Abstract
Introduction
To date, the resistance of infectious agents has been assessed by widely varying criteria in different countries. Therefore, published data on resistance often cannot be meaningfully compared. In Germany, different laboratories can potentially report different results for identical microorganisms, since there is no uniform system for categorization. This situation is unsatisfactory.
Methods
Selective literature review and evaluation of committee reports.
Results
The new ISO standard 20776 for determination of the resistance of infectious agents and the harmonized evaluation system of the European Society for Clinical Microbiology and Infectious Diseases provide a new basis for susceptibility testing. The categorization of infectious agents as "susceptible," "intermediate," or "resistant" to particular antibiotics will become more reliable and will be consistent throughout Europe.
Discussion
For a number of antibiotics, the criteria for evaluation of infectious agents as "susceptible," "intermediate", or "resistant" will change. Comparability with earlier resistance data will be compromised. However, the new evaluation criteria reflect current knowledge on the pharmacokinetics and pharmacodynamics of antimicrobial substances.
Keywords: resistance testing, antibiotic, pharmakokinetics, in-vitro diagnostic agent, bacteriological testing
In the microbiology laboratory, the identified infectious organisms are usually tested for their degree of resistance to various anti-infective substances in order to prevent the administration of ineffective treatments. The treating physician is usually informed of the test results with a report in which the activity of individual drugs against the isolated organism is categorized by one of the three terms "susceptible" (earlier term: "sensitive"), "intermediate," and "resistant." This information can be used to optimize treatment for the individual patient, while, in the aggregate, data of this type can be used to form a picture of the degree of resistance to each drug in the population at large. The latter is, in turn, an important criterion in the selection of antibiotics for the initial ("empirical") treatment of infectious diseases (1, 2). As will be described in this article, the classification system for antibiotic effectiveness has recently been modified to enable the detection of more resistance mechanisms and to take better account of recent discoveries in the pharmacokinetics and pharmacodynamics of antimicrobial chemotherapeutic agents. The degree of certainty of the assessment has also been improved.
This review article will describe the basic elements in the determination of the drug resistance of infectious organisms and will point out the major changes that were recently introduced in an attempt to unify the criteria of assessment across Europe. It is based on a selective review of the relevant literature, and it reports on the results of the working sessions of three relevant committees: the CEN/ISO (Comité Européen de Normalisation/International Organization for Standardization) task force, the DIN subcommittee on chemotherapeutic testing methods, and the EUCAST (European Committee on Antimicrobial Susceptibility Testing).
The determination of microbial sensitivity
An important task of medical microbiology is the phenotypic in vitro testing of antimicrobial substances for their effectiveness against infectious organisms. A variety of tests have been developed for this purpose. Thus, for example, the bactericidal activity of antibiotics can be described by the investigation of bactericidal kinetics or by the determination of the minimal bactericidal concentration of a particular antibiotic against a particular bacterial strain (3, 4). Such tests generally provide a basic characterization of the interactions between the substance and the microorganism.
Data on the minimum inhibitory concentration (MIC) of various antibiotics used against the detected organism generally suffice for the assessment of therapeutic options. The MIC is defined as the minimum concentration of an antibiotic that is just barely able to prevent the further growth of the infectious organism in vitro. Testing techniques must be standardized to make the test results reproducible, because parameters such as the culture medium, microorganism inoculum size, and incubating temperature and duration can all influence the result (5). In Germany, the subcommittee on chemotherapeutic testing methods of the Medical Standards Committee (Normenausschuss Medizin, NAMed), a section of the German Institute for Standardization (Deutsches Institut für Normung, DIN), has devoted itself to this task for more than 40 years. Nonetheless, other standardized methods are used in Germany as well, namely those of the Clinical Laboratory Sciences Institute (CLSI) of the United States, formerly known as the National Committee on Clinical Laboratory Standards (NCCLS). In the last 3 years, and on the initiative of the DIN, a norm has been worked out and approved by the International Organization for Standardization (ISO 20776-1) (6) that is now considered valid all over the world. Microbouillon dilution has been chosen as the reference method for the determination of MIC (table 1). A further ISO norm has also been approved concerning quality criteria for derived testing procedures (ISO 20776-2) (7). This norm was necessary because other automatized and simplified tests are often performed instead of MIC determinations, particularly for reasons of cost.
Table 1. Schematic representation of a microtitration plate for the determination of minimal inhibitory concentrations (MICs).
| mg/L | mg/L | mg/L | mg/L | mg/L | mg/L | mg/L | mg/L | |
| Ampicillin | 0.25 | 0.5 | 1 | 2 | 4 | 8 | 16 | 32 |
| Ampicillin/sulbactam | 0.25 | 0.5 | 1 | 2 | 4 | 8 | 16 | 32 |
| Piperacillin/tazobactam | 0.5/2 | 1 | 2 | 4 | 8 | 16 | 32 | 64 |
| Cefuroxime | 0.125 | 0.25 | 0.5 | 1 | 2 | 4 | 8 | 16 |
| Cefotaxime | 0.125 | 0.25 | 0.5 | 1 | 2 | 4 | 8 | 16 |
| Imipenem | 0.125 | 0.25 | 0.5 | 1 | 2 | 4 | 8 | 16 |
| Gentamicin | 0.125 | 0.25 | 0.5 | 1 | 2 | 4 | 8 | 16 |
| Doxycyclin | 0.125 | 0.25 | 0.5 | 1 | 2 | 4 | 8 | GC |
| Cotrimoxazole | 1 | 2 | 4 | 8 | 16 | 32 | 64 | 128 |
| Ciprofloxacin | 0.03 | 0.06 | 0.125 | 0.25 | 0.5 | 1 | 2 | 4 |
| Levofloxacin | 0.03 | 0.06 | 0.125 | 0.25 | 0.5 | 1 | 2 | 4 |
| Moxifloxacin | 0.03 | 0.06 | 0.125 | 0.25 | 0.5 | 1 | 2 | 4 |
The antibiotics and concentrations indicated are the ones that are usually selected. The results for a particular tested strain of E. coli are indicated by gray highlighting.
The MIC values for this strain and the sensitivity ratings that will be assigned to them henceforward by EUCAST are: ampicillin = 4 mg/L (i); ampicillin/sulbactam = 1 mg/L (i); piperacilin/tazobactam = 2 mg/L (s); cefuroxime = 4 mg/L (i); cefotaxime = 0.125 mg/L (s); imipenem = 0.5 mg/L (s); gentamicin = 0.25 mg/L (s); doxycycline = 8 mg/L (Ø); cotrimoxazole >128 mg/L (r); ciprofloxacin <0.03 mg/L (s); levofloxacin <0.03 mg/L (s); moxifloxacin = 0.06 mg/L (s); GC, growth control; Ø, no data, because the combination of organism and antibiotic is unsuitable; i, intermediate; s, susceptible; r, resistant.
The result of MIC testing for a particular antibiotic used against a particular infectious organism is expressed as a concentration in mg/L. This piece of information, together with the known pharmacokinetic properties of the substance, can be used to assess the presumed degree of therapeutic effectiveness of the drug. In individual cases, additional special characteristics of the patient can be taken into account. This, however, requires knowledge of the relevant pharmacokinetics. For the purpose of simplification, a standardized, threshold-based assessment scheme has been introduced in which the degree of drug effectiveness is characterized as "susceptible," "intermediate," or "resistant," depending on the MIC value. According to the new ISO 20776-1 standard, which is valid all over the world, these terms are defined as follows:
Susceptible (s): A bacterial strain is said to be susceptible to a given antibiotic when it is inhibited in vitro by a concentration of this drug that is associated with a high likelihood of therapeutic success.
Intermediate (i): The sensitivity of a bacterial strain to a given antibiotic is said to be intermediate when it is inhibited in vitro by a concentration of this drug that is associated with an uncertain therapeutic effect.
Resistant (r): A bacterial strain is said to be resistant to a given antibiotic when it is inhibited in vitro by a concentration of this drug that is associated with a high likelihood of therapeutic failure.
A variety of circumstances may necessitate an adjustment of breakpoint values, e.g., changes in the usual dosage of the drug or the appearance of new mechanisms of resistance. The classification "intermediate" means that the organism may well be eliminated in body compartments that are easily accessible by the drug, e.g., the urinary tract, while the same antibiotic may not be adequately effective against the same organism if it is located at other sites, eg, the meninges. If drugs that have been found to possess only intermediate effectiveness against an infectious organism are to be used, then dose. This category also serves as a "buffer zone" to prevent the fluctuating interpretation of test results as susceptible at some times and resistant at others merely because of minor, random variations in testing conditions.
Breakpoint determination
Thresholds are determined on the basis of a large amount of data. For each substance, at least the following information must be taken into account:
Indications
Dosage
-
Pharmacokinetics
Pharmacodynamics
Concentration-dependent toxicity, if any
Results of clinical testing
Analysis of cases of therapeutic failure.
Individual antibiotics are sometimes used for a very wide variety of indications, ranging from urinary and respiratory tract infections to intra-abdominal infections (8). Antibiotic concentrations can be very different in different tissues and organs, however, and this complicates the determination of therapeutic thresholds. If new indications arise in the course of time, the breakpoint values may need to be reconsidered. Many antibiotics can be used in different doses (9). The "intermediate" category was devised in order to take this fact into account, among other reasons. Infectious organisms whose susceptibility to a particular drug has been rated as intermediate should, therefore, be treated with a high dose of this drug.
Important elements of pharmacokinetics include the half-life of the drug, its degree of protein binding, and the temporal course of the drug concentration in different bodily tissues. At present, data from human subjects are extrapolated to large groups of individuals with Monte Carlo simulations in order to obtain a more accurate impression of inter-individual variation (10). These simulations serve the purpose of stochastic evaluation of complex distributions.
The important elements of pharmocodynamics include the following:
The MIC distributions for the infectious organisms within the area of indication of the drug
MIC compared to minimal bactericidal concentration
Microbial death kinetics
Inoculum effects
Data from animal experiments.
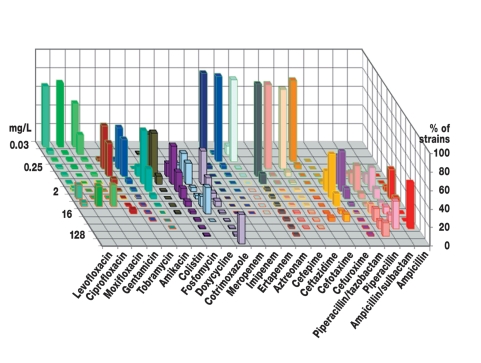
The MICs for a particular species of infectious organism often show a bimodal distribution, in which so-called wild-type strains without any resistance mechanisms for the drug have low MIC values that are characteristic of the species, while resistant strains have markedly higher MIC values (figure). The MIC distribution alone, however, is not a surefire indication of clinical success or failure. In order to set the breakpoint values appropriately, a combination of Monte Carlo simulations (pharmacokinetics) and data from animal experiments (pharmacodynamics) must be taken into account (11). Clinical testing enables an analysis of the relationship between the MIC values of the infectious organisms and the clinical and microbiological results of treatment (12).
Figure.
The distribution of MIC values of E. coli isolates cultured from patient specimens at the Leipzig University Clinic in 2006 (n = 2224). The EUCAST ratings (which have already been published for this data set) are indicated by colored borders: red = r, yellow = i, green = s. Bimodal distributions can be seen, in particular, with the penicillins (e.g., wild types for piperacillin up to MIC = 8 mg/L, resistant strains starting at MIC = 16 mg/L), cotrimoxazole (wild types up to MIC = 8 mg/L, resistant strains starting at MIC = 128 mg/L), and the quinolones (e.g., wild types for ciprofloxacin up to MIC = 0.25 mg/L, strains with clinically relevant resistance mechanisms starting at MIC = 4 mg/L).
In view of the extensive quantity and large variety of data that must be considered, it is obvious that the setting of thresholds is not an exact science, but rather a task in which arguments must be weighed against one another and substances must be assigned their proper places in an existing array of breakpoints. It should come as no surprise, therefore, that different organizations that have taken this task upon themselves have arrived at varying results. Thus, an organism classified as susceptible in the USA by the CLSI criteria might perhaps be classified as resistant in Germany by the DIN 58940 criteria.
Adjustment of threshold values in Europe
In Germany, the subcommittee on chemotherapeutic testing methods of the German Institute for Standardization (Deutsches Institut für Normung, DIN) established the breakpoints necessary for tripartite classification. These were published in Supplement 1 to DIN 58940-4 and updated as needed (13). In addition to the DIN subcommittee, there are other active groups in Europe that have published breakpoints, sometimes with marked differences between them. This unfortunate state of affairs led the European Society for Clinical Microbiology and Infectious Diseases (ESCMID) to establish the European Committee on Antimicrobial Susceptibility Testing (EUCAST), whose task is to standardize the breakpoints across Europe. The EUCAST executive committee has now released uniform European breakpoints for the assessment of the MIC values of major antibiotic drugs; these can be downloaded from the Internet at http:/www.escmid.org/sites/index_f.aspx?par=2.4 (table 2). Furthermore, the EUCAST has reached an agreement with the European Agency for the Evaluation of Medicinal Products (EMEA) according to which it will play a role in the approval process for new antimicrobial drugs. The breakpoints will be stated in the physicians’ information sheets that will be provided across Europe.
Table 2. EUCAST threshold value assignments for the carbapenems as implemented in the DIN supplement.
| Carbapenems | Species-independent ratings*1 (MIC in mg/L) | Organism-specific threshold value assignments (s ≤/r >) | ||||||||||||
| S | I | R | Enterobacteriaceae | Pseudomonas | Acinetobacter | Enterococcus | Streptococcus A, B, C, G*3, 4 | Streptococcus pneumoniae *3, 4 | Haemophilus influenzae M. Catarrhalis3,4 | N. gonorrhoeae | N. meningitidis | Gram-negative anaerobes | Gram-positive anaerobes | |
| Ertapenem | ≤ 0.5 | > 0.5/≤ 1 | > 1 | 0.5/1 | – | – | – | 0.5/0.5 | 0.5/0.5 | 0.5/0.5 | IE | – | 1/1*7 | 0.5/1 |
| Imipenem | ≤ 2 | > 2/≤ 8 | > 8 | 2/8*2 | 4/8*6 | 2/8 | 4/8*6 | 2/2 | 2/2 | 2/2 | IE | – | 2/8 | 2/8 |
| Meropenem | ≤ 2 | > 2/≤ 8 | > 8 | 2/8 | 2/8 | 2/8 | – | 2/2 | 2/2 | 2/2 | IE | 0.25/0.25*4. 5 | 2/8 | 2/8 |
*1 Species-independent threshold values were assigned mainly on the basis of pharmacokinetic and pharmacodynamic data and are independent of the distribution of individual bacterial species. They may be used for organisms that are not mentioned in the table, but not for organisms for which testing is not recommended (as designated in the table by – or IE.
*2 The effectiveness of imipenem against Proteus spp. and Morganella spp. is limited regardless of the in vitro test result.
*3 Imipenem and ertapenem are not suitable for the treatment of meningitis. The threshold values for the treatment of meningitis due to Streptococcus pneumoniae or Haemophilus influenzae with meropenem are 0.25/1 mg/L.
*4 Strains with minimal inhibitory concentrations above the s/i threshold value have been observed only rarely or not at all to date. If such strains should arise, it is recommended that the species identification and resistance testing should be rechecked and the strain should be sent to a reference laboratory.
Since clinical experience in how to treat these strains is currently lacking they should be classified as resistant, in accordance with the threshold value (in bold).
*5 The threshold values listed for meropenem and Neisseria meningitidis are valid only for the treatment of meningitis.
*6 The s/i threshold value for imipenem and Pseudomonas spp. / Entereococcus spp. was raised from 4 to 8 mg/L in order not to overlap with the wild-type strain MIC distribution.
*7 The s/i threshold value for ertapenem and gram-negative anaerobes was raised from 0.5 to 1.0 mg/L in order not to overlap with the wild-type strain MIC distribution.
Testing not recommended, because the substance is not recommended for treatment; IE, inadequate data are available regarding clinical treatment results; EUCAST, European Committee on Antimicrobial Susceptibility.
The DIN subcommittee has actively followed these developments and will act in accordance with the new determinations. This means that the EUCAST proposals will be implemented in revised DIN standards (autumn 2008). Some important changes will come about in Germany as a result. All organisms have been assessed until now according to uniform breakpoint criteria. This has often caused problems because, for many antibiotic drugs, the two peaks of the bimodal distribution of MIC values are found at different concentrations for different species of infectious microorganism. Now, breakpoints have been set for individual classes of microorganism, e.g., for staphylococci, pneumococci, enterococci, and so forth (table 2). This allows the breakpoints for individual classes of microorganism to be adjusted so that contiguous clusters of infectious organisms (e.g., wild types without resistance mechanisms) are not artificially divided from each other by breakpoints.
The changes in relation to the breakpoints that were applicable until now according to DIN 58940-4, Supplement 1, affect the cephalosporins in particular. In this area, the breakpoint between "susceptible" and "intermediate" for the Enterobacteriaceae had to be lowered so that organisms with clinically relevant extended spectrum beta-lactamases (ESBL) would not be incorrectly classified as susceptible. Clinical experience has shown that infections with strains possessing ESBLs cannot be treated very effectively with cephalosporins, or cannot be treated with them at all (14). Aside from this case, the changes relative to the previously applicable DIN breakpoints are slight. Nonetheless, any comparison of old and new resistance rates will be unreasonable, because spurious trends will arise that will be due solely to changed breakpoints rather than to changes in the antibiotic susceptibility of the infectious organisms.
Differences between Europe and the USA
The differences between the European and American breakpoints are particularly striking (see the example of cefotaxime illustrated in table 3). When the breakpoints were set, one of the facts that were taken into account was that, one hour after 1 g of cefotaxime is given intravenously, the blood level is approximately 12 mg/L, while the half-life of the drug is one hour (15). The DIN breakpoint assignments and the EUCAST assignments by which they have now been replaced are, in general, much more cautious than those of the CLSI, yet the two standards (DIN and CLSI) are now in use in Germany simultaneously. Regrettably, practicing clinicians are usually unaware which of the two standards was used in reporting the microbiological findings and how large the differences between them actually are for many organism-drug combinations. In extreme cases, e.g., Enterobacteriaceae-cefotaxime, the different assessments reflect a 16-fold difference in dosing. The same holds for published epidemiological data. Often, it is not even stated which breakpoints were used. It thus seems more reasonable to present MIC distributions, as in the figure shown in this paper, because otherwise there may be no basis for comparison of the microbiological data obtained by different research groups.
Table 3. A comparison of the European and American CLSI threshold values for cefotaxime.
| Antibacterial substance | Organism-specific threshold value assignments | |||||||||||
| Entero-bacteriaceae | Pseudomonas | Acinetobacter | Staphylococcus | Enterococcus | Streptococcus A, B, C, G | Streptococcus pneumoniae | Haemophilus influenzae M. catarrhalis | N. gonorrhoeae | N. meningitidis | Anaerobes | ||
| CLSI | Cefotaxim | ≤ 8/≥ 64 | ≤ 8/≥ 64 | ≤ 8/≥ 64 | ≤ 16/≥ 64 | – | ≤ 0.5/– | ≤ 1/>4 | ≤ 2/– | ≤ 0.5/– | – | |
| EUCAST | Cefotaxim | ≤ 1/≥ 2 | – | – | * | – | ≤ 0.5/> 0.5 | ≤ 0.5/> 2 | ≤ 0.12/> 0.12 | ≤ 0.12/> 0.12 | ≤ 0.12/> 0.12 | – |
* The test results for staphylococci and methicillin (as well as oxacillin and cefoxitin) are applicable to all intravenously administered cephalosporins with the exception of ceftazidime, which should not be used to treat staphylococcal infections.
CLSI, Clinical Laboratory Standards Institute; EUCAST, European Committee on Antimicrobial Susceptibility.
The manufacturers of automatized testing systems state that they will provide test kits meeting the EUCAST specifications. It will be difficult to implement the new breakpoints for other derived testing procedures, particularly the agar diffusion test. Because the MIC breakpoints have changed, the corresponding breakpoint diameters for zones of inhibition must be changed as well. Regrettably, many of the correlations underlying the DIN breakpoints that have been in use up to the present were established many years ago and are inadequately documented. Therefore, if the EUCAST breakpoints are to be implemented, extensive new studies are required in order to correlate the MIC values with inhibitory zone diameters for each and every antibiotic. In addition, the requirements of the new ISO 20776-2 standard must be met as well. In view of the likelihood that the agar diffusion test will also be standardized across Europe in the near future, particularly with respect to the inoculum that should be used, it would seem inadvisable to go to this great effort at present for the procedure that has been standardized according to DIN 58940-3. It follows that test results corresponding to the EUCAST/DIN set of breakpoints will not be available from this technique in the foreseeable future. This, in turn, will cause problems with accreditation with external quality control systems. Nor does it seem reasonable to go back to the CLSI breakpoints, because this would only heighten the differences that are already present. EUCAST is now attempting to establish a European standard for the agar diffusion test, including breakpoints for inhibitory zone diameters. The results are not expected until late 2009.
Summary
Like the older criteria that were in use up to the present, the new EUCAST/DIN criteria for the assessment of antibiotic effectiveness in the tripartite classification scheme—"susceptible," "intermediate," and "resistant"—are independent of the particular indication for which the antibiotic is to be used. For a number of drugs, however, the indications for which the drug is approved will have an influence on the breakpoints. Thus, an antibiotic that has been approved solely for the treatment of urinary tract infections should be assessed differently from one that is also used to treat respiratory tract infections. When the breakpoints were set for Neisseria meningitidis, for example, account was taken of the fact that this organism usually causes an infection in the central nervous system. On the other hand, for a number of organism-drug combinations, no breakpoints were determined. In some cases, this was because the organism did not belong to the spectrum of efficacy of the drug; in other cases, no breakpoint was set because of the lack of adequate published clinical data on the particular combination in question.
The new standards (ISO, EUCAST/DIN) introduce a new level of complexity to the determination of antibiotic resistance of infectious microorganisms. The newly standardized breakpoints across Europe will, however, improve the reliability of categorization as "susceptible," "intermediate," and "resistant." This in no way relieves treating physicians of the duty to consider the available therapeutic options carefully when prescribing antibiotics.
Acknowledgments
Translated from the original German by Ethan Taub, M.D.
Footnotes
Conflict of interest statement
Professor Müller has received financial support from the following companies: Astra Zeneca, Sanofi-Aventis Germany, Bayer Vital GmbH, Biosyn Arzneimittel GmbH, Biotest AG, Fresenius Medical Care, MSD Sharp & Dohme GmbH, Novartis Pharma, Pfizer Pharma GmbH, and Wyeth Pharma. He is a member of the Advisory Boards for caspofungin (MSD Sharp & Dohme GmbH; Merck USA), voriconazole (Pfizer Pharma GmbH), anidulafungin (Pfizer Germany, Pfizer Europe), and posaconazole (Essex Pharma, invited).
Professor Rodloff has received financial support for consulting, lecture fees, and travel costs from the following companies: Biomerieux, Dade Behring, MSD, Pfizer, Optimer, Sanofi-Aventis, Wyeth, Novartis, Bayer, and Chameleon.
PD Dr. Bauer, Professor Ewig, and Professor Kujath declare that they have no conflict of interest as defined by the guidelines of the International Committee of Medical Journal editors.
References
- 1.Rello J, Diaz E. Pneumonia in the intensive care unit. Crit Care Med. 2003;31:2544–2551. doi: 10.1097/01.CCM.0000089928.84326.D2. [DOI] [PubMed] [Google Scholar]
- 2.Beardsley JR, Williamson JC, Johnson JW, Ohl CA, Karchmer TB, Bowton DL. Using local microbiologic data to develop institution-specific guidelines for the treatment of hospital-acquired pneumonia. Chest. 2006;130:787–793. doi: 10.1378/chest.130.3.787. [DOI] [PubMed] [Google Scholar]
- 3.Kiem S, Schentag JJ. Relationship of minimal inhibitory concentration and bactericidal activity to efficacy of antibiotics for treatment of ventilator-associated pneumonia. Semin Respir Crit Care Med. 2006;27:51–67. doi: 10.1055/s-2006-933674. [DOI] [PubMed] [Google Scholar]
- 4.Lichtenstein SJ, Wagner RS, Jamison T, Bell B, Stroman DW. Speed of bacterial kill with a fluoroquinolone compared with nonfluoroquinolones: clinical implications and a review of kinetics of kill studies. Adv Ther. 2007;24:1098–1111. doi: 10.1007/BF02877716. [DOI] [PubMed] [Google Scholar]
- 5.Gould IM. Determinants of response to antibiotic therapy. J Chemother. 1998;10:347–353. doi: 10.1179/joc.1998.10.5.347. [DOI] [PubMed] [Google Scholar]
- 6.DIN EN ISO 20776-1: Labormedizinische Untersuchungen und In-vitro-Diagnostika-Systeme - Empfindlichkeitsprüfung von Infektionserregern und Evaluation von Geräten zur antimikrobiellen Empfindlichkeitsprüfung - Teil 1: Referenzmethode zur Testung der In-vitro-Aktivität von antimikrobiellen Substanzen gegen schnell wachsende aerobe Bakterien, die Infektionskrankheiten verursachen (ISO 20776-1). Deutsche Fassung EN ISO 20776-1: DIN Deutsches Institut für Normung e.V. Berlin, Wien, Zürich: Beuth Verlag; 2006. [Google Scholar]
- 7.DIN EN ISO 20776-2, Labormedizinische Untersuchungen und In-vitro-Diagnostika-Systeme - Empfindlichkeitsprüfung von Infektionserregern und Evaluation von Geräten zur antimikrobiellen Empfindlichkeitsprüfung - Teil 2: Evaluation der Leistung einer Vorrichtung zur antimikrobiellen Empfindlichkeitsprüfung (ISO 20776-2). Deutsche Fassung EN ISO 20776-2: DIN Deutsches Institut für Normung e.V. Berlin, Wien, Zürich: Beuth Verlag; 2007. [Google Scholar]
- 8.Eliopoulos GM. Antimicrobial agents for treatment of serious infections caused by resistant Staphylococcus aureus and enterococci. Eur J Clin Microbiol Infect Dis. 2005;24:826–831. doi: 10.1007/s10096-005-0055-1. [DOI] [PubMed] [Google Scholar]
- 9.Rodloff AC, Goldstein EJ, Torres A. Two decades of imipenem therapy. J Antimicrob Chemother. 2006;58:916–929. doi: 10.1093/jac/dkl354. [DOI] [PubMed] [Google Scholar]
- 10.Eagye KJ, Kuti JL, Dowzicky M, Nicolau DP. Empiric therapy for secondary peritonitis: a pharmacodynamic analysis of cefepime, ceftazidime, ceftriaxone, imipenem, levofloxacin, piperacillin/tazobactam, and tigecycline using Monte Carlo simulation. Clin Ther. 2007;29:889–899. doi: 10.1016/j.clinthera.2007.05.018. [DOI] [PubMed] [Google Scholar]
- 11.Drusano GL. Pharmacokinetics and pharmacodynamics of antimicrobials. Clin Infect Dis. 2007;15(45 Suppl 1):89–95. doi: 10.1086/518137. [DOI] [PubMed] [Google Scholar]
- 12.Pai MP, Turpin RS, Garey KW. Association of fluconazole area under the concentration-time curve/MIC and dose/MIC ratios with mortality in nonneutropenic patients with candidemia. Antimicrob Agents Chemother. 2007;51:35–39. doi: 10.1128/AAC.00474-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.DIN 58940-4 Beiblatt 1: Medizinische Mikrobiologie - Empfindlichkeitsprüfung von mikrobiellen Krankheitserregern gegen Chemotherapeutika - Teil 4: Bewertungsstufen für die minimale Hemmkonzentration; MHK-Grenzwerte von antibakteriellen Wirkstoffen; DIN Deutsches Institut für Normung e. V. Berlin, Wien, Zürich: Beuth Verlag; 2004. [Google Scholar]
- 14.Paterson DL, Ko WC, Von Gottberg A, et al. Outcome of cephalosporin treatment for serious infections due to apparently susceptible organisms producing extended-spectrum beta-lactamases: implications for the clinical microbiology laboratory. J Clin Microbiol. 2001;39:2206–2212. doi: 10.1128/JCM.39.6.2206-2212.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stille W, Brodt HR, Groll AH, Just-Nübling G. Antibiotika-Therapie. Stuttgart, New York: Schattauer; 2005. 81 pp. [Google Scholar]