Abstract
Erythropoietin receptor (EpoR) binding mediates neuroprotection by endogenous Epo or by exogenous recombinant human (rh)Epo. The level of EpoR gene expression may determine tissue responsiveness to Epo. Thus, harnessing the neuroprotective power of Epo requires an understanding of the Epo–EpoR system and its regulation. We tested the hypothesis that neuronal expression of EpoR is required to achieve optimal neuroprotection by Epo. The ventral limbic region (VLR) in the rat brain was used because we determined that its neurons express minimal EpoR under basal conditions, and they are highly sensitive to excitotoxic damage, such as occurs with pilocarpine-induced status epilepticus (Pilo-SE). We report that (i) EpoR expression is significantly elevated in nearly all VLR neurons when rats are subjected to 3 moderate hypoxic exposures, with each separated by a 4-day interval; (ii) synergistic induction of EpoR expression is achieved in the dorsal hippocampus and neocortex by the combination of hypoxia and exposure to an enriched environment, with minimal increased expression by either treatment alone; and (iii) rhEpo administered after Pilo-SE cannot rescue neurons in the VLR, unless neuronal induction of EpoR is elicited by hypoxia before Pilo-SE. This study thus demonstrates using environmental manipulations in normal rodents, the strict requirement for induction of EpoR expression in brain neurons to achieve optimal neuroprotection. Our results indicate that regulation of EpoR gene expression may facilitate the neuroprotective potential of rhEpo.
Keywords: hypoxia, environmental enrichment, epilepsy, limbic system, enviromimetic
Recombinant human erythropoietin (rhEpo) is now recognized as a promising molecule to prevent or protect against neurodegeneration in a wide variety of experimental neurological disorders (1–3). Also, encouraging results on the neuroprotective efficacy of rhEpo in humans have been obtained from clinical trials involving stroke patients (4), patients with chronic schizophrenia (5), and patients with chronic progressive multiple sclerosis (6).
The level of Epo receptor (EpoR) expression in brain tissue has been proposed to determine the cytoprotective effects of Epo (7). In vivo, all neurons may not be prone to the protective effects of Epo, based on previous results showing that constitutive EpoR gene expression is heterogeneous in the rat central nervous system (8). Also, all brain areas do not exhibit the same neuronal vulnerability to excitotoxic injury; compared with the dorsal regions of the brain, the ventral limbic region (VLR) is subjected to intense neuronal death in response to a pilocarpine-induced status epilepticus (Pilo-SE) (9). Thus, the present study was aimed at finding physiological conditions making it possible to increase expression of EpoR in neurons of the VLR, and to test the hypothesis that increased neuronal expression of EpoR is required to achieve optimal neuroprotection by rhEpo after excitotoxicity induced by Pilo-SE.
In vitro studies showed that hypoxic exposure increases EpoR gene expression in cultured neurons (10–12). However, in the adult mouse brain, a single hypoxic exposure in vivo failed to increase EpoR gene expression (13, 14). Thus, we hypothesized that in rats, EpoR gene induction in neurons may require repetitive hypoxic challenges. First, we show that 3 hypoxic exposures significantly increase neuronal expression of EpoR; and second, that EpoR induction is required for rhEpo to counteract neurodegenerative processes in the VLR after Pilo-SE.
Results
Constitutive Expression of EpoR Is Low in VLR Neurons.
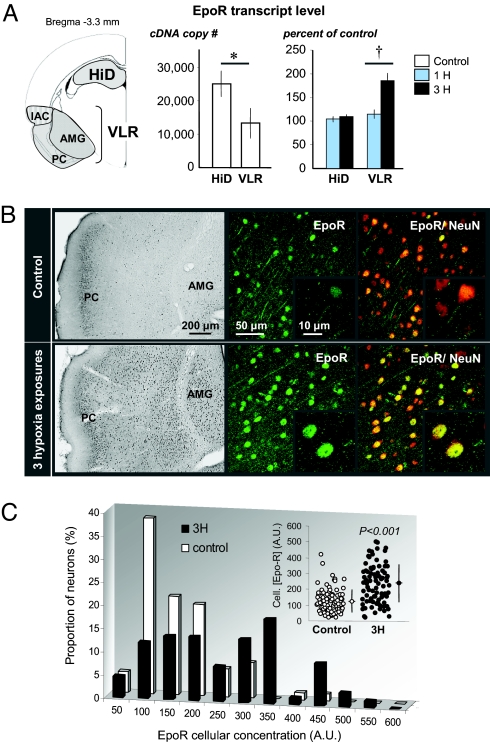
The EpoR gene is expressed at different levels in the adult rat hippocampus (Hi), neocortex (NC), and spinal cord (8). Here, we have refined the analysis of EpoR gene expression by examining the VLR, which includes the insular agranular cortex (IAC), the amygdala (AMG), and the piriform cortex (PC). EpoR gene expression has been analyzed at both the transcript and protein level, by targeting the full-length-EpoR isoform involved in intracellular signaling (15–17). Therefore, the PCR primers and the antibody used in this study are specific for the C-terminal cytoplasmic domain of EpoR cDNA and protein, respectively. We provide evidence that (i) EpoR transcript level in the VLR is lower than that measured in the dorsal Hi (HiD) (Fig. 1A); (ii) EpoR protein is detected with ease by using colorimetric immunohistochemistry in the pyramidal layer of the PC, whereas it is expressed at a barely detectable level in the other areas of the VLR (Fig. 1B); and (iii) EpoR protein is exclusively detected in neurons when dual immunofluorescent labeling of EpoR with NeuN is used (Fig. 1B).
Fig. 1.
Repeated hypoxic exposures activate EpoR gene expression in the VLR. (A) EpoR transcript level measured by reverse transcription quantitative PCR (RT-qPCR) in the HiD and the VLR of control rats revealed that EpoR-mRNA level was lower in the VLR than that measured in the HiD (*, P < 0.05). When measured at reoxygenation time in rats subjected to either 1 (1H) or 3 (3H) hypoxic episodes, a significant increase in EpoR-mRNA level was found in the VLR after 3H only (†, P < 0.05, compared with 1H). All bars represent mean ± SEM (n = 4 in each group). (B) Three days after reoxygenation time in rats subjected to 3H, the number of cells in which EpoR was detected increased compared with controls, as shown on representative sections stained for chromogenic detection of EpoR. In sections processed for dual EpoR and NeuN immunofluorescent detection, all EpoR-positive cells appeared to be neurons (NeuN+), as illustrated in the IAC: EpoR is in green and NeuN in red. (C) After 3H, the increased number of cells detected by chromogenic immunohistochemistry in the IAC was associated with an increased EpoR cellular concentration index, which was determined as the intensity of the immunofluorescent labeling (n = 132 neurons in controls; n = 130 neurons after 3H). The illustration represents all neurons measured and the mean ± SD for each group.
Repeated Hypoxic Exposures Activate Neuronal Expression of EpoR in the VLR.
In regard to the faint expression of EpoR in most of the neurons of the VLR, we explored the possibility of activating EpoR gene expression above detection threshold in these neurons. Hypoxia had already been shown to induce EpoR gene expression in cultured neurons (10, 11, 18), but not in vivo. Here, we show that a single hypoxic exposure (1H) has no effect on the EpoR transcript level in HiD or the VLR immediately after hypoxia (Fig. 1A) or 1, 2, or 8 days after reoxygenation (Fig. S1 A and B). However, by repeating hypoxic exposure on 3 occasions (3H) with each separated by 4 days, we demonstrated a significant increase (+85%) in EpoR transcript level in the VLR, which was observed at the time of reoxygenation after the last hypoxic exposure (Fig. 1A); the basal level recovered 1 day later (Fig. S1D). The increased EpoR transcript level was associated 3 days after 3H with an increased number of cells expressing EpoR protein above detection threshold in the PC, the AMG, and the IAC. All EpoR-positive cells appeared to be neurons (Fig. 1B). Quantitative analysis of EpoR immunofluorescent labeling over all NeuN-positive neurons revealed that (i) fluorescent labeling was detected in all neurons, with the lowest values ranging from 25 to 43 arbitrary units (A.U.; this concentration was found in 5% of total neurons both in controls and after 3H); (ii) neurons with a concentration >250 A.U. represented 11% of total neurons in controls and 48% after 3H; and (iii) the average cellular concentration of EpoR increased by 74% in VLR neurons after 3H (Fig. 1C).
Hypoxia-Induced Expression of EpoR in VLR Neurons Is Associated with Induction of Epo.
In vivo, a single hypoxic exposure (1H) is well known to increase Epo gene expression in the brain of rodents (19). Here, we show that, after 1H, Epo transcript level was increased at the time of reoxygenation to the same extent in the 2 brain regions studied (HiD and VLR), and was further increased after 3H in the VLR only (Fig. 2A). After either 1H or 3H, the apparent peak of Epo mRNA was observed at the time of reoxygenation only, basal level being recovered 1 day later (Fig. S2).
Fig. 2.
Repeated hypoxic exposures induce Epo gene expression in the VLR. (A) Constitutive level of Epo transcript measured by RT-qPCR was similar in the HiD and the VLR. At reoxygenation time after 1H, Epo-mRNA level was significantly increased to the same extent in the 2 brain areas (P < 0.001 between control and 1H). However, at reoxygenation time after 3H, Epo-mRNA level was superinduced in the VLR only (†, P < 0.05; †††, P < 0.001 between 1H and 3H). All bars represent mean ± SEM (n = 4 in each group). (B) Three days after reoxygenation time in rats subjected to 3H, the number of cells in which Epo was detected increased compared with controls, as shown on representative sections stained for chromogenic detection of Epo. In sections processed for dual EpoR and NeuN immunofluorescent detection, all Epo-positive cells appeared to be neurons (NeuN+), as illustrated in the IAC: Epo is in green and NeuN in red. (C) After 3H, the increased number of cells detected by chromogenic immunohistochemistry in the IAC was associated with an increased Epo cellular concentration index, which was determined as the intensity of the immunofluorescent labeling (n = 141 neurons in controls; n = 153 neurons after 3H). Illustration represents all neurons measured and the mean ± SD for each group.
The greater increase in Epo mRNA observed after 3H was, 3 days after reoxygenation, associated with an increased number of cells expressing Epo protein above detection threshold in the PC, the AMG, and the IAC, and all Epo+ cells appeared to be neurons (Fig. 2B). Quantitative analysis of Epo immunofluorescent labeling over all NeuN-positive neurons revealed that (i) 100% of VLR neurons expressed an Epo concentration >100 A.U. after 3 H, compared with 26% in controls; and (ii) the average cellular concentration of Epo increased by 349% in VLR neurons after 3H (Fig. 2C). Our results demonstrate that repeated hypoxic exposures elevate both Epo and EpoR gene expression in neurons in the VLR.
Hypoxia-Induced Expression of EpoR Is Not Associated with Degenerative Processes.
Increased EpoR expression within the central nervous system was reported in pathological conditions in humans (20–23), and in rodent models of neurodegeneration (8, 24–26). Here, after 3H, we did not detect any degenerating neurons, either by Fluorojade B staining (Fig. S3), or by using terminal deoxynucleotidyltransferase-mediated dUTP nick end-labeling of DNA breaks (Fig. S3). These results indicate that induced expression of EpoR protein after repeated hypoxic exposures was unrelated to neurodegenerative events in our system.
Environmental Enrichment Extends Hypoxia-Induced EpoR Gene Expression Beyond the VLR.
Environmental enrichment refers to housing conditions with enhanced sensory, cognitive, and motor stimulation. It has various what are considered beneficial effects on structural brain plasticity and behavior (27, 28). These effects in brain are likely sustained by modulation of the expression patterns of many different genes (29). We report here that rats housed in MARLAU enriched cages (EC) had greater EpoR transcript level in HiD and HiV, and the VLR, compared with rats housed in “standard” cages (SC; Fig. 3A). Maximal EpoR transcript levels in the NC, the HiD, and the VLR were reached in rats reared in EC and subjected to 3H (Fig. 3A). In the VLR of rats raised in EC, the maximal level of EpoR transcript was associated with an increased number of neurons in which EpoR was detected at 3 days after 3H (Fig. 3B). In the HiV, the lowest EpoR transcript level was observed in rats raised in SC, and the maximal level was attained in rats raised in EC or subjected to 3H, independently of the rearing condition (Fig. 3A). Rats raised in EC displayed increased Epo transcript level in the Hi (HiD and HiV) and the VLR (Fig. 3C), compared with rats housed in SC. However, responsiveness of Epo transcript level to 3H in all brain areas studied was similar in the 2 housing conditions (Fig. 3D).
Fig. 3.
Environmental enrichment increases brain EpoR and Epo gene expression. (A) Level of EpoR transcript was measured in the NC, the HiD, the HiV, and the VLR of rats raised in SC or EC, and subjected or not to 3H. In 3H groups, tissues were collected at reoxygenation time of the last hypoxic exposure (*, P < 0.001, compared with SC; †, P < 0.001, compared with EC). (B) Three days after reoxygenation time in rats housed in EC and subjected to 3H, the number of cells in which EpoR was detected increased, compared with rats raised in SC. (C) Constitutive expression of Epo was increased in the HiD, the HiV, and the VLR in rats raised in EC compared with SC (*, P < 0.05). (D) Brain reactivity of Epo gene expression to 3H, measured at transcript level, was not affected by housing conditions. All bars represent mean ± SEM (n = 4 in each group).
Induced EpoR Gene Expression in the VLR Determines the Neuroprotective Effect of rhEpo After Pilo-SE.
High dose (5,000 international units/kg) of rhEpo (administered immediately, 1 and 3 days after Pilo-SE), which is known to induce neuroprotective effects in the HiD (Fig. S4) (8), failed to protect neurons in the VLR (Fig. 4 A–C). This lack of rhEpo effect in the VLR cannot be attributed to weak passage of rhEpo across the brain barrier, because parenchymal rhEpo concentration was greater in the VLR than in the HiD (Fig. 4D).
Fig. 4.
Neuroprotective effects of rhEpo in the VLR are observed only in rats subjected to 3H after Pilo-SE. (A) Neuronal density in the VLR was measured at anatomical planes corresponding to interaural (IA) +6.44 and +5.40 mm, according to ref. 42. Because the anatomical plane itself had no effect, and no significant interaction was found between “anatomical plane” and “treatment condition,” results for neuronal density were collapsed over the anatomical plane factor. Neither 3H alone nor rhEpo alone had neuroprotective effects in the VLR after Pilo-SE. However, rhEpo administered in rats subjected to 3H significantly protected VLR neurons against neurodegeneration after Pilo-SE. (*, P < 0.001, compared with controls; †, P < 0.05) (n = 6 in each group). (B) Immunohistochemical detection of NeuN 6 days after Pilo-SE in the whole VLR indicates that the lesioned area (dotted lines) is considerably reduced only in rats subjected to 3H before Pilo-SE. (C) Enlarged illustrations of NeuN-immunohistochemical detection in the IAC. (D) Parenchymal uptake of rhEpo was greater in the VLR than that measured in the HiD (P < 0.001, ANOVA 2 in both control and Pilo-SE groups; factor 1 is “brain area,” factor 2 is “time after rhEpo injection”), both in controls and in rats subjected to Pilo-SE. Each bar represents the mean ± SEM (n = 3 in each group). ‡, time of termination.
We then tested whether neuroprotective effects of rhEpo could be achieved in the VLR after 3H-induced neuronal expression of EpoR. We first verified that the way rats entered into sustained SE after pilocarpine administration was not altered by 3H. Continuous convulsions were attained 24.6 ± 2.9 and 21.6 ± 1.0 min after pilocarpine administration in control rats and in rats subjected to 3H, respectively. We observed that rhEpo exerted neuroprotective effects in the VLR of rats subjected to 3H (Fig. 4 A–C). However, the intense induction in endogenous Epo measured after 3H alone (Fig. 2A) was not sufficient to protect vulnerable neurons in the VLR after Pilo-SE (Fig. 4 A–C). Interestingly, in the HiD, where 3H did not induce EpoR gene expression, we observed identical neuroprotective effects of rhEpo between rats subjected or not to 3H (Fig. S4), suggesting that lack of EpoR gene induction after 3H prevented rhEpo from exhibiting optimal neuroprotective effects.
Repeated Hypoxic Exposures Do Not Alter IGF-1 and Tpo/TpoR Transcript Levels in the VLR.
Endogenous factors can either act in synergy with Epo, as is the case with IGF-1 (30), or interfere with Epo, as is the case with thrombopoietin (Tpo) (10). Interestingly, hypoxia was shown to decrease Tpo and TpoR expression in cultured neurons at both the transcript and protein level (10). We expected that 3H would elevate the expression of IGF-1 and/or down-regulate that of Tpo and its receptor TpoR. We show that Tpo and TpoR transcript levels tended to be decreased up to 1 day after reoxygenation at the end of 3H, whereas IGF-1 mRNA level remained stable (Fig. 5).
Fig. 5.
Repeated hypoxic exposures do not alter IGF-1, Tpo, and TpoR transcript levels in the VLR. Controls and rats subjected to 3H were killed immediately after (R0), R1, and R2 days after the last hypoxic exposure. Levels of transcripts were measured by RT-qPCR. Each bar represents the mean ± SEM (n = 4 in each group).
Discussion
Although advances have been made in the understanding of the mechanisms that contribute to premature brain cell death, efforts to discover and implement effective neuroprotection strategies have lagged behind. Our study provides evidence that EpoR gene expression is up-regulated in the adult rat brain under physiological conditions in which the brain attempts to decrease vulnerability. This up-regulation that occurs predominantly in neurons is required for rhEpo to exert any neuroprotective effect. Thus, EpoR up-regulation appears to be an effective way to increase the neuroprotective efficacy of rhEpo.
We have also refined previous findings (8) that not all brain areas similarly express EpoR gene by showing, for example, that the level of EpoR is greater in the Hi than in the VLR. Consistent with the hypothesis that the tissue level of EpoR determines the tissue response to Epo (31), our data indicate that not all brain areas respond similarly to rhEpo. Indeed, we show in the Pilo-SE model that rhEpo significantly protects hippocampal neurons from degeneration, but is ineffective in protecting neurons of the VLR. This observation led us to search for physiological conditions that might enhance EpoR gene expression within the VLR.
Induction of EpoR has been proposed as a tissue-protective response to injury (2, 8, 25), and the only physiological response reported so far to enhance brain EpoR protein has been acclimation to ambient heat (32). Sublethal exposures to extreme environmental conditions, known to increase brain tolerance to a subsequent damaging event (33, 34), may also increase brain EpoR gene expression. Here, we show that nondeleterious repetition of hypoxic exposures dramatically increases EpoR gene expression at both the transcript and protein level, primarily in neurons of the VLR. We confirmed that EpoR gene expression remains unchanged in the brain of adult rodents after a single hypoxic exposure (13, 14). Hypoxia has been shown in the adult rat spleen to elevate the expression of EpoR (16), and the reason why repetition of hypoxic exposures induces EpoR in the VLR is not known. The superinduction of Epo, occurring exclusively in the VLR after hypoxia repetition, may have a role, involving the transcription factor GATA-3, previously linked with induced EpoR transcript level in cultured neurons in an Epo-dependent fashion (12). Intriguingly, we demonstrate that extreme environmental manipulations are not the only condition inducing EpoR gene expression in the brain. Indeed, environmental enrichment significantly elevated EpoR transcript level in the HiD and the VLR, and rendered dorsal brain areas (HiD and NCx) sensitive to repeated hypoxia. By contrast, maximal EpoR gene expression in the ventral brain areas (HiV and VLR) was attained after repeated hypoxia exposures, independently of whether rats were raised in standard or enriched housing conditions. There are likely to be other physiological interventions that will selectively raise EpoR in specific brain areas, but remain unidentified.
Consistent with the notion that enhanced EpoR gene expression confers increased tissue response to rhEpo, we ascertained that rhEpo protects VLR neurons after excitotoxic injury in rats subjected to repeated hypoxic exposure only. This effect is mainly explained by the increased expression of EpoR gene in VLR neurons, because neither rhEpo nor repeated hypoxic exposures were sufficient to induce neuroprotective effects. Also, this effect is very likely independent of other adaptive mechanisms activated by the repetition of hypoxic exposures because (i) in the HiD of rats subjected to 3H that showed no induction of EpoR, rhEpo had no additional protective effects; and (ii) the expression of IGF-1, known to potentiate rhEpo effects in vitro (30), was not modified by 3H. Altogether, our results indicate that induction of EpoR gene expression in vulnerable brain areas by 3H is a prerequisite to optimize neuroprotective effects of rhEpo. Unfortunately, this concept could not be tested in the HiD, where EpoR gene expression is enhanced in rats raised in EC and subjected to 3H, due to the inhibitory effect of environmental enrichment on the development of brain excitability and SE (35, 36).
Epo, which is a molecule induced by hypoxia, is considered to have a key role in the enhancement of brain robustness by hypoxia (19). Thus, rhEpo can be considered as an “enviromimetic,” defined as any exogenous molecule that mimics the beneficial effects of environmental changes (27). Here, we show that repeated hypoxic exposures rendered rhEpo effective in the VLR by induced EpoR. These results are in line with the concept that optimization of the effect of neuroprotective agent may require the preliminary induction of its targeted receptor (37). Concerning rhEpo, future studies should elucidate mechanisms promoting trafficking of EpoR toward the cell surface (38), and the mechanisms selectively involved in the induction of EpoR after environmental manipulations, to develop drugs capable of inducing EpoR.
Materials and Methods
Animals.
All animal experiments were in compliance with the guidelines of the European Union (directive 86/609), taken into the French law (decree 87/848), regulating animal experimentation. All efforts were made to minimize animal suffering and to reduce the number of animals used. Sprague–Dawley male rats were used throughout the study. For more detailed information, see SI Materials and Methods.
Hypoxic Exposure.
Hypoxia was realized by introducing rats within a chamber (Biospherix), the oxygen (O2) proportion of which decreased progressively from 21% to 8% in 1 h. Each hypoxia exposure was maintained at 8% O2 for 6 h. O2 proportion was automatically regulated by the Pro-Ox system (Biospherix). The 3 hypoxia exposures were carried out 4 days apart.
Administration of rhEpo.
rhEpo (Eprex, generously provided by Janssen-Cilag) was administered at 5,000 international uunits/kg (i.p.). For more detailed information, see SI Materials and Methods.
Environmental Enrichment.
We engineered a cage (MARLAU cage, patent no. FR09/00544) promoting standardization of the procedures of enrichment. This cage (Fig. S5) allows increased social interactions (12 rats per cage), increased voluntary exercise (large surface area and presence of 3 running wheels), “diverting” activities (red tunnel, ladder, and toboggan slide), and cognitive stimulations using labyrinths, the configuration of which is changed 3 times a week. Standard rats were housed in groups of 6 from weaning to adulthood in type “E” cages (Charles River).
Pilo-SE.
Scopolamine methyl nitrate (1 mg/kg, s.c.; Sigma) was administered 30 min before pilocarpine hydrochloride (350 mg/kg, i.p.; Sigma). SE was stopped 2 h after its onset by i.p. injection of 20 mg/kg diazepam (Valium; Roche), as previously described (8, 39).
Ex Vivo Procedures.
All rats were deeply anesthetized with a lethal dose of pentobarbital (250 mg/kg) before being killed. For biochemical analysis, brain structures were rapidly microdissected, frozen in liquid nitrogen, and stored at −80 °C. For immunohistochemistry analysis, animals were transcardially perfused with chilled 4% paraformaldehyde in 0.1 M phosphate buffer. After cryoprotection in 25% sucrose, brains were frozen at −40 °C in isopentane and stored at −80 °C. For ELISA measurement, rats were intracardially perfused for 2 min with chilled 0.9% NaCl. After brain removal, the VLR was dissected, weighed, frozen in liquid nitrogen, and stored at −80 °C.
RT-qPCR.
Variations in transcript levels were determined by real-time PCR amplification of cDNAs of interest after reverse transcription of total mRNAs, as previously detailed (8). For more detailed information on primers used for PCR, see SI Materials and Methods.
Quantitative Determination of rhEpo by Using ELISA.
rhEpo was measured by using an ELISA kit (R&D Systems), as previously described (8).
Immunohistochemistry.
Free-floating sections of fixed tissue were used for colorimetric or fluorescent labeling of Epo and EpoR, in combination or not with labeling of either NeuN or GFAP. Images were captured by a TCS SP2 confocal microscopy system (Leica). For more detailed information about antibody characterization, see SI Materials and Methods and ref. 40.
Labeling of Neuronal Degeneration.
Fluoro-Jade B (Chemicon) was used to identify degenerating neurons after Pilo-SE in rats (41). Cell death occurring with DNA breaks was detected by using terminal deoxynucleotidyltransferase-mediated dUTP nick end-labeling (TUNEL) of DNA breaks (Roche).
Image Analysis.
Measurements of neuronal density and fluorescent intensity were performed by using an image analysis system (Visilog, Noesis). For more detailed information, see SI Materials and Methods.
Statistical Analysis.
Data are expressed as mean ± SEM of the different variables analyzed (mRNA level, neuronal density, and brain uptake of rhEpo), and were compared among groups by using 1- or 2-way ANOVA followed by Fisher's protected least significant difference test.
Supplementary Material
Acknowledgments.
We thank D. Ressnikoff and Y. Tourneur from the Centre Commun de Quantimétrie (University of Lyon 1) for their assistance in the use of the confocal microscope. This work was supported by grants from the Centre National de la Recherche Scientifique and the University of Lyon 1. P.E.S. is a fellow from the Délégation Générale pour l'Armement, Ministère Français de la Défense.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0901840106/DCSupplemental.
References
- 1.Brines M, Cerami A. Emerging biological roles for erythropoietin in the nervous system. Nat Rev Neurosci. 2005;6:484–494. doi: 10.1038/nrn1687. [DOI] [PubMed] [Google Scholar]
- 2.Maiese K, Chong ZZ, Li F, Shang YC. Erythropoietin: Elucidating new cellular targets that broaden therapeutic strategies. Prog Neurobiol. 2008;85:194–213. doi: 10.1016/j.pneurobio.2008.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Noguchi CT, Asavaritikrai P, Teng R, Jia Y. Role of erythropoietin in the brain. Crit Rev Oncol Hematol. 2007;64:159–171. doi: 10.1016/j.critrevonc.2007.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ehrenreich H, et al. Erythropoietin therapy for acute stroke is both safe and beneficial. Mol Med. 2002;8:495–505. [PMC free article] [PubMed] [Google Scholar]
- 5.Ehrenreich H, et al. Improvement of cognitive functions in chronic schizophrenic patients by recombinant human erythropoietin. Mol Psychiatry. 2007;12:206–220. doi: 10.1038/sj.mp.4001907. [DOI] [PubMed] [Google Scholar]
- 6.Ehrenreich H, et al. Exploring recombinant human erythropoietin in chronic progressive multiple sclerosis. Brain. 2007;130:2577–2588. doi: 10.1093/brain/awm203. [DOI] [PubMed] [Google Scholar]
- 7.Chen ZY, Warin R, Noguchi CT. Erythropoietin and normal brain development: Receptor expression determines multi-tissue response. Neurodegener Dis. 2006;3:68–75. doi: 10.1159/000092096. [DOI] [PubMed] [Google Scholar]
- 8.Nadam J, et al. Neuroprotective effects of erythropoietin in the rat hippocampus after pilocarpine-induced status epilepticus. Neurobiol Dis. 2007;25:412–426. doi: 10.1016/j.nbd.2006.10.009. [DOI] [PubMed] [Google Scholar]
- 9.Covolan L, Mello LE. Temporal profile of neuronal injury following pilocarpine or kainic acid-induced status epilepticus. Epilepsy Res. 2000;39:133–152. doi: 10.1016/s0920-1211(99)00119-9. [DOI] [PubMed] [Google Scholar]
- 10.Ehrenreich H, et al. A hematopoietic growth factor, thrombopoietin, has a proapoptotic role in the brain. Proc Natl Acad Sci USA. 2005;102:862–867. doi: 10.1073/pnas.0406008102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu R, Suzuki A, Guo Z, Mizuno Y, Urabe T. Intrinsic and extrinsic erythropoietin enhances neuroprotection against ischemia and reperfusion injury in vitro. J Neurochem. 2006;96:1101–1110. doi: 10.1111/j.1471-4159.2005.03597.x. [DOI] [PubMed] [Google Scholar]
- 12.Yu X, et al. Erythropoietin receptor signalling is required for normal brain development. Development. 2002;129:505–516. doi: 10.1242/dev.129.2.505. [DOI] [PubMed] [Google Scholar]
- 13.Bernaudin M, et al. Neurons and astrocytes express EPO mRNA: Oxygen-sensing mechanisms that involve the redox-state of the brain. Glia. 2000;30:271–278. [PubMed] [Google Scholar]
- 14.Soliz J, Gassmann M, Joseph V. Soluble erythropoietin receptor is present in the mouse brain and is required for the ventilatory acclimatization to hypoxia. J Physiol. 2007;583:329–336. doi: 10.1113/jphysiol.2007.133454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu C, Shen K, Liu Z, Noguchi CT. Regulated human erythropoietin receptor expression in mouse brain. J Biol Chem. 1997;272:32395–32400. doi: 10.1074/jbc.272.51.32395. [DOI] [PubMed] [Google Scholar]
- 16.Shams I, Nevo E, Avivi A. Erythropoietin receptor spliced forms differentially expressed in blind subterranean mole rats. FASEB J. 2005;19:1749–1751. doi: 10.1096/fj.05-3975fje. [DOI] [PubMed] [Google Scholar]
- 17.Yamaji R, et al. The intron 5-inserted form of rat erythropoietin receptor is expressed as a membrane-bound form. Biochim Biophys Acta. 1998;1403:169–178. doi: 10.1016/s0167-4889(98)00037-8. [DOI] [PubMed] [Google Scholar]
- 18.Chin K, et al. Production and processing of erythropoietin receptor transcripts in brain. Mol Brain Res. 2000;81:29–42. doi: 10.1016/s0169-328x(00)00157-1. [DOI] [PubMed] [Google Scholar]
- 19.Sharp FR, Bernaudin M. HIF1 and oxygen sensing in the brain. Nat Rev Neurosci. 2004;5:437–448. doi: 10.1038/nrn1408. [DOI] [PubMed] [Google Scholar]
- 20.Assaraf MI, et al. Brain erythropoietin receptor expression in Alzheimer disease and mild cognitive impairment. J Neuropathol Exp Neurol. 2007;66:389–398. doi: 10.1097/nen.0b013e3180517b28. [DOI] [PubMed] [Google Scholar]
- 21.Ehrenreich H, et al. Erythropoietin: A candidate compound for neuroprotection in schizophrenia. Mol Psychiatr. 2004;9:42–54. doi: 10.1038/sj.mp.4001442. [DOI] [PubMed] [Google Scholar]
- 22.Eid T, et al. Increased expression of erythropoietin receptor on blood vessels in the human epileptogenic hippocampus with sclerosis. J Neuropathol Exp Neurol. 2004;63:73–83. doi: 10.1093/jnen/63.1.73. [DOI] [PubMed] [Google Scholar]
- 23.Siren AL, et al. Erythropoietin and erythropoietin receptor in human ischemic/hypoxic brain. Acta Neuropathol. 2001;101:271–276. doi: 10.1007/s004010000297. [DOI] [PubMed] [Google Scholar]
- 24.Bernaudin M, et al. A potential role for erythropoietin in focal permanent cerebral ischemia in mice. J Cereb Blood Flow Metab. 1999;19:643–651. doi: 10.1097/00004647-199906000-00007. [DOI] [PubMed] [Google Scholar]
- 25.Grasso G, et al. Erythropoietin and erythropoietin receptor expression after experimental spinal cord injury encourages therapy by exogenous erythropoietin. Neurosurgery. 2005;56:821–827. doi: 10.1227/01.neu.0000156493.00904.7e. [DOI] [PubMed] [Google Scholar]
- 26.Malhotra S, Savitz SI, Ocava L, Rosenbaum DM. Ischemic preconditioning is mediated by erythropoietin through PI-3 kinase signaling in an animal model of transient ischemic attack. J Neurosci Res. 2006;83:19–27. doi: 10.1002/jnr.20705. [DOI] [PubMed] [Google Scholar]
- 27.Nithianantharajah J, Hannan AJ. Enriched environments, experience-dependent plasticity and disorders of the nervous system. Nat Rev Neurosci. 2006;7:697–709. doi: 10.1038/nrn1970. [DOI] [PubMed] [Google Scholar]
- 28.van Praag H, Kempermann G, Gage FH. Neural consequences of environmental enrichment. Nat Rev Neurosci. 2000;1:191–198. doi: 10.1038/35044558. [DOI] [PubMed] [Google Scholar]
- 29.Rampon C, et al. Effects of environmental enrichment on gene expression in the brain. Proc Natl Acad Sci USA. 2000;97:12880–12884. doi: 10.1073/pnas.97.23.12880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Digicaylioglu M, Garden G, Timberlake S, Fletcher L, Lipton SA. Acute neuroprotective synergy of erythropoietin and insulin-like growth factor I. Proc Natl Acad Sci USA. 2004;101:9855–9860. doi: 10.1073/pnas.0403172101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen ZY, Asavaritikrai P, Prchal JT, Noguchi CT. Endogenous erythropoietin signaling is required for normal neural progenitor cell proliferation. J Biol Chem. 2007;282:25875–25883. doi: 10.1074/jbc.M701988200. [DOI] [PubMed] [Google Scholar]
- 32.Shein NA, Horowitz M, Alexandrovich AG, Tsenter J, Shohami E. Heat acclimation increases hypoxia-inducible factor 1alpha and erythropoietin receptor expression: Implication for neuroprotection after closed head injury in mice. J Cereb Blood Flow Metab. 2005;25:1456–1465. doi: 10.1038/sj.jcbfm.9600142. [DOI] [PubMed] [Google Scholar]
- 33.Gidday JM. Cerebral preconditioning and ischaemic tolerance. Nat Rev Neurosci. 2006;7:437–448. doi: 10.1038/nrn1927. [DOI] [PubMed] [Google Scholar]
- 34.Ruscher K, et al. Erythropoietin is a paracrine mediator of ischemic tolerance in the brain: Evidence from an in vitro model. J Neurosci. 2002;22:10291–10301. doi: 10.1523/JNEUROSCI.22-23-10291.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Auvergne R, et al. Delayed kindling epileptogenesis and increased neurogenesis in adult rats housed in an enriched environment. Brain Res. 2002;954:277–285. doi: 10.1016/s0006-8993(02)03355-3. [DOI] [PubMed] [Google Scholar]
- 36.Young D, Lawlor PA, Leone P, Dragunow M, During MJ. Environmental enrichment inhibits spontaneous apoptosis, prevents seizures and is neuroprotective. Nat Med. 1999;5:448–453. doi: 10.1038/7449. [DOI] [PubMed] [Google Scholar]
- 37.Lipton SA. Pathologically activated therapeutics for neuroprotection. Nat Rev Neurosci. 2007;8:803–808. doi: 10.1038/nrn2229. [DOI] [PubMed] [Google Scholar]
- 38.Ravid O, et al. An extracellular region of the erythropoietin receptor of the subterranean blind mole rat Spalax enhances receptor maturation. Proc Natl Acad Sci USA. 2007;104:14360–14365. doi: 10.1073/pnas.0706777104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Morales A, et al. Unexpected expression of orexin-B in basal conditions and increased levels in the adult rat hippocampus during pilocarpine-induced epileptogenesis. Brain Res. 2006;1109:164–175. doi: 10.1016/j.brainres.2006.06.075. [DOI] [PubMed] [Google Scholar]
- 40.Sanchez PE, et al. Erythropoietin receptor expression is concordant with erythropoietin but not with common beta chain expression in the rat brain throughout the life span. J Comp Neurol. 2009;514:403–414. doi: 10.1002/cne.22020. [DOI] [PubMed] [Google Scholar]
- 41.Schmued LC, Hopkins KJ. Fluoro-Jade B: A high affinity fluorescent marker for the localization of neuronal degeneration. Brain Res. 2000;874:123–130. doi: 10.1016/s0006-8993(00)02513-0. [DOI] [PubMed] [Google Scholar]
- 42.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 4th Ed. New York: Academic; 1998. p. 256. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.