Abstract
The innovation of reprogramming somatic cells to induced pluripotent stem cells provides a possible new approach to treat β-thalassemia and other genetic diseases such as sickle cell anemia. Induced pluripotent stem (iPS) cells can be made from these patients' somatic cells and the mutation in the β-globin gene corrected by gene targeting, and the cells differentiated into hematopoietic cells to be returned to the patient. In this study, we reprogrammed the skin fibroblasts of a patient with homozygous β0 thalassemia into iPS cells, and showed that the iPS cells could be differentiated into hematopoietic cells that synthesized hemoglobin. Prenatal diagnosis and selective abortion have been effective in decreasing the number of β-thalassemia births in some countries that have instituted carrier screening and genetic counseling. To make use of the cells from the amniotic fluid or chorionic villus sampling that are used for prenatal diagnosis, we also showed that these cells could be reprogrammed into iPS cells. This raises the possibility of providing a new option following prenatal diagnosis of a fetus affected by a severe illness. Currently, the parents would choose either to terminate the pregnancy or continue it and take care of the sick child after birth. The cells for prenatal diagnosis can be converted into iPS cells for treatment in the perinatal periods. Early treatment has the advantage of requiring much fewer cells than adult treatment, and can also prevent organ damage in those diseases in which damage can begin in utero or at an early age.
Keywords: amniocentesis, chorionic villus sampling, hematopoietic differentiation, hemoglobin
Worldwide, thalassemia is one the most common genetic diseases. It affects people who originate from the Mediterranean areas through the Middle East to the Indian subcontinent and Southeast Asia (1). The thalassemia syndromes are broadly classified into 2 groups: α-thalassemia, in which the α-globin chain synthesis is defective; and β-thalassemia, which results from defective β-globin synthesis. α-thalassemia is most commonly caused by gene deletions that affect one or both of the duplicated α-globin loci. The severe form of α-thalassemia, common in Southeast Asia, is caused by the deletion of the duplicated α-globin genes, and the majority of homozygous fetuses die at the third trimester of pregnancy or at birth. In β-thalassemia, the common molecular defects are the results of point mutations or small deletions that affect the transcription, splicing, or translation of the mRNA. Newborns with homozygous β-thalassemia are healthy, but as the hemoglobin switches from fetal to adult hemoglobin after birth, the lack of β-globin results in progressive anemia. Although the degree of anemia may vary depending on the degree of β-globin deficiency and the amount of fetal hemoglobin synthesis, many patients with β-thalassemia have severe anemia and require life-long blood transfusion. Because the blood given by transfusion is rich in iron, the patients require iron chelation therapy throughout life. Hence, β-thalassemia poses a severe health and economic burden to families at risk.
Cure for β-thalassemia can be achieved by bone marrow or cord blood transplantations if histocompatible donors are available (2, 3). However, because these patients' families are usually small, donors are not commonly found. Furthermore, graft versus host diseases of variable severity commonly follow. Prevention of new births with homozygous β-thalassemia has been achieved with prenatal diagnosis. In countries such as Italy, Greece, and Cyprus, carrier screening, genetic counseling, prenatal diagnosis, and selective abortion of the homozygous fetuses have effectively decreased the number of births with homozygous β-thalassemia.
An experimental approach to treatment is gene therapy of β-thalassemia and sickle cell anemia. Using lentiviral vectors expressing the β-globin gene to transduce hematopoietic cells ex vivo, several groups of investigators have successfully treated mouse models of β-thalassemia or sickle cell anemia (4–6). A human trial is said to be proceeding in Europe (7).
The reprogramming (8–10) of somatic cells into induced pluripotent stem (iPS) cells opens a new approach of treating β-thalassemia. iPS cells have been made from a variety of tissues, including skin fibroblasts, hepatocytes, stomach, testicular, and neural cells (11–14). In addition, iPS cells have also been made from the somatic cells of patients with a number of diseases including adenosine deaminase deficiency, Gaucher disease, muscular dystrophies, Parkinson disease, Huntington disease, type 1 diabetes mellitus, amyotrophic lateral sclerosis, and Down and Lesch-Nyhan syndromes (15–17). These iPS cells provide a means of studying pathophysiology as well as approaches to testing therapeutic agents for diseases. As β-thalassemia is an extremely common genetic disease, we investigated the reprogramming of somatic cells from patients with β-thalassemia into iPS cells. β-thalassemia, like sickle cell anemia, appears to be a good candidate for iPS cell therapy. The β-globin gene mutations of most patients are known, and in sickle cell anemia, they can be corrected by gene targeting techniques (18, 19), and the iPS cells differentiated into hematopoietic cells that, in turn, can be returned to the patients for treatment. Indeed, sickle cell anemia was cured in this manner in a mouse model (20).
Another opportunity for iPS cell therapy lies in the perinatal period. When the prenatal diagnosis of a genetic disease associated with severe illness is made, the parents choose either to terminate the pregnancy or to continue it and take care of the child with a life-long illness. In most cases, the parents would choose termination. The cells from the amniotic fluid (AF) or chorionic villus sample (CVS) used for the diagnosis are eventually discarded. If these cells can be reprogrammed into iPS cells, they may be used for early treatment of the affected fetus during the perinatal period.
In this study, we investigated (i) the generation of iPS cells from the skin fibroblasts from a patient with homozygous β-thalassemia and (ii) a method of differentiating the iPS cells into hematopoietic cells that synthesize hemoglobin. In addition, we also studied the feasibility of reprogramming AF and CVS cells into iPS cells that can be used for early treatment of thalassemia, sickle cell anemia, and other genetic diseases.
Results
Reprogramming β-Thalassemia Fibroblasts.
Fibroblasts cultured from skin biopsy were from a patient with β0-thalassemia who was homozygous for the codon 41/42 4-bp (CTTT) deletion. This mutation causes a frameshift and no β-globin chain is made. Cells from CVS and amniocentesis were obtained anonymously from the clinical laboratories at University of California San Francisco.
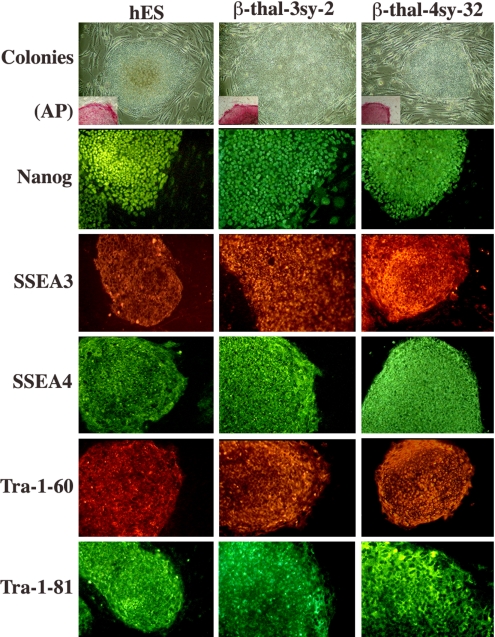
Two types of infection were created in the generation of iPS cells from the skin fibroblasts by retroviral vectors. In one, the skin fibroblasts were infected with retroviral vectors containing the 4 transcription factor genes (Oct4, Sox2, Klf4, and cMyc), and in the other with 3 genes omitting cMyc. With the addition of valproic acid in the culture medium, approximately 450 and 50 tightly packed colonies similar to human ES cell colonies began to appear at 2 to 3 weeks (21) after infection of 1 × 105 cells with 4 and 3 factor genes, respectively. Forty of 4 factors-induced colonies (4sy series) and 30 of 3 factors-induced colonies (3sy series) were picked at 30 to 35 d after infection. Thirty-five of 4 factors-infected colonies and 28 of the 3 factors-infected colonies could be expanded and showed the same morphology as human embryonic stem (ES) cells. They expressed the pluripotency markers, such as Nanog, SSEA3, SSEA4, Tra-1–60, and Tra-1–81, as well as alkaline phosphatase, similar to human ES cells (Fig. 1). Western blot analysis of these colonies revealed a similar level of Nanog expression compared with human ES cells (data not shown). These cells could be maintained in an undifferentiated state on Matrigel-coated plates in mouse embryonic fibroblast-conditioned human ES cell medium. Some of the colonies have been cultured for more than 4 to 7 months in our laboratory.
Fig. 1.
iPS cell colonies reprogrammed with 3 factors (3sy) or 4 factors (4sy) from the skin fibroblasts of a patient with homozygous β–thalassemia (β-thal) compared with hESC colonies (hES). They were stained for alkaline phosphatase (AP, Inset) and ES cell markers Nanog, SSEA3, SSEA4, Tra-1–60, and Tra-1–81.
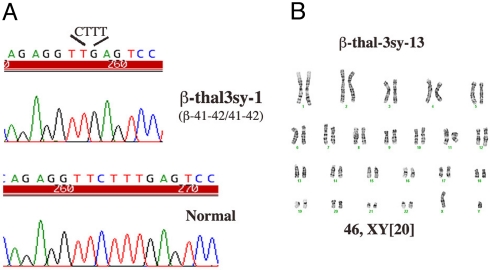
To confirm that the generated iPS cell lines were derived from the β-thalassemia patient's skin fibroblasts, genomic DNA sequencing analyses were performed in 5 individual iPS cell lines. Homozygous codon 41/42 4-bp (CTTT) deletion was verified in all 5, identical to the sequence of the skin fibroblasts (Fig. 2A). Karyotypes of 5 individual iPS lines after 5 passages were normal, and 1 cultured for 15 additional passages was also found to maintain the normal karyotype (Fig. 2B).
Fig. 2.
Sequence analysis (A) and karyotype (B) of iPS cells reprogrammed from fibroblasts of the patient with β-thalassemia.
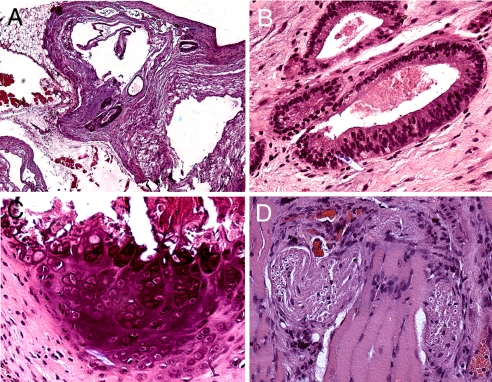
To examine the pluripotency of the generated iPS cells in vivo, we injected 106 iPS cells intramuscularly into the hind leg of immunodeficient (NOD-SCID) mice. Seven weeks after injection, tumor formation was observed. Histological analysis of the tumors revealed that they contained tissues representative of all 3 germ layers including ciliary respiratory and gut-like epithelia (i.e., endoderm), bone and muscle (i.e., mesoderm), and nerve and sebaceous glands (i.e., ectoderm; Fig. 3).
Fig. 3.
Photomicrographs of H&E stain of teratoma formed in NOD-SCID mice after injection of iPS cells from the patient with β-thalassemia patient shows (A) heterogeneous tissue under low power (magnification ×4), (B) respiratory epithelium, (C) bone, and (D) neural tissues (magnification ×20).
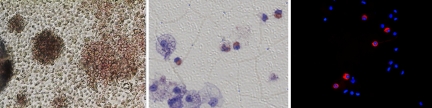
We investigated the ability of the iPS cells to differentiate into hematopoietic cells. Single cells were made from one of the β-thalassemia iPS colonies with the enzyme Accutase. After washing, the cells were resuspended in the first differentiation medium, which is the basal medium, SFM supplemented with human recombinant bone morphogenetic protein 4, human VEGF (hVEGF), human stem cell factor (hSCF), human Flt3, human IL3, human IGF-II, and TPO (22). Embryoid bodies (EBs) were made by plating these cells in a V-bottom 96-well plate and centrifuged according to the method of Ng et al. (23, 24). Initially, we optimized the formation of EB by plating 5,000, 10,000, and 20,000 cells in the wells. EBs were formed in the well, where the cell number is greater than 10,000. After incubation at 37 °C and 5% CO2 for 10 to 14 d, EBs were then singly placed in a gelatinized tissue culture plate and allowed to differentiated further into hematopoietic lineage in the secondary differentiation medium, which is SFM-supplemented with hVEGF, hSCF, human Flt3, human IL6 and human erythropoietin. Some fibroblast-like cells grew and spread out from the EBs right away and some round, non-adherent cells showed up in some wells after 4 to 5 d incubation and “hemoglobinized” colonies were evident with longer incubation. After 2 weeks, these non-adherent cells were removed, spun down on the slides, and stained with hemoglobin F antibody. The cells from the wells that contain hemoglobinized colonies showed positive staining with antibody against hemoglobin F (Fig. 4).
Fig. 4.
Differentiation of iPS cells into hematopoietic cells. (Left) Hematopoietic colonies (magnification ×4); (Middle) Giemsa stain showing hemoglobinization of some of the cells; (Right) Superimposition of DAPI and anti-hemoglobin F antibody stains (magnification ×40).
Reprogramming of AF and CVS Cells.
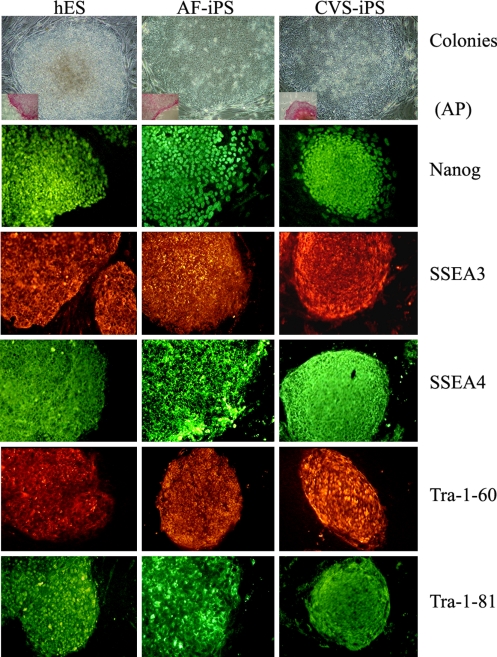
To see if the CVS and AF cells used for prenatal diagnosis can be used for potential therapy, we explored if these cells can be reprogrammed into iPS cells. We obtained anonymous cells that were used for routine prenatal diagnosis of chromosomal abnormalities. The cells cultured from the CVS were infected with retroviral vectors carrying the 4 transcription factor genes. As iPS cells have been reported to be generated with fewer than 4 transcription factors, we infected the AF cells with 2 vectors carrying the Oct3/4 and Sox2 genes or 3 vectors carrying the Oct3/4, Sox2, and Klf4 genes. iPS cell colonies were successfully reprogrammed from all 3 samples of CVS and AF cells, 5 from CVS, and 50 and 34, respectively, from 105 AF cells. They expressed the pluripotency markers as in human ES cells (Fig. 5).
Fig. 5.
Human ES cells compared with iPS cell colonies reprogrammed from cells from AF and CVS.
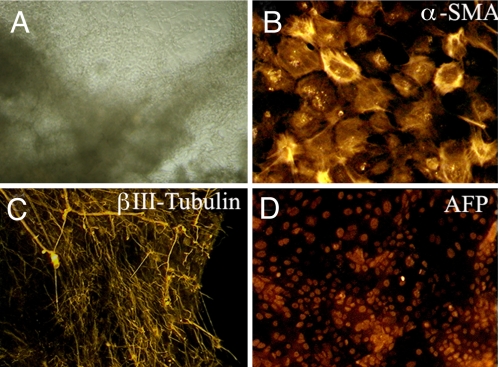
We tested the ability of the iPS cell prepared from prenatal diagnosis to differentiate into cells of the 3 germ layers in vitro. We used the EB-mediated protocol to differentiate the iPS cells (25). The iPS colonies were treated with collagenase and cultured in human ES medium lacking basic FGF (bFGF). After 8 d in suspension culture, EBs were formed. The EBs were transferred to gelatin-coated plates and further cultured for 8 d. We observed cells with diverse morphology (Fig. 6A). Immunocytochemistry analysis of these cells was positive for α-smooth muscle actin (marker for mesoderm), βIII-tubulin (ectoderm), and α-fetoprotein (endoderm), representative of all 3 germ layers (Fig. 6 B–D). These results indicate that the cells used for prenatal diagnosis have the potential to be reprogrammed to pluripotent stem cells that may be used for early treatment of genetic diseases.
Fig. 6.
Embryoid body-mediated differentiation of iPS cells derived from amniotic fluid cells. (A) Photomicrograph of differentiated iPS cells on day 16 (magnification ×4) and immuno-stained with (B) α-smooth muscle actin, (C) βIII-tubulin, and (D) α-fetoprotein (magnification ×20).
Discussion
In this study we showed that iPS cells can be reprogrammed from the skin fibroblasts of a patient with homozygous β-thalassemia. The iPS cells are pluripotent, as they can differentiate into cells of the 3 germ layers and maintain their normal karyotypes as well. We also demonstrated that, like human ES cells, human iPS cells from this patient can be differentiated into hematopoietic cells, which synthesize fetal hemoglobin in vitro.
These results raise the possibility of using iPS cells to treat patients with homozygous β-thalassemia. A similar strategy of treatment may also be applied to sickle cell anemia, as has been demonstrated in a mouse model (20). Somatic cells such as skin cells can be cultured from the patients and reprogrammed into iPS cells. The mutation in the β-globin gene in β-thalassemia or sickle cell anemia can be corrected in the cultured fibroblasts or following their reprogramming into iPS cells by the gene targeting method. The iPS cells can then be differentiated into hematopoietic cells for the treatment of the patients. As the cells are the patient's own, immune rejection is avoided.
There are some hurdles that need to be overcome before iPS treatment of β-thalassemia and other genetic diseases can be contemplated. At present, the most efficient method of reprogramming somatic cells into iPS cells is to use retroviral vectors to introduce the transcription factor genes. However, retroviral integration may disturb gene functions. Recent reports using lentiviral vectors, which require fewer integration sites, may minimize this problem (26, 27). In addition, the floxed vector can be removed by cre recombinase after reprogramming when the expression of the transcription factors is no longer needed (17). Non-integrating adenoviral vectors have also been used successfully to reprogram iPS cells (28). A method using transposon to deliver the transcription factor genes has also been described (29–31). Other non-viral or chemical methods have also been investigated (32).
Another refinement needed is to avoid using animal feeder layers such as mouse embryonic fibroblasts or animal products such as bovine fetal serum as they could introduce animal pathogens into human cells, which will not be suitable for treatment. Recently, serum-free media and feeder layer methods have been described for human ES cell cultures. It has to be determined if they will prove to be as useful for human iPS cells.
We have also shown that the cells from AF and CVS used for prenatal diagnosis can be reprogrammed into iPS cells. If the diagnosis of the homozygous states of a genetic disease is made, reprogramming these cells into iPS cells can begin during the pregnancy right after diagnosis so as to offer early treatment in the neonatal period. Hence, in addition to the 2 options currently available to the parents, a new option can be offered to them if the fetus is diagnosed to have a severe genetic illness.
Early treatment of genetic diseases has 2 distinct advantages. First, the number of cells required for treatment is much smaller. As the processes of reprogramming, mutation correction, and cell differentiation require many steps, preparing enough cells to treat an adult may prove challenging. A second advantage of early treatment applies to diseases in which organ damage may have begun in utero. For example, bone marrow or cord blood stem cell therapy in inherited metabolic diseases such as mucopolysaccharidosis is associated with mortality and morbidity resulting from graft versus host disease as well as organ damage that has already begun (33, 34). iPS cell therapy not only will avoid graft versus host disease but also may forestall organ damage when given in utero or in the neonatal period.
In conclusion, these studies show that iPS cells can offer a new approach for treatment of β-thalassemia and other genetic diseases. Early treatment following prenatal diagnosis will provide a new option to families at risk and may yield better outcomes.
Materials and Methods
Cell Cultures.
Fibroblasts culture from skin biopsy was provided anonymously from a patient with homozygous β0 thalassemia resulting from a 4-bp deletion frameshift mutation (-CTTT). AF and CVS cells were obtained anonymously from clinical samples.
Skin fibroblast cells were maintained in DMEM (Invitrogen) containing 10% FBS (HyClone), 2 mM L-glutamine (Invitrogen), and 50 U/mL penicillin/50 mg/mL streptomycin.
AF cells were obtained by centrifugation from 10 to 15 mL AF in a centrifuge tube at 1,000 rpm for 10 min. The supernatant was then removed and the cell resuspended in 2 mL of AminoMax (Invitrogen), which was transferred to 2 T-25 flasks, with the volume made up to 5 mL each, and cultured at 37 °C with 5% CO2.
Chorionic villi were placed in a Petri dish containing 2.5 mL aspiration media (1% sodium heparin, 1% L-Glu, and 1% Pen-Strp in RPMI-1640) and thoroughly cleaned by removing the attached decidua and blood clots under a dissecting microscope. The medium was replaced with 2 mL of 10× Trypsin-EDTA (Invitrogen) and incubated at 37 °C for 25 min. The trypsin solution was replaced with 2 mL of collagenase II (500 U/mL; Worthington) and incubated at 37 °C for 45 min. The cells in suspension were centrifuged and suspended in AmnioMax and treated and cultured in T-25 flasks as for the AF cells.
Retroviral Vectors Production and Infection of Cells.
293FT cells for retroviral production were maintained in retroviral infection medium [DMEM containing 10% FBS (HyClone), 2 mM L-glutamine (Invitrogen), and 50 U/mL penicillin and 50 mg/mL streptomycin]. They were co-transfected with pMXs retroviral vectors containing the human cDNAs for Oct3/4, Sox2, Klf4 and cMyc, VSV-G, and Gag-Pol (pUVMC) (all from Addgene) with Fugene 6 (Roche). Forty-eight hours after transfection, the medium was collected, filtered, and concentrated by ultracentrifugation at 70,000 × g for 1.5 h at 4 °C. The titer was adjusted to 1 × 108 infectious units/mL in DMEM.
Skin fibroblast, CVS, and AF cells were seeded at 1 × 105/well of a 6-well plate, and 6 h later the medium was replaced with the retroviral infection medium supplemented with 5 μg/mL of protamine sulfate and containing the retroviral vectors Oct4, Sox2, Klf4, and cMyc for fibroblast cells and CVS cells; Oct4, Sox2, and Klf4 for fibroblast and AF cells; and Oct4 and Sox2 for AF cells at the multiplicity of infection of 10. Twenty-four hours later, the culture was replaced with the same medium without the viral vectors. Four to 5 days after infection, the cells were harvested by trypsinization and re-plated at 1 × 105 cells per 10-cm gelatin-coated dish on mouse embryonic fibroblast feeder layer in retroviral infection medium. The medium was replaced the next day with human ES medium (DMEM/F12 medium supplemented with 20% knockout serum replacement serum, 1 mM L-glutamine, 100 μM nonessential amino acids, 100 μM β-mercaptoethanol, 50 U/mL penicillin and 50 mg/mL streptomycin, 4 ng/mL bFGF; Invitrogen) and supplemented with 2 mM valproic acid (Sigma). One week later, valproic acid was removed and cells were cultured with daily change of ES medium. Thirty to 35 d after transduction, colonies were picked up and transferred onto mouse embryonic fibroblast feeder layer in 24-well plates. Five to 7 d later, colonies were expanded by mechanical dissociation or treated with collagenase and processed for analyses of marker gene expression and pluripotency.
ES Cell Marker Identification.
Alkaline phosphatase staining was performed with a kit from Millipore. For immunocytochemistry, iPS cells grown on feeder cells were fixed with 4% paraformaldehyde for 15 min. After washing with PBS solution, the cells were treated with PBS solution containing 5% normal goat or donkey serum (Vector), 1% BSA (Sigma), and 0.1% Triton X-100 for 45 min at room temperature. They were incubated with primary antibodies against SSEA3, TRA 1–81, TRA 1–60 (Millipore), Nanog (R&D Systems), or SSEA4 (Abcam) overnight at 4 °C, followed the next day by 1 h at room temperature with secondary antibodies, Alexa Fluor 555-conjugated goat anti-mouse IgM, Alexa Fluor 488–conjugated goat anti-mouse IgM, Alexa Fluor 546-conjugated goat anti-rabbit IgG, or Alexa Fluor 488–conjugated donkey anti-goat IgG (Invitrogen). The mounting solution (Vector) containing DAPI was used to counterstain the nuclei. Images were taken by using a PixCell II (Arcturus) inverted fluorescence microscope and processed and analyzed using Adobe Photoshop software.
Cytogenetic Analysis.
A minimum of 20 metaphases were examined by experienced and certified cytogeneticists. The karyotypes were captured and documented by using Apply Imaging System for cytogenetic analysis
In Vivo Differentiation of iPS Cells.
The iPS cells are tested for their ability to form teratoma. Cells were harvested by collagenase IV treatment, collected by centrifugation, and washed once with DMEM/F12. Approximately 106 cells (i.e., approximately one 10-cm dish) were resuspended in a mixture of DMEM/F12 and Matrigel at the ratio of 2:1. The cell mixtures were injected intramuscularly into the hind legs of Nod-SCID mouse. Tumors were dissected at 7 to 10 weeks after injection and fixed with 10% formalin, embedded in paraffin, sectioned, and stained with H&E.
In Vitro Differentiation of iPS Cells.
iPS cells were harvested by treating with collagenase IV (Invitrogen). Clumps of the cells were transferred to a poly(2-hydroxyrthyl methacrylate)-coated dish in human ES medium without bFGF. The medium was changed every other day. After 8 d culture in suspension, the EBs formed were transferred into a gelatin-coated plate and cultured in the same medium for another 8 to 10 d. Differentiated cells were photographed and stained with antibodies against α-fetoprotein (R&D Systems), βIII-tubulin (Covance), and α-smooth muscle actin (Sigma).
Hematopoietic Cell Differentiation.
Human iPS cells were routinely passed by the standard method by using collagenase treatment. For EB formation, iPS cells on a 10-cm plate were washed once with PBS solution. Four milliliters of Accutase (Chemicon) was then added, and the cells incubated at 37 °C, 5% CO2, for 2 to 4 min until single cells in the colonies were evident. Accutase was removed by aspiration and the cells were washed with PBS solution once. Cells were harvested by adding 10 mL of PBS solution, and the cell suspension was centrifuged at 1,000 rpm (Eppendorf centrifuge 5804, rotor A-2-MTP) for 5 min. Cell pallets were collected and washed with PBS solution again and resuspended in differentiation medium, which contained the basal medium SFM described by Johansson and Wiles (22) and the same cytokines. SFM comprised a 1:1 ratio of IMDM and Ham F-12 (Gibco/Invitrogen), 5 mg/mL BSA (A3311; Sigma), 1:100 synthetic lipids (Gibco 11905–031; Invitrogen), 450 μM α-monothioglycerol (M-1753; Sigma), 1:100 insulin-transferrin-selenium (ITS-X, Gibco 51500–056; Invitrogen), 2 mM Glutamax II (Gibco 35050–061; Invitrogen), 5% protein-free hybridoma mix (PFHMII, Gibco 12040–077; Invitrogen), and 50 μg/mL ascorbic acid-2-phosphate (A-8960; Sigma). EB formation was induced by seeding the desired number (i.e., 5,000–20,000) of hESCs in 100 μL of SFM supplemented with hBMP4 10 ng/mL, hVEGF 5 ng/mL, hSCF 20 ng/mL, hFlt3 ligand 5 ng/mL, hIL-6 5 ng/mL, and hIGF-II 5 ng/mL (all from R & D Systems) in each well of 96-well V-shaped bottom, low-attachment plates (Nunc) and centrifuging the plates at 1,500 rpm (Eppendorf centrifuge 5804, rotor A-2-MTP) for 5 min at room temperature to aggregate the cells. The cell aggregates, left undisturbed for 2 d in the wells, were pooled in a 10-cm bacterial dish and incubated further at 37 °C and 5% CO2 with half of the medium changed every 2 to 3 d. After 10 to 12 d, the EBs were transferred to 96-well flat-bottomed tissue culture plates (Nunc) pre-coated with gelatin, in SFM supplemented with hVEGF 5 ng/mL, hSCF 20 ng/mL, hFlt3 ligand 5 ng/mL, hIL-3 5 ng/mL, hTPO 5 ng/mL (R & D Systems), and human erythropoietin 5 U/mL (StemCell Technologies) and allowed to differentiate further at 37 °C and 5% CO2 for 14 d or longer with medium changed every 2 to 3 d. The non-adherent cells in each well were harvested, spun down onto the slides, and stained with antibodies against hemoglobin F as previously described (35).
Acknowledgments.
We thank Dr. T.S. Benedict Yen of the Pathology Department for reviewing the teratoma sections. This research was supported by Louis K. Diamond, Viola Schroeder, and Horton Funds.
Footnotes
The authors declare no conflict of interest.
References
- 1.Weatherall D, Clegg J. The Thalassaemia Syndromes. 2nd Ed. New York: Wiley-Blackwell; 2001. [Google Scholar]
- 2.Giardini C, Lucarelli G. Bone marrow transplantation for beta-thalassemia. Hematol Oncol Clin North Am. 1999;13:1059–1064. doi: 10.1016/s0889-8588(05)70109-x. [DOI] [PubMed] [Google Scholar]
- 3.Locatelli F, et al. Related umbilical cord blood transplantation in patients with thalassemia and sickle cell disease. Blood. 2003;101:2137–2143. doi: 10.1182/blood-2002-07-2090. [DOI] [PubMed] [Google Scholar]
- 4.Rivella S, May C, Chadburn A, Riviere I, Sadelain M. A novel murine model of Cooley anemia and its rescue by lentiviral-mediated human beta-globin gene transfer. Blood. 2003;101:2932–2939. doi: 10.1182/blood-2002-10-3305. [DOI] [PubMed] [Google Scholar]
- 5.Pawliuk R, et al. Correction of sickle cell disease in transgenic mouse models by gene therapy. Science. 2001;294(5550):2368–2371. doi: 10.1126/science.1065806. [DOI] [PubMed] [Google Scholar]
- 6.Pestina TI, et al. Correction of murine sickle cell disease using gamma-globin lentiviral vectors to mediate high-level expression of fetal hemoglobin. Mol Ther. 2009;17:245–252. doi: 10.1038/mt.2008.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bank A, Dorazio R, Leboulch P. A phase I/II clinical trial of beta-globin gene therapy for beta-thalassemia. Ann N Y Acad Sci. 2005;1054:308–316. doi: 10.1196/annals.1345.007. [DOI] [PubMed] [Google Scholar]
- 8.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 9.Takahashi K, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 10.Yu J, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 11.Park IH, et al. Reprogramming of human somatic cells to pluripotency with defined factors. Nature. 2008;451:141–146. doi: 10.1038/nature06534. [DOI] [PubMed] [Google Scholar]
- 12.Aoi T, et al. Generation of pluripotent stem cells from adult mouse liver and stomach cells. Science. 2008;321:699–702. doi: 10.1126/science.1154884. [DOI] [PubMed] [Google Scholar]
- 13.Conrad S, et al. Generation of pluripotent stem cells from adult human testis. Nature. 2008;456:344–349. doi: 10.1038/nature07404. [DOI] [PubMed] [Google Scholar]
- 14.Kim JB, et al. Oct4-induced pluripotency in adult neural stem cells. Cell. 2009;136:411–419. doi: 10.1016/j.cell.2009.01.023. [DOI] [PubMed] [Google Scholar]
- 15.Park IH, et al. Disease-specific induced pluripotent stem cells. Cell. 2008;134:877–886. doi: 10.1016/j.cell.2008.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dimos JT, et al. Induced pluripotent stem cells generated from patients with ALS can be differentiated into motor neurons. Science. 2008;321:1218–1221. doi: 10.1126/science.1158799. [DOI] [PubMed] [Google Scholar]
- 17.Soldner F, et al. Parkinson's disease patient-derived induced pluripotent stem cells free of viral reprogramming factors. Cell. 2009;136:964–977. doi: 10.1016/j.cell.2009.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smithies O, Gregg RG, Boggs SS, Koralewski MA, Kucherlapati RS. Insertion of DNA sequences into the human chromosomal beta-globin locus by homologous recombination. Nature. 1985;317:230–234. doi: 10.1038/317230a0. [DOI] [PubMed] [Google Scholar]
- 19.Thomas KR, Capecchi MR. Site-directed mutagenesis by gene targeting in mouse embryo-derived stem cells. Cell. 1987;51:503–512. doi: 10.1016/0092-8674(87)90646-5. [DOI] [PubMed] [Google Scholar]
- 20.Hanna J, et al. Treatment of sickle cell anemia mouse model with iPS cells generated from autologous skin. Science. 2007;318:1920–1923. doi: 10.1126/science.1152092. [DOI] [PubMed] [Google Scholar]
- 21.Huangfu D, et al. Induction of pluripotent stem cells by defined factors is greatly improved by small-molecule compounds. Nat Biotechnol. 2008;26:795–797. doi: 10.1038/nbt1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johansson BM, Wiles MV. Evidence for involvement of activin A and bone morphogenetic protein 4 in mammalian mesoderm and hematopoietic development. Mol Cell Biol. 1995;15:141–151. doi: 10.1128/mcb.15.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ng ES, Davis RP, Azzola L, Stanley EG, Elefanty AG. Forced aggregation of defined numbers of human embryonic stem cells into embryoid bodies fosters robust, reproducible hematopoietic differentiation. Blood. 2005;106:1601–1603. doi: 10.1182/blood-2005-03-0987. [DOI] [PubMed] [Google Scholar]
- 24.Ng ES, Davis RP, Hatzistavrou T, Stanley EG, Elefanty AG. Directed differentiation of human embryonic stem cells as spin embryoid bodies and a description of the hematopoietic blast colony forming assay. Curr Protoc Stem Cell Biol. 2008 doi: 10.1002/9780470151808.sc01d03s4. Chapter 1:Unit 1D 3. [DOI] [PubMed] [Google Scholar]
- 25.Itskovitz-Eldor J, et al. Differentiation of human embryonic stem cells into embryoid bodies compromising the three embryonic germ layers. Mol Med. 2000;6:88–95. [PMC free article] [PubMed] [Google Scholar]
- 26.Sommer CA, et al. iPS cell generation using a single lentiviral stem cell cassette. Stem Cells. 2008 doi: 10.1634/stemcells.2008-1075. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carey BW, et al. Reprogramming of murine and human somatic cells using a single polycistronic vector. Proc Natl Acad Sci USA. 2009;106:157–162. doi: 10.1073/pnas.0811426106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stadtfeld M, Nagaya M, Utikal J, Weir G, Hochedlinger K. Induced pluripotent stem cells generated without viral integration. Science. 2008;322:945–949. doi: 10.1126/science.1162494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Woltjen K, et al. piggyBac transposition reprograms fibroblasts to induced pluripotent stem cells. Nature. 2009;458:766–770. doi: 10.1038/nature07863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kaji K, et al. Virus-free induction of pluripotency and subsequent excision of reprogramming factors. Nature. 2009;458:771–775. doi: 10.1038/nature07864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yusa K, Rad R, Takeda J, Bradley A. Generation of transgene-free induced pluripotent mouse stem cells by the piggyBac transposon. Nat Methods. 2009;6:363–369. doi: 10.1038/nmeth.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Okita K, Nakagawa M, Hyenjong H, Ichisaka T, Yamanaka S. Generation of mouse induced pluripotent stem cells without viral vectors. Science. 2008;322:949–953. doi: 10.1126/science.1164270. [DOI] [PubMed] [Google Scholar]
- 33.Rovelli AM, Steward CG. Hematopoietic cell transplantation activity in Europe for inherited metabolic diseases: open issues and future directions. Bone Marrow Transplant. 2005;35(suppl 1):S23–S26. doi: 10.1038/sj.bmt.1704839. [DOI] [PubMed] [Google Scholar]
- 34.Prasad VK, et al. Unrelated donor umbilical cord blood transplantation for inherited metabolic disorders in 159 pediatric patients from a single center: influence of cellular composition of the graft on transplantation outcomes. Blood. 2008;112:2979–2989. doi: 10.1182/blood-2008-03-140830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chang JC, Ye L, Kan YW. Correction of the sickle cell mutation in embryonic stem cells. Proc Natl Acad Sci USA. 2006;103:1036–1040. doi: 10.1073/pnas.0510177103. [DOI] [PMC free article] [PubMed] [Google Scholar]