Abstract
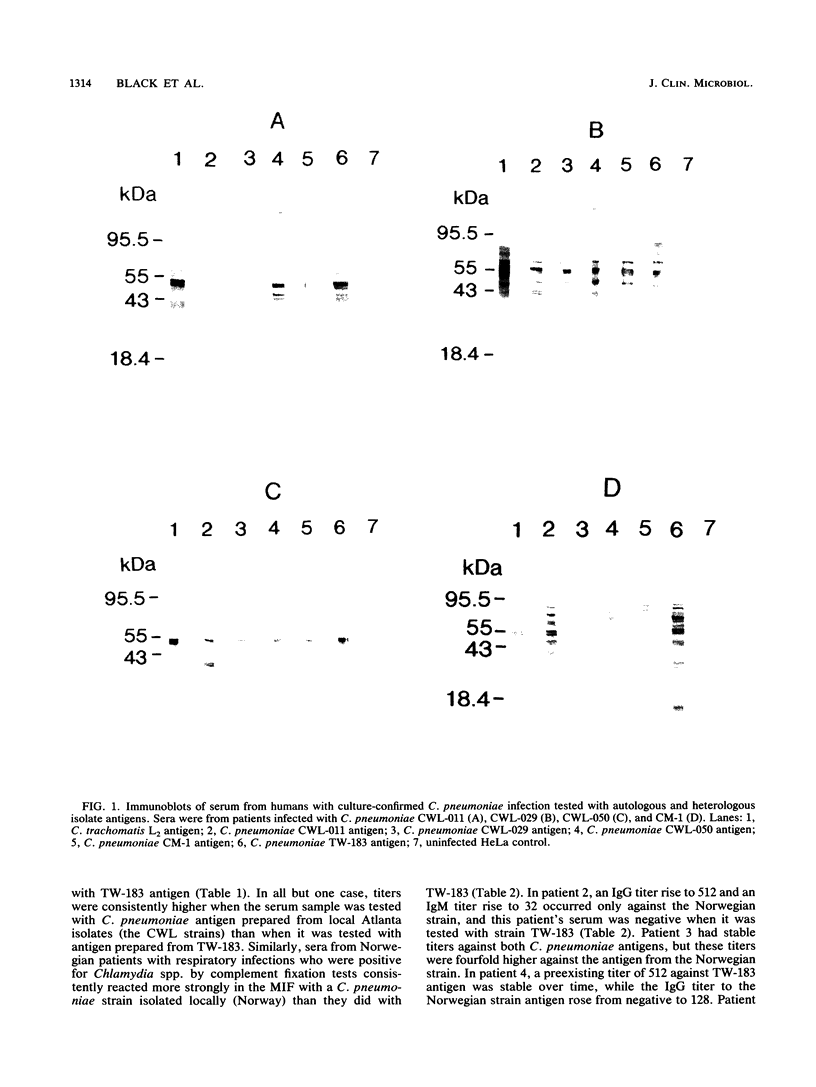
The antigenic profiles of six strains of Chlamydia pneumoniae were analyzed by the microimmunofluorescence test (MIF) and immunoblotting with human serum and murine monoclonal antibody. MIF-derived antibody titers in serum samples from culture-positive patients were four- to eightfold higher against autologous isolate antigen than they were against the prototype antigen strain TW-183. Sera of patients with respiratory illness that were culture negative and complement fixation positive for Chlamydia spp. produced higher titers by MIF against a strain of C. pneumoniae isolated in the area than they did against TW-183. For two of five cases, the criteria for establishing the diagnosis of acute infection were met only with use of the antigen from the local strain; TW-183 was inadequate for this purpose. Immunoblot profiles revealed antigenic differences between strains that varied with the human serologic response; i.e., unique antigens were recognized by the sera of some individuals and not by the sera of others. Using the reactivity of a genus-specific monoclonal antibody against a major outer membrane protein, we found that strain CWL-011, isolated in Atlanta, Ga., may possess a major outer membrane protein with a molecular mass between those of C. trachomatis L2 and other C. pneumoniae strains. These data provide evidence of several new and unique serotypes of C. pneumoniae and suggest that the serologic diagnosis of C. pneumoniae infection may require the use of antigens from more than one strain of this species.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Caldwell H. D., Kromhout J., Schachter J. Purification and partial characterization of the major outer membrane protein of Chlamydia trachomatis. Infect Immun. 1981 Mar;31(3):1161–1176. doi: 10.1128/iai.31.3.1161-1176.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell L. A., Kuo C. C., Grayston J. T. Structural and antigenic analysis of Chlamydia pneumoniae. Infect Immun. 1990 Jan;58(1):93–97. doi: 10.1128/iai.58.1.93-97.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell L. A., Kuo C. C., Wang S. P., Grayston J. T. Serological response to Chlamydia pneumoniae infection. J Clin Microbiol. 1990 Jun;28(6):1261–1264. doi: 10.1128/jcm.28.6.1261-1264.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi E. Y., Kuo C. C., Grayston J. T. Unique ultrastructure in the elementary body of Chlamydia sp. strain TWAR. J Bacteriol. 1987 Aug;169(8):3757–3763. doi: 10.1128/jb.169.8.3757-3763.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grayston J. T., Wang S. P., Kuo C. C., Campbell L. A. Current knowledge on Chlamydia pneumoniae, strain TWAR, an important cause of pneumonia and other acute respiratory diseases. Eur J Clin Microbiol Infect Dis. 1989 Mar;8(3):191–202. doi: 10.1007/BF01965260. [DOI] [PubMed] [Google Scholar]
- Ladany S., Black C. M., Farshy C. E., Ossewaarde J. M., Barnes R. C. Enzyme immunoassay to determine exposure to Chlamydia pneumoniae (strain TWAR). J Clin Microbiol. 1989 Dec;27(12):2778–2783. doi: 10.1128/jcm.27.12.2778-2783.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newhall W. J., Batteiger B., Jones R. B. Analysis of the human serological response to proteins of Chlamydia trachomatis. Infect Immun. 1982 Dec;38(3):1181–1189. doi: 10.1128/iai.38.3.1181-1189.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S. P., Grayston J. T. Human serology in Chlamydia trachomatis infection with microimmunofluorescence. J Infect Dis. 1974 Oct;130(4):388–397. doi: 10.1093/infdis/130.4.388. [DOI] [PubMed] [Google Scholar]