Abstract
The active site A-cluster in the α subunit of the title enzyme consists of an Fe4S4 cluster coordinated to a [Nip Nid] subcomponent. The cluster must be activated for catalysis using low-potential reductants such as Ti(III) citrate. Relative to the inactive {[Fe4S4]2+ Nip2+ Nid2+} state, the activated state appears to be 2-electrons more reduced, but the location of these electrons within the A-cluster is uncertain, with {[Fe4S4]2+ Nip0 Nid2+} and {[Fe4S4]1+ Nip1+ Nid2+} configurations proposed. Recombinant apo-α subunits oligomerize after activation with NiCl2. The dimer fraction, upon reduction with excess Ti(III)citrate, exhibited Mössbauer spectra consisting of 2 quadrupole doublets representing 51% and 21% of the Fe, with parameters indicating [Fe4S4]1+ states. Spectra recorded in strong magnetic fields were typical of diamagnetic systems, indicating an exchange-coupled S = 0 {[Fe4S4]1+ Nip1+} state. Additional treatment with CO altered the doublet Mössbauer parameters, suggesting an interaction with CO, but maintaining the cluster in the {[Fe4S4]1+ Nip1+} state. Reduction with sub-stoichiometric equivalents of Ti(III) citrate afforded an EPR signal typical of Ni1+ ions, with g∥ = 2.10 and g⊥ = 2.02. Addition of more Ti caused the signal intensity to decline, suggesting that it arises from the semi-reduced {[Fe4S4]2+ Nip1+} state.
Acetyl-coenzyme A synthase/carbon monoxide dehydrogenase is an α2β2 tetramer, found in anaerobic archaea and bacteria that grow chemoautotrophically on CO or CO2.1 The active site A-cluster in the synthase α subunit consists of an Fe4S4 cluster coordinated through a bridging cysteinate to a [Nip Nid] subcomponent (Chart 1).2 The oxidized state of the cluster assembly, Aox, can be formulated as {[Fe4S4]2+ Nip2+ Nid2+}.3-5 Aox does not accept, in the presumed first catalytic step, a methyl cation from the corrinoid-iron-sulfur protein (CH3-Co3+FeSP).6 However, methyl transfer occurs when Aox is treated with low-potential reductants such as Ti(III) citrate. The electronic configuration for the reductively activated Ared-act has not been established but the state is probably 2 electrons more reduced than Aox.4 Reduction of the square-planar distal Nid2+ with amide N coordination is unlikely,7 and stopped-flow evidence disfavors the reduction of the [Fe4S4]2+ cluster,6 leaving the proximal Nip2+ as the only site that might accept electrons. Attainment of a Nip0 state has been suggested,5 and this idea is supported by a report that a Ni0 phosphine complex can accept a methyl cation from a CH3-Co3+ complex.8 However, independent DFT studies by Brunold and Field argue against this suggestion and support a {[Fe4S4]1+ Nip1+} configuration for Ared-act.9, 10 This hypothetical state was not observed in our previous Mössbauer study where it was found that Ti(III) citrate-reduced Ni-activated recombinant α subunits exhibited two major species: ∼ 70 % of spectral intensity originated from {[Fe4S4]1+ Nip2+} centers while ∼ 30 % reflected a diamagnetic state containing an [Fe4S4]2+ moiety.4 Given that the enzyme is known to be heterogeneous, with ∼ 30 % functional and ∼ 70 % nonfunctional A-centers,11 this result was consistent with a {[Fe4S4]2+ Nip0} state in functional subunits.
Chart 1.
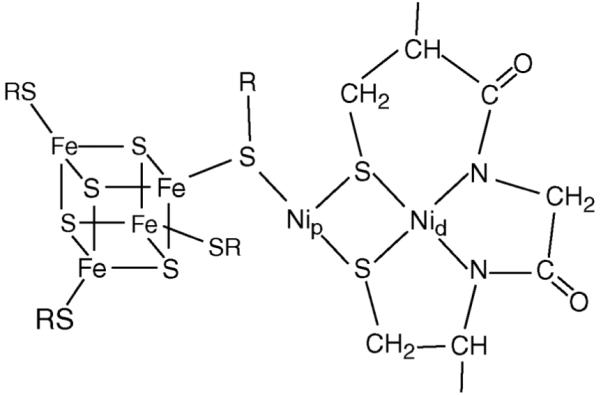
Structure of the A-cluster. SR represents cysteinyl residues.
We recently altered our procedure for preparing recombinant α subunit by adding NiCl2 to purified apo-α subunit (containing the Fe4S4 cluster but lacking the Ni subcomponent) rather than including it in the growth medium.11 Gel filtration FPLC of Ni-activated α subunits indicated Ni-dependent oligomerization into dimers and other forms.12 Here we report Mössbauer evidence that α dimer samples prepared in this manner, when reduced by Ti(III) citrate, contain A-clusters in the {[Fe4S4]1+ Nip1+} state. We also report a new EPR signal that appears to arise from the semi-reduced {[Fe4S4]2+ Nip1+} state.
The 4.2 K Mössbauer spectrum of Ni-activated α dimers reduced with Ti(III) citrate (Figure 1A) exhibits three components. A paramagnetic component (≈ 20-30 % of Fe), extending from −2 mm/s to +2 mm/s Doppler velocity, most probably belongs to {[Fe4S4]1+ Nip2+} clusters. The central portion of the spectrum exhibits two doublets (1 and 2) with ΔEQ1 = 1.21(4) mm/s, δ1 = 0.56(2) mm/s, 51(3) % of Fe, and ΔEQ2 = 0.47(2) mm/s, δ2 = 0.55(2) mm/s, 21(2) %. The relative intensities of 1 and 2 are ≈ 2.4:1 rather than 2:2 or 3:1 as one might expect if doublets 1 and 2 would represent subsites in one [Fe4S4] cluster. The [Fe4S4]2+ cubane of the A-cluster has δ = 0.45(2) mm/s in all states examined,3 while [Fe4S4]1+ clusters typically have an average δ ∼ 0.54-0.59 mm/s.13, 14 The Sc = ½ [Fe4S4]1+ cluster of apo-α has δ = 0.55 mm/s and ΔEQ = 0.92 mm/s (Figure S1). Thus, the values for δ1 and δ2 indicate the [Fe4S4]1+ state.
Figure 1.

4.2 K Mössbauer spectra of α dimer taken in applied fields as indicated. (A) Spectrum of 1 mM Ti(III) citrate-reduced α dimer. Red solid line is a superposition of doublets 1 and 2, using the parameters quoted in the text; blue and green solid lines show doublets separately. (B) Spectrum of (A) after removing the line width contribution of the 57Co source by a Fourier deconvolution. (C) Spectrum of 1 mM Ti(III) citrate-reduced α dimer after exposure to CO. The solid line is a simulation for doublets 3 and 4 superimposed on a simulation for the Ared-CO species (dashed line above data, using published parameters3). (D) 7.0 T spectrum (parallel applied field) of the sample of (A) after removal of a 25 % contribution of apo-α. The solid line is a spectral simulation assuming that 1 and 2 are diamagnetic; raw data and all parameters are given in Supplementary Information.
Magnetically isolated Sc = ½ [Fe4S4]1+ clusters generally exhibit paramagnetic hyperfine structure at 4.2 K (e.g. Figure S1), reflecting hyperfine fields up to ≈ 12 T. The observation of doublets 1 and 2 at 4.2 K suggests A-clusters with integer or zero electronic cluster spin. This argument is supported by spectra recorded in applied fields of 0.1 T, 8 T and 7.0 T (Figure 1D), which show that 1 and 2 belong to diamagnetic A-clusters, strongly suggesting exchange coupling between an Sc = ½ [Fe4S4]1+ cluster and an SNi = ½ Nip1+ site.
Figure 1C shows a 4.2 K spectrum of Ti (III) citrate-reduced α dimers after incubation with CO. This treatment produces the S = ½ NiFeC signal, the signature of the exchange coupled, {[Fe4S4]2+ Nip1+-CO}, Ared-CO state.3, 15 In one preparation, we obtained by EPR 0.39(4) spins/α together with the corresponding Mössbauer component, representing 34(3) % of the Fe. For a second preparation (Figure 1C) 31(2) % of the A-clusters were in the Ared-CO state (dashed line drawn above Figure 1C). The remainder of the sample exhibits two doublets, 3 and 4, again reflecting [Fe4S4]1+ clusters albeit with parameters that differ from the Ti(III) citrate-reduced sample: ΔEQ3 = 0.96 mm/s, δ3 = 0.51 mm/s, 42(3) % of Fe, and ΔEQ4 = 1.88 mm/s, δ4 = 0.60 mm/s, 21(2) %. Interestingly, addition of CO changed all spectral components. We speculate that 3 and 4 reflect a {[Fe4S4]1+ Nip1+-CO} cluster.16 The data of Figure 1C also imply that all α subunits in the sample have the proximal site occupied by Ni. Studies at 8.0 T applied field (Figure S3) show that 3 (and probably 4) originates from a diamagnetic site.
The fractions quoted for 1,2 and 3,4 do not reveal whether doublets 1,2 (and 3,4) belong to the same cluster; we defer this problem to future studies. Here we discuss aspects of the electronic structure of the A-cluster implied by the observation of diamagnetic species with δ ≈ 0.55-0.60 mm/s, using 1 as an example.
[Fe4S4]1+ clusters occur in various spin states, mostly with Sc = ½ and 3/2. We ignore Sc = 3/2 configurations here as they do not yield a diamagnetic ground state for {[Fe4S4]1+ Nip1+}. Frequently, the Sc = ½ ground state of [Fe4S4]1+ clusters yields a diferrous and mixed-valence pair, distinguishable by their ΔEQ and δ values.13 However, some [Fe4S4]1+ clusters,17 like that of apo-α of Figure S1, have the same ΔEQ and δ for all sites. The exchange-coupling pathway between the Sc = ½ [Fe4S4]1+ cluster and the SNi = ½ Nip1+ involves the cysteinate that links one cluster site, previously labeled FeD, to the Ni1+ (ℋ = JSD•SNi, where SD is the local spin of cluster site D).3 The J-term acts in first-order between the two S = ½ ground manifolds and mixes in second-order excited [Fe4S4]1+ cluster states into the ground manifold. The second-order term is relevant for understanding the Ared-CO state3 but we ignore it here. Coupling then yields a singlet (A-cluster spin S = 0) and a triplet state, split by KDJ, where KD is a spin projection factor (|KD| ≤ 1.5 18) that depends on the internal coupling of the [Fe4S4]1+ cluster and the nature of FeD. For KDJ > 0 the ground state is diamagnetic. The solid line in Figure 1D is a spectral simulation assuming that 1 and 2 are diamagnetic.
In a series of preliminary experiments we observed a new EPR signal when samples of Ni-activated α were treated with sub-stoichiometric amounts of Ti(III) citrate. This axial signal (Figure 2A) has g∥ = 2.10 and g⊥ = 2.02, values typical of Ni1+ species. The intensity of the axial signal increases as Ti(III) citrate is initially added (Figure 2B) and maximizes at ∼ 0.2 equivalents/α of Ti(III) citrate (See also Supplementary Information). The qualitative behavior of the titration plot suggests that the signal originates from a semi-reduced S = ½ {[Fe4S4]2+ Nip1+} state which is then further reduced to the {[Fe4S4]1+ Nip1+} state.
Figure 2.
(A) EPR spectrum of Ni-activated α dimer reduced with 0.2 Ti(III) citrate/α monomer. Conditions: 9.482 GHz; 1 mT modulation; T = 10 K. (B) Plot of EPR signal intensity (amplitude of derivative feature) versus amount of Ti(III) citrate added.
After the Mössbauer spectra were collected, the two samples of Figure 1 were analyzed for their ability to accept a methyl group from CoFeSP. In each case, 0.3 methyl groups per α were transferred, indicating that only ∼ 30 % of the α subunits in these samples arise from the functional Ared-act state.
In summary, our results demonstrate that Ti(III) citrate can reduce the isolated Ni-activated α subunit of the title enzyme to form the {[Fe4S4]1+ Nip1+} state, and what appears to be the semi-reduced {[Fe4S4]2+ Nip1+} state. The former state has been suggested by DFT calculations, and the latter has been proposed as a catalytic intermediate1 but here we provide the first experimental evidence for the existence of these states. Although further studies are required to reveal which of the Mössbauer spectral components exhibited by the Ti(III) citrate-treated sample represent the Ared-act state, binding of a methyl cation to {[Fe4S4]1+ Nip1+} could result, after internal electron transfer from the [Fe4S4]1+ moiety to Nip, in the methyl-bound state {[Fe4S4]2+ Nip2+-CH3}.4
Supplementary Material
Acknowledgment
This work was supported by National Institutes of Health grant GM46441 (PAL) and the National Science Foundation grant MCB-042 4494 (EM).
Supporting Information: Sample preparations. Mössbauer and EPR spectra of apo - α. Details of Mössbauer analyses and comments on exchange coupling. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Ragsdale SW. J. Inorg. Biochem. 2007;101:1657–1666. doi: 10.1016/j.jinorgbio.2007.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Darnault C, Volbeda A, Kim EJ, Legrand P, Vernede X, Lindahl PA, Fontecilla-Camps JC. Nat. Struct. Biol. 2003;10:271–279. doi: 10.1038/nsb912. [DOI] [PubMed] [Google Scholar]
- 3.Xia J, Hu Z, Popescu CV, Lindahl PA, Münck E. J. Am. Chem. Soc. 1997;119:8301–8312. [Google Scholar]
- 4.Bramlett MR, Stubna A, Tan X, Surovtsev IV, Münck E, Lindahl PA. Biochemistry. 2006;45:8674–8685. doi: 10.1021/bi060003+. [DOI] [PubMed] [Google Scholar]
- 5.Lindahl PA. J. Biol. Inorg. Chem. 2004;9:516–524. doi: 10.1007/s00775-004-0564-x. [DOI] [PubMed] [Google Scholar]
- 6.Tan X, Sewell C, Yang Q, Lindahl PA. J. Am. Chem. Soc. 2003;125:318–319. doi: 10.1021/ja028442t. [DOI] [PubMed] [Google Scholar]
- 7.Kruger HJ, Peng G, Holm RH. Inorg. Chem. 1991;30:734–742. [Google Scholar]
- 8.Eckert NA, Dougherty WG, Yap GPA, Riordan CG. J. Am. Chem. Soc. 2007;129:9286–9287. doi: 10.1021/ja072063o. [DOI] [PubMed] [Google Scholar]
- 9.Schenker RP, Brunold TC. J. Am. Chem. Soc. 2003;125:13962–13963. doi: 10.1021/ja037893q. [DOI] [PubMed] [Google Scholar]
- 10.Amara P, Volbeda A, Fontecilla-Camps JC, Field MJ. J. Am. Chem. Soc. 2005;127:2776–2784. doi: 10.1021/ja0439221. [DOI] [PubMed] [Google Scholar]
- 11.Loke H-K, Tan X, Lindahl PA. J. Am. Chem. Soc. 2002;124:8667–8672. doi: 10.1021/ja025924w. [DOI] [PubMed] [Google Scholar]
- 12.Tan X, Kagiampakis I, Surovtsev IV, Demeler B, Lindahl PA. Biochemistry. 2007;46:11606–11613. doi: 10.1021/bi7014663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lindahl PA, Day EP, Kent TA, Orme-Johnson WH, Munck E. J. Biol. Chem. 1985;260:11160–11173. [PubMed] [Google Scholar]
- 14.Bertini I, Ciurli S, Luchinat C. Struct. Bond. 1995;83:1–53. [Google Scholar]
- 15.Ragsdale SW, Wood HG, Antholine WE. PNAS. 1985;82:6811–6814. doi: 10.1073/pnas.82.20.6811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.The effect of CO is rather complex: for our earlier preparations we observed that adding CO caused oxidation of [Fe4S4]1+ clusters to the 2+ state, see ref. 3 and 4.
- 17.Ragsdale SW, Lindahl PA, Münck E. J. Biol. Chem. 1987;262:14289–14297. [PubMed] [Google Scholar]
- 18.Noodleman L. Inorg. Chem. 1991;30:246–256. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



