Abstract
Plastid development in seedlings of Arabidopsis thaliana is affected by the transfer of 1O2-mediated retrograde signals from the plastid to the nucleus and changes in nuclear gene expression during late embryogenesis. The potential impact of these mechanisms on plastid differentiation is maintained throughout seed dormancy and becomes effective only after seed germination. Inactivation of the 2 nuclear-encoded plastid proteins EXECUTER1 and EXECUTER2 blocks 1O2-mediated retrograde signaling before the onset of dormancy and impairs normal plastid formation in germinating seeds. This long-term effect of 1O2 retrograde signaling depends on the recruitment of abscisic acid (ABA) during seedling development. Unexpectedly, ABA acts as a positive regulator of plastid formation in etiolated and light-grown seedlings.
Keywords: 1O2 signaling, seed development
Higher plants are characterized by the formation of seeds that originate from fertilized ovules and include embryos and maternally derived tissues. Embryogenesis can be divided into a morphogenetic phase, characterized by rapid cell division, and a maturation phase, during which cells discontinue dividing, grow in size, and accumulate storage material (1, 2). This second phase ends with the onset of desiccation and the dispersal of dried seeds. Seeds remain dormant until stimuli are right for germination (1, 3, 4). Seedlings derived from seeds buried in the soil grow heterotrophically on seed reserves. On reaching the soil surface and being exposed to light, they undergo a rapid transition from heterotrophic to autotrophic growth that requires the light-induced formation of functional chloroplasts and chlorophyll accumulation (5).
Evidence implicating 1O2-mediated retrograde signaling with the control of plastid differentiation during seedling development was derived from the analysis of executer (ex) mutations that had previously been identified in the flu mutant of Arabidopsis (5–8). After being shifted to the dark, the conditional flu mutant accumulates free protochlorophyllide (Pchlide) (5). On reillumination of the mutant, this tetrapyrrole intermediate acts as a potent photosensitizer and generates 1O2 by energy transfer (5, 6). On exposure to a dark/light shift, flu seedlings bleach and die, and mature flu plants stop growing. The genetic basis of these 1O2-mediated stress reactions was revealed by the discovery of 2 nuclear genes encoding the closely related plastid proteins, EXECUTER1 (EX1) and EXECUTER2 (EX2), required for transmittal of 1O2-dependent signals from the plastid to the nucleus (7, 8). Inactivation of EX1 attenuates, but does not fully eliminate, the up-regulation of 1O2-responsive nuclear genes, whereas inactivation of both EX proteins suppresses these changes. As shown in the present study, EX-dependent 1O2-signaling operates not only in flu but also in wild-type (WT) Arabidopsis and, before seed dormancy, predetermines the differentiation of plastids during seedling development.
Results
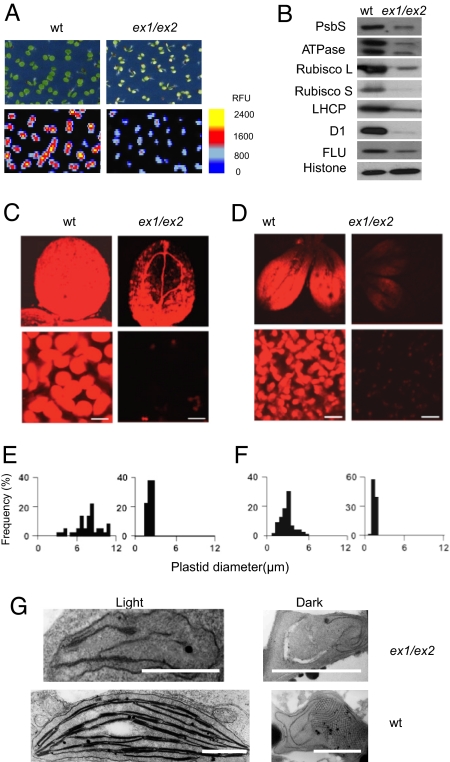
Chloroplast development was significantly disturbed in seedlings of the ex1/ex2 double mutant. The chlorophyll (Chl) content and fluorescence of cotyledons were reduced (Fig. 1A), and chloroplasts of ex1/ex2 seedlings were much smaller than those of WT seedlings (Fig. 1 C and E) and resembled undifferentiated proplastids (Fig. 1G). The impaired plastid differentiation in ex1/ex2 was not evenly distributed across the cotyledon, but rather occurred primarily within its apical part, as indicated by the Chl fluorescence confined to the basal zone of the cotyledon (Fig. 1C). The disturbed chloroplast development also is reflected in the reduced levels of chloroplast proteins (Fig. 1B). All of these deficiencies were observed in the cotyledons but not in true leaves of the mutant (data not shown). The arrest of plastid differentiation in cotyledons of ex1/ex2 mutant seedlings indicates that 1O2-mediated signaling may be involved in controlling plastid development. Generation of 1O2 in plastids by energy transfer occurs only in the light. Because the impaired plastid differentiation in ex1/ex2 mutant seedlings also affected etioplast differentiation in dark-grown seedlings (Fig. 1D, F, and G), EX1/EX2-dependent signaling is likely to occur not during seedling development, but rather at an earlier developmental stage, when growing embryos are exposed to light.
Fig. 1.
Chloroplast development in 4-day-old seedlings of Arabidopsis depends on the activity of the nuclear encoded plastid proteins EX1 and EX2. (A) Chlorophyll (Chl) accumulation and Chl fluorescence images of 4-day-old WT and ex1/ex2 seedlings. (B) The impact of ex1/ex2 on the accumulation of chloroplast proteins. PsbS, photosystem II (PSII) subunit S; ATPase, β-subunit of chloroplast ATPase; rubisco L/S, large (L)/small (S) subunits of the ribulose-1.5-bisphosphate carboxylase; LHCP, light-harvesting Chl a/b protein; D1, D1 reaction center protein of PSII; FLU, “fluorescent” protein, mediating feedback control of Chl biosynthesis. As a loading control, the relative concentration of histone was determined. (C) Chl fluorescence images of cotyledons and chloroplasts of light-grown WT and ex1/ex seedlings. (Scale bar: 10 μm.) (D) Pchlide fluorescence images of cotyledons and etioplasts of etiolated seedlings of WT and ex1/ex2. (Scale bar: 10 μm.) (E) Size distribution of plastids from light-grown seedlings. (F) Size distribution of plastids from etiolated seedlings. (G) Transmission electron micrographs of plastids from 4-day-old etiolated (dark) and light-grown (light) seedlings of WT and ex1/ex2 mutants. (Scale bar: 1 μm.)
Infiltration methods used previously to determine 1O2 in leaves of the flu mutant could not be applied to measure 1O2 production during seed development, because of the small size and delicate structure of immature seeds (6, 9). Instead, the release of 1O2 during late seed development was probed in vivo with a 1O2-specific reporter gene cassette consisting of the promoter of an AAA-ATPase gene (At3g28580) fused to the luciferase gene (LUC) (Fig. 2A) (10). Using this reporter gene in WT and flu plants, generation of 1O2 was assessed noninvasively at 4 different stages of embryogenesis: 4 days after anthesis (DAA), during the morphogenetic phase (“pale”); 7 DAA, when embryos start to green and develop functional chloroplasts (“pale-green”); 13 DAA, with embryos accumulating storage reserves and having fully developed photoheterotrophic chloroplasts (“green”); and s17 DAA, when seed coats are no longer transparent but turn brownish (“brown”). A total of 50 seeds of each stage were collected under dim green safe light, placed on agar plates, and probed for luciferase activity. In AAA:LUC WT plants grown under continuous light, only “green” seeds showed luciferase activity (Fig. 2B). When these immature seeds were shifted to the dark to stop 1O2 generation and 1O2-dependent AAA:LUC expression, luciferase activity was no longer detected after 4 h in the dark (Fig. 2B). Similar responses also were seen when “green” immature seeds remained attached to the opened siliques (data not shown). To rule out the possibility that developmental factors others than the release of 1O2 were responsible for the stage specificity of luciferase activity, immature seeds obtained at different DAA from siliques of light-grown AAA:LUC/flu plants were transferred to the dark to induce the accumulation of excess Pchlide that in the light acts as a potent photosensitizer and generates 1O2. In this case, not only “green” seeds, but also “pale” and “pale-green” seeds, accumulated Pchlide and demonstrated luciferase activity after predarkened immature seeds were reexposed to light (Fig. 2C). When 10 DAA seeds of ex1/ex2 and WT were germinated precociously, plastids developed normally in both lines and were indistinguishable [supporting information (SI) Fig. S1]. Thus, 1O2-mediated and EX1/EX2-dependent control of plastid development are not associated with germination, but define a specific stage of seed development before dormancy.
Fig. 2.
The noninvasive detection of 1O2 during embryogenesis using the 1O2-specific AAA-LUC reporter gene. (A) 1O2-induced expression of LUC in plants. Transgenic flu plants grown under continuous light were either shifted to the dark for 8 h and reexposed to light (D/L) or kept under continuous light (LL). The image of the D/L-treated plant was taken after 1 h of reillumination. (B) Luciferase activity at different stages of embryogenesis of transgenic WT plants expressing AAA:LUC. Immature seeds were taken from siliques at 4, 7, 13, and 17 DAA and kept under dim light (LL) or transferred to the dark for 4 h (D). Luciferase activity was detectable in immature “green” seeds at 13 DAA, when they were exposed to dim light but not after they had been kept in the dark. Similar results were obtained with seeds still attached to opened siliques. (C) Luciferase activity at different stages of embryogenesis of transgenic flu plants expressing AAA:LUC. Seeds were taken from siliques at 4, 7, 13, and 17 DAA and kept under dim light (LL), transferred to the dark for 4 h (D), or reexposed to light after 4 h of dark treatment (D/L). In the dark, free Pchlide accumulated that during reillumination acted as a photosensitizer and generated 1O2 in immature flu seeds taken from siliques at 4, 7, and 13 DAA.
Because 1O2 production begins in “green” seeds of WT plants, 1O2-mediated and EX1- and EX2-dependent signaling should affect gene expression only during the late stage of seed development. Total RNA was extracted from 6–7 DAA and 15–16 DAA immature seeds of WT and ex1/ex2 plants, respectively, and analyzed on Affymetrix ATH1 gene chips, which represent a major part of the Arabidopsis genome. Comparing the global gene expression profiles of the 6–7 DAA and 15–16 DAA immature seeds revealed drastic changes in gene expression affecting more than 13,000 genes, seemingly reflecting reported major differences in cell differentiation and metabolism of these 2 stages of seed development (11) (Fig. S2A and B). Inactivation of EX1 and EX2 had almost no detectable effect on the expression of nuclear genes in the 6–7 DAA immature seeds (Fig. S2C), whereas in the 15–16 DAA immature seeds, these mutations affected the expression of a small and significant group of 103 genes (Fig. S2D; Table S1A,B). Twenty-nine of these genes were at least 3-fold up-regulated in WT relative to ex1/ex2 (Table S1B), whereas 74 genes were up-regulated in ex1/ex2 relative to WT (Table S1A). Only a minor fraction of these genes had previously been found among the 1O2-responsive genes expressed in leaves of the flu mutant (6).
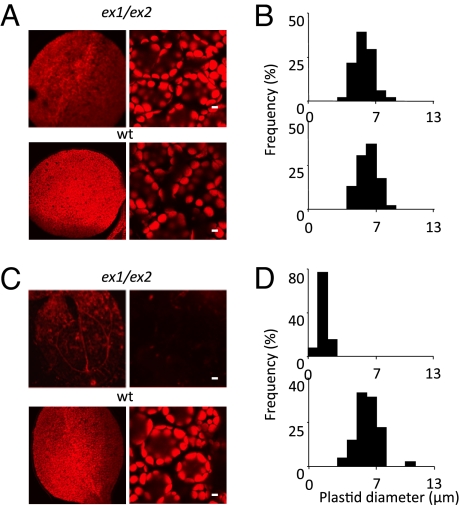
Because generation of 1O2 during embryogenesis occurs only in the light, the proposed impact of 1O2-mediated signaling on plastid differentiation in seedlings should be suppressed not only by inactivating EX1 and EX2, blocking 1O2-mediated plastid signaling, but also by keeping developing seeds away from light to prevent 1O2 production. Half of the siliques of the primary inflorescence of light-grown WT plants that had initially been kept for the first 10 DAA under continuous light were covered with aluminum foil, with the second half kept under continuous illumination, until seed maturation was complete. Contrary to what was predicted, proplastid differentiation was not disturbed in WT seedlings derived from seeds that had been transferred to the dark during embryogenesis to prevent 1O2 production (Fig. 3 A and B); however, plastid differentiation in ex1/ex2 seedlings derived from seeds transferred to the dark during development was no longer impaired, very much in contrast to the arrest of proplastid differentiation in seedlings derived from seeds of the same plant that had developed under continuous illumination (Fig. 3 C and D). Collectively, these data indicate that in light-grown plants lacking EX1 and EX2 proteins, generation of 1O2 before seed dormancy results in a severe subsequent disturbance of plastid formation during seedling development, while maintenance of EX1/EX2-dependent signaling in these plants suppresses this negative impact of 1O2. In immature seeds transferred to the dark, however, 1O2 production does not occur, and plastid differentiation during postembryonic development is not impaired regardless of the genetic background of the seeds. Changes during late embryogenesis that affect plastid differentiation in seedlings and are associated with the light-dependent release of 1O2 seem to be controlled by 2 different signaling routes, one that proceeds independently of EX1 and EX2 and impedes plastid differentiation during postembryonic development and the other that depends on EX1 and EX2 and suppresses the negative impact of the former on plastid differentiation (Fig. S3). This seemingly complex interaction of 2 opposing signaling events may help the plant coordinate and separate 2 conflicting developmental steps that occur during late embryogenesis in the same cell, chloroplast disintegration and chlorophyll catabolism on the one hand and the conservation and protection of proplastids on the other hand.
Fig. 3.
Restoration of plastid differentiation in ex1/ex2 seedlings after siliques were placed in the dark. (A and B) Siliques of ex1/ex2 and WT plants grown under continuous light were covered with aluminum foil from 10 DAA to the end of seed maturation. (C and D) In contrast to ex1/ex2 control plants kept under continuous light, 4-day-old ex1/ex2 seedlings derived from seeds taken from darkened siliques contained normal chloroplasts with Chl content (A) and size distributions (B) similar to those of WT chloroplasts. In (A) and (C), cotyledons (Left Panels) and chloroplasts in cotyledon mesophyll cells (Right Panels) are shown. (Scale bar: 5 μm.)
Plastid differentiation in seedlings may be due to the activation of transcripts during seed germination that had already been synthesized during late embryogenesis and stored in quiescent embryos before dormancy. In this case, differences in transcript levels of immature seeds between WT and ex1/ex2 would be expected to persist after the beginning of seed germination. Total RNA was extracted after seeds had been placed on agar plates at room temperature for 48 h in the dark and radicles started to break the seed coats. Global expression profiles of these 48-h-old dark-grown seedlings of ex1/ex2 and WT turned out to be remarkably similar. In particular, transcripts of genes that had been differentially affected before seed dormancy reached the same levels in emerging seedlings. The expression of 116 new genes was deregulated in ex1/ex2 seedlings relative to WT, however (Table S1C,D); 111 of these genes were at least 2-fold down-regulated in ex1/ex2 relative to WT (Table S1D). More than 95% of these genes are known to encode plastid proteins. Plastid genes encode roughly one-third of these proteins, whereas nuclear genes encode the remaining two-thirds (Table S1D). This remarkable selectivity and specificity of transcript changes in seedlings associated with the inactivation of EX1/EX2-dependent signaling during late seed development points to factor(s) stored throughout dormancy in quiescent seeds and remaining active immediately after the end of dormancy. Because the arrest of proplastid differentiation occurs only in ex1/ex2 seedlings derived from light-exposed seeds, formation of this regulator may be linked to gene transcripts that are selectively up-regulated during late embryogenesis of light-grown ex1/ex2 plants but not in WT plants. Genes whose expression during late embryogenesis was enhanced in light-grown ex1/ex2 relative to WT were reexamined. Transcripts of 12 of them reached at least 10-fold higher levels in ex1/ex2 than in WT (Table S1A). One of these, At5g45340, is of particular interest. It forms part of a small family of 4 genes encoding cytochrome P450 proteins with ABA 8′-hydroxylase activity considered to be catabolic enzymes regulating ABA levels (12, 13). ABA had been implicated previously with the control of chloroplast formation in young seedlings (14, 15). Cyp707A1, CYP707A2, and Cyp707A3 are expressed in developing seeds, whereas CYP707A4 expression occurs within siliques (16). Inactivation of CYP707A1 and CYP707A2 genes leads to drastically enhanced ABA levels in dried seeds, whereas ABA levels in seeds of cyp707a3 knockout mutant plants reach similar levels as in WT, suggesting that changes in ABA levels during seed development are affected mainly by CYP707A1 and CYP707A2. CYP707A2 also controls seed germination, whereas CYP707A1 and CYP707A3 regulate postgermination growth (16).
Among the 3 cytochrome P450 genes expressed in seeds, only CYP707A3 was differentially affected during late embryogenesis, reaching a more than 10-fold greater expression level in ex1/ex2 (Fig. S4A and Table S1A). Despite this drastic up-regulation of CYP707A3, ABA reached similar levels in immature and dried seeds of ex1/ex2 as in WT (Fig. S5), in line with the control of ABA levels during seed development by CYP707A1 and CYP707A2, but not CYP707A3 (16). Up-regulation of CYP707A3 during seed development of ex1/ex2 had a clear impact on seed germination and postembryonic development, however. Inhibition of radicle emergence and root hair appearance by exogenous ABA was significantly weaker in ex1/ex2 than in WT (Fig. S6).
To test whether the enhanced expression of CYP707A3 in ex1/ex2 during late embryogenesis impacts plastid differentiation in light-grown ex1/ex2 seedlings, a T-DNA knockout mutant of CYP707A3 was crossed with ex1/ex2 and triple mutants were identified among the segregating F2 progeny of this cross (Fig. 4A). In the T-DNA line transcripts of CYP707A3 were not detectable (Fig. 4A). Comparing seedlings derived from seeds of light-grown triple mutants with seedlings of the parental ex1/ex2 line showed clear differences in plastid differentiation (Fig. 4B). Inactivation of CYP707A3 in ex1/ex2 partially restored the capacity of proplastids to differentiate into chloroplasts, as indicated by the enlargement of plastids in cotyledons of the triple mutant to the size of those in WT (Fig. 4B) and a 2- to 3-fold increase in chlorophyll content (data not shown). To explore the physiological impact of ABA on plastid differentiation in ex1/ex2 seedlings more directly, seeds were placed on agar plates in the absence or presence of different ABA concentrations and allowed to germinate in either the dark or the light. Seeds of both lines kept in the light germinated equally well at 0.5 μM ABA (Fig. S6A). The size distribution and Chl content of chloroplasts in ex1/ex2 seedlings grown on ABA plates were similar to those of WT seedlings, in contrast to ex1/ex2 control seedlings that were kept on agar plates without ABA (Fig. 4C; data not shown). A similar ABA-dependent recovery of plastid formation also was observed in etiolated ex1/ex2 seedlings except that seed germination in the dark occurred at 0.2 μM ABA (data not shown).
Fig. 4.
The impact of ABA on plastid differentiation in 7-day-old light-grown seedlings derived from seeds of plants grown under continuous light. (A) Characterization of the T-DNA insertion mutant cyp707a3. Transcripts of EX1, EX2, and CYP707A3 in WT (1), ex1/ex2 double mutant (2), and ex1/ex2/cyp707a3 triple mutant (3). Profilin 1 (PRF1) amplification was used as a control. (B) Size distribution of plastids in cotyledons of seedlings of WT (1), ex1/ex2 (2), and ex1/ex2/cyp707a3 (3). (C) Size distribution of plastids in cotyledons of WT and ex1/ex2 seedlings grown in the light on agar plates in the absence (-) or presence (+) of ABA (0.5 μM). Note that the average size of plastids in 7-day-old ex1/ex2 seedlings is larger than that in the 4-day-old seedlings shown in Fig. 1. (D) ABA-induced restoration of transcript levels of genes encoding plastid proteins that are suppressed in ex1/ex2 seedlings (Table S1D). Seeds were harvested from ex1/ex2 plants grown under continuous light and germinated in the dark for 2 days on agar plates in the absence or presence of ABA (0.2 μM). Transcript levels of 4 nuclear genes encoding plastid proteins (1, At1g14150; 2, At3g16250; 3, At3g63140; 4, At4g28750), 4 plastid genes (5, PsbB; 6, PsbD; 7, RbcL; 8, PsaA), and 3 control genes not affected by the ex1/ex2 mutations (9, At3g01790; 10, At5g60390; 11, At3g54280) were measured by real-time PCR using gene-specific primers and expressed relative to WT levels (-ABA). Values represent mean and SD of 3 independent measurements.
A likely reason for the recovery of plastid differentiation in ABA-treated ex1/ex2 seedlings is the readjusted expression to the WT level of genes that encode plastid proteins and are down-regulated at the start of seedling development of ex1/ex2 relative to WT. Among the affected genes, 4 nuclear genes and 4 plastid genes were selected randomly to determine the impact of exogenous ABA on their transcript levels in etiolated 2-day-old ex1/ex2 seedlings. Adding ABA to the growth medium restored the expression of the 4 nuclear genes close to the WT level, whereas the expression of the 4 plastid genes also was up-regulated, but to a lesser extent. The expression of 3 control genes that had not been differentially affected by the inactivation of EX1 and EX2 was not altered grossly in response to ABA (Fig. 4D). These results contrast strikingly with the suppression of photosynthesis-associated nuclear gene expression by ABA in isolated embryos reported earlier (14); however, in this latter experiment, a 100-fold higher concentration of ABA was used. Thus, ABA seems to affect proplastid differentiation in opposing ways, either by suppressing synthesis of nuclear-encoded plastid proteins at higher ABA concentrations that also prevent seed germination (14) or by selectively promoting the expression of nuclear-encoded plastid proteins at much lower concentrations that allow seed germination to proceed.
Discussion
The present study identifies 1O2-mediated retrograde signaling as an as-yet unknown determinant of plastid differentiation that acts in immature seeds before the onset of dormancy, while they are still part of the maternal plant. Our work provides new insights but also raises new questions concerning the control of seedling development and plastid differentiation. During seed development, proplastids in embryonic cells may differentiate into chloroplasts. These generally have been thought to enhance the seed's oxygen supply needed for the synthesis and deposition of storage material through photosynthesis (17). Developing seeds are entrapped in green siliques and thus receive only very little photosynthetically active light. Placing siliques in the dark resulted in no visible impairment of weight, growth, viability, or germination rate (Fig. S7), even though the lipid content of immature seeds of both WT and ex1/ex2 was reduced in the absence of light (Fig. S8). Collectively, these results suggest that in the absence of photosynthesis, developing seeds may attract sufficient nutrients from other parts of the plant. This raises the question of why plants invest in the costly transformation of proplastids into chloroplasts and the accumulation of Chl that are catabolized soon after, before seed dormancy.
One consequence of the transient accumulation and catabolism of chlorophyll is the release of 1O2 during seed development. Our present work assigns a regulatory role to this reactive oxygen species that forms an integral part of seed development and affects plastid formation during postgermination growth. 1O2-mediated retrograde signals during late embryogenesis change nuclear gene expression. The impact of these gene expression changes on plastid differentiation is maintained throughout seed dormancy and becomes effective only once seeds start to germinate. This long-term effect of 1O2-mediated signaling depends on the recruitment of ABA, which acts as a positive regulator of plastid formation in etiolated and light-grown seedlings.
Materials and Methods
Plant Material.
The ex1 and ex2 mutants used in this study have been described previously (8). Seeds of the CYP707A3 (At5g45340) T-DNA insertion line (SALK_078170) were obtained from the European Arabidopsis Stock Centre. The ex1/ex2 double-mutant lines were generated by crossing ex1 and ex2 mutants. The ex1/ex2/cyp707a3 triple mutant was obtained by crossing ex1/ex2 and cyp707a3 mutant lines. Within the segregating F2 population, triple mutants were identified by PCR-based genotyping.
Determination of Chlorophyll Autofluorescence and Plastid Size.
The chlorophyll autofluorescence of cotyledons and leaves was recorded with a FluorCam system (Photon Systems Instruments) and a TCS-NT confocal laser scanning microscope (Leica Microsystems). To measure plastid size, sections of cotyledons and leaves were digitally scanned using a Leica Microsystems confocal laser scanning microscope. These size measurements were repeated and confirmed in transgenic lines expressing the plastid-localized marker protein SSU-GFP (18). Image version 1.6.587 (Leica Microsystems) was used to trace plastid outlines and determine the diameter of each plastid.
RNA and Protein Analysis.
Details of RNA and protein analysis are provided in SI Materials and Methods. Ttransgenic lines overexpressing the AAA:LUC reporter gene were used to detect 1O2 during seed development (10). Details of the assay are given in SI Materials and Methods.
Transmission Electron Microscopy.
Cotyledons of 3-day-old light- or dark-grown seedlings of WT and ex1/ex2 were used. The cotyledons were prefixed in 4% paraformaldehyde and 5% glutaraldehyde for 1 h, and then fixed in 1% OsO4 for 1 h. After dehydration through an acetone series (10 steps from 25% to 100%), the samples were embedded in Agar resin 100 and sectioned using a glass knife.
Determination of ABA and Total Esterified Fatty Acids in Seeds.
Details of measurements of ABA and total fatty acids of seeds are provided in SI Materials and Methods.
Supplementary Material
Acknowledgments.
We thank F. Landgraf and M. Ruppel for electronmicrograph preparation, Lorraine Johnson for language corrections, and U. Wobus and C. Laloi for their comments on the manuscript. This work was supported by the Swiss National Science Foundation, ETH-Zürich, the Functional Genomic Center Zürich, the Boyce Thompson Institute for Plant Research, and the German Research Council.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0901315106/DCSupplemental.
References
- 1.Vicente-Carbajosa J, Carbonero P. Seed maturation: Developing an intrusive phase to accomplish a quiescent state. Int J Dev Biol. 2005;49:645–651. doi: 10.1387/ijdb.052046jc. [DOI] [PubMed] [Google Scholar]
- 2.Goldberg RB, de Paiva G, Yadegari R. Plant embryogenesis: Zygote to seed. Science. 1994;266:605–614. doi: 10.1126/science.266.5185.605. [DOI] [PubMed] [Google Scholar]
- 3.Braybrook SA, et al. Genes directly regulated by LEAFY COTYLEDON2 provide insight into the control of embryo maturation and somatic embryogenesis. Proc Natl Acad Sci USA. 2006;103:3468–3473. doi: 10.1073/pnas.0511331103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Possingham JV, Moye DV, Anderson AJ. Influence of elevated shoot and root temperature on nitrogen fixation. Plant Physiol. 1964;39:561–563. doi: 10.1104/pp.39.4.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Meskauskiene R, et al. FLU: A negative regulator of chlorophyll biosynthesis in Arabidopsis thaliana. Proc Natl Acad Sci USA. 2001;98:12826–12831. doi: 10.1073/pnas.221252798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.op den Camp RG, et al. Rapid induction of distinct stress responses after the release of singlet oxygen in Arabidopsis. Plant Cell. 2003;15:2320–2332. doi: 10.1105/tpc.014662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wagner D, et al. The genetic basis of singlet oxygen–induced stress responses of Arabidopsis thaliana. Science. 2004;306:1183–1185. doi: 10.1126/science.1103178. [DOI] [PubMed] [Google Scholar]
- 8.Lee KP, Kim C, Landgraf F, Apel K. EXECUTER1- and EXECUTER2-dependent transfer of stress-related signals from the plastid to the nucleus of Arabidopsis thaliana. Proc Natl Acad Sci USA. 2007;104:10270–10275. doi: 10.1073/pnas.0702061104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Flors C, et al. Imaging the production of singlet oxygen in vivo using a new fluorescent sensor, Singlet Oxygen Sensor Green (R) J Exp Bot. 2006;57:1725–1734. doi: 10.1093/jxb/erj181. [DOI] [PubMed] [Google Scholar]
- 10.Baruah A, Simkova K, Apel K, Laloi C. Arabidopsis mutants reveal multiple singlet oxygen signaling pathways involved in stress response and development. Plant Mol Biol. 2009 doi: 10.1007/s11103-009-9491-0. in press. [DOI] [PubMed] [Google Scholar]
- 11.Ruuska SA, Girke T, Benning C, Ohlrogge JB. Contrapuntal networks of gene expression during Arabidopsis seed filling. Plant Cell. 2002;14:1191–1206. doi: 10.1105/tpc.000877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Millar AA, et al. Seed dormancy and ABA metabolism in Arabidopsis and barley: the role of ABA 8′-hydroxylase. Plant J. 2006;45:942–954. doi: 10.1111/j.1365-313X.2006.02659.x. [DOI] [PubMed] [Google Scholar]
- 13.Umezawa T, et al. CYP707A3, a major ABA 8′-hydroxylase involved in dehydration and rehydration response in Arabidopsis thaliana. Plant J. 2006;46:171–182. doi: 10.1111/j.1365-313X.2006.02683.x. [DOI] [PubMed] [Google Scholar]
- 14.Penfield S, Li Y, Gilday AD, Graham S, Graham IA. Arabidopsis ABA INSENSITIVE4 regulates lipid mobilization in the embryo and reveals repression of seed germination by the endosperm. Plant Cell. 2006;18:1887–1899. doi: 10.1105/tpc.106.041277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rohde A, et al. ABI3 affects plastid differentiation in dark-grown Arabidopsis seedlings. Plant Cell. 2000;12:35–52. doi: 10.1105/tpc.12.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Okamoto M, et al. CYP707A1 and CYP707A2, which encode abscisic acid 8′-hydroxylases, are indispensable for proper control of seed dormancy and germination in Arabidopsis. Plant Physiol. 2006;141:97–107. doi: 10.1104/pp.106.079475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weber H, Borisjuk L, Wobus U. Molecular physiology of legume seed development. Annu Rev Plant Biol. 2005;56:253–279. doi: 10.1146/annurev.arplant.56.032604.144201. [DOI] [PubMed] [Google Scholar]
- 18.Kim C, Apel K. Substrate-dependent and organ-specific chloroplast protein import in planta. Plant Cell. 2004;16:88–98. doi: 10.1105/tpc.015008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.