Abstract
The hypothalamic-pituitary-adrenal/interrenal axis couples serotonergic activity in the brain to the peripheral regulators of energy balance and response to stress. The regulation of peripheral systems occurs largely through the release of peptide hormones, especially the melanocortins (adrenocorticotropic hormone [ACTH] and alpha melanocyte stimulating hormone [α–MSH]), and beta-endorphin. Once in circulation, these peptides regulate a wide range of processes; α–MSH in particular regulates behaviors and physiologies with sexual and social functions. We investigated the role of the HPI and melanocortin peptides in regulation of electric social signals in the gymnotiform electric fish, Brachyhypopomus pinnicaudatus. We found that corticotropin releasing factor, thyrotropin-releasing hormone, and α–MSH, three peptide hormones of the HPI/HPA, increased electric signal waveform amplitude and duration when injected into free-swimming fish. A fourth peptide, a synthetic cyclic-α–MSH analog attenuated the normal circadian and socially-induced EOD enhancements in vivo. When applied to the electrogenic cells (electrocytes) in vitro, only α–MSH increased the amplitude and duration of the electrocyte discharge similar to the waveform enhancements seen in vivo. The cyclic-α–MSH analog had no effect on its own, but blocked or attenuated α–MSH-induced enhancements in the single-cell discharge parameters, demonstrating that this compound functions as a silent antagonist at the electrocyte. Overall, these results strongly suggest that the HPI regulates the EOD communication signal, and demonstrate that circulating melanocortin peptides enhance the electrocyte discharge waveform.
The melanocortin peptides serve a broad range of functions in the central nervous system and the periphery (Hadley and Haskell-Luevano, 1999). This family of peptides includes adrenocorticotropic hormone (ACTH) and alpha-, beta-, and gamma-melanocyte stimulating hormones (MSHs), which bind with varying affinities to five G-protein coupled melanocortin receptors (MC1R–MC5R). Among the melanocortin peptides, ACTH and α–MSH play prominent roles in the periphery when released from the pituitary into general circulation. While α–MSH does not induce cortisol release in most vertebrates, it nonetheless regulates key peripheral processes including energy homeostasis, melanocyte dispersion, melanin synthesis, exocrine activity, inflammation, sexual receptivity, sexual performance, and sexual signaling (rev. Strand, 1999). Alpha –MSH appears to be a key regulator of vertebrate social signals, such as aggression pheromones in mice (Morgan, Thomas and Cone, 2004) and socially-induced skin darkening response in subordinate Arctic charr (Hoglund et al., 2000). Expanding the suite of known social signal functions for melanocortins, we have shown that melanocortin peptides are capable of regulating the electric navigation/communication signals in some electric fish by modulating the activity of the excitable cells that produce the electric signals (Markham and Stoddard, 2005).
The electric organ discharge (EOD) of weakly electric gymnotiform fish is a dual-purpose signal used to navigate and communicate in total darkness. The EOD is a model system particularly well suited for investigating neuroendocrine control of communication signals in response to social stimuli (Stoddard et al., 2006). The EOD is produced by electrocytes, specialized excitable cells in the peripheral electric organ. The EOD waveform of Brachyhypopomus pinnicaudatus is a biphasic sinusoidal pulse that varies in amplitude and in the duration of the second phase. Dynamic variation in EOD waveform is readily observed between and among individuals on a circadian rhythm, and in response to social and environmental stimuli (Franchina et al., 2001; Stoddard et al., 2007). Social encounters and environmental stimuli modulate the EOD waveform by altering the membrane biophysics and discharge waveforms of the electrocytes, resulting in either an enhanced or diminished waveform (Franchina et al., 2001; Hagedorn and Zelick, 1989).
We have shown that peripheral injections of serotonin (5-HT) or the melanocortin peptide adrenocorticotropic hormone (ACTH) modulate the EOD waveform in a manner similar to social encounters in B. pinnicaudatus, but only ACTH acts directly on electrocytes to modify their discharge waveforms (Allee et al., 2008; Markham and Stoddard, 2005; Stoddard et al., 2003). Serotonin’s lack of direct effect on the electrocytes led us to hypothesize that 5-HT modulates the EOD indirectly, acting centrally to stimulate the release of melanocortin peptides into peripheral circulation. We proposed that the hypothalamic-pituitary-interrenal (HPI) axis is the circuit connecting central serotonin activity and modulation of signals emitted by the electrocytes in the periphery.
In teleost fishes, 5-HT interacts specifically with 5HT1A and 5HT2A/2C receptors in the hypothalamus and/or pituitary to stimulate the HPI axis, inducing the biosynthesis and release of corticotropin-releasing factor (CRF) and thyrotropin-releasing hormone (TRH) (Calogero et al., 1990; Calogero et al., 1993; Contesse et al., 2000; Hoglund et al., 2002; Larsen et al., 1998; Van de Kar et al., 2001; Winberg et al., 1997). These peptidergic hormones then stimulate pituitary corticotropes and melanotropes to release into circulation ACTH and α–MSH, respectively (Rotllant et al., 2000). The presence of ACTH in turn stimulates the production and release of glucocorticosteroids (cortisol), adjusting peripheral responses to stressors. Alpha–MSH may act as an alternate corticotrope in some teleosts, but this function for α–MSH is not universal and its taxonomic range is unknown (Lamers et al., 1992; Lamers et al., 1994; Metz et al., 2005). Glucocorticosteroids, the terminal products of the HPI cascade, regulate situation-appropriate behaviors and promote the allocation and distribution of energy required to sustain those behaviors. This connection between brain 5-HT and cortisol production defines the HPI as a key neuroendocrine pathway connecting central brain serotonergic activity and the expression of behaviors within social contexts, particularly in those where dominance and subordination are expressed. Responsiveness of the EOD waveform to social environment is consistent with the hypothesis that 5-HT is involved in producing contextually appropriate changes seen in the EOD waveform via activation of the HPI.
Our objective in this study was to investigate the role of the HPI in regulation of electric social signals. Based on the direct actions of ACTH on the electrocytes, we predicted that α–MSH, the other dominant melanocortin, and CRF and TRH, the upstream hypothalamic secretagogues of endogenous pituitary melanocortins, should also augment the waveform by acting in the pituitary to release melanocortins into the periphery. We tested the ability of these peptide hormones to modulate the EOD waveform in vitro, applied to electrocytes in a dish, and in vivo by peripheral injection into free-swimming fish. All three peptides modulated the EOD waveform in vivo, but only α–MSH modulated the electrocyte discharge. We also found that a fourth peptide, a synthetic cyclic-MSH analog, inhibits circadian and socially-induced EOD enhancements in vivo, and blocks the effects of α–MSH on the electrocyte discharge in vitro. We conclude from these data that the HPI mediates EOD enhancements by regulating release of peripheral melanocortins, and that circulating melanocortin peptides contribute to EOD modulation by enhancing the electrocyte discharge.
Materials and Methods
Animals
We used captive-bred adult male Brachyhypopomus pinnicaudatus from colonies maintained at Florida International University (Miami, FL) and The University of Texas at Austin. Experiments were approved in advance by the FIU IACUC and the UT IACUC, and complied with the “Principles of Animal Care” publication No. 86-23, revised 1985, of the National Institutes of Health.
Solutions and Reagents
The normal saline for injections and in vitro recordings contained (in mM): 114 NaCl, 2 KCl, 4 CaCl2•2H20, 2 MgCl2•6H20, 5 HEPES, 6 glucose; pH to 7.2 with NaOH. We purchased thyrotropin releasing hormone (TRH), corticotropin releasing factor (CRF), and alpha-melanocyte stimulating hormone (α–MSH) from Sigma-Aldrich (St. Louis, MO). We obtained the cyclic-α–MSH analog, [Ac-Cys4,DPhe7,Cys10] α–MSH(4-13)-amide (hereafter cyclic-MSHa), from American Peptide (Sunnyvale, CA). All peptides were dissolved in water at a stock concentration of 100 μM, stored in single use aliquots at −20 C, then thawed and diluted in saline to working concentrations immediately before use.
EOD Recordings, Injections, and Social Challenges
Our system for recording calibrated EODs from freely swimming fish and procedures for injecting fish are described in detail elsewhere (Stoddard et al., 2003). Fish were placed in an automated measurement tank, 120 × 44 × 44 cm, located in a light- and temperature-controlled room on a 12L:12D light cycle. EODs were amplified and digitized from carbon electrodes at opposite ends of the tank only when the fish passed through or was resting in an unglazed ceramic tube centered between the recording electrodes. EODs were recorded at intervals of ~1 min round the clock. We recorded baseline EODs for ~24 h before performing injections or social challenges.
We prepared the injection solutions to produce the desired dose when injected intramuscularly at 1 ul g−1 body weight (bw). Saline injections (1 ul g−1 bw) served as a control condition for handling and injection effects. We selected dosages for each of the pharmacological compounds based our our earlier experiments
All injections of CRF, TRH, and α–MSH were given midday (12:00–15:00). We selected dosages for these compounds based on our previous finding (Stoddard et al., 2003) that EOD response to serotonin saturates at 2.5 nM g−1 body weight (bw). Accordingly, the dosages of CRF and TRH were 2.5 nM g−1 and α–MSH was delivered at a dose of 3 nM g−1 bw. We injected cyclic-MSHa at a much higher dose (10 nM g−1 bw) to allow this compound to compete with the endogenous melanocortin ligands. Fish were quickly netted from the recording tank, injected in the hypaxial muscle, and then returned to the recording tank where EOD recordings continued at ~1 min intervals. Handling time from capture to replacement in the tank was usually less than 30 s.
To determine involvement of endogenous melanocortins with waveform enhancement that accompanies social encounters (Franchina et al., 2001) we initiated social challenges midday (13:00) when EOD parameters are low. We injected the focal fish with cyclic-MSHa or saline control, returned it to its ceramic hiding/recording tube. Within 15 min we added a second male fish from the breeding colony to the same hiding tube, blocking the ends with polyester filter fiber to keep the fish from relocating. After 45 minutes the challenger was removed while the focal fish remained in the recording tank to record ongoing changes in its waveform. Individuals were distinguished by 5–10% differences in body length. Their waveform recordings were distinguished by continuity from the time before the 2nd fish was added. In one of six trials, the waveform amplitude recordings were confounded by the presence of the 2nd fish and the trial was deleted in the final analysis. To determine effects of endogenous melanocortins on the evening rise, we injected focal fish 50–60 min before the normal lights-out time with cyclic-MSHa or saline control, then recorded data for an additional 5 hours. For both social and evening rise effects, each of the 6 male subjects served as its own control, with half of the individuals receiving cyclic-MSHa and half receiving saline. Injection dates were spaced two days apart to allow recovery from treatment. Amplitude recordings for one of the evening rise trials were removed from the final analysis due to excessive missing data points.
Electrophysiology
We recorded the discharges of single electrocytes (μEODs) and electrocyte action potentials (APs) as described in detail elsewhere (Markham and Stoddard, 2005). Electrocytes of B. pinnicaudatus are large disc-shaped cells (approx. 250μm width × 800μm diameter) innervated at the apex of the posterior membrane (Hopkins et al., 1990) (Fig. 1). Both the posterior- and anterior-face membranes are excitable and the sequential APs of these two excitable membranes in series produce the μEOD (Bennett, 1961). The nerve initiates the μEOD, which is produced by sequential APs: the posterior face spikes first (AP1), followed within 80 μs by a spike from the non-innervated anterior face (AP2). These two APs sum to produce the biphasic μEOD recorded differentially across the whole cell. The single-membrane APs are typical monophasic spikes, while the μEOD is a biphasic sinusoidal discharge that resembles the EOD.
Figure 1.
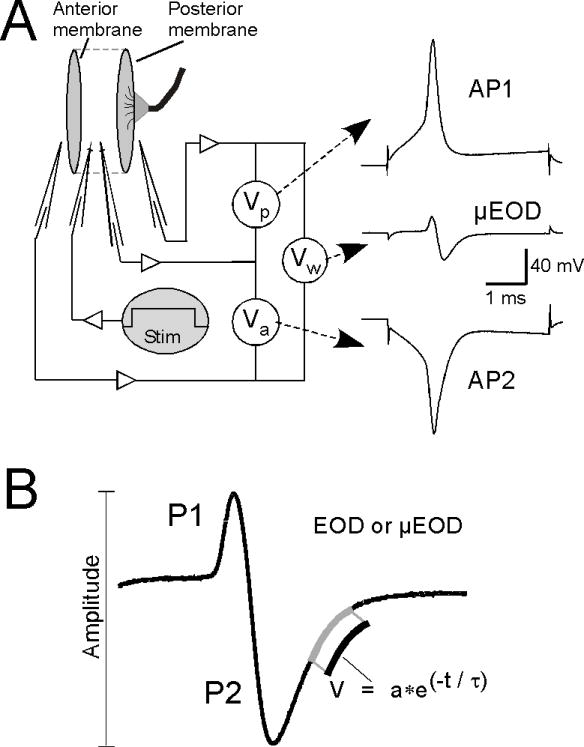
Recording of electrocyte action potentials and μEODs and analysis of EOD waveform parameters. A) We delivered intracellular current steps to electrocytes in vitro to elicit μEODs while simultaneously recording voltage from one intracellular and two extracellular micropipettes. Sequential action potentials on the innervated posterior face (AP1) and the noninnervated anterior face (AP2) of the electrocyte sum to produce the biphasic μEOD. Potentials are recorded from one intracellular micropipette and two extracellular pipettes. Offline subtraction of the posterior extracellular record from the intracellular record yields the posterior-membrane voltage (Vp) and AP1, the posterior-face action potential. Subtraction of the anterior extracellular record from the intracellular record yields the anterior-membrane voltage (Va) and AP2, the anterior-face action potential. Subtraction of the posterior extracellular record from the anterior extracellular record yields the whole-cell voltage (Vw) and the μEOD. B) EOD and μEOD amplitude was measured peak-to-peak. The time constant of the second phase (P2) repolarization, τP2, was estimated by exponential fit to the repolarization segment of P2.
We cut short sections (1.0 – 1.5 cm) of the tail filament, removed the skin on one side to expose the cells of the electric organ, and pinned the tissue in a Sylgard-coated recording chamber (800 μl total volume) containing normal saline at room temperature (23 ± 1°C).
We recorded AP1, AP2, and the μEOD with multi-electrode current clamp procedures detailed elsewhere (Bennett, 1961; Markham and Stoddard, 2005). A depolarizing current step passed through one intracellular pipette initiates the μEOD. A second intracellular pipette records intracellular potential, and two extracellular pipettes placed within 50 μm of the anterior and posterior membranes yield recordings of the extracellular anterior and posterior potentials. Offline subtraction of the posterior extracellular record from the intracellular record yields AP1, subtraction of the anterior extracellular record from the intracellular record yields AP2, and subtraction of the posterior extracellular record from the anterior extracellular record yields the μEOD.
Sharp micropipettes were pulled from thin wall borosilicate glass to resistances of 0.8–1.2 MΩ when filled with 3M KCl. Extracellular pipettes were broken to resistances of 400–600 KΩ filled with normal saline. For intracellular stimulation and recording we used an Axoclamp 900A amplifier (Molecular Devices, Union City, CA) in current clamp mode. Extracellular recordings were made using both channels of a Dagan TEV200A amplifier (Dagan Corp., Minneapolis, MN) in current clamp mode. We controlled the experiment and acquired data at 125 KHz with a Digidata 1440 digitizer and pClamp 10 software (Molecular Devices, Union City, CA). Data were analyzed with custom-developed code and resident functions in MATLAB (The Mathworks Inc, Natick, MA).
Recording and Perfusion
We recorded only from cells with stable resting potentials (Vrest < −90 mV) and stable input resistances. After placement of all electrodes, depolarizing current steps of 6 ms were delivered and the current magnitude was adjusted manually until the current step reliably elicited the μEOD. We then elicited μEODs at 60 s intervals for the remainder of each experiment. The recording chamber was perfused at a rate of ~5 ml/h with normal saline or with saline containing one of the test compounds. We changed solutions during the interstimulus interval by rapidly perfusing 5 ml of the new solution then slowing perfusion of the new solution to the normal flow rate of 5 ml/h. We first recorded at least 20 min of baseline data in normal saline before changing the solution to one of the test solutions. Saline controls were exposed to continued perfusion of normal saline.
Data Treatment and Analysis
The calibrated EOD recorded in vivo is a biphasic sinusoidal pulse (Fig. 1) that varies in amplitude and duration (Franchina and Stoddard, 1998), with duration changing primarily in the second phase (Hopkins et al., 1990). We analyzed in vivo EODs as described previously (Stoddard et al., 2003; Stoddard et al., 2007). We measured amplitude peak-to-peak and measured duration of P2 as the repolarization time constant of the second phase (P2) by fitting an exponential curve to the P2 repolarization segment from 50% amplitude to 5% amplitude (hereafter τP2).
We measured amplitude and τP2 of μEODs recorded in vitro as done for the EOD in vivo. Amplitudes of AP1 and AP2 were measured from peak to resting potential. We also measured AP spike width at half-amplitude (half-width), and the delay between AP1 and AP2 (AP1-AP2 delay) as the interval between the peak of AP1 and the peak of AP2.
Changes in the EOD waveform resulting from in vivo injections of CRF, TRH, and α–MSH are superimposed upon endogenous circadian cycles in waveform parameters (Stoddard et al., 2007). We therefore mathematically isolated drug-induced changes in these measures as reported previously (Stoddard et al., 2003). This analysis allowed us to extract the changes in EOD amplitude and τP2that were caused by the challenge trials and not a function of the normal circadian modulations in EOD. Consistent with previous reports, we quantified EOD amplitude and τP2 responses as the peak increase in each waveform parameter within 2h following injection (Stoddard et al., 2003). Statistical analyses and data plotting were performed with MATLAB (Mathworks, Natick, MA) or Prism (Graphpad, La Jolla, CA). Averaged data are reported as mean ± SEM. All statistical analyses were compared to a significance level set at p < 0.05. Experiments with only two treatment conditions were analyzed with Student’s t-tests. Experiments with three or more experimental conditions were analyzed first with one-way ANOVA. Significant omnibus ANOVAs were further analyzed with post-hoc pairwise comparisons of each treatment condition against a single control condition (saline carrier control) using Dunnett’s pairwise multiple comparison t-test (Dunnett, 1955; 1964) to maintain experiment-wise alpha at 0.05.
For social treatments, changes in EOD waveform following cyclic-MSHa and saline control were normalized to the values at the time of injection and social treatment. For evening treatments, these changes were normalized to values at the time of lights-out. These data were compared at 5-minute intervals from 60–90 min post treatment using repeated measures analysis of variance, then again at the center of this period (75 min post treatment) using a paired T-test. P-values are one-tailed following a prediction of decline in values for treatment with the antagonist.
Results
Effects of hypothalamic and pituitary peptide hormones on EOD waveform
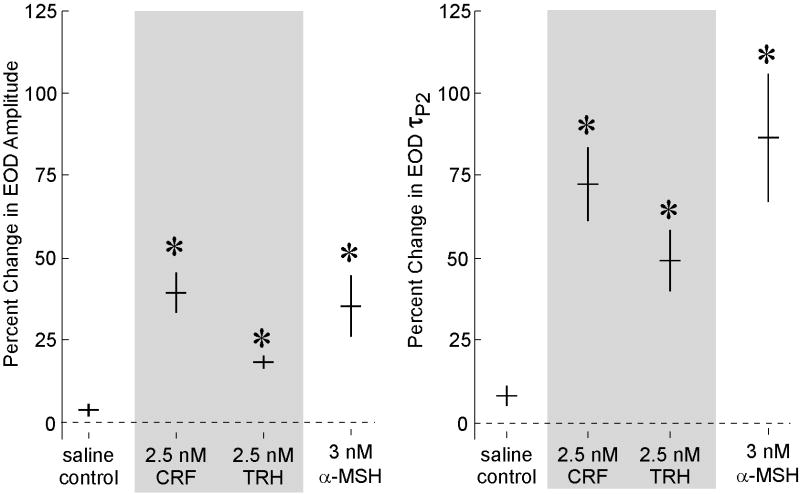
Intramuscular injection of the hypothalamic releasing hormones CRF (2.5 nM g−1; n=6) and TRH (2.5 nM g−1; n = 8), as well as the melanocortin α–MSH (3 nM g−1; n = 8) elicited marked increases in EOD amplitude compared to saline control injections (n = 7; Fig. 2; F[3, 26] = 19.28, p < 0.0001). Injection of CRF, TRH, and α–MSH also increased EOD τP2 compared to saline controls (Fig. 2; F[3, 26] = 19.33, p < 0.0001).
Figure 2.
Injections of CRF, TRH, and α–MSH enhance EOD waveforms in vivo. Injections of saline caused little or no change in amplitude or τP2, whereas injections of CRF, TRH, and α–MSH caused clear increases in EOD amplitude (left panel) and τP2 (right panel). Horizontal bars represent means and vertical bars represent SEM. Asterisks indicate conditions statistically different from saline (p < 0.01),
Cyclic-MSHa attenuates social and circadian modulation of the EOD in vivo
We were surprised to find during pilot experiments that cyclic-MSHa, previously shown to function as a potent and long-lasting MCR agonist at the mammalian MC1R (Cody et al., 1985), appeared to suppress rather than enhance endogenous EOD modulation in B. pinnicaudatus. We hypothesized that this compound was functioning as an antagonist of endogenous melanocortin activity, and therefore tested whether injections of cyclic-MSHa indeed would suppress circadian and socially-induced EOD enhancements in vivo.
Injecting cyclic-MSHa 60 min prior to lights-out caused an initial decrease in EOD amplitude (n = 5) and τP2 (n = 6) compared to saline controls (Fig. 3). Following lights out, fish pretreated with cyclic-MSHa showed a delayed and diminished increase in τP2 compared to saline controls (MANOVA 60–90 min: F[5, 1, 83]=6.626, P=0.025; paired T-test at 75 min: T=3.22, P=0.012). In contrast, EOD amplitude showed only a delayed increase in cyclic-MSHa treated animals with a non-significant mean difference (albeit in the predicted direction) in the 60-90 min comparison window (F[4, 1, 69]=2.08, P=0.11; T=1.37, P=0.16). Cyclic-MSHa significantly reduced socially-induced EOD enhancements. Injecting the focal fish midday with cyclic-MSHa 15 min prior to adding a second fish to the recording tube depressed EOD τP2 relative to saline controls (F[5, 1, 83]=11.45, P=0.01; T=4.09, P=0.005). Cyclic-MSHa depressed EOD amplitude relative to saline, again with effects not as clearly pronounced as for τP2 (F[4, 1, 69]=3.50, P=0.07; T=2.40, P=0.03).
Figure 3.
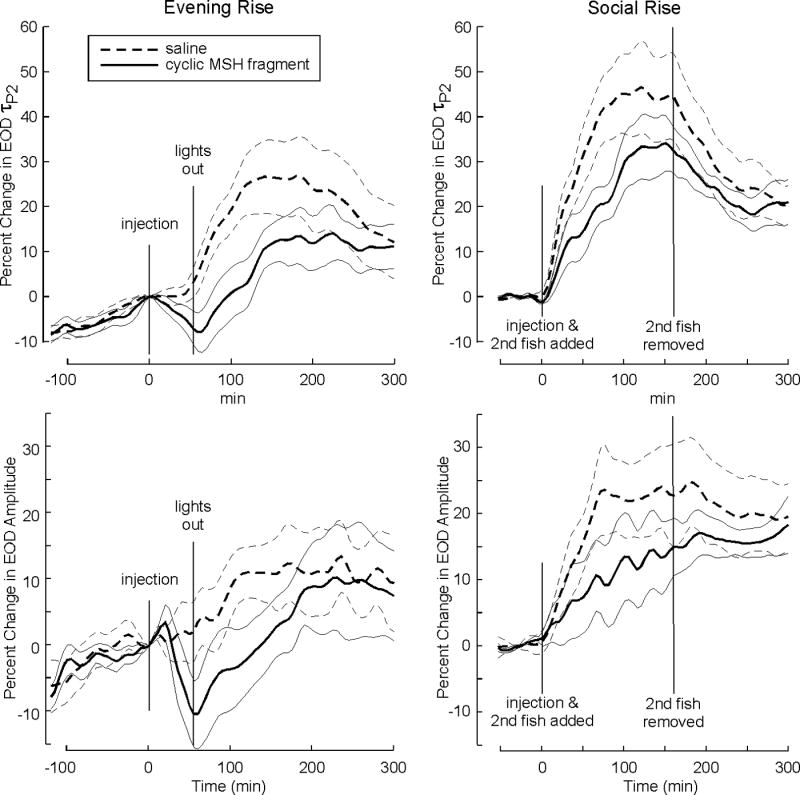
Injections of cyclic-MSHa in vivo attenuate circadian and socially-induced EOD waveform enhancements. Bold lines represent means and dashed lines 95% confidence intervals. Left panels show that injection of cyclic-MSHa 60 min before lights out delays and attenuates the circadian rise in EOD τP2 (top) and amplitude (bottom). Right panels show that cyclic-MSHa injections just before addition of a conspecific delay and attenuate the socially- induced increases in EOD τP2 (top) and amplitude (bottom). All records are rescaled to the magnitude at the time of injection. Over the entire 350 min record, the parameter values show an overall rise rather than a return to baseline: the rise reflects the endogenous circadian rhythm (Stoddard et al., 2007) in the parameter after the passing of the treatment effect.
Effects of hypothalamic and pituitary peptide hormones on electrocyte discharges in vitro
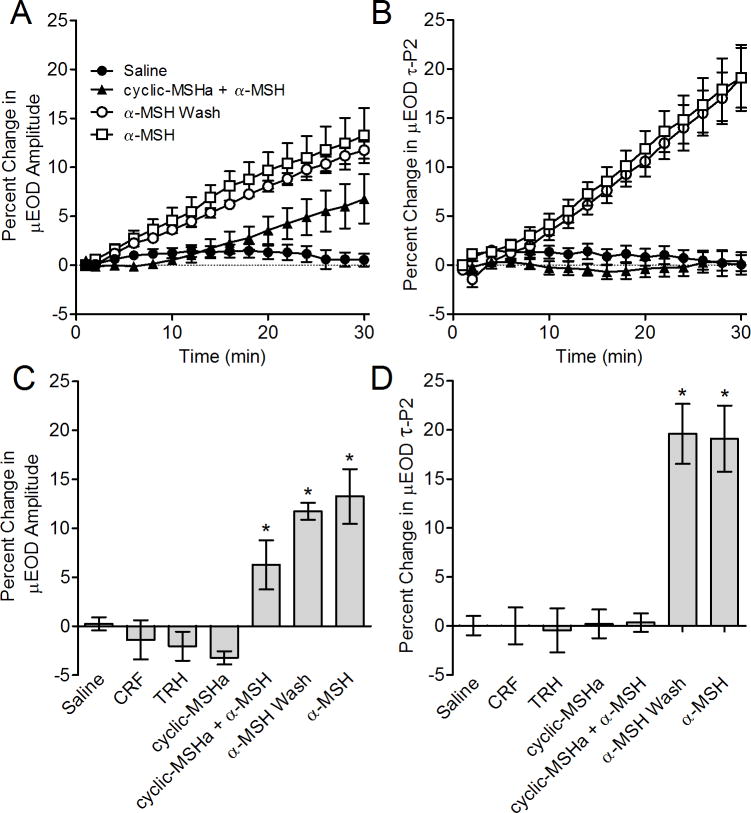
Application of CRF (100 nM; n = 6) and TRH (100 nM; n = 6) had no effect on μEOD waveform whereas α–MSH (100 nM; n = 6) produced rapid and pronounced increases in μEOD amplitude compared to saline controls (Fig. 4c; F[6, 36] = 15.23, p < 0.0001). We found a similar pattern for τP2. Cells treated with α–MSH showed significantly higher τP2 than saline controls whereas cells treated with CRF or TRH showed no difference in τP2 compared to controls (Fig. 4d; F[6, 36] = 19.59, p < 0.0001).
Figure 4.
Effects of peptide and peptidergic factors on the μEOD in vitro. Symbols or bars represent means and error bars show SEM. A) Time course of μEOD amplitude changes in single electrocytes. Alpha–MSH caused rapid μEOD amplitude increases. Cells pretreated with cyclic-MSHa showed a delayed and attenuated μEOD amplitude response in pretreated cells. Following washout of cyclic-MSHa, application of α–MSH rapidly increased μEOD amplitude. B) Time course of μEOD τP2 changes. Alpha–MSH caused rapid and large increases in μEOD τP2. Cells pretreated with cyclic-MSHa showed no change in τP2 in response to α–MSH application. Following washout of cyclic-MSHa, application of α–MSH increases μEOD amplitude and τP2 in a manner indistinguishable from cells that were not pretreated with cyclic-MSHa. C) Percent change in μEOD amplitude after 30 minutes exposure to experimental conditions. Responses to TRH, CRF and cyclic-MSHa were indistinguishable from saline controls after 30 minutes. In contrast, α–MSH caused a large increase in μEOD amplitude. Cells pretreated with cyclic-MSHa showed no change in τP2 in response to α–MSH application, whereas α–MSH produced an attenuated μEOD amplitude response in pretreated cells. Following washout of cyclic-MSHa, application of α–MSH increased μEOD amplitude. D) Percent change in μEOD τP2 after 30 minutes exposure to experimental conditions. Responses to TRH, CRF and cyclic-MSHa were indistinguishable from saline controls after 30 minutes. A–MSH caused a large increase in μEOD τP2, whereas cells pretreated with cyclic-MSHa showed no change in τP2 in response to α–MSH application. Following washout of cyclic-MSHa, application of α–MSH increased μEOD τP2.
Cyclic-MSHa antagonized the effects of α–MSH action on the electrocyte discharge. When applied to electrocytes, cyclic-MSHa alone (200 nM; n=6) did not alter the μEOD amplitude or τP2 (Fig. 4). In contrast, pretreating electrocytes with cyclic-MSHa for 30 min (200 nM; n=6) completely blocked the ability of α–MSH to augment μEOD τP2 (combined bath of 100 nM α–MSH and 200 nM cyclic-MSHa) (n = 6; Fig. 4b,d). In contrast, cyclic-MSHa reduced but did not completely abolish the effects of α–MSH on μEOD amplitude. A delayed and attenuated increase in μEOD amplitude occurred after approximately 20 minutes (n = 6; Fig. 4a,c).
The antagonist effects of cyclic-MSHa (100nM) were quickly and completely reversible on washout. After pretreating cells for 30 min with cyclic-MSHa, switching to a bath solution containing only α–MSH produced increases in μEOD amplitude and τP2 comparable in timecourse and magnitude to cells that were not pretreated with cyclic-MSHa (Fig. 4).
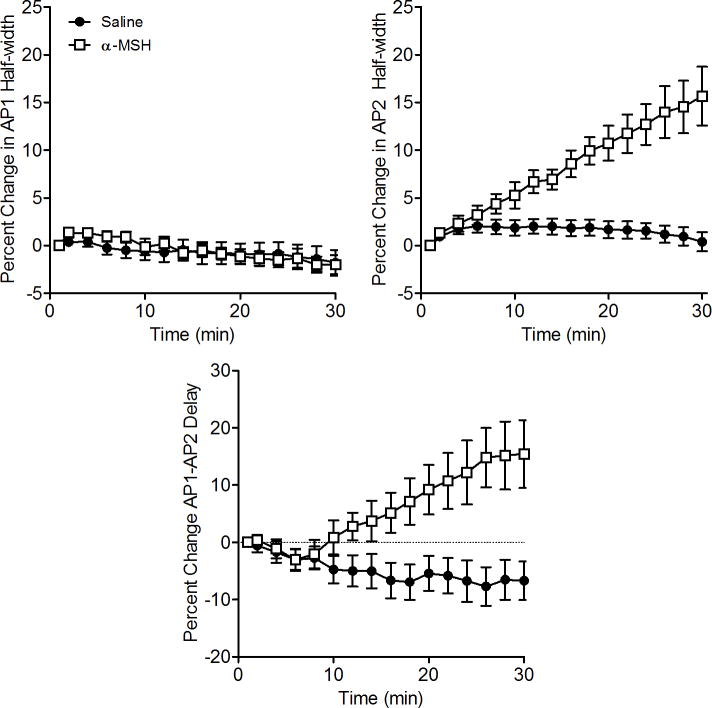
The changes in AP timing and waveform underlying the α–MSH-induced increases in μEOD amplitude and τP2 are the same as we reported for ACTH-induced modulations of the μEOD (Markham and Stoddard, 2005). As with ACTH, application of α–MSH selectively broadened the width of AP2 but not AP1 (Fig. 5), the change responsible for the increase in P2 amplitude and τP2 seen in the whole-cell μEOD. The half-width of AP2 increased significantly in the presence of α–MSH compared to saline controls (t = 5.33, df = 12, p < 0.001), whereas AP1 half-width was not affected by ACTH (Fig. 5; unpaired t-test: p > 0.9). The enhanced μEOD P1 amplitude occurs through an increase in the delay between the electrocyte’s two action potentials (AP1-AP2 Delay; Fig. 5), unblocking AP1, which is otherwise partially attenuated by temporal overlap with AP2. We found that α–MSH increased AP1-AP2 delay compared to saline controls (t = 3.47, df = 12, p < 0.01).
Figure 5.
Alpha-MSH selectively broadens the electrocyte AP2 waveform and increases the temporal offset of AP1 and AP2. The half width of AP1 spikes remained constant in electrocytes treated with α–MSH or saline with no differences between groups even after 30 minutes. Symbols represent means and error bars show SEM.
Discussion
Taken together these results support our hypothesis that the hypothalamic-pituitary portion of the hypothalamic-pituitary-interrenal axis dynamically regulates the EOD waveform of the gymnotiform electric fish B. pinnicaudatus. First, intramuscular administration of the hypothalamic hormones corticotropin-releasing factor (CRF) and thyrotropin-releasing hormone (TRH) led to rapid and dramatic enhancements of EOD amplitude and τP2. The action of these hypothalamic peptides to augment the EOD in vivo without affecting electrocytes in vitro proves that endogenous downstream target hormones (presumably melanocortins) are capable of augmenting the waveforms. Second, the pituitary melanocortin α–MSH also induced large waveform enhancements, concurring with our previous finding that the melanocortin ACTH is a potent enhancer of EOD waveform both in vivo and in vitro (Markham and Stoddard, 2005). These data collectively demonstrate that the hormones found at hypothalamic and pituitary levels of the HPI cascade are pharmacologically relevant modulators of the EOD in our fish. Third, injection of cyclic-MSHa in vivo attenuated EOD enhancements that normally follow lights-out or interaction with conspecifics. Because cyclic-MSHa acts as a silent antagonist of α–MSH activity in vitro, this result supports our hypothesis (Markham and Stoddard, 2005) that endogenous melanocortin action is at least partially responsible for the circadian and socially induced enhancement of the EOD documented previously (Franchina and Stoddard, 1998; Franchina et al., 2001; Silva et al., 1999; Stoddard et al., 2007). We should note that we have never been able to induce in B. pinnicaudatus any waveform modulations with cortisol over a wide range of doses, which indicates that the HPI fills a unique role in signaling behavior of some electric fish apart from its role as a regulator of cortisol release.
It is not entirely surprising that cyclic-MSHa functioned as a potent antagonist of α–MSH in vitro but did not completely block circadian and socially induced EOD enhancements in vivo. Our in vitro data strongly suggest that cyclic-MSHa is competitively binding with α–MSH at the electrocyte melanocortin receptor (MCR). Despite pretreating cells with cyclic-MSHa then applying it together with α–MSH at a 2:1 concentration ratio, we still observed a moderate increase in the μEOD amplitude. Furthermore, cyclic-MSHa appears to bind to electrocyte melanocortin receptors with low affinity. The effects of this compound were eliminated completely within minutes after washout. It is possible then, that during our in vivo experiments excretion or metabolism of cyclic-MSHa from a single bolus dose quickly reduced its plasma concentration while concentrations of endogenous α–MSH simultaneously increased in response to lights out or social challenge. Such an account might also explain why we observed an initial drop in EOD amplitude and τP2 during the 60 minutes between the cyclic-MSHa injection and lights out, but not during the shorter 15-min interval between injection of cyclic-MSHa and the social challenges (Fig. 3). Alternatively, circulating hormones other than melanocortins might contribute to circadian and socially induced EOD enhancements, a possibility given that other 7-transmebrane receptors can induce the cAMP-PKA increase previously shown to drive intracellular changes in electrocyte action potentials of B. pinnicaudatus (Markham and Stoddard, 2005).
We have frequently observed that EOD amplitude and τP2 do not change synchronously in vivo, suggesting that these waveform parameters are somehow independently regulated. In particular, amplitude typically begins its nocturnal rise shortly after noon while τP2 is still in decline (Franchina and Stoddard, 1998). The present experiments provide our first evidence that these parameters can be dissociated in vitro. Exposing electrocytes to a saturating concentration of α–MSH increased both μEOD amplitude and τP2 with identical time courses indicating that this peptide is responsible for modulating both parameters. However, pretreatment with cyclic-MSHa completely blocked the α–MSH induced τP2 increase, but only delayed and attenuated the increase in μEOD amplitude. We have shown previously that ACTH increases both amplitude and τP2 via the cyclic-AMP/protein kinase A (PKA) pathway, consistent with the known signal transduction pathways regulated by MCRs. It seems unlikely, therefore, that the differential response of μEOD amplitude and τP2 would occur through different MCRs or transduction pathways. Instead, we speculate that the cellular mechanisms responsible for setting μEOD amplitude are sensitive to lower concentrations of PKA than the mechanisms that control τP2.
Taken together, our results solidify roles for the HPI axis and circulating melanocortins in regulating the EOD waveform. That CRF and TRH modulate the EOD waveform is consistent with our proposal that the HPI mediates serotonergic control of EOD waveform (Allee et al., 2008), since serotonin is a confirmed regulator of these peptide hormones in teleosts (Winberg et al., 1997). Moreover, the EOD is an energetically expensive signal and enhancing waveform parameters in response to social stimuli is particularly costly (Salazar and Stoddard, 2008). Therefore, any stimulus that results in reduction or enhancement of the EOD likely involves the HPI to regulate simultaneously the shape of the electric waveform and the energy made available for adaptive social response.
While the present experiments and our previous reports confirm that the pituitary melanocortins ACTH and α–MSH both can modulate the EOD and μEOD in a similar manner, we have yet to show which endogenous melanocortins are responsible for EOD modulation in vivo, since both are co-released by CRF and TRH in other teleosts (Rotllant et al., 2000). In addition, we have not confirmed which of the melanocortin receptor isoforms is responsible for μEOD modulation. Preliminary evidence from ongoing cloning and gene expression studies points to MC5R as the functional receptor (Stoddard et al., 2006; J. Tackney & P. Stoddard, unpublished observations). Further, the compound SHU9119 enhances the EOD in vitro (Stoddard et al., 2006) and has been shown to act as an agonist at the MC5R and an antagonist at the MC4R of rainbow trout (Haitina et al., 2004). In addition, electrocytes are derived from myocytes during development so MC5R receptors, present in skeletal muscle (Fathi et al., 1995), are likely candidates for expression in electrocytes.
Activation of MC5R in skeletal myocytes upregulates fatty acid oxidation (An et al., 2007). Melanocortin peptides also exert neurotropic effects at the neuromuscular junction, increasing amplitude of compound muscle action potentials by enhancing excitability and neurotransmitter release at the motor endplate (Davies and Smith, 1994; Gonzalez and Strand, 1981). These roles of melanocortins in the skeletal muscle system suggest that they might, under stressful conditions, simultaneously increase energy availability in the muscle while enhancing neurotransmission at the neuromuscular junction, a combined effect that might help to overcome muscle fatigue. This suggests the interesting question of whether electric fish have harnessed these mechanisms to enhance the μEOD discharge in myogenic electrocytes.
It is noteworthy, but not unusual, that cyclic-MSHa is reported to be a potent agonist of MC1R receptors (Cody et al., 1985), but functions as a silent antagonist of melanocortin activity at electrocytes. Synthetic MCR ligands often have agonist effects at one receptor subtype while functioning as antagonists for other subtypes as is the case for the widely used α-MSH analog SHU9119 mentioned above.
While the hypothalamic-pituitary-gonadal axis (HPG) is known to be an important mediator of sexual and aggressive signaling, the hypothalamic-pituitary-interrenal/adrenal axis (HPI/HPA) has surprised us with its prominence. Melanocortins, intermediate hormones in the HPI/HPA axis, are known to regulate (1) synthesis and release of aggression-mediating preputial pheromones in nocturnal rodents (Morgan, Thomas and Cone, 2004; Morgan, Thomas, Ma et al., 2004; Nowell and Wouters, 1975), (2) skin pigmentation in teleosts, frogs, and lizards (e.g., Castrucci et al., 1984; Hoglund et al., 2000), and now (3) electric waveform characters in a gymnotiform electric fish. Given the morphologic and taxonomic variation in these signal structures, it appears that evolution has persistently reused melanocortins for control of social signals. It will be interesting to explore common life history characteristics that favor use of melanocortins for control of social signaling. Clues may be found in their pleiotropic effects, in particular their deep involvement in the physiology of energy management.
Acknowledgments
Financial support and equipment were provided by NIH grants MBRS GM08205 (PKS) and K01MH064550 (MRM). Carol Curtis assisted with early experiments that led to the discovery that cyclic-MSHa served as a melanocortin antagonist. Susan Pasquale carried out some of the MSH injections. Vicky Salazar assisted with fish breeding and construction of apparatus. We thank David Berman, Ana Hernandez, Jennifer Herrick, Vance Hodge, Maggie Patay, Michael Couto, and Joel Baez for their assistance with this project. This paper is contribution number XXX to the Tropical Biology Program at Florida International University.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allee SJ, Markham MR, Salazar VL, Stoddard PK. Opposing actions of 5HT1A and 5HT2-like serotonin receptors on modulations of the electric signal waveform in the electric fish Brachyhypopomus pinnicaudatus. Horm Behav. 2008;53:481–488. doi: 10.1016/j.yhbeh.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An JJ, Rhee Y, Kim SH, Kim DM, Han DH, Hwang JH, Jin YJ, Cha BS, Baik JH, Lee WT, Lim SK. Peripheral effect of alpha-melanocyte-stimulating hormone on fatty acid oxidation in skeletal muscle. J Biol Chem. 2007;282:2862–2870. doi: 10.1074/jbc.M603454200. [DOI] [PubMed] [Google Scholar]
- Bennett MVL. Modes of operation of electric organs. Ann N Y Acad Sci. 1961;94:458–509. [Google Scholar]
- Calogero AE, Bagdy G, Szemeredi K, Tartaglia ME, Gold PW, Chrousos GP. Mechanims of serotonin receptor agonist-induced activation of the hypothalamic-pituitary-adrenal axis in the rat. Endocrinology. 1990;126:1888–1894. doi: 10.1210/endo-126-4-1888. [DOI] [PubMed] [Google Scholar]
- Calogero AE, Bagdy G, Moncada ML, Dagata R. Effect of selective serotonin agonists on basal corticotropin-releasing hormone-induced and vasopressin-induced ACTH release in vitro from rat pituitary cells. J Endocrinol. 1993;136:381–387. doi: 10.1677/joe.0.1360381. [DOI] [PubMed] [Google Scholar]
- Castrucci AM, Hadley ME, Hruby VJ. Melanotropin bioassays: in vitro and in vivo comparisons. Gen Comp Endocrinol. 1984;55:104–111. doi: 10.1016/0016-6480(84)90134-5. [DOI] [PubMed] [Google Scholar]
- Cody WL, Mahoney M, Knittel JJ, Hruby VJ, Castrucci AM, Hadley ME. Cyclic melanotropins. 9 7-D-Phenylalanine analogues of the active-site sequence. J Med Chem. 1985;28:583–588. doi: 10.1021/jm50001a008. [DOI] [PubMed] [Google Scholar]
- Contesse V, Lefebvre H, Lenglet S, Kuhn JM, Delarue C, Vaudry H. Role of 5-HT in the regulation of the brain-pituitary-adrenal axis: effects of 5-HT on adrenocortical cells. Can J Physiol Pharmacol. 2000;78:967–983. [PubMed] [Google Scholar]
- Davies DA, Smith ME. ACTH (4-10) increases quantal content at the mouse neuromuscular junction. Brain Res. 1994;637:328–330. doi: 10.1016/0006-8993(94)91254-8. [DOI] [PubMed] [Google Scholar]
- Dunnett CW. A multiple comparison procedure for comparing several treatments with a control. J Am Stat Assoc. 1955;50:1096–1121. [Google Scholar]
- Dunnett CW. New tables for multiple comparisons with a control. Biometrics. 1964;20:482–491. [Google Scholar]
- Fathi Z, Iben LG, Parker EM. Cloning, expression, and tissue distribution of a fifth melanocortin receptor subtype. Neurochem Res. 1995;20:107–113. doi: 10.1007/BF00995160. [DOI] [PubMed] [Google Scholar]
- Franchina CR, Stoddard PK. Plasticity of the electric organ discharge waveform of the electric fish Brachyhypopomus pinnicaudatus - I. Quantification of day-night changes. J Comp Physiol [A] 1998;183:759–768. doi: 10.1007/s003590050299. [DOI] [PubMed] [Google Scholar]
- Franchina CR, Salazar VL, Volmar CH, Stoddard PK. Plasticity of the electric organ discharge waveform of male Brachyhypopomus pinnicaudatus. II Social effects. J Comp Physiol [A] 2001;187:45–52. doi: 10.1007/s003590000176. [DOI] [PubMed] [Google Scholar]
- Gonzalez ER, Strand FL. Neurotropic action of MSH/ACTH 4-10 on neuromuscular function in hypophysectomized rats. Peptides. 1981;2(Suppl 1):107–113. doi: 10.1016/0196-9781(81)90064-4. [DOI] [PubMed] [Google Scholar]
- Hadley ME, Haskell-Luevano C. The proopiomelanocortin system. Ann N Y Acad Sci. 1999;885:1–21. doi: 10.1111/j.1749-6632.1999.tb08662.x. [DOI] [PubMed] [Google Scholar]
- Hagedorn M, Zelick R. Relative dominance among males is expressed in the electric organ discharge characteristics of a weakly electric fish. Anim Behav. 1989;38:520–525. [Google Scholar]
- Haitina T, Klovins J, Andersson J, Fredriksson R, Lagerstrom MC, Larhammar D, Larson ET, Schioth HB. Cloning, tissue distribution, pharmacology and three-dimensional modelling of melanocortin receptors 4 and 5 in rainbow trout suggest close evolutionary relationship of these subtypes. Biochem J. 2004;380:475–486. doi: 10.1042/BJ20031934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoglund E, Balm PH, Winberg S. Skin darkening, a potential social signal in subordinate arctic charr (Salvelinus alpinus): the regulatory role of brain monoamines and pro-opiomelanocortin-derived peptides. J Exp Biol. 2000;203:1711–1721. doi: 10.1242/jeb.203.11.1711. [DOI] [PubMed] [Google Scholar]
- Hoglund E, Balm PHM, Winberg S. Stimulatory and inhibitory effects of 5-HT1A receptors on adrenocorticotropic hormone and cortisol secretion in a teleost fish, the Arctic charr (Salvelinus alpinus) Neurosci Lett. 2002;324:193–196. doi: 10.1016/s0304-3940(02)00200-8. [DOI] [PubMed] [Google Scholar]
- Hopkins CD, Comfort NC, Bastian J, Bass AH. Functional analysis of sexual dimorphism in an electric fish, Hypopomus pinnicaudatus, order Gymnotiformes. Brain Behav Evol. 1990;35:350–367. doi: 10.1159/000115880. [DOI] [PubMed] [Google Scholar]
- Lamers AE, Flik G, Atsma W, Wendelaar Bonga SE. A role for di-acetyl alpha-melanocyte-stimulating hormone in the control of cortisol release in the teleost Oreochromis mossambicus. J Endocrinol. 1992;135:285–292. doi: 10.1677/joe.0.1350285. [DOI] [PubMed] [Google Scholar]
- Lamers AE, Flik G, Wendelaar Bonga SE. A specific role for TRH in release of diacetyl alpha-MSH in tilapia stressed by acid water. Am J Physiol Regul Integr Comp Physiol. 1994;267:R1302–R1308. doi: 10.1152/ajpregu.1994.267.5.R1302. [DOI] [PubMed] [Google Scholar]
- Larsen DA, Swanson P, Dickey JT, Rivier J, Dickhoff WW. In vitro thyrotropin-releasing activity of corticotropin-releasing hormone-family peptides in Coho salmon, Oncorhynchus kisutch. Gen Comp Endocrinol. 1998;109:276–285. doi: 10.1006/gcen.1997.7031. [DOI] [PubMed] [Google Scholar]
- Markham MR, Stoddard PK. Adrenocorticotropic hormone enhances the masculinity of an electric communication signal by modulating the waveform and timing of action potentials within individual cells. J Neurosci. 2005;25:8746–8754. doi: 10.1523/JNEUROSCI.2809-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metz JR, Geven EJW, van den Burg EH, Flik G. ACTH, α-MSH, and control of cortisol release: cloning, sequencing, and functional expression of the melanocortin-2 and melanocortin-5 receptor in Cyprinus carpio. Am J Physiol. 2005;289:R814–826. doi: 10.1152/ajpregu.00826.2004. [DOI] [PubMed] [Google Scholar]
- Morgan C, Thomas RE, Cone RD. Melanocortin-5 receptor deficiency promotes defensive behavior in male mice. Horm Behav. 2004;45:58–63. doi: 10.1016/j.yhbeh.2003.08.004. [DOI] [PubMed] [Google Scholar]
- Morgan C, Thomas RE, Ma W, Novotny MV, Cone RD. Melanocortin-5 receptor deficiency reduces a pheromonal signal for aggression in male mice. Chem Senses. 2004;29:111–115. doi: 10.1093/chemse/bjh011. [DOI] [PubMed] [Google Scholar]
- Nowell NW, Wouters A. Proceedings: Release of aggression-promoting pheromone by male mice treated with alpha-melanocyte-stimulating hormone. J Endocrinol. 1975;65:36P–37P. [PubMed] [Google Scholar]
- Rotllant J, Balm PHM, Ruane NM, Perez-Sanchez J, Wendelaar-Bonga SE, Tort L. Pituitary proopiomelanocortin-derived peptides and hypothalamus-pituitary-interrenal axis activity in the gilthead sea bream (Sparus aurata) during prolonged crowding stress: differential regulation of adrenocorticotropin hormone and α-melanocyte-stimulating hormone release by corticotropin-releasing hormone and thyrotropin-releasing hormone. Gen Comp Endocrinol. 2000;119:152–163. doi: 10.1006/gcen.2000.7508. [DOI] [PubMed] [Google Scholar]
- Salazar VL, Stoddard PK. Sex differences in energetic costs explain sexual dimorphism in the circadian rhythm modulation of the electrocommunication signal of the gymnotiform fish Brachyhypopomus pinnicaudatus. J Exp Biol. 2008;211:1012–1020. doi: 10.1242/jeb.014795. [DOI] [PubMed] [Google Scholar]
- Silva A, Quintana L, Galeano M, Errandonea P, Macadar O. Water temperature sensitivity of EOD waveform in Brachyhypopomus pinnicaudatus. J Comp Physiol [A] 1999;185:187–197. [Google Scholar]
- Stoddard PK, Markham MR, Salazar VL. Serotonin modulates the electric waveform of the gymnotiform electric fish Brachyhypopomus pinnicaudatus. J Exp Biol. 2003;206:1353–1362. doi: 10.1242/jeb.00252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoddard PK, Zakon HH, Markham MR, McAnelly L. Regulation and modulation of electric waveforms in gymnotiform electric fish. J Comp Physiol [A] 2006;192:613. doi: 10.1007/s00359-006-0101-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoddard PK, Markham MR, Salazar VL, Allee S. Circadian rhythms in electric waveform structure and rate in the electric fish Brachyhypopomus pinnicaudatus. Physiol Behav. 2007;90:11–20. doi: 10.1016/j.physbeh.2006.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strand FL. New vistas for melanocortins. Finally, an explanation for their pleiotropic functions. Ann N Y Acad Sci. 1999;897:1–16. doi: 10.1111/j.1749-6632.1999.tb07874.x. [DOI] [PubMed] [Google Scholar]
- Van de Kar LD, Javed A, Zhang YH, Serres F, Raap DK, Gray TS. 5-HT2A receptors stimulate ACTH, corticosterone, oxytocin, renin, and prolactin release and activate hypothalamic CRF and oxytocin-expressing cells. J Neurosci. 2001;21:3572–3579. doi: 10.1523/JNEUROSCI.21-10-03572.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winberg S, Nilsson A, Hylland P, Soderstom V, Nilsson GE. Serotonin as a regulator of hypothalamic-pituitary-interrenal activity in teleost fish. Neurosci Lett. 1997;230:113. doi: 10.1016/s0304-3940(97)00488-6. [DOI] [PubMed] [Google Scholar]