Abstract
Background
Previous clinical studies have identified the cervical facet joint, including the capsular ligaments, as sources of pain in whiplash patients. The goal of this study was to determine whether whiplash caused increased capsular ligament laxity by applying quasi-static loading to whiplash-exposed and control capsular ligaments.
Methods
A total of 66 capsular ligament specimens (C2/3 to C7/T1) were prepared from 12 cervical spines (6 whiplash-exposed and 6 control). The whiplash-exposed spines had been previously rear impacted at a maximum peak T1 horizontal acceleration of 8 g. Capsular ligaments were elongated at 1 mm/s in increments of 0.05 mm until a tensile force of 5 N was achieved and subsequently returned to neutral position. Four pre-conditioning cycles were performed and data from the load phase of the fifth cycle were used for subsequent analyses. Ligament elongation was computed at tensile forces of 0, 0.25, 0.5, 0.75, 1.0, 2.5, and 5.0 N. Two factor, non-repeated measures ANOVA (P<0.05) was performed to determine significant differences in the average ligament elongation at tensile forces of 0 and 5 N between the whiplash-exposed and control groups and between spinal levels.
Findings
Average elongation of the whiplash-exposed capsular ligaments was significantly greater than that of the control ligaments at tensile forces of 0 and 5 N. No significant differences between spinal levels were observed.
Interpretation
Capsular ligament injuries, in the form of increased laxity, may be one component perpetuating chronic pain and clinical instability in whiplash patients.
Keywords: Whiplash, biomechanics, capsular ligament, injury, laxity
1. Introduction
Whiplash injuries of the neck, caused by relative acceleration between the head and thorax during motor vehicle collisions, produce acute and chronic neck pain, headache, dizziness, vertigo, and parasthesias in the upper extremities (Barnsley et al., 1994; Spitzer et al., 1995; Sterner and Gerdle, 2004). MRI and autopsy studies have correlated chronic symptoms with injuries to the cervical discs, ligaments, and facet joints in whiplash patients (Jonsson et al., 1991; Kaale et al., 2005a, b; Krakenes and Kaale, 2006; Pettersson et al., 1997). Previous clinical studies have targeted the cervical facet joint and capsule, including the capsular ligament (CL), as sources of chronic pain in whiplash patients (Barnsley et al., 1995; Lord et al., 1996a). These studies administered blockage of the facet joint afferents, including the CL nerves, in whiplash patients. Results demonstrated pain relief in up to 60% of the patients. Single or cumulative micro-trauma due to overstretching of CLs causing subfailure injuries and increased ligament laxity have been hypothesized to injure embedded ligament mechanoreceptors (Panjabi, 2006). The effect of injured ligament mechanoreceptors on spine stability has not been studied. However, in vivo animal models have demonstrated that stimulation of spinal ligaments initiated activity of spinal musculature (Indahl et al., 1997; Solomonow et al., 2002; Solomonow et al., 1998). Corrupted signals from the injured mechanoreceptors may potentially elicit abnormal muscle response patterns causing excessive facet loading and CL strains, further increasing the CL laxity and injury and preventing or delaying ligament healing.
Previous in vitro biomechanical studies have investigated potential neck ligament injuries due to whiplash (Panjabi et al., 2006; Pearson et al., 2004; Stemper et al., 2005; Tominaga et al., 2006). Tominaga et al (2006) compared the high-speed mechanical properties of cervical bone-ligament-bone preparations between whiplash-exposed and control cervical spines. They found that the average failure force and average energy absorption capacity of the whiplash-exposed ligaments were significantly less than those of the control ligaments. The effect of whiplash on ligament laxity was not investigated. Others have documented potentially injurious CL strains and abnormal facet kinematics due to simulated whiplash loading (Pearson et al., 2004; Stemper et al., 2005). Although implied, neither study documented actual injury or increased laxity of CLs due to whiplash. Lastly, Panjabi et al (2006) applied quasi-static physiological loading to cervical spine specimens prior to and following simulated rear impacts and documented injuries at the middle and lower cervical spine and increased injury risk due to rotated head posture at the time of impact, as compared to forward facing. Injuries to specific ligaments were not identified.
In vivo animal studies have investigated the relation between painful chronic symptoms and injurious CL strains (Lee et al., 2004; Lee et al., 2006). Using a rat model, Lee et al (2004) determined the CL strain threshold for behavioral hypersensitivity as measured by mechanical allodynia up to 2 weeks following application of injurious CL strain. This CL strain injury threshold was used to demonstrate that the CL mechanical properties at failure differed significantly from those at the onset of subfailure strain injury (Lee et al., 2006). Others have used a goat model to measure and correlate CL nerve root activity, load, and strain during CL elongation (Chen et al., 2005; Lu et al., 2005a, b). Nonetheless, these aforementioned animal studies have limitations. The tensile loading used to produce the CL strain does not fully represent the complex neck loading experienced by those involved in automobile collisions (Ivancic et al., 2006). Behavioral hypersensitivity was measured for up to only 2 weeks and these animal results have yet to be correlated with chronic symptoms in whiplash patients.
To our knowledge, no previous clinical or biomechanical studies have identified CL injuries due to whiplash as determined by increased ligament laxity. These data may aid understanding of the mechanism causing painful chronic symptoms in whiplash patients. Our goal was to determine whether whiplash caused increased CL laxity by applying quasi-static loading to whiplash-exposed and control facet-CL-facet preparations.
2. Methods
2.1 Specimen preparation
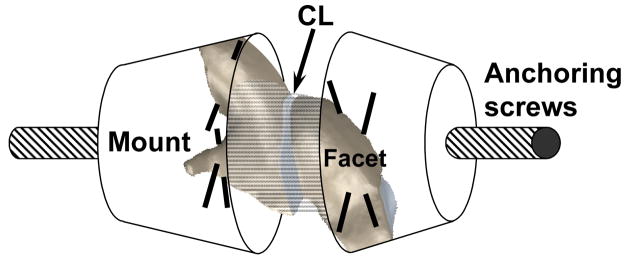
Facet-CL-facet specimens were prepared from 12 osteoligamentous whole cervical spines (6 whiplash-exposed: average age of 70.8 years, range, 52 to 84 years; 6 control: average age of 80.6 years, range, 71 to 92 years) (Tominaga et al., 2006). There were four male and two female donors in each group. The whiplash-exposed spines had been previously rear impacted using experimental methodology described in detail elsewhere (Ivancic et al., 2005). Briefly, this protocol entailed mounting of the occiput and T1 vertebra in resin mounts. A surrogate head was rigidly attached to the occipital mount while the T1 mount was fixed to a trauma sled. The sled was mounted on linear bearings. The surrogate head and spine were stabilized using muscle force replication. To simulate rear impact loading, the trauma sled and thus the T1 vertebra, were accelerated anteriorly using the incremental trauma protocol up to a maximum peak T1 horizontal acceleration of 8 g. The control spines and the whiplash-exposed spines, following the 8 g impact, were frozen at −20°C prior to preparation for mechanical testing (Panjabi et al., 1985). All spines had no history of any disease that could have affected the osteoligamentous structures. The spines were divided into two equal groups with each group consisting of three whiplash-exposed and three control spines. Facet-CL-facet specimens were prepared using C2/3, C4/5, and C6/7 spinal levels in the first group and C3/4, C5/6, and C7/T1 spinal levels in the second group. Left and right facet-CL-facet specimens from each spinal level were prepared separately. Each preparation was then mounted for mechanical testing (Figure 1). To ensure rigid anchoring of the facets within quick setting bondo mounts (Evercoat Z-Grip, Fibre Glass-Evercoat, Cincinnati, OH), two perpendicular thru-holes were drilled into each facet in which 19 gauge needles were inserted. Each mount contained an anchoring screw for subsequent attachment to the testing apparatus. In total, 66 facet-CL-facet specimens were prepared (Table 1). Each specimen was mounted in a custom designed, displacement-controlled mechanical testing apparatus which was controlled by a computer (Panjabi et al., 1996). The main components of the bench-top apparatus included a stationary, horizontal frame, stepper motor which controlled a movable platform (resolution 0.01 mm), and load cell (222 N capacity, model LCCA-50, Omega, Stamford, CT). To achieve neutral CL position, the apparatus allowed ligament alignment in the plane perpendicular to the axis of CL elongation. One end of the specimen was rigidly fixed to the horizontal frame by means of the load cell, while the other end was fixed to the movable platform. To standardize the neutral CL position, the facets were preloaded to 1 N of compression immediately prior to testing and this was defined as zero CL elongation.
Figure 1.
Schematic showing the orientation of the capsular ligament (CL) within the facet-CL-facet specimen.
Table 1. Sample sizes for cervical capsular ligament (CL) preparations.
A total of 66 facet-CL-facetspecimens were analyzed.
| Whiplash-exposed | Control | |
|---|---|---|
| C2/3 | 6 | 3 |
| C3/4 | 4 | 6 |
| C4/5 | 6 | 5 |
| C5/6 | 6 | 6 |
| C6/7 | 6 | 6 |
| C7/T1 | 6 | 6 |
|
| ||
| Totals | 34 | 32 |
2.2 Experimental protocol
The facet-CL-facet specimen was elongated at 1 mm/s in increments of 0.05 mm until a tensile force of 5 N was achieved and subsequently was returned to neutral position. Force and elongation data were recorded at each motion step following a 0.5 s rest period. Four pre-conditioning cycles were performed and data from the load phase of the fifth cycle were used for subsequent analyses. Preliminary experimentation demonstrated that four load/unload cycles were adequate for preconditioning, and that force-elongation curves among subsequent cycles showed little change.
2.3 Data analyses
CL elongation was computed at tensile forces of 0, 0.25, 0.5, 0.75, 1.0, 2.5, and 5.0 N using the equation of the line through the two data points that encompassed the desired force. This uneven distribution provided more readings in the early phase of the average force-elongation curves, when changes occurred most rapidly.
Statistics
Data from left and right CLs were combined within each group. Two factor, non-repeated measures ANOVA was used to determine differences in the average CL elongation at tensile forces of 0 and 5 N between the groups (whiplash-exposed vs. control) and between spinal levels (C2/3 though C7/T1). The significance level was set at P<0.05. If differences between spinal levels were observed, pair-wise Bonferroni post-hoc tests were performed with adjusted P-values computed based upon the number of post-hoc tests performed.
3. Results
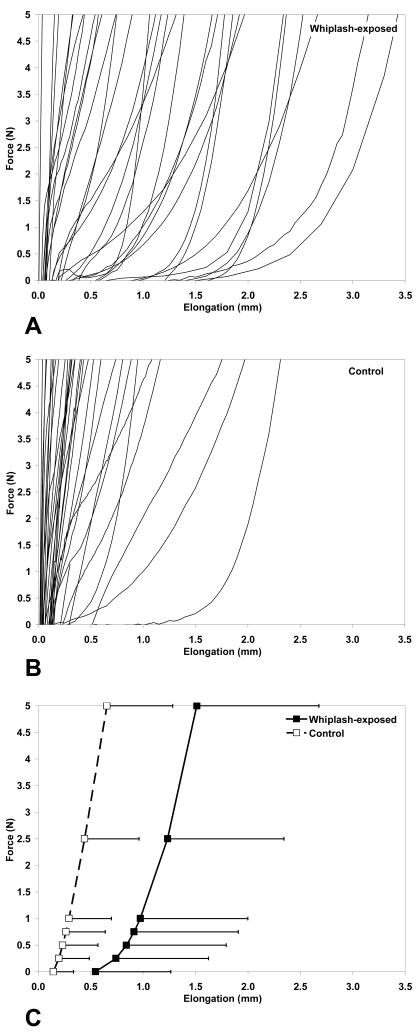
The force-elongation curve is shown for each whiplash-exposed CL (Figure 2A) and control CL (Figure 2B) along with the average curves with standard deviations (Figure 2C). As expected, the average force-elongation curves were nonlinear, with greater flexibility at low forces and increasing stiffness at higher forces. Greater flexibility was generally observed in the whiplash-exposed CLs, as compared to the control CLs, particularly at low forces. The difference between the average elongation of the whiplash-exposed and control CLs progressively increased from 0.4 mm at 0 N to 0.9 mm at 5 N. The standard deviations for the average elongations of the whiplash-exposed CLs were generally larger than those of the control CLs.
Figure 2.
Force-elongation curves for A) whiplash-exposed capsular ligaments (CLs), B) control CLs, and C) averages with standard deviations. The average CL elongation for each group was computed at tensile forces of 0, 0.25, 0.5, 0.75, 1.0, 2.5, and 5.0 N.
The average elongation of the whiplash-exposed CLs was significantly greater than that of the control CLs at tensile forces of 0 N (Table 2A) and 5 N (Table 2B). No significant differences between spinal levels were observed at either tensile force. No significant interactions were found (tensile force of 0 N: P= 0.711; tensile force of 5 N: P=0.777).
Table 2. Comparisons of elongation between whiplash-exposed and control capsular ligaments (CLs).
Average (SD) elongations (mm) at tensile forces of A) 0 N and B) 5 N were calculated by combining data from left and right CLs within each group. Statistical differences are indicated as *P<0.05.
| A) CL Elongations at Tensile Force of 0 N | |||||||
|---|---|---|---|---|---|---|---|
| C2/3 | C3/4 | C4/5 | C5/6 | C6/7 | C7/T1 | Spinal Level | |
| Whiplash-exposed | 0.3 (0.2) | 0.5 (0.6) | 0.6 (0.7) | 1.0 (1.3) | 0.4 (0.3) | 0.3 (0.6) | P=0.643 |
| Control | 0.2 (0.2) | 0.1 (0.0) | 0.3 (0.4) | 0.1 (0.1) | 0.1 (0.1) | 0.1 (0.1) | |
|
| |||||||
| Whiplash-exposed vs. Control | P=0.007* | ||||||
| B) CL Elongations at Tensile Force of 5 N | |||||||
|---|---|---|---|---|---|---|---|
| C2/3 | C3/4 | C4/5 | C5/6 | C6/7 | C7/T1 | Spinal Level | |
| Whiplash-exposed | 1.3 (0.5) | 1.2 (1.3) | 1.7 (1.1) | 2.1 (1.8) | 1.7 (0.8) | 0.8 (0.8) | P=0.336 |
| Control | 0.9 (0.7) | 0.4 (0.2) | 0.7 (0.9) | 1.0 (0.6) | 0.5 (0.3) | 0.6 (0.8) | |
|
| |||||||
| Whiplash-exposed vs. Control | P=0.002* | ||||||
4. Discussion
Whiplash causes soft tissue neck injuries that result in substantial societal costs (Spitzer et al., 1995). There is controversy as to which anatomical components are injured during whiplash and even greater debate regarding the specific cause and source of the chronic symptoms reported by whiplash patients. These chronic symptoms include neck pain, headache, dizziness, vertigo, and parasthesias in the upper extremities. Previous clinical and biomechanical studies have identified the cervical facet joint, including the capsular ligaments (CLs), as sources of pain due to whiplash loading (Barnsley et al., 1995; Lee et al., 2004; Lord et al., 1996a; Pearson et al., 2004). While subfailure ligament injuries are common, non-destructive methods for detecting and quantifying these injuries in specific ligaments are not well established. The present study utilized a new methodology for quantifying ligament laxity using quasi-static, non-destructive loading of facet-CL-facet specimens. The force-elongation curves of whiplash-exposed and control CLs were compared at and in the vicinity of the neutral zone (Panjabi, 1992). Significant increases were observed in the laxity of the whiplash-exposed CLs, as compared to the controls. Chronic symptoms reported by whiplash patients may be caused by elongation-induced CL strains during trauma which produce subfailure tears in some or all CL fibers and injuries to embedded mechanoreceptors (Panjabi, 2006). The present biomechanical results may help to explain, at least in part, the chronicity of painful symptoms in whiplash patients.
The limitations of the present study should be considered before interpreting the results. The average age of whiplash-exposed spines, 70.8 years, was older than the younger population most likely to suffer whiplash injuries. Being an in vitro biomechanical study, the in vivo tissue responses of healing and adaptation were not modeled. Histological evaluation or scanning electron microscopy techniques were not performed as they were outside the scope of this study. Tensile force up to 5 N was applied to the facet-CL-facet specimens. This peak load was large enough to evaluate the CL laxity in the vicinity of the neutral zone, but small enough not to injure the specimens. The latter was demonstrated by reproducible force-elongation curves following the four preconditioning cycles. Although only six whole cervical spines were available in each of the whiplash-exposed and control groups, a total of 34 and 32 facet-CL-facet specimens were analyzed in each of the respective groups. In order to determine the effect of whiplash on CL laxity, data from left and right CLs were combined within each group. No significant differences in CL laxity were observed between spinal levels, most likely due to the limited sample sizes. Increased sample sizes may have demonstrated significantly greater CL laxity at the lower, as compared to upper and mid cervical spine regions. Greatest high-speed CL strains during simulated rear impact have been documented at the lower cervical spine, C5/6 and C6/7, indicating that these spinal levels may be at greatest risk of injury due to whiplash (Pearson et al., 2004). Also due to the limited sample sizes, we did not evaluate the laxity of other cervical ligaments, such as the anterior longitudinal ligament and annular fibers, however these ligaments may also be injured due to excessive strains during whiplash (Ivancic et al., 2004; Panjabi et al., 2004).
The variability of the average whiplash-exposed CL elongation was between 85% and 275% larger than that of the average control CL elongation, as measured by standard deviation (Figure 2C). This percentage difference in variability of CL elongation was greatest at the neutral zone (0 N tensile force) and least at 5 N tensile force. This result corresponds with in vivo studies that have reported greater variability in neck range of motion, position sense, and postural activity of whiplash patients, as compared with controls (Feipel et al., 1999; Madeleine et al., 2004). These cumulative results demonstrate that whiplash loading may cause CL subfailure injuries that vary in severity from microscopic tears of a few CL fibers to near complete rupture of all fibers. These CL subfailure injuries may potentially cause injury of varying severity to embedded mechanoreceptors. The effects of injured CL mechanoreceptors on neck stability are not well understood. Animal models have shown that stimulation of spinal ligaments initiated activity of spinal muscles (Indahl et al., 1997; Solomonow et al., 2002; Solomonow et al., 1998). Varying injury severity of the mechanoreceptors embedded in the CLs may potentially lead to a wide range of corrupted transducer signals received by the neuromuscular control unit. It has been hypothesized that these corrupted signals may produce the varied abnormal muscle response patterns that lead to the decreased neck mobility and proprioception in whiplash patients (Panjabi, 2006).
The present data, which conclusively demonstrates CL injury in the form of increased laxity due to whiplash, builds upon previously reported findings. Decreased strength of whiplash-exposed human neck ligaments has been observed due to high-speed elongation following simulated rear impacts, as compared to controls (Tominaga et al., 2006). Multiple studies have documented non-physiological CL strains during simulated whiplash loading of human cadaveric spines (Cusick et al., 2001; Pearson et al., 2004; Siegmund et al., 2001; Stemper et al., 2005; Winkelstein et al., 2000; Yoganandan et al., 2002). An increase in behavioural sensitivity in excess of three-fold was observed using an in vivo rat model that underwent 34% CL strain, as compared to 11% strain. This composite evidence of CL injuries due to whiplash-type loading provides a biomechanical basis to explain the chronic symptoms reported by whiplash patients.
Increased laxity and subfailure injuries of CLs and embedded mechanoreceptors may be the origin of at least some neck neuromuscular abnormalities observed in whiplash patients, such as repositioning error (Heikkila and Astrom, 1996; Loudon et al., 1997), altered range of motion (Antonaci et al., 2002; Bonelli et al., 2000; Madeleine et al., 2004), and muscle spasm (Norris and Watt, 1983; Ronnen et al., 1996). While neck muscle injuries may explain short term neuromuscular abnormalities, they most likely do not lead to chronic symptoms due the abundant blood supply of muscles which enables relative quick healing. Ligaments, in contrast, heal slowly and poorly and may scar (Osti et al., 1990; Osti et al., 1992). Panjabi (2006) hypothesized that abnormal mechanoreceptor transducer signals produced from injured CLs may cause corrupted neck muscle response patterns, hindering the neck proprioception of whiplash patients. Reduced active neck motion in whiplash patients may be a response of the neuromuscular control system to stiffen the injured neck to prevent excessive spinal motions, nerve tissue impingement, and further CL injuries. This may also result in the muscle spasm observed in the whiplash patients.
Increased CL laxity in whiplash patients may be one component perpetuating chronic neck pain and clinical instability. As the CL contains both mechanoreceptive and nociceptive nerve endings, CL injury during whiplash causing increased laxity may potentially injure these structures causing inflammation and pain. Increased CL laxity may cause residual instability, altered loading patterns, and nerve tissue impingement. Partial injury of embedded CL mechanoreceptors due to subfailure stretching of CLs may potentially cause abnormal transducer signals leading to altered muscle response patterns, muscle spasm, repositioning errors, and altered neck range of motion.
5. Conclusions
This well-controlled biomechanical study, utilizing quasi-static, non-destructive tensile loading of whiplash-exposed and control facet-CL-facet specimens, documented injury in the form of significant increases in the laxity of whiplash-exposed CLs, as compared to controls. Our results are consistent with several clinical studies that have reported pain relief in whiplash patients following nerve block and radiofrequency ablation of facet joint afferents (Lord et al., 1995; Lord et al., 1996b). The present biomechanical data improves our understanding of injury and increased laxity of CLs due to whiplash.
Acknowledgments
This research was supported by NIH Grant 1 RO1 AR45452 1A2.
Footnotes
Conflict of Interest Statement
We declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Antonaci F, Bulgheroni M, Ghirmai S, Lanfranchi S, Dalla Toffola E, Sandrini G, Nappi G. 3D kinematic analysis and clinical evaluation of neck movements in patients with whiplash injury. Cephalalgia. 2002;22:533–542. doi: 10.1046/j.1468-2982.2002.00405.x. [DOI] [PubMed] [Google Scholar]
- Barnsley L, Lord S, Bogduk N. Whiplash injury. Pain. 1994;58:283–307. doi: 10.1016/0304-3959(94)90123-6. [DOI] [PubMed] [Google Scholar]
- Barnsley L, Lord SM, Wallis BJ, Bogduk N. The prevalence of chronic cervical zygapophysial joint pain after whiplash. Spine. 1995;20:20–25. doi: 10.1097/00007632-199501000-00004. discussion 26. [DOI] [PubMed] [Google Scholar]
- Bonelli A, Donati P, Maltoni G, Puglisi F, Norelli GA. Neck motion evaluation after whiplash: a radiographic and kinematic protocol. Ital J Anat Embryol. 2000;105:51–62. [PubMed] [Google Scholar]
- Chen C, Lu Y, Cavanaugh JM, Kallakuri S, Patwardhan A. Recording of neural activity from goat cervical facet joint capsule using custom-designed miniature electrodes. Spine. 2005;30:1367–1372. doi: 10.1097/01.brs.0000166193.39389.21. [DOI] [PubMed] [Google Scholar]
- Cusick JF, Pintar FA, Yoganandan N. Whiplash syndrome: kinematic factors influencing pain patterns. Spine. 2001;26:1252–1258. doi: 10.1097/00007632-200106010-00015. [DOI] [PubMed] [Google Scholar]
- Feipel V, Rondelet B, LePallec JP, DeWitte O, Rooze M. The use of disharmonic motion curves in problems of the cervical spine. Int Orthop. 1999;23:205–209. doi: 10.1007/s002640050351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heikkila H, Astrom PG. Cervicocephalic kinesthetic sensibility in patients with whiplash injury. Scand J Rehabil Med. 1996;28:133–138. [PubMed] [Google Scholar]
- Indahl A, Kaigle AM, Reikeras O, Holm SH. Interaction between the porcine lumbar intervertebral disc, zygapophysial joints, and paraspinal muscles. Spine. 1997;22:2834–2840. doi: 10.1097/00007632-199712150-00006. [DOI] [PubMed] [Google Scholar]
- Ivancic PC, Panjabi MM, Ito S. Cervical spine loads and intervertebral motions during whiplash. Traffic Inj Prev. 2006;7:389–399. doi: 10.1080/15389580600789127. [DOI] [PubMed] [Google Scholar]
- Ivancic PC, Panjabi MM, Ito S, Cripton PA, Wang JL. Biofidelic whole cervical spine model with muscle force replication for whiplash simulation. Eur Spine J. 2005;14:346–355. doi: 10.1007/s00586-004-0758-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivancic PC, Pearson AM, Panjabi MM, Ito S. Injury of the anterior longitudinal ligament during whiplash simulation. Eur Spine J. 2004;13:61–68. doi: 10.1007/s00586-003-0590-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonsson H, Jr, Bring G, Rauschning W, Sahlstedt B. Hidden cervical spine injuries in traffic accident victims with skull fractures. J Spinal Disord. 1991;4:251–263. doi: 10.1097/00002517-199109000-00001. [DOI] [PubMed] [Google Scholar]
- Kaale BR, Krakenes J, Albrektsen G, Wester K. Head position and impact direction in whiplash injuries: associations with MRI-verified lesions of ligaments and membranes in the upper cervical spine. J Neurotrauma. 2005a;22:1294–1302. doi: 10.1089/neu.2005.22.1294. [DOI] [PubMed] [Google Scholar]
- Kaale BR, Krakenes J, Albrektsen G, Wester K. Whiplash-associated disorders impairment rating: neck disability index score according to severity of MRI findings of ligaments and membranes in the upper cervical spine. J Neurotrauma. 2005b;22:466–475. doi: 10.1089/neu.2005.22.466. [DOI] [PubMed] [Google Scholar]
- Krakenes J, Kaale BR. Magnetic resonance imaging assessment of craniovertebral ligaments and membranes after whiplash trauma. Spine. 2006;31:2820–2826. doi: 10.1097/01.brs.0000245871.15696.1f. [DOI] [PubMed] [Google Scholar]
- Lee KE, Davis MB, Mejilla RM, Winkelstein BA. In vivo cervical facet capsule distraction: mechanical implications for whiplash and neck pain. Stapp Car Crash J. 2004;48:373–395. doi: 10.4271/2004-22-0016. [DOI] [PubMed] [Google Scholar]
- Lee KE, Franklin AN, Davis MB, Winkelstein BA. Tensile cervical facet capsule ligament mechanics: failure and subfailure responses in the rat. J Biomech. 2006;39:1256–1264. doi: 10.1016/j.jbiomech.2005.03.018. [DOI] [PubMed] [Google Scholar]
- Lord SM, Barnsley L, Bogduk N. Percutaneous radiofrequency neurotomy in the treatment of cervical zygapophysial joint pain: a caution. Neurosurgery. 1995;36:732–739. doi: 10.1227/00006123-199504000-00014. [DOI] [PubMed] [Google Scholar]
- Lord SM, Barnsley L, Wallis BJ, Bogduk N. Chronic cervical zygapophysial joint pain after whiplash. A placebo-controlled prevalence study. Spine. 1996a;21:1737–1744. doi: 10.1097/00007632-199608010-00005. discussion 1744-1735. [DOI] [PubMed] [Google Scholar]
- Lord SM, Barnsley L, Wallis BJ, McDonald GJ, Bogduk N. Percutaneous radio-frequency neurotomy for chronic cervical zygapophyseal-joint pain. N Engl J Med. 1996b;335:1721–1726. doi: 10.1056/NEJM199612053352302. [DOI] [PubMed] [Google Scholar]
- Loudon JK, Ruhl M, Field E. Ability to reproduce head position after whiplash injury. Spine. 1997;22:865–868. doi: 10.1097/00007632-199704150-00008. [DOI] [PubMed] [Google Scholar]
- Lu Y, Chen C, Kallakuri S, Patwardhan A, Cavanaugh JM. Development of an in vivo method to investigate biomechanical and neurophysiological properties of spine facet joint capsules. Eur Spine J. 2005a;14:565–572. doi: 10.1007/s00586-004-0835-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Chen C, Kallakuri S, Patwardhan A, Cavanaugh JM. Neurophysiological and biomechanical characterization of goat cervical facet joint capsules. J Orthop Res. 2005b;23:779–787. doi: 10.1016/j.orthres.2005.01.002. [DOI] [PubMed] [Google Scholar]
- Madeleine P, Prietzel H, Svarrer H, Arendt-Nielsen L. Quantitative posturography in altered sensory conditions: a way to assess balance instability in patients with chronic whiplash injury. Arch Phys Med Rehabil. 2004;85:432–438. doi: 10.1016/j.apmr.2003.03.003. [DOI] [PubMed] [Google Scholar]
- Norris SH, Watt I. The prognosis of neck injuries resulting from rear-end vehicle collisions. J Bone Joint Surg Br. 1983;65:608–611. doi: 10.1302/0301-620X.65B5.6643566. [DOI] [PubMed] [Google Scholar]
- Osti OL, Vernon-Roberts B, Fraser RD. 1990 Volvo Award in experimental studies. Anulus tears and intervertebral disc degeneration. An experimental study using an animal model. Spine. 1990;15:762–767. doi: 10.1097/00007632-199008010-00005. [DOI] [PubMed] [Google Scholar]
- Osti OL, Vernon-Roberts B, Moore R, Fraser RD. Annular tears and disc degeneration in the lumbar spine. A post-mortem study of 135 discs. J Bone Joint Surg Br. 1992;74:678–682. doi: 10.1302/0301-620X.74B5.1388173. [DOI] [PubMed] [Google Scholar]
- Panjabi MM. A hypothesis of chronic back pain: ligament subfailure injuries lead to muscle control dysfunction. Eur Spine J. 2006;15:668–676. doi: 10.1007/s00586-005-0925-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panjabi MM. The stabilizing system of the spine. Part II. Neutral zone and instability hypothesis. J Spinal Disord. 1992;5:390–396. doi: 10.1097/00002517-199212000-00002. [DOI] [PubMed] [Google Scholar]
- Panjabi MM, Ito S, Pearson AM, Ivancic PC. Injury mechanisms of the cervical intervertebral disc during simulated whiplash. Spine. 2004;29:1217–1225. doi: 10.1097/00007632-200406010-00011. [DOI] [PubMed] [Google Scholar]
- Panjabi MM, Ivancic PC, Maak TG, Tominaga Y, Rubin W. Multiplanar cervical spine injury due to head-turned rear impact. Spine. 2006;31:420–429. doi: 10.1097/01.brs.0000199940.61373.d5. [DOI] [PubMed] [Google Scholar]
- Panjabi MM, Krag M, Summers D, Videman T. Biomechanical time-tolerance of fresh cadaveric human spine specimens. J Orthop Res. 1985;3:292–300. doi: 10.1002/jor.1100030305. [DOI] [PubMed] [Google Scholar]
- Panjabi MM, Yoldas E, Oxland TR, Crisco JJ., 3rd Subfailure injury of the rabbit anterior cruciate ligament. J Orthop Res. 1996;14:216–222. doi: 10.1002/jor.1100140208. [DOI] [PubMed] [Google Scholar]
- Pearson AM, Ivancic PC, Ito S, Panjabi MM. Facet joint kinematics and injury mechanisms during simulated whiplash. Spine. 2004;29:390–397. doi: 10.1097/01.brs.0000090836.50508.f7. [DOI] [PubMed] [Google Scholar]
- Pettersson K, Hildingsson C, Toolanen G, Fagerlund M, Bjornebrink J. Disc pathology after whiplash injury. A prospective magnetic resonance imaging and clinical investigation. Spine. 1997;22:283–287. doi: 10.1097/00007632-199702010-00010. [DOI] [PubMed] [Google Scholar]
- Ronnen HR, de Korte PJ, Brink PR, van der Bijl HJ, Tonino AJ, Franke CL. Acute whiplash injury: is there a role for MR imaging?--a prospective study of 100 patients. Radiology. 1996;201:93–96. doi: 10.1148/radiology.201.1.8816527. [DOI] [PubMed] [Google Scholar]
- Siegmund GP, Myers BS, Davis MB, Bohnet HF, Winkelstein BA. Mechanical evidence of cervical facet capsule injury during whiplash: a cadaveric study using combined shear, compression, and extension loading. Spine. 2001;26:2095–2101. doi: 10.1097/00007632-200110010-00010. [DOI] [PubMed] [Google Scholar]
- Solomonow M, Zhou B, Baratta RV, Zhu M, Lu Y. Neuromuscular disorders associated with static lumbar flexion: a feline model. J Electromyogr Kinesiol. 2002;12:81–90. doi: 10.1016/s1050-6411(01)00032-3. [DOI] [PubMed] [Google Scholar]
- Solomonow M, Zhou BH, Harris M, Lu Y, Baratta RV. The ligamento-muscular stabilizing system of the spine. Spine. 1998;23:2552–2562. doi: 10.1097/00007632-199812010-00010. [DOI] [PubMed] [Google Scholar]
- Spitzer WO, Skovron ML, Salmi LR, Cassidy JD, Duranceau J, Suissa S, Zeiss E. Scientific monograph of the Quebec Task Force on Whiplash-Associated Disorders: redefining “whiplash” and its management. Spine. 1995;20:1S–73S. [PubMed] [Google Scholar]
- Stemper BD, Yoganandan N, Gennarelli TA, Pintar FA. Localized cervical facet joint kinematics under physiological and whiplash loading. J Neurosurg Spine. 2005;3:471–476. doi: 10.3171/spi.2005.3.6.0471. [DOI] [PubMed] [Google Scholar]
- Sterner Y, Gerdle B. Acute and chronic whiplash disorders--a review. J Rehabil Med. 2004;36:193–209. doi: 10.1080/16501970410030742. quiz 210. [DOI] [PubMed] [Google Scholar]
- Tominaga Y, Ndu AB, Coe MP, Valenson AJ, Ivancic PC, Ito S, Rubin W, Panjabi MM. Neck ligament strength is decreased following whiplash trauma. BMC Musculoskelet Disord. 2006;7:103. doi: 10.1186/1471-2474-7-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkelstein BA, Nightingale RW, Richardson WJ, Myers BS. The cervical facet capsule and its role in whiplash injury: a biomechanical investigation. Spine. 2000;25:1238–1246. doi: 10.1097/00007632-200005150-00007. [DOI] [PubMed] [Google Scholar]
- Yoganandan N, Pintar FA, Cusick JF. Biomechanical analyses of whiplash injuries using an experimental model. Accid Anal Prev. 2002;34:663–671. doi: 10.1016/s0001-4575(01)00066-5. [DOI] [PubMed] [Google Scholar]