Abstract
Cytolytic CD8+ T cells (CTLs) kill virally infected cells, tumor cells, or other potentially autoreactive T cells in a calcium-dependent manner. To date, the molecular mechanism that leads to calcium intake during CTL differentiation and function has remained unresolved. We demonstrate that desmoyokin (AHNAK1) is expressed in mature CTLs, but not in naive CD8+ T cells, and is critical for calcium entry required for their proper function during immune response. We show that mature AHNAK1-deficient CTLs exhibit reduced Cav1.1 α1 subunit expression (also referred to as L-type calcium channels or α1S pore-forming subunits), which recently were suggested to play a role in calcium entry into CD4+ T cells. AHNAK1-deficient CTLs show marked reduction in granzyme-B production, cytolytic activity, and IFN-γ secretion after T cell receptor stimulation. Our results demonstrate an AHNAK1-dependent mechanism controlling calcium entry during CTL effector function.
Calcium plays critical roles in T-cell differentiation and function, such as activation, proliferation, and cytokine production (1, 2). Cytotoxic CD8+ effector T cells (CTLs) primarily use the perforin/granule exocytosis pathway to kill virus-infected and tumor cells (3). The role of calcium in CTL-mediated cytolysis has been studied extensively, mainly by using calcium antagonists (e.g., see ref. 4). The exact requirements for calcium and its molecular mode of entry during CTL function still are largely uncertain (4, 5).
The calcium release-activated calcium channel (CRAC) pathway is the most studied plasma membrane store operated calcium (SOC) channel through which calcium enters after T-cell stimulation. Surprisingly, ORAI1 (also known as “CRACM1” or “TMEM142A”)-deficient CTLs indeed show reduced calcium entry but only partial IFN-γ production and normal granzyme-B expression, suggesting that other pore subunits, possibly ORAI2 or ORAI3, or different channels altogether, are involved in this process (6).
In addition to CRAC channels (7), we and others (8, 9), found that L-type calcium channel (Cav1) subunits α1, α2, β, γ, and δ, which constitute the major route of calcium entry in excitable cells and take up calcium in response to membrane depolarization, also are expressed by T cells (10). Previously, we have shown that CD4+ T cells express α1 subunits of the Cav1 family and that functional Cav β4 and β3 regulatory subunits are necessary for normal TCR-triggered calcium response, nuclear factor of activated T cells (NFAT) nuclear translocation, and cytokine production (8, 11).
The AHNAK family of scaffold PDZ proteins consists of 2 giant proteins (700 kDa), AHNAK1 (desmoyokin) and AHNAK2 (12–14). AHNAK1 is involved with calcium signaling through protein–protein interactions (15–18). Furthermore, in cardiomyocytes, AHNAK1 associates with the β-subunit of cardiac Cav channels at the plasma membrane and is phosphorylated by protein kinase A (PKA) in response to β-adrenoreceptor stimulation (19).
Using AHNAK1-deficient mice (14), we recently described a novel mechanism for the regulation of calcium signaling through Cav1.1 α1 subunits mediated by AHNAK1 in peripheral CD4+ T cells. AHNAK1 is associated with the regulatory β2 subunit of Cav1 channels and is required for normal expression of the Cav1.1 α1 subunit and intact calcium influx following TCR cross-linking (11). Here, we demonstrate that CTLs employ AHNAK1 to mediate calcium entry required for cytolytic activity late in primary TCR stimulation through the regulation of Cav1.1 channels.
Results
AHNAK1 Is Expressed in Mature CTLs.
Previously, we have shown that AHNAK1 is required for differentiation of naive CD4+ T cells (11). In CD8+ T cells, we found that AHNAK1 was not expressed in naive CD8+ T cells (Fig. S1A) but was highly expressed in mature CTLs 5 days after primary stimulation in vitro (Fig. S1A). Further examination of AHNAK1 expression showed that it was highly expressed late after primary differentiation in vitro, starting on day 4, throughout day 5, and 24 h after restimulation, as shown by densitometry of immunoblot analysis (Fig. S1B).
Ahnak1−/− mice showed normal thymocyte development and normal lymphoid composition in their thymus and spleen (11) as well as normal CD8+ T cell numbers in the spleen (Fig. S2). In addition, using CD44, CD62L, and CD69 as markers, we found that CD8+ T cells exhibited normal numbers of naive and memory populations as well as normal activation in culture. (Fig. S3A shows an analysis of unstimulated cells and purified CD8+ T cells 48 h after primary stimulation.)
Naive Ahnak1−/− CD8+ T cells proliferated normally, as determined by the 5 (and 6)-carboxyfluorescein diacetate succinimidyl ester (CFSE) assay 72 h after primary TCR stimulation (Fig. S3B), and secreted normal amounts of IL-2 and IFN-γ, as measured 24 h (not shown) and 48 h after activation (Fig. S3 C and D). Collectively, our observations indicate that AHNAK1 is neither expressed nor required for early stages of in vitro CD8+ T-cell differentiation.
Ahnak1−/− CTLs Display Reduced Cav1.1 α1 Subunit Expression and Calcium Influx.
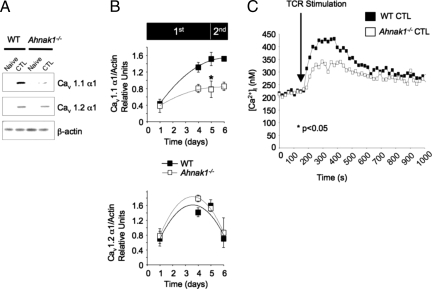
AHNAK1 has been shown previously to be required for calcium influx after TCR activation of naive CD4+ T cells (8, 11). AHNAK1 is highly expressed in naive CD4+ T cells and seems to be required for the targeting and/or stabilization of Cav1.1 α1 subunits at the plasma membrane. Reduced expression of Cav1.1 in CD4+ T cells from both Ahnak1−/− and β4−/− mice resulted in diminished calcium entry and consequent functional abnormalities after TCR stimulation (8, 11). We therefore examined whether Cav1.1 α1 also was expressed in CTLs (Fig. 1 A and B). Interestingly, unlike expression in CD4+ T cells, Cav1.1 and Cav1.2 α1 subunits were not expressed in naive wild-type CD8+ T cells (Fig. 1A); instead, their expression was detected only in CTLs after primary stimulation (Fig. 1A).
Fig. 1.
Reduced Cav1.1 α1 subunit expression and calcium entry in response to TCR stimulation in Ahnak1−/− CTLs. (A) The expression of Cav1.1 and Cav1.2 was examined in purified naive CD8+ T cells and in mature CTLs 5 days after primary stimulation using plate-bound anti-CD3 and anti-CD28 (amount of antibodies is described in Fig. S1A) in wild-type and Ahnak1−/− cells. (B) Kinetics of Cav1.1 and Cav1.2 expression by immunoblot analysis [using anti-α1S (Cav1.1) and anti-α1C (Cav1.2)] was as described in Fig. S1B. Expression of Cav1.1 and Cav1.2 α1 subunit was normalized to β-actin for each time point. This final densitometry plot of Cav1.1 and Cav1.2 α1 subunit expression is an average of 3 independent experiments. There is a statistically significant difference in Cav1.1 expression, but not in Cav1.2, α1 subunit expression, between wild-type and Ahnak1−/− cells (*P < 0.05) at day 5 after primary stimulation. Experiment B was performed in addition to and was independent of experiment A. (C) CTLs from wild-type and Ahnak1−/− mice, obtained after 5 days of primary stimulation as in Fig. S1A, were incubated with anti-CD3 (10 μg/mL) for 30 min on ice and subsequently were cross-linked by goat anti-hamster at the indicated time (TCR stimulation). Then [Ca2+]i was measured by a ratiometric method using Fura-2 as a probe. An average of 3 independent experiments is shown (The individual experiments are shown in Fig. S4). There is statistically significant difference between wild-type and Ahnak1−/− cells (P < 0.05).
Fig. 1B shows the expression, measured by immunoblot assay and densitometry, of Cav1.1 and Cav1.2 at various time points during primary stimulation, which lasted for 5 days, followed by restimulation for 24 h. Cav1.1 but not Cav1.2 α1 subunit expression, which correlated remarkably with AHNAK1 protein expression, was up-regulated by day 4 after primary stimulation, and remained highly expressed after restimulation (cf. Fig. 1B and Fig. S1B). Although expression of a second pore-forming subunit, Cav1.2, was up-regulated late during primary stimulation, its expression was drastically down-regulated after restimulation (Fig. 1B Lower). AHNAK1- deficient CTLs showed reduced induction of Cav1.1 but not of Cav1.2, α1 subunit expression (Fig. 1B), possibly because peak amounts of AHNAK1 were required. These data suggest that AHNAK1 is likely to regulate Cav1.1 α1 protein expression in CTLs in a way similar to its role in CD4+ T cells.
Reduced Cav1.1 α1 subunit expression correlated with reduced calcium entry into CD4+ T cells after TCR cross-linking (11). Because Cav1.1 expression also was reduced in Ahnak1−/− CTLs after primary stimulation, we examined calcium entry after TCR stimulation in these cells. Intracellular calcium concentration in response to TCR stimulation was measured in wild-type and Ahnak1−/− CTLs by a ratiometric method using Fura-2-acetoxymethyl ester (Fura-2) as a probe. In 3 independent experiments, averaged in Fig. 1C and shown individually in Fig. S4, we found significant reduction (P < 0.05) in Ahnak1−/− CTL calcium response to TCR cross-linking compared with wild-type T cells.
Ahnak1−/− CTLs Show Reduced Killing Activity.
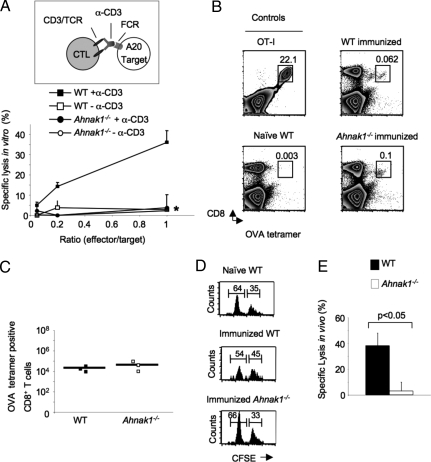
Because CTL require calcium entry for the killing activity, we next examined CTL activity in the absence of AHNAK1 during late stages after primary stimulation in vitro. Therefore, we collected CTLs from wild-type and Ahnak1−/− mice late after primary stimulation (on day 5, when AHNAK1 is highly expressed) and tested their lytic activity in the presence or absence of anti-CD3 using A20 cells as targets in a redirected lysis assay in vitro. Ahnak1−/− CTLs showed marked impairment in their cytolytic activity in a standard 4-h assay (Fig. 2A).
Fig. 2.
Ahnak1−/− CTLs show deficient cytolytic activity in vitro and in vivo. (A) Calcein-AM-loaded A20 were used as targets in the calcein-AM retention CTL assay, with wild-type or Ahnak1−/− CTLs at the noted effector/target ratios and redirected with anti-CD3. Results shown are mean (SD) for 3 individual mice tested per group. We obtained similar results from 2 similar independent experiments. There is a statistically significant difference between wild-type and Ahnak1−/− CTL killing (*, P < 0.05). (B and C) Wild-type dendritic cells, pulsed with H-2Kb-binding SIINFEKL peptide (OVA), were transferred into wild-type and Ahnak1−/− mice. Splenocytes were analyzed for CTL expansion in vivo by H-2kb/OVA257–264 (SIINFEKL) peptide tetramer staining 7 days later. A representative mouse is shown in B. An OT-I mouse is shown as positive control for the tetramer staining, and a naive wild-type mouse is shown as negative control. (C) An average absolute cell number was calculated for 3 individual mice. (D and E) Wild-type and Ahnak1−/− mice were immunized as in B. After 7 days, we co-transferred SIINFEKL peptide (OVA)-loaded CFSE-low-labeled (0.5 μM) mixed with unloaded CFSE-high (5 μM) target wild-type splenocytes. Splenocytes were harvested 3 h later and were analyzed by flow cytometry. Representative naive unimmunized wild-type control mice, immunized wild-type mice, and Ahnak1−/− mice are shown in D. The left and the right peaks shown in the histogram represent the peptide-loaded and unloaded target cells, respectively. Specific lysis was calculated from the ratio between peptide-loaded and unloaded target cells in unimmunized wild-type control and immunized wild-type or Ahnak1−/− mice. An average of 7 mice from each group is shown in E. Significant differences were found between wild-type and Ahnak1−/− mice (P < 0.05).
We next tested Ahnak1−/− CTL function in vivo using a previously described model system (20–22). For this purpose, wild-type dendritic cells pulsed with H-2Kb-binding SIINFEKL peptide (OVA257–264) were transferred into wild-type and Ahnak1−/− mice. Under these conditions, after 7 days, we observed normal expansion of the SIINFEKL peptide (OVA257–264)-specific CTLs in vivo in both wild-type and Ahnak1−/− mice as detected by staining with an H-2Kb/OVA257–264 peptide tetramer. (Fig. 2B shows representative mice, and Fig. 2C demonstrates average SIINFEKL peptide (OVA257–264)-specific cell numbers from 3 individual mice.) As controls we used OT-I TCR transgenic mice, which express a TCR specific for the SIINFEKL (OVA257–264) peptide, or naive, unimmunized wild-type mice (Fig. 2B) (23). This result corroborated our in vitro proliferation results (Fig. S3) and further suggests that in vivo differentiation and expansion of CTLs does not require AHNAK1.
We examined the cytotoxicity of SIINFEKL peptide (OVA257–264)-specific CTLs to transferred CFSE-labeled (0.5 μM, CFSE-low) SIINFEKL peptide (OVA257–264)-loaded target cells 7 days after immunization of wild-type or Ahnak1−/− mice with peptide-pulsed dendritic cells. As control, we co-transferred CFSE-labeled (5 μM, CFSE-high) naive, unloaded target cells. We observed a typical 40% antigen-specific lysis in wild-type mice (Fig. 2E), measured as a ratio between unloaded CFSE-high and peptide-loaded CFSE-low target cells in each individual mouse (Fig. 2D). In contrast to wild-type animals, Ahnak1−/− mice showed significantly reduced antigen-specific cytotoxicity, consistent with the in vitro results in Fig. 2A.
Altogether, we observed a consistent and significant reduction in cytotoxicity (roughly 30%–40%) by Ahnak1−/− CTLs in vitro and in vivo.
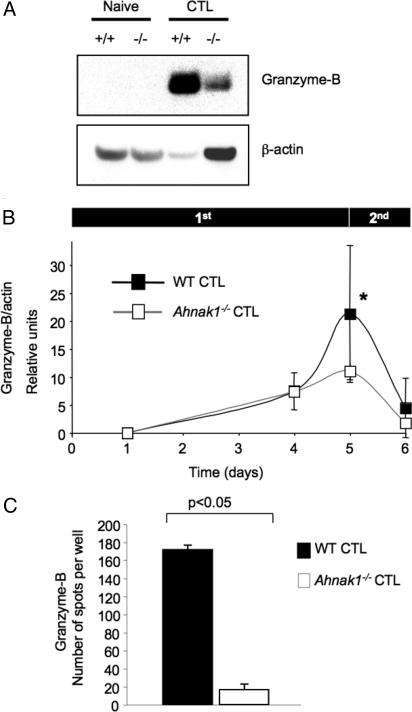
Reduced Expression of Granzyme B by AHNAK1-Deficient CTLs.
To examine why Ahnak1−/− CTLs display deficient cytotoxic activity, we tested the production of granzyme B, the main granzyme produced by CTLs during primary stimulation (24, 25). We used protein extracts from purified naive CD8+ T cells as well as from wild-type and Ahnak1−/− CTLs to examine the expression of granzyme B by immunoblot. We detected very low granzyme-B expression in Ahnak1−/− CTLs obtained by primary TCR stimulation in vitro for 5 days (Fig. 3A). Fig. 3B shows the expression of granzyme B at various time points during primary stimulation (which lasted for 5 days) followed by restimulation for 24 h measured by immunoblot assay followed by densitometry. We found that granzyme-B expression correlated with AHNAK1 expression (Fig. 3B). Although Ahnak1−/− effector CD8+ T cells up-regulated granzyme-B expression normally during the first 4 days after stimulation (Fig. 3B), they displayed significant (P < 0.05) reduction in granzyme-B expression on the fifth day after primary stimulation. Granzyme B was neither produced (Fig. 3 A and B) nor secreted by Ahnak1−/− CTLs on the fifth day after primary stimulation, as determined by ELISPOT assay (Fig. 3C).
Fig. 3.
Reduced granzyme-B production in the absence of AHNAK1. (A) The expression of granzyme B was examined in wild-type and Ahnak1−/−-naive CD8+ T cells versus CTLs. (B) Kinetics of granzyme-B expression were obtained as in Fig. S1B. β-actin was used as the loading control. We performed 3 independent experiments. There is a statistically significant difference in granzyme-B expression in wild-type and Ahnak1−/− cells on the fifth day after primary stimulation (*, P < 0.05). Experiment B was performed in addition to and is independent of experiment A. (C) The granzyme-B ELISPOT assay was performed using wild-type or Ahnak1−/− CTLs, stimulated for 5 days as described in Fig. S1. We performed 3 independent experiments. There is a statistically significant difference in granzyme-B secretion in wild-type and Ahnak1−/− cells (P < 0.05).
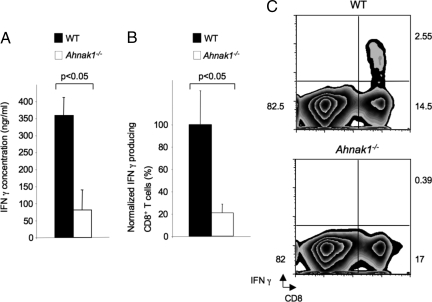
Low Levels of IFN-γ Are Produced by AHNAK1-Deficient CTLs.
Because Ahnak1−/− CTLs showed severely reduced cytolytic activity, we further examined their ability to secrete IFN-γ (26). As shown in Fig. S3, AHNAK1 was not required for IFN-γ production during the early stages of primary stimulation. Interestingly, we found that Ahnak1−/− CTLs (obtained after 5-day stimulation in vitro) were defective in IFN-γ secretion following in vitro TCR re-stimulation for 24 h (Fig. 4A). In addition, we found that Ahnak1−/− CTLs produced normal IFN-γ mRNA levels; therefore AHNAK1 probably is required for posttranscriptional events (Fig. S5). AHNAK1-deficient CTLs also exhibited normal expression of T-Bet and Eomesodermin, which regulate IFN-γ mRNA production (Fig. S5) (27–29).
Fig. 4.
In vivo and in vitro deficient IFN-γ production in the absence of AHNAK1. (A) Wild-type and Ahnak1−/− CTLs were obtained as in Fig. S1A, were washed, and were restimulated using plate-bound anti-CD3 only (2 μg/mL) for 24 h. Supernatants from in vitro-stimulated CD8+ T cells were collected, and IFN-γ production was measured by ELISA. Results shown are mean (SD) for 3 mice per group. There is a statistically significant difference between wild-type and Ahnak1−/− cells (P < 0.05). We obtained similar results with 2 other similar experiments. (B) Wild-type dendritic cells pulsed with H-2Kb-binding SIINFEKL peptide (OVA) were transferred into wild-type and Ahnak1−/− mice. Splenocytes were purified and restimulated in vitro with OVA for 6 h, and 7 days later CD8 and IFN-γ expression was analyzed by flow cytometry. The normalized percentages of IFN-γ-producing CD8+ T cells are shown. Results shown are mean (SD) for 6 mice per group. We performed 2 independent experiments. There is a statistically significant difference between wild-type and Ahnak1−/− cells (*P < 0.05). (C) Results from one representative wild-type and Ahnak1−/− are shown.
To test IFN-γ production by CD8+ T cells in the absence of AHNAK1 in vivo, we used the model system described earlier (see in vivo CTL assay, Fig. 2). Thus, wild-type dendritic cells primed with H-2Kb-binding SIINFEKL peptide (OVA257–264) were transferred to wild-type and Ahnak1−/− mice. We examined the ability of antigen-specific CTLs to produce IFN-γ 7 days after transfer. As shown in Fig. 4 B and C, the wild-type response in the spleen resulted in 2–3% of antigen-specific IFN-γ-producing CTLs. In contrast, Ahnak1−/− CTLs showed a significant deficiency in IFN-γ production, consistent with the in vitro results shown in Fig. 4A. In addition, this finding suggests that the deficiency in IFN-γ production in Ahnak1−/− CTLs is cell autonomous, because wild-type dendritic cells were used for the transfers in this assay.
Discussion
For decades, numerous studies have shown that calcium is required for CTL-mediated killing. Most of these studies were performed using calcium antagonists, which significantly inhibited the killing by CTLs (e.g., see ref. 4). However, the mechanism responsible for calcium entry required for the killing process is largely unknown.
The functional presence of Cav1 channels in T lymphocytes has been suggested previously (30–35). Cav1.1 α1 subunits are critical for TCR-induced calcium entry into CD4+ T cells, as suggested by our recent studies of Cav1 β4−/− and Ahnak1−/− mice (8, 11). In all the cases reported thus far, reduced Cav1.1 α1 subunit expression results in deficient calcium entry through the plasma membrane and does not affect calcium release from intracellular stores (11).
In the present study, we demonstrate that AHNAK1 is required for CTL function, probably by regulating Cav1.1 α1 subunit expression. In T cells, AHNAK1 interacts with Cav1.1 α1 subunits and regulates their membrane expression through its interaction with the regulatory β2 subunit (11). AHNAK1 and Cav1.1 α1 subunits are expressed only in CTLs, but not in naive CD8+ T cells, days after primary stimulation. This expression pattern explains our finding that, unlike CD4+ T cells, Ahnak1−/−-naive CD8+ T cells do not differ from their wild-type counterparts in activation and proliferation following primary TCR stimulation both in vitro and in vivo. Our observations suggest that AHNAK1 is required after CD8+ T cells differentiate to become CTLs. When the requirement for AHNAK1 by CTLs arises, a phenotype is revealed in Ahnak1−/− CTLs, and they display severe abnormality in Cav1.1 α1 subunit expression levels and deficient calcium entry after TCR restimulation, resulting in deficient CTL activity and IFN-γ and granzyme-B production.
Collectively, CD4+ and CD8+ T cells display differential requirements for AHNAK1 and Cav1.1 α1 subunits during the immune response. We therefore conclude that T cells probably require multiple calcium pathways, including CRAC, AHNAK1, Cav, or others, to mediate calcium entry during various differentiation stages in the course of an immune response. Further studies will be required to identify those pathways and to clarify their functional requirement during the immune response. Here we describe a critical function for AHNAK1 during CTL response, which normally is required to fight cancer or viral infections.
Materials and Methods
Reagents and Antibodies.
For reagents and antibodies we used anti-β-actin (sc-1616, Santa Cruz Biotechnology), anti-CD3 (145–2C11), anti-CD28 (37.1), anti-AHNAK1-C2 (a kind gift from the Hasse-H Max Delbrück Center for Molecular Medicine), Anti-Granzyme-B (R&D, AF1865), anti-Cav1.1 (Santa Cruz), anti-Cav1.2 (Alomone), anti-CD44 CyChrome, anti-CD62L FITC, anti-CD69 FITC, anti-TCRβ (H57) PE, and anti-CD8 PE CyChrome (all PharMingen), and H-2Kb/OVA257–264 peptide tetramer (kindly provided by Tim Willinger).
Mice.
Ahnak1−/− mice were previously described (14). Wild-type littermates were used as control. Mice within experiments were age and sex matched. Mice were cared for in accordance with protocols approved by the Institutional Animal Care and Use Committee at the Yale University School of Medicine Animal Facility.
Immunoblotting.
Cell lysis and immunoblotting were performed as previously described (11).
ELISPOT Assay.
The ELISPOT assay was performed using the granzyme-B ELISPOT assay kit (R&D, EL1865)
In Vitro T-Cell Activation/Differentiation Experiments.
Splenic CD8+ T cells were isolated from 6- to 8-week-old mice by MACS sorting using anti-CD8-coupled beads and columns (Miltenyi Biotec). Cells were cultured in Bruff's medium (10% FCS, penicillin, streptomycin, and L-glutamine). T cells were stimulated using coated plates as described previously (36). Plates were coated for 2 h at 37 °C with anti-CD3 and anti-CD28 (2 μg/mL of each) in PBS. Typically, 2 million T cells/well were plated in a 24-well plate. All effector CD8+ T cells used in this study were incubated as described earlier in this article for a 5-day period.
In Vitro Cytotoxicity Assay.
Cytotoxicity analysis was performed by using calcein-AM retention assays (37). Briefly, A20 target cells plated in U-bottomed microtiter plates at a concentration of 2 × 104 cells per well were washed once with PBS and labeled with 0.02 μg/mL calcein-AM (Molecular Probes) in serum- and phenol red-free medium (GIBCO BRL) for 30 min at 37 °C. Labeling efficiency was assessed initially by using a fluorescence plate reader. Effector cells were added at different effector/target ratios in quadruplicate. Phenol red-free medium was added to a 6-well set of target cells for estimation of retention of calcein-AM in medium alone. Anti-CD3 was added at 10 μg/mL, and a control without anti-CD3 was included. Maximal lysis was determined by solubilizing 6 wells of target cells in lysis buffer (50 mM sodium botate 0.1% Triton X-100, pH 9.0). After 4 h of incubation at 37 °C, the assays were terminated by washing the plates twice, and the remaining fluorescence was read. The percentage of specific cytotoxicity was calculated as follows: % cytotoxicity = [(retention experimental well − retention maximal lysis)/(retention in medium − retention maximal lysis)] × 100. Retention values were calculated by normalizing measured fluorescence with initial labeling of the same well.
ELISA.
Analysis of Intracellular Calcium Concentration.
Calcium concentration was measured using Fura2/AM (Molecular Probes) as described previously (8, 11).
5 (and 6)-Carboxyfluorescein Diacetate Succinimidyl Ester (CFSE) Labeling.
CFSE (Molecular Probes) was added to purified CD8+ T cells to a final concentration of 3 μM, and cells were incubated for 25 min at 37 °C. At the end of the incubation period, the cells were washed immediately 3 times in PBS containing 10% FCS. Cells then were stimulated for the 72-h period, and CFSE staining was measured by flow cytometry.
Real-Time PCR.
In Vivo Priming of CD8+ T Cells, CTL Assay, and IFN-γ Production.
The method for generating bone marrow dendritic cells was adapted from Lutz et al (21). Briefly, bone marrow cells were harvested from wild-type mice and cultured for 7 days in petri dishes in complete Bruff's medium containing GM-CSF. On day 7, cells were centrifuged at 300 × g for 5 min and were resuspended in complete Bruff's medium containing GM-CSF. Cells then were incubated overnight with H-2Kb-binding SIINFEKL peptide (OVA257–264) (10 μg/mL) and LPS (200 ng/mL). On the next day, cells were washed 3 times with sterile PBS and finally were resuspended in PBS for injection.
In Vivo IFN-γ Production.
We injected 1 million cells in 200 μL PBS i.p. into wild-type and Ahnak1−/− mice. After 7 days mice were killed, spleens were harvested, and splenocytes were stimulated in vitro with SIINFEKL peptide (10 μg/mL) or with Phorbol Myristate Acetate (PMA) (100 ng/mL) plus ionomycin (1 μM) or were left unstimulated in complete Bruff's medium for 6 h in the presence of Golgi stop (BD Biosciences, Cat # 554715). Cells were stained with CD8 and then were fixed and permeabilized for intracellular staining of IFN-γ. Stained cells were analyzed by flow cytometry on a FACSCalibur and analyzed using CellQuest software from BD Biosciences or Flowjo software from Treestar.
In Vivo CTL Assay.
Wild-type and Ahnak1−/− mice were immunized as described previously for 7 days, followed by co-transfer of SIINFEKL peptide (OVA)-loaded CFSE-low-labeled (0.5 μM) (Molecular Probes) or unloaded CFSE-high (5 μM) target wild-type splenocytes. Splenocytes were harvested 3 h later and analyzed by flow cytometry. We injected 5 million mixed target cells per mouse.
The percentage of specific lysis was calculated as {1 − [(ratio in a naive mouse: unpulsed CFSE-high/pulsed CFSE-low)/(ratio in an immunized mouse: unpulsed CFSE high/pulsed CFSE low)]} × 100, as described previously (21).
Statistical Analysis.
Results are shown as average (SD). Statistical differences were determined by an analysis of 2-tailed Student's t test. Values of P < 0.05 were considered statistically significant.
Supplementary Material
Acknowledgments.
The authors thank F. Manzo for manuscript preparation. D.M. and R.A.F. thank Prof. Gillian M. Griffiths for helpful discussions related to this work. R.A.F. is an Investigator of the Howard Hughes Medical Institute and a recipient of grants from the National Institutes of Health (NIH). D.M. and S.S. were supported by Cancer Research Institute postdoctoral fellowships. D.M. is currently supported by a fellowship from the Israeli Ministry of Immigrant Adsorption. S.S. also was supported by NIH Grants CA121974 and DK051665. A.B. was supported by the Arthritis National Research Foundation. M.K.J. is supported by an Arthritis Foundation postdoctoral fellowship. T.W. is supported by a James Hudson Brown-Alexander Brown Coxe postdoctoral fellowship. A.A. is supported by a Richard K. Gershon fellowship.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0902844106/DCSupplemental.
References
- 1.Cantrell D. T cell antigen receptor signal transduction pathways. Annu Rev Immunol. 1996;14:259–274. doi: 10.1146/annurev.immunol.14.1.259. [DOI] [PubMed] [Google Scholar]
- 2.Lewis RS. Calcium signaling mechanisms in T lymphocytes. Annu Rev Immunol. 2001;19:497–521. doi: 10.1146/annurev.immunol.19.1.497. [DOI] [PubMed] [Google Scholar]
- 3.Berke G. Unlocking the secrets of CTL and NK cells. Immunol Today. 1995;16:343–346. doi: 10.1016/0167-5699(95)80152-9. [DOI] [PubMed] [Google Scholar]
- 4.Esser MT, Haverstick DM, Fuller CL, Gullo CA, Braciale VL. Ca2+ signaling modulates cytolytic T lymphocyte effector functions. J Exp Med. 1998;187:1057–1067. doi: 10.1084/jem.187.7.1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lyubchenko TA, Wurth GA, Zweifach A. Role of calcium influx in cytotoxic T lymphocyte lytic granule exocytosis during target cell killing. Immunity. 2001;15:847–859. doi: 10.1016/s1074-7613(01)00233-3. [DOI] [PubMed] [Google Scholar]
- 6.Gwack Y, et al. Hair loss and defective T- and B-cell function in mice lacking ORAI1. Mol Cell Biol. 2008;28:5209–5222. doi: 10.1128/MCB.00360-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zweifach A. Target-cell contact activates a highly selective capacitative calcium entry pathway in cytotoxic T lymphocytes. J Cell Biol. 2000;148:603–614. doi: 10.1083/jcb.148.3.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Badou A, et al. Critical role for the beta regulatory subunits of Cav channels in T lymphocyte function. Proc Natl Acad Sci USA. 2006;103:15529–15534. doi: 10.1073/pnas.0607262103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kotturi MF, Hunt SV, Jefferies WA. Roles of CRAC and Cav-like channels in T cells: More than one gatekeeper? Trends Pharmacol Sci. 2006;27:360–367. doi: 10.1016/j.tips.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 10.Catterall WA. Structure and regulation of voltage-gated Ca2+ channels. Annu Rev Cell Dev Biol. 2000;16:521–555. doi: 10.1146/annurev.cellbio.16.1.521. [DOI] [PubMed] [Google Scholar]
- 11.Matza D, et al. A scaffold protein, AHNAK1, is required for calcium signaling during t cell activation. Immunity. 2008;28:64–74. doi: 10.1016/j.immuni.2007.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shtivelman E, Bishop JM. The human gene AHNAK encodes a large phosphoprotein located primarily in the nucleus. J Cell Biol. 1993;120:625–630. doi: 10.1083/jcb.120.3.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kudoh J, et al. Localization of the human AHNAK/desmoyokin gene (AHNAK) to chromosome band 11q12 by somatic cell hybrid analysis and fluorescence in situ hybridization. Cytogenet Cell Genet. 1995;70:218–220. doi: 10.1159/000134037. [DOI] [PubMed] [Google Scholar]
- 14.Komuro A, et al. The AHNAKs are a class of giant propeller-like proteins that associate with calcium channel proteins of cardiomyocytes and other cells. Proc Natl Acad Sci USA. 2004;101:4053–4058. doi: 10.1073/pnas.0308619101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sekiya F, Bae YS, Jhon DY, Hwang SC, Rhee SG. AHNAK, a protein that binds and activates phospholipase C-gamma1 in the presence of arachidonic acid. J Biol Chem. 1999;274:13900–13907. doi: 10.1074/jbc.274.20.13900. [DOI] [PubMed] [Google Scholar]
- 16.Lee IH, et al. Ahnak protein activates protein kinase C (PKC) through dissociation of the PKC-protein phosphatase 2A complex. J Biol Chem. 2008;283:6312–6320. doi: 10.1074/jbc.M706878200. [DOI] [PubMed] [Google Scholar]
- 17.Gentil BJ, et al. The giant protein AHNAK is a specific target for the calcium- and zinc-binding S100B protein: Potential implications for Ca2+ homeostasis regulation by S100B. J Biol Chem. 2001;276:23253–23261. doi: 10.1074/jbc.M010655200. [DOI] [PubMed] [Google Scholar]
- 18.Benaud C, et al. AHNAK interaction with the annexin 2/S100A10 complex regulates cell membrane cytoarchitecture. J Cell Biol. 2004;164:133–144. doi: 10.1083/jcb.200307098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haase H, et al. Ahnak is critical for cardiac Ca(V)1.2 calcium channel function and its beta-adrenergic regulation. FASEB J. 2005;19:1969–1977. doi: 10.1096/fj.05-3997com. [DOI] [PubMed] [Google Scholar]
- 20.Song S, et al. Augmented induction of CD8+ cytotoxic T-cell response and antitumor effect by DCs pulsed with virus-like particles packaging with CpG. Cancer Lett. 2007;256:90–100. doi: 10.1016/j.canlet.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 21.Lutz MB, et al. An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J Immunol Methods. 1999;223:77–92. doi: 10.1016/s0022-1759(98)00204-x. [DOI] [PubMed] [Google Scholar]
- 22.Ingulli E. Tracing tolerance and immunity in vivo by CFSE-labeling of administered cells. Methods in Molecular Biology. 2007;380:365–376. doi: 10.1007/978-1-59745-395-0_23. [DOI] [PubMed] [Google Scholar]
- 23.Hogquist KA, et al. T cell receptor antagonist peptides induce positive selection. Cell. 1994;76:17–27. doi: 10.1016/0092-8674(94)90169-4. [DOI] [PubMed] [Google Scholar]
- 24.Heusel JW, Wesselschmidt RL, Shresta S, Russell JH, Ley TJ. Cytotoxic lymphocytes require granzyme B for the rapid induction of DNA fragmentation and apoptosis in allogeneic target cells. Cell. 1994;76:977–987. doi: 10.1016/0092-8674(94)90376-x. [DOI] [PubMed] [Google Scholar]
- 25.Revell PA, et al. Granzyme B and the downstream granzymes C and/or F are important for cytotoxic lymphocyte functions. J Immunol. 2005;174:2124–2131. doi: 10.4049/jimmunol.174.4.2124. [DOI] [PubMed] [Google Scholar]
- 26.Fruh K, Yang Y. Antigen presentation by MHC class I and its regulation by interferon gamma. Curr Opin Immunol. 1999;11:76–81. doi: 10.1016/s0952-7915(99)80014-4. [DOI] [PubMed] [Google Scholar]
- 27.Glimcher LH, Townsend MJ, Sullivan BM, Lord GM. Recent developments in the transcriptional regulation of cytolytic effector cells. Nature Reviews. Immunology. 2004;4:900–911. doi: 10.1038/nri1490. [DOI] [PubMed] [Google Scholar]
- 28.Mayer KD, et al. Cutting edge: T-bet and IL-27R are critical for in vivo IFN-gamma production by CD8 T cells during infection. J Immunol. 2008;180:693–697. doi: 10.4049/jimmunol.180.2.693. [DOI] [PubMed] [Google Scholar]
- 29.Pearce EL, et al. Control of effector CD8+ T cell function by the transcription factor Eomesodermin. Science. 2003;302:1041–1043. doi: 10.1126/science.1090148. [DOI] [PubMed] [Google Scholar]
- 30.Badou A, et al. HgCl2-induced interleukin-4 gene expression in T cells involves a protein kinase C-dependent calcium influx through L-type calcium channels. J Biol Chem. 1997;272:32411–32418. doi: 10.1074/jbc.272.51.32411. [DOI] [PubMed] [Google Scholar]
- 31.Savignac M, et al. Protein kinase C-mediated calcium entry dependent upon dihydropyridine sensitive channels: A T cell receptor-coupled signaling pathway involved in IL-4 synthesis. FASEB J. 2001;15:1577–1579. doi: 10.1096/fj.00-0733fje. [DOI] [PubMed] [Google Scholar]
- 32.Kotturi MF, Carlow DA, Lee JC, Ziltener HJ, Jefferies WA. Identification and functional characterization of voltage-dependent calcium channels in T lymphocytes. J Biol Chem. 2003;278:46949–46960. doi: 10.1074/jbc.M309268200. [DOI] [PubMed] [Google Scholar]
- 33.Savignac M, et al. Dihydropyridine receptors are selective markers of Th2 cells and can be targeted to prevent Th2-dependent immunopathological disorders. J Immunol. 2004;172:5206–5212. doi: 10.4049/jimmunol.172.9.5206. [DOI] [PubMed] [Google Scholar]
- 34.Stokes L, Gordon J, Grafton G. Non-voltage-gated L-type Ca2+ channels in human T cells: Pharmacology and molecular characterization of the major alpha pore-forming and auxiliary beta-subunits. J Biol Chem. 2004;279:19566–19573. doi: 10.1074/jbc.M401481200. [DOI] [PubMed] [Google Scholar]
- 35.Kotturi MF, Jefferies WA. Molecular characterization of L-type calcium channel splice variants expressed in human T lymphocytes. Mol Immunol. 2005;42:1461–1474. doi: 10.1016/j.molimm.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 36.Guerder S, Carding SR, Flavell RA. B7 costimulation is necessary for the activation of the lytic function in cytotoxic T lymphocyte precursors. J Immunol. 1995;155:5167–5174. [PubMed] [Google Scholar]
- 37.Lichtenfels R, Biddison WE, Schulz H, Vogt AB, Martin R. CARE-LASS (calcein-release-assay), an improved fluorescence-based test system to measure cytotoxic T lymphocyte activity. J Immunol Methods. 1994;172:227–239. doi: 10.1016/0022-1759(94)90110-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.