Abstract
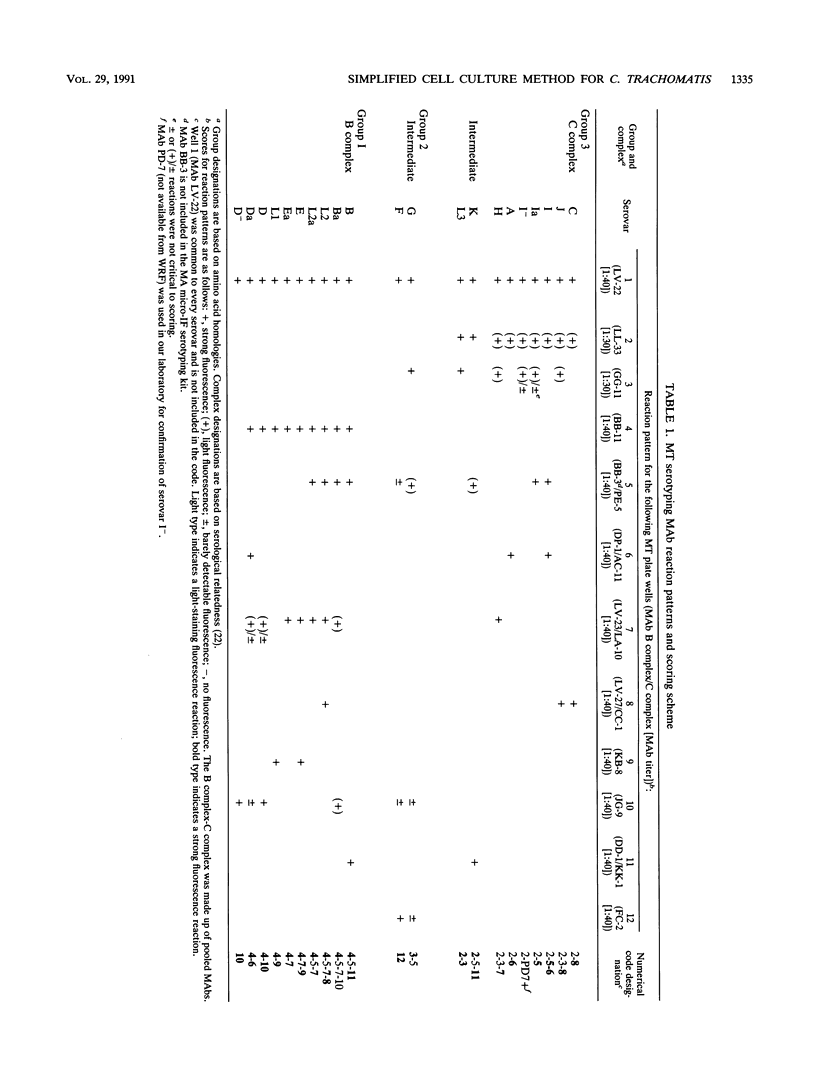
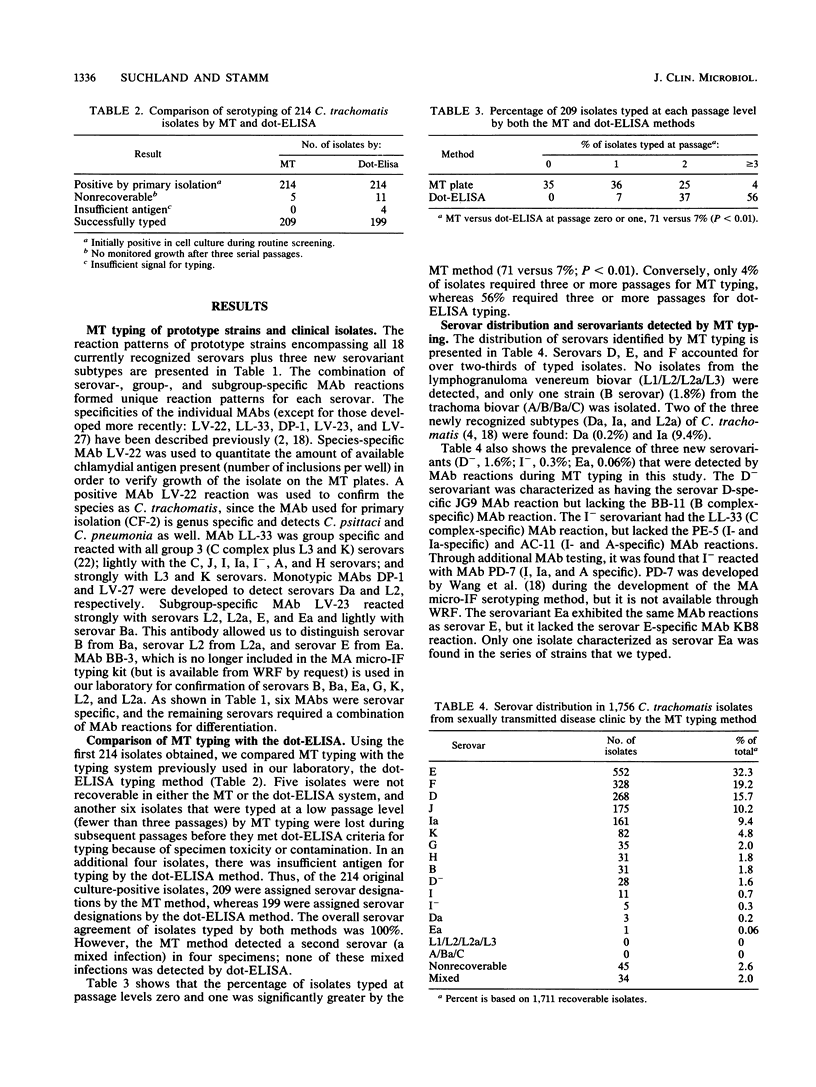
Serotyping of Chlamydia trachomatis strains usually requires three to six serial passages in shell vials to attain sufficient antigen for typing procedures. To circumvent this problem, we developed a rapid low-passage method for serotyping of C. trachomatis clinical isolates. Isolates with an inclusion count of greater than or equal to 500 per well in primary isolation were inoculated directly onto cell culture monolayers in microtiter plates for typing. Primary isolates with a lower initial inclusion count were passed one to two times in shell vials until there were greater than or equal to 20 inclusions per well and were then inoculated onto plates for typing. Inclusions were grown to maturity and reacted with a panel of 17 C. trachomatis-specific monoclonal antibodies in pools. Wells were then reacted with a fluorescein isothiocyanate conjugate and read by FA microscopy, and the reaction patterns were compared with prototype strain reaction patterns to determine the serotype. By the microtiter method, we successfully typed 1,711 consecutive C. trachomatis isolates; 1,215 isolates (71%) were typed with no or with one passage. The first 209 isolates typed by the microtiter method were also typed by the dot-enzyme-linked immunosorbent assay serotyping method; 100% agreement was demonstrated among strains that were typeable by both methods. We conclude that the microtiter method is extremely useful for accurate serotyping of large numbers of isolates and requires greatly reduced technician time.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnes R. C., Suchland R. J., Wang S. P., Kuo C. C., Stamm W. E. Detection of multiple serovars of Chlamydia trachomatis in genital infections. J Infect Dis. 1985 Nov;152(5):985–989. doi: 10.1093/infdis/152.5.985. [DOI] [PubMed] [Google Scholar]
- Barnes R. C., Wang S. P., Kuo C. C., Stamm W. E. Rapid immunotyping of Chlamydia trachomatis with monoclonal antibodies in a solid-phase enzyme immunoassay. J Clin Microbiol. 1985 Oct;22(4):609–613. doi: 10.1128/jcm.22.4.609-613.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batteiger B. E., Lennington W., Newhall W. J., Katz B. P., Morrison H. T., Jones R. B. Correlation of infecting serovar and local inflammation in genital chlamydial infections. J Infect Dis. 1989 Aug;160(2):332–336. doi: 10.1093/infdis/160.2.332. [DOI] [PubMed] [Google Scholar]
- Howard L., Orenstein N. S., King N. W. Purification on renografin density gradients of Chlamydia trachomatis grown in the yolk sac of eggs. Appl Microbiol. 1974 Jan;27(1):102–106. doi: 10.1128/am.27.1.102-106.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo C. C., Wang S. P., Holmes K. K., Grayston J. T. Immunotypes of Chlamydia trachomatis isolates in Seattle, Washington. Infect Immun. 1983 Aug;41(2):865–868. doi: 10.1128/iai.41.2.865-868.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köhler G., Milstein C. Derivation of specific antibody-producing tissue culture and tumor lines by cell fusion. Eur J Immunol. 1976 Jul;6(7):511–519. doi: 10.1002/eji.1830060713. [DOI] [PubMed] [Google Scholar]
- Newhall W. J., 5th, Terho P., Wilde C. E., 3rd, Batteiger B. E., Jones R. B. Serovar determination of Chlamydia trachomatis isolates by using type-specific monoclonal antibodies. J Clin Microbiol. 1986 Feb;23(2):333–338. doi: 10.1128/jcm.23.2.333-338.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens R. S., Kuo C. C., Tam M. R. Sensitivity of immunofluorescence with monoclonal antibodies for detection of Chlamydia trachomatis inclusions in cell culture. J Clin Microbiol. 1982 Jul;16(1):4–7. doi: 10.1128/jcm.16.1.4-7.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens R. S., Tam M. R., Kuo C. C., Nowinski R. C. Monoclonal antibodies to Chlamydia trachomatis: antibody specificities and antigen characterization. J Immunol. 1982 Mar;128(3):1083–1089. [PubMed] [Google Scholar]
- WANG S. P., GRAYSTON J. T. CLASSIFICATION OF TRACHOMA VIRUS STRAINS BY PROTECTION OF MICE FROM TOXIC DEATH. J Immunol. 1963 Jun;90:849–856. [PubMed] [Google Scholar]
- Wagenvoort J. H., Suchland R. J., Stamm W. E. Serovar distribution of urogenital Chlamydia trachomatis strains in The Netherlands. Genitourin Med. 1988 Jun;64(3):159–161. doi: 10.1136/sti.64.3.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S. P., Kuo C. C., Barnes R. C., Stephens R. S., Grayston J. T. Immunotyping of Chlamydia trachomatis with monoclonal antibodies. J Infect Dis. 1985 Oct;152(4):791–800. doi: 10.1093/infdis/152.4.791. [DOI] [PubMed] [Google Scholar]
- Wang S. P., Kuo C. C., Grayston J. T. A simplified method for immunological typing of trachoma-inclusion conjunctivitis-lymphogranuloma venereum organisms. Infect Immun. 1973 Mar;7(3):356–360. doi: 10.1128/iai.7.3.356-360.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoder B. L., Stamm W. E., Koester C. M., Alexander E. R. Microtest procedure for isolation of Chlamydia trachomatis. J Clin Microbiol. 1981 Jun;13(6):1036–1039. doi: 10.1128/jcm.13.6.1036-1039.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Y., Zhang Y. X., Watkins N. G., Caldwell H. D. Nucleotide and deduced amino acid sequences for the four variable domains of the major outer membrane proteins of the 15 Chlamydia trachomatis serovars. Infect Immun. 1989 Apr;57(4):1040–1049. doi: 10.1128/iai.57.4.1040-1049.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y. X., Stewart S., Joseph T., Taylor H. R., Caldwell H. D. Protective monoclonal antibodies recognize epitopes located on the major outer membrane protein of Chlamydia trachomatis. J Immunol. 1987 Jan 15;138(2):575–581. [PubMed] [Google Scholar]


