3), CD3 PerCPcy5 (145–2C11), CD11c APC (N418), CD11b PE (M1/70), CD5 PE-Cy5 (53–7.3 or Ly-1), B220APC (RA3–6B2) or B220Pacific Blue (RA3–6B2), CD21FITC (7G6), and CD23PE (B3B4) (All from E-Bioscience) and assessed on a FACScalibur or CyAn ADP Flow Cytometer and analyzed using FlowJo 8.8.3. (TreeStar). B1b chimeras were generated as described in ref. 13, with modifications. WT mice were immunized with porins for 4 days and B1b cells (B220int CD5−) sorted. Cells (2 × 105) were transferred into IgH−/− mice and 3 days later chimeras immunized i.p. with 20 μg of porins. Transfer success was confirmed by detecting IgM and anti-porin Ab 20 days later before challenge with STm.
Statistics.
Statistics were calculated using the Mann–Whitney test; significance was accepted at P ≤ 0.05 (4).
Acknowledgments.
We thank Agata Wasik for help on immunoblotting, Drs. Tim Dafforn and MingQi Fan for help with AUC, the Birmingham Medical Services Unit for technical help and Dr Cal MacLennan for useful suggestions. This work was funded by the Medical Resource Council New Investigator Research Grant, Biotechnology and Biological Sciences Research Council New Investigator Award, Royal Society project grants, and a Wellcome Trust VIP award (to A.F.C.); a Medical Research Council program grant (to I.C.M.M.), a Medical Research Council PhD studentship (to S.B.) and by Consejo Nacional de Ciencia y Tecnología Grants SEP-2003-CO2-45261 and SALUD 2004-01-132 and Instituto Mexicano del Seguro Social Project No. FIS/IMSS/PROT/C2007/049 (to C.G.-C. and C.L.-M.).
Footnotes
Conflict of interest statement: The results of this work have led to OmpD being patented as potential vaccine candidate against selected Salmonella infections.
This article is a PNAS Direct Submission.
References
- 1.Gordon MA. Salmonella infections in immunocompromised adults. J Infect. 2008;56:413–422. doi: 10.1016/j.jinf.2008.03.012. [DOI] [PubMed] [Google Scholar]
- 2.Graham SM, et al. Nontyphoidal Salmonella infections of children in tropical Africa. Pediatr Infect Dis J. 2000;19:1189–1196. doi: 10.1097/00006454-200012000-00016. [DOI] [PubMed] [Google Scholar]
- 3.Mastroeni P, Menager N. Development of acquired immunity to Salmonella. J Med Microbiol. 2003;52:453–459. doi: 10.1099/jmm.0.05173-0. [DOI] [PubMed] [Google Scholar]
- 4.Cunningham AF, et al. Salmonella induces a switched antibody response without germinal centers that impedes the extracellular spread of infection. J Immunol. 2007;178:6200–6207. doi: 10.4049/jimmunol.178.10.6200. [DOI] [PubMed] [Google Scholar]
- 5.MacLennan C, et al. Interleukin (IL)-12 and IL-23 are key cytokines for immunity against Salmonella in humans. J Infect Dis. 2004;190:1755–1757. doi: 10.1086/425021. [DOI] [PubMed] [Google Scholar]
- 6.Fieschi C, et al. Low penetrance, broad resistance, and favorable outcome of interleukin 12 receptor 1 deficiency: Medical and immunological implications. J Exp Med. 2003;197:527–535. doi: 10.1084/jem.20021769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.MacLennan CA, et al. The neglected role of antibody in protection against nontyphoidal salmonella bacteremia in African children. J Clin Invest. 2008;118:1553–1562. doi: 10.1172/JCI33998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guzman CA, et al. Vaccines against typhoid fever. Vaccine. 2006;24:3804–3811. doi: 10.1016/j.vaccine.2005.07.111. [DOI] [PubMed] [Google Scholar]
- 9.Nikaido H. Molecular basis of bacterial outer membrane permeability revisited. Microbiol Mol Biol Rev. 2003;67:593–656. doi: 10.1128/MMBR.67.4.593-656.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Santiviago CA, Toro CS, Bucarey SA, Mora GC. A chromosomal region surrounding the ompD porin gene marks a genetic difference between Salmonella typhi and the majority of Salmonella serovars. Microbiology. 2001;147:1897–1907. doi: 10.1099/00221287-147-7-1897. [DOI] [PubMed] [Google Scholar]
- 11.Secundino I, et al. Salmonella porins induce a sustained, lifelong specific bactericidal antibody memory response. Immunology. 2006;117:59–70. doi: 10.1111/j.1365-2567.2005.02263.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ghosn EE, Yang Y, Tung J, Herzenberg LA, Herzenberg LA. CD11b expression distinguishes sequential stages of peritoneal B-1 development. Proc Natl Acad Sci USA. 2008;105:5195–5200. doi: 10.1073/pnas.0712350105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hsu MC, Toellner KM, Vinuesa CG, Maclennan IC. B cell clones that sustain long-term plasmablast growth in T-independent extrafollicular antibody responses. Proc Natl Acad Sci USA. 2006;103:5905–5910. doi: 10.1073/pnas.0601502103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sprent J, Schaefer M, Hurd M, Surh CD, Ron Y. Mature murine B and T cells transferred to SCID mice can survive indefinitely and many maintain a virgin phenotype. J Exp Med. 1991;174:717–728. doi: 10.1084/jem.174.3.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Benz R, Ishii J, Nakae T. Determination of ion permeability through the channels made of porins from the outer membrane of Salmonella typhimurium in lipid bilayer membranes. J Membr Biol. 1980;56:19–29. doi: 10.1007/BF01869348. [DOI] [PubMed] [Google Scholar]
- 16.Santiviago CA, Toro CS, Hidalgo AA, Youderian P, Mora GC. Global regulation of the Salmonella enterica serovar typhimurium major porin, OmpD. J Bacteriol. 2003;185:5901–5905. doi: 10.1128/JB.185.19.5901-5905.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dorman CJ, Chatfield S, Higgins CF, Hayward C, Dougan G. Characterization of porin and ompR mutants of a virulent strain of Salmonella typhimurium: ompR mutants are attenuated in vivo. Infect Immun. 1989;57:2136–2140. doi: 10.1128/iai.57.7.2136-2140.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meyer PN, Wilmes-Riesenberg MR, Stathopoulos C, Curtiss R., III Virulence of a Salmonella typhimurium OmpD mutant. Infect Immun. 1998;66:387–390. doi: 10.1128/iai.66.1.387-390.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Singh SP, Upshaw Y, Abdullah T, Singh SR, Klebba PE. Structural relatedness of enteric bacterial porins assessed with monoclonal antibodies to Salmonella typhimurium OmpD and OmpC. J Bacteriol. 1992;174:1965–1973. doi: 10.1128/jb.174.6.1965-1973.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matsui K, Arai T. Protective immunities induced by porins from mutant strains of Salmonella typhimurium. Microbiol Immunol. 1990;34:917–927. doi: 10.1111/j.1348-0421.1990.tb01070.x. [DOI] [PubMed] [Google Scholar]
- 21.Singh SP, Williams YU, Benjamin WH, Klebba PE, Boyd D. Immunoprotection by monoclonal antibodies to the porins and lipopolysaccharide of Salmonella typhimurium. Microb Pathog. 1996;21:249–263. doi: 10.1006/mpat.1996.0059. [DOI] [PubMed] [Google Scholar]
- 22.Hornick RB, et al. Typhoid fever: Pathogenesis and immunologic control. 2. N Engl J Med. 1970;283:739–746. doi: 10.1056/NEJM197010012831406. [DOI] [PubMed] [Google Scholar]
- 23.Freer G, et al. Vesicular stomatitis virus Indiana glycoprotein as a T-cell-dependent and -independent antigen. J Virol. 1994;68:3650–3655. doi: 10.1128/jvi.68.6.3650-3655.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Colombo MJ, Alugupalli KR. Complement factor H-binding protein, a putative virulence determinant of Borrelia hermsii, is an antigenic target for protective B1b lymphocytes. J Immunol. 2008;180:4858–4864. doi: 10.4049/jimmunol.180.7.4858. [DOI] [PubMed] [Google Scholar]
- 25.Alugupalli KR, et al. B1b lymphocytes confer T cell-independent long-lasting immunity. Immunity. 2004;21:379–390. doi: 10.1016/j.immuni.2004.06.019. [DOI] [PubMed] [Google Scholar]
- 26.Haas KM, Poe JC, Steeber DA, Tedder TF. B-1a and B-1b cells exhibit distinct developmental requirements and have unique functional roles in innate and adaptive immunity to S. pneumoniae. Immunity. 2005;23:7–18. doi: 10.1016/j.immuni.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 27.Racine R, Chatterjee M, Winslow GM. CD11c expression identifies a population of extrafollicular antigen-specific splenic plasmablasts responsible for CD4 T-independent antibody responses during intracellular bacterial infection. J Immunol. 2008;181:1375–1385. doi: 10.4049/jimmunol.181.2.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cunningham AF, Serre K, Mohr E, Khan M, Toellner KM. Loss of CD154 impairs the Th2 extrafollicular plasma cell response but not early T cell proliferation and interleukin-4 induction. Immunology. 2004;113:187–193. doi: 10.1111/j.1365-2567.2004.01951.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Salazar-Gonzalez RM, et al. Induction of cellular immune response and anti-Salmonella enterica serovar typhi bactericidal antibodies in healthy volunteers by immunization with a vaccine candidate against typhoid fever. Immunol Lett. 2004;93:115–122. doi: 10.1016/j.imlet.2004.01.010. [DOI] [PubMed] [Google Scholar]
- 30.Alugupalli KR. A distinct role for B1b lymphocytes in T cell-independent immunity. Curr Top Microbiol Immunol. 2008;319:105–130. doi: 10.1007/978-3-540-73900-5_5. [DOI] [PubMed] [Google Scholar]
- 31.Guinamard R, Okigaki M, Schlessinger J, Ravetch JV. Absence of marginal zone B cells in Pyk-2-deficient mice defines their role in the humoral response. Nat Immunol. 2000;1:31–36. doi: 10.1038/76882. [DOI] [PubMed] [Google Scholar]
- 32.Datsenko KA, Wanner BL. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci USA. 2000;97:6640–6645. doi: 10.1073/pnas.120163297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kelly SM, Jess TJ, Price NC. How to study proteins by circular dichroism. Biochim Biophys Acta. 2005;1751:119–139. doi: 10.1016/j.bbapap.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 34.Schuck P. Size-distribution analysis of macromolecules by sedimentation velocity ultracentrifugation and lamm equation modeling. Biophys J. 2000;78:1606–1619. doi: 10.1016/S0006-3495(00)76713-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cunningham AF, et al. Responses to the soluble flagellar protein FliC are Th2, while those to FliC on Salmonella are Th1. Eur J Immunol. 2002;169:2900–2906. doi: 10.1002/eji.200425403. [DOI] [PubMed] [Google Scholar]