Abstract
Therapeutic methods directed at alleviating the basic pathological processes of normal-tension glaucoma (NTG) are yet to be established. Although there seems to be little doubt that intraocular pressure (IOP) represents a risk factor in most patients, reduction of IOP does not prevent progression in every patient with NTG, indicating that factors other than elevated IOP are involved in glaucoma progression. New avenues of treatment under investigation include agents that could improve blood flow to the eye and neuroprotective drugs. The major components of the renin-angiotensin system have been identified in ocular tissue. Angiotensin-converting enzyme (ACE) inhibitors are widely used to treat systemic hypertension. ACE inhibitors are inhibitors of kininase II and thus prevent breakdown of bradykinin. Bradykinin displays protective actions against glutamate neurotoxicity through bradykinin-B2 receptors in cultured retinal neurons. ACE inhibitors blocked the liberation of angiotensin II from angiotensin I. Lower angiotensin II levels may have beneficial effects on outcomes by lowering vascular superoxide anion production. The effects of ACE inhibitor as a potential antiglaucoma therapy deserve intense scrutiny.
Keywords: glaucoma, angiotensin-converting enzyme inhibitor, bradykinin, neuroprotection, ACE inhibitor
Introduction
Normal-tension glaucoma (NTG) refers to a glaucomatous optic nerve head change and corresponding glaucomatous visual field defects without elevated intraocular pressure (IOP). A long-term collaborative study conducted in North America and Europe revealed that a 30% reduction in IOP exerted positive effects on the progression of visual field loss in NTG (Collaborative Normal-Tension Glaucoma Study Group 1998a). Treatment goals for open-angle glaucoma have focused almost exclusively on lowering IOP using drugs, laser therapy or surgery. However, many investigators believe that IOP is not the only factor causally related to glaucomatous optic nerve changes and that some factors unrelated to IOP play significant roles in at least some NTG cases. Patients with glaucoma in whom IOP is lowered to within normal range often continue to suffer further progressive damage (Mao et al 1991; Nouri-Mahdavi et al 1995). A recent 10-year follow-up study showed a direct correlation between IOP levels and stabilization of the optic disc and visual field (Araujo et al 1995). Still, 10% of patients in that study with a mean final IOP of 13 mmHg continued to show disease progression.
The association of glaucoma with various systemic vascular diseases including low systemic blood pressure, transient nocturnal decreases in blood pressure, hypertension, migraine, vasospasm and diabetes has been reported (Flammer et al 1999; Hayreh 1999; Bonomi et al 2000; Drance et al 2001). Many patients with chronic open-angle glaucoma present with coexisting vascular disorders, the most common of which is systemic hypertension, which occurs in 48% of the total chronic open-angle glaucoma population (Gottfredsdottir et al 1997). Pharmacological treatment of non-IOP-dependent mechanisms in glaucoma has largely been limited to the use of calcium-channel blockers, which are widely used in the treatment of systemic hypertension, coronary artery diseases, stroke and arrhythmias. The jury is still out on the contrasting results for systemic calcium-channel blockers used on human glaucoma patients. Calcium-channel blockers may increase blood flow to the optic nerve head (Tomita et al 1999) and might be particularly useful in patients with NTG (Netland et al 1993; Kancllopoulos et al 1996). However, one study showed no significant difference in progression of glaucoma in patients using or not using systemic calcium-channel blockers (Liu et al 1996). Systemic calcium channel blockers can also have adverse cardiac effects, particularly if the patient is being treated with topical β-blockers (Kancllopoulos et al 1996).
Neuroprotection refers to the post-injury protection of neurons that were initially undamaged or only marginally damaged by a particular insult, but are at risk from toxic stimuli released by damaged cells, causing secondary degeneration (Yoles and Schwartz 1998). Secondary degeneration refers to the spread of degeneration to apparently healthy neurons that escape the primary insult, but are adjacent to injured neurons and thus exposed to the degenerative milieu that results (Yoles and Schwartz 1998).
Renin-angiotensin system
The renin-angiotensin system (RAS) plays an important role in vasoconstriction, regulation of electrolyte balance and vascular remodeling. Local renin-angiotensin regulation is present in the eye (Danser et al 1994; Wagner et al 1996). Since the initial application of angiotensin-converting enzyme (ACE) inhibitors as therapeutic agents for the treatment of hypertension, several additional clinical indications have been identified and approved (Brown and Vaughan 1998). ACE inhibitor activity reportedly improves endothelial function and stimulates vascular remodeling, in addition to attenuating progression of arteriosclerosis and the occurrence of cardiovascular events in humans (Mancini et al 1996; Yusuf et al 2000). The identification of ACE as a signaling molecule, which can be activated by the binding of ACE inhibitor, may account for some of the beneficial effects of this class of compounds on the cardiovascular system.
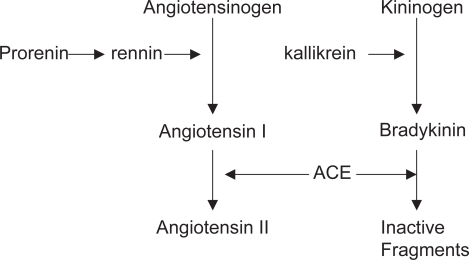
The RAS is an enzymatic cascade that generates a range of angiotensin peptides with varying biological actions. Definitive evidence that an RAS exists within the eye has been provided by molecular biological techniques such as real-time PCR and RNAse protection assays, which have revealed that components of the RAS are synthesized in ocular tissue. Renin is expressed in the pigmented epithelium and retinal Müller cells (Berka et al 1995; Wagner et al 1996). Angiotensinogen is the sole precursor of angiotensin peptides and is cleaved to generate angiotensin I by renin and aspartyl proteases (Figure 1). Angiotensin II can be liberated from angiotensin I by ACE or serine proteases. Angiotensin II is the main effector peptide of the RAS and acts on two main receptor subtypes: angiotensin type I (AT1); and angiotensin type II (AT2). Gene expression for AT1 and AT2 receptors has been identified in several ocular tissues, including the retina (Brandt et al 1994; Murata et al 1997; Wheeler-Schilling et al 1999; Sarlos et al 2003). AT1 receptors have been localized to the ganglion cell layer and inner nuclear layer in rat retina (Wheeler-Schilling et al 1999). AT1 receptor elicits most of the known physiological actions of angiotensin II, including vasoconstriction, electrolyte homeostasis, modulation of drinking behavior and stimulation of pituitary hormone release (Culman et al 1995; Ito et al 1995; Aguilera and Kiss 1996). In tissue pathology such as cardiovascular disease, nephropathies, liver cirrhosis and cancer, AT1 receptor is implicated in a wide variety of cell events including cell growth, differentiation and migration, fibrosis and inflammation and angiogenesis (Yoshiji et al 2001; Yoshiji et al 2002; Brewster et al 2003; Gilbert et al 2003; Muller et al 2003). Actions of the AT2 receptor are less well defined, but possibly oppose actions of the AT1 receptor, including vasodilation and apoptosis (Chung et al 1998). However, evidence in a number of tissues suggests that the AT2 receptor displays similar actions to the AT1 receptor, promoting cell growth and angiogenesis (Levy et al 1996; Cao et al 2000; Sarlos et al 2003).
Figure 1.
The kallikrein-kinin system
In general, the kallikrein-kinin system is thought to counterbalance the activities of the RAS. Kinin peptides are generated from kininogens by kallikrein, a serine protease (Figure 1). The actions of kinin peptides include promoting inflammation, leakage of plasma proteins, pain and more recently, angiogenesis.
Components of the kallikrein-kinin system have been identified in the eye. Kallikrein and kininase II activity are found in the retina, choroid and ciliary body of swine eyes, with the highest activity of kininase I in aqueous humor (Igic 1985). Bradykinin is a vasodilating nonapeptide that is degraded by ACE (Hornig and Drexler 1997). Plasma bradykinin levels are increased in patients treated with ACE inhibitor (Cugno et al 2005). Bradykinin displays a wide range of actions, mediated through at least two subtypes of receptor: B1 and B2 (Regoli and Barabe 1980). B1 receptor has been implicated in angiogenesis, as bradykinin stimulates endothelial cell proliferation in vitro via activation of the B1 receptor cAMP pathway (Morbiddelli et al 1998). Most biological actions are mediated by B2 receptors. Bradykinin-B2 receptors are reportedly abundantly distributed in vascular tissues and smooth muscles cell, and also in human brain (Raidoo et al 1996) and retinal (Ma et al 1996) neurons. In human retina, tissue kallikrein, low molecular weight kininogen and B1 and B2 receptors are expressed in neuronal cells of the outer nuclear layer, inner nuclear layer and ganglion cell layer, and on the retinal vasculature (Ma et al 1996). In addition, mRNA for the B2 receptor has been identified in the retinal ganglion cell layer and in a population of cells adjacent to the sclerocorneal junction in rats (Takeda et al 1999). In B2 receptor-deficient mice with hindlimb ischemia, ACE inhibition increased vessel density and capillary number in the ischemic leg (Silvestre et al 2001). This was not observed in the ischemic hindlimb of mice lacking B2 receptor (Silvestre et al 2001).
Blood flow
AT1 receptor antagonist ameliorates impaired optic nerve head blood flow in rabbits (Inoue et al 2003). The decreased flow velocity in hypertensive patients improved with an oral ACE inhibitor (Steigerwalt et al 1998). ACE inhibitor may act in several ways to increase the blood flow in the eye. The L-arginine nitric oxide pathway is an important local regulator of vascular tone. In the ophthalmic circulation, bradykinin is a potent activator of this pathway, and it causes pronounced endothelium-dependent relaxations in isolated blood vessels (Yao et al 1991; Haefliger et al 1992, 1994), which releases nitric oxide through B2 receptors (Meyer et al 1995), and increases in ophthalmic flow in the isolated perfused eye (Meyer et al 1993). Stimulation of the L-arginin nitric oxide pathway by bradykinin not only leads vasodilation but also to the inhibition of migration (Nyborg et al 1990) and proliferation of vascular smooth muscle cells (Garg and Hassid 1989; Dubey 1994).
It must be emphasized that angiotensin II is also assumed to play a role in cerebral myogenic autoregulation (Strandgaard and Paulsen 1992). Myogenic autoregulation may also be present in the choroidal vasculature (Kiel 1994). Hence angiotensin receptor blockade may be useful to prevent abnormal cerebral and ocular autoregulation in patients with activated RAS.
ACE inhibitors and diabetic retinopathy
Diabetic retinopathy is a major cause of blindness in those of working age (Goldstein et al 1993). Diabetic retinopathy progresses from a mild non-proliferative form characterized by vascular permeability to moderate or severe non-proliferative diabetic retinopathy in which retinal vessels close, and finally to proliferative diabetic retinopathy that comprises new blood vessel growth typically between the retina and posterior surface of the vitreous.
Angiotensin II is a known stimulus for the expression of vascular endothelial growth factor (VEGF), which is associated with the induction of retinal neovascularization (Aiello et al 1995; Gilbert et al 1998; Otani et al 2000; Okada et al 2001). Vitreous levels of both angiotensin II and VEGF are significantly higher in eyes with active proliferative diabetic retinopathy (PDR) than in eyes with quiescent PDR, suggesting that upregulated angiotensin II may be involved in the progression of neovascularization (Funatsu et al 2002). In experimental diabetic models, inhibition of angiotensin II formation with ACE inhibition results in suppression of VEGF expression (Moravski et al 2000; Higgins et al 2003). Vitreous VEGF levels were higher in patients with PDR than in patients without diabetes, but these high levels were significantly reduced after ACE inhibitor treatment (Hogeboom et al 2002). A growing number of both clinical and experimental studies suggest that inhibition of the RAS can provide protective effects against diabetic retinopathy in the absence of hypertension. The most widely cited clinical evidence for a role of the RAS in diabetic retinopathy in the absence of hypertension was produced in the EURODIAB Controlled Trial of Lisinopril in Insulin-Dependent Diabetic Mellitus study (EUCLID), which reported that ACE inhibition with lisinopril reduced progression of retinopathy in type I diabetic patients who were normotensive (Chaturvedi et al 1998). This 2-year multicenter study showed that progression of retinopathy was reduced by at least one level in the lisinopril-treated group compared with that in placebo-treated control individuals after adjustment for center. This study also showed a decreased rate of progression to PDR in the lisinopril-treated group after adjustment for glycemic control. At 1 month, mean diastolic blood pressure was 74 mmHg for the lisinopril-treated group and 77 mmHg in the placebo group and this difference was maintained over the course of study (EUCLID Study Group 1997). The normotensive Appropriate Blood Pressure Control in Diabetes (ABCD) study compared the effects on progression of diabetic retinopathy obtained with moderate and intensive control of blood pressure in normotensive type 2 diabetic patients treated with enalapril or nisoldipine (Schrier et al 2002). Follow-up blood pressure was 128 mmHg for the intensive treatment group and 137 mmHg for the moderate control group. At 5 years, this prospective study showed that progression of diabetic retinopathy was 34% in the intensively treated group, compared with 46% in the moderately treated group. No significant difference in response was seen between enalapril- and nisoldipine-treated patients. These results showed that a decrease in blood pressure in normotensive patients with type 2 diabetes was associated with reductions in progression of diabetic retinopathy. The results of both the EUCLID and normotensive ABCD studies show significant beneficial effects of ACE inhibition on progress of diabetic retinopathy in diabetic patients in the absence of hypertension, but the mechanisms that contribute to this response have yet to be identified. Both studies reported a significant reduction in systemic blood pressure. These results may suggest that reduction of blood pressure within the normotensive range could contribute to the decreases in progression of diabetic retinopathy. However, inhibition of the RAS has also been shown to effect a number of circulating factors, including reactive oxygen species and inflammatory cytokines, which could indirectly contribute to vascular effects (Koh et al 2003). In addition, mechanical stretch can activate the AT1 receptor (Zou et al 2004) and upregulate the VEGF pathway in retinal endothelial cells (Suzuma et al 2001), suggesting that blood pressure itself may affect actions of the AT1 receptor. The relative contributions of reduced systemic blood pressure within the normotensive range and other systemic or local ocular effects of RAS inhibitors to the progression of diabetic retinopathy remain unknown.
Potential use of ACE inhibitors in glaucoma treatment
Elevated IOP is the most important risk factor for glaucomatous damage, but it is still only a risk factor. Visual damage in glaucoma results from a combination of elevated IOP and IOP-independent risk factors. Although hypothesizing that NTG has an underlying mechanism independent of IOP is tempting, use of hypotensive treatment strategies does appear to slow the progression of NTG (Collaborative Normal-Tension Glaucoma Study Group 1998a). However, the findings of the Collaborative Normal Tension Glaucoma Study Group showed no significant association between mean IOP change and visual field progression (Collaborative Normal-Tension Glaucoma Study Group 1998a; Collaborative Normal-Tension Glaucoma Study Group 1998b).
Even more recently, application of neuroprotection to the treatment of non-IOP-dependent glaucomatous damage has received increasingly intensive attention. If glaucoma is actually a collection of diseases that all result in a common optic neuropathy, therapy aimed at preventing the process of damage would show enormous therapeutic potential.
ACE inhibitors have recently attracted attention as a new class of drugs for the treatment of glaucoma. ACE inhibitors have been shown to lower IOP in patients with ocular hypertension or primary open-angle glaucoma (Constad et al 1988). Lotti and Pawlowski (Lotti and Pawlowski 1990) proposed the involvement of prostaglandins in the ocular hypotensive effect of enalaprilat. This conclusion was based on the finding that indomethacin blocked the IOP-lowering effect of enalaprilat. ACE inhibitors are also inhibitors of kininase II and thus prevent the breakdown of bradykinin. Increased bradykinin levels promote prostaglandin synthesis. Prostaglandin, particularly PGF2α, is known to increase uveoscleral outflow of aqueous humor (Crawford and Kaufman 1987).
Apoptosis has been shown to be at least one of the mechanisms for retinal ganglion cell death in monkey models of pressure-induced glaucoma (Quigley et al 1995). Evidence for apoptosis in Alzheimer’s disease has been provided from both animal models and human materials (Schmechel 1999). Longitudinal intervention studies for hypertension, such as the Systolic Hypertension in Europe (SYST-EUR) study and the Peridopril Protection Against Recurrent Stroke Study (PROGRESS) trial, have reported a significant inverse relationship between antihypertensive treatments and risk of vascular-related dementia (Forette et al 2002; Tzourio et al 2003). For example, SYST-EUR data from the 8-year follow-up phase of the program have suggested a 55% reduction in risk for dementia during long-term active treatment with the ACE inhibitor enalapril (Forette et al 2002). Similarly, the PROGRESS study indicated that a substantially reduced risk for dementia (34%) was confirmed by treatment with the ACE inhibitor perindopril. Long-term use of ACE inhibitors may have a protective role against the development of Alzheimer’s disease (Ohrui et al 2004).
Bradykinin has a protective action against glutamate neurotoxicity through bradykinin-B2 receptors in cultured retinal neurons (Yasuyoshi et al 2004). We have previously reported that NTG patients were more sensitive to exogenous bradykinin than normal subjects (Hirooka et al 2002). These data suggest that endogenous bradykinin levels may be lower in NTG patients than in normal subjects. Angiotensin I-converting enzyme and endothelial cell kininase are thought to represent one and the same enzyme (Mombouli and Vanhoutte 1995). As a kininase, this enzyme is responsible for the degradation of bradykinin. ACE inhibitors are thus capable of prolonging the half-life of any bradykinin in the proximity of endothelium. Long-term treatment with ACE inhibitors increases plasma bradykinin levels (Cugno et al 2005). Yasuyoshi et al (2000) recently reported that bradykinin has protective effects on neurotoxicity induced by glutamate through bradykinin B2 receptors in cultured retinal neurons. The protective action of bradykinin is mediated by the opening of the mitochondrial adenosine triphosphate-sensitive potassium channel (Yamauchi et al 2003).
Inhibition of bradykinin degradation by ACE inhibitors may increase the activity of superoxide dismutase and modulate the production of nitric oxide, leading to the inactivation of reactive oxygen species, while also inhibiting various pro-oxidative mechanisms within the vasculature (Ehring et al 1994). Lower angiotensin II levels, such as seen during treatment with ACE inhibitor, may have beneficial effects on outcome by lowering vascular superoxide anion production (Rajagopalan and Harrison 1996). ACE inhibitor may benefit vascular function. A previous study reported average rates of decline in NTG as −0.41 dB per year (Anderson et al 2001) with a mean follow-up of 4.5 years. In subjects with a significant decline, mean rate of loss across the entire field was −0.90 dB/year (Anderson et al 2001). In subjects who had not progressed, mean rate of loss across the entire field was −0.14 dB/year (Anderson et al 2001). We retrospectively reviewed a total of 38 patients with NTG (Hirooka et al 2006), divided into 3 groups: control subjects with no previous history of hypertension; NTG hypertension patients receiving ACE inhibitor; and NTG hypertension patients receiving other antihypertensive drug treatment. In the ACE inhibitor-treated group, mean deviation (MD) change per year was 0.48 ± 0.19 dB, compared to −0.38 ± 0.23 dB in control subjects, and −0.50 ± 0.39 dB in the other antihypertensive drug-treated group. These results suggest that ACE inhibitors might exert favorable effects on the visual field in patients with NTG (Hirooka et al 2006). ACE inhibitors have numerous properties that theoretically should be beneficial in treating non-IOP-dependent mechanisms in glaucoma.
Randomized, controlled clinical trials have not been performed to clarify neuroprotective effects of ACE inhibitors in glaucoma yet. In the absence of such trials, further studies in various animal models of glaucoma are also needed.
References
- Aguilera G, Kiss A. Regulation of the hypothalamic-pituitary-adrenal axis and vasopressin secretion. Role of angiotensin II. Adv Exp Med Biol. 1996;396:105–12. doi: 10.1007/978-1-4899-1376-0_11. [DOI] [PubMed] [Google Scholar]
- Aiello LP, Pierce EA, Foley ED, et al. Suppression of retinal neovascularization in vivo by inhibition of vascular endothelial growth factor (VEGF) using soluble VEGF-receptor chimeric proteins. Proc Natl Acad Sci USA. 1995;92:10457–61. doi: 10.1073/pnas.92.23.10457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson DR, Drance SM, Schulzer M, Collaborative Normal-Tension Glaucoma Study Group Natural history of normal-tension glaucoma. Ophthalmology. 2001;108:247–53. doi: 10.1016/s0161-6420(00)00518-2. [DOI] [PubMed] [Google Scholar]
- Araujo SV, Spaeth GL, Roth SM, et al. A ten-year follow-up on a prospective, randomized trial of postoperative corticosteroids after trabeculectomy. Ophthalmology. 1995;102:1753–9. doi: 10.1016/s0161-6420(95)30797-x. [DOI] [PubMed] [Google Scholar]
- Berka JL, Stubbs AJ, Wang DZ, et al. Renin-containing Müller cells of the retina display endocrine features. Invest Ophthalmol Vis Sci. 1995;36:1450–8. [PubMed] [Google Scholar]
- Bonomi L, Marchini G, Marraffa M, et al. Vascular risk factors for primary open angle glaucoma: the Enga-Neumarkt Study. Ophthalmology. 2000;107:1287–93. doi: 10.1016/s0161-6420(00)00138-x. [DOI] [PubMed] [Google Scholar]
- Brandt CR, Pumfery AM, Micales B, et al. Renin mRNA is synthesized locally in rat ocular tissues. Curr Eye Res. 1994;13:755–63. doi: 10.3109/02713689409047011. [DOI] [PubMed] [Google Scholar]
- Brewster UC, Setaro JF, Perazella MA. The rennin-angiotensinaldosterone system: cardiorenal effects and implications for renal and cardiovascular diseases states. Am J Med Sci. 2003;326:15–24. doi: 10.1097/00000441-200307000-00003. [DOI] [PubMed] [Google Scholar]
- Brown NJ, Vaughan DE. Angiotensin-converting enzyme inhibitions. Circulation. 1998;97:1411–20. doi: 10.1161/01.cir.97.14.1411. [DOI] [PubMed] [Google Scholar]
- Cao Z, Kelly DJ, Cox A, et al. Angiotensin type 2 receptor is expressed in the adult rat kidney and promotes cellular proliferation and apoptosis. Kidney Int. 2000;58:2437–51. doi: 10.1046/j.1523-1755.2000.00427.x. [DOI] [PubMed] [Google Scholar]
- Chaturvedi N, Sjolle AK, Stephenson JM, et al. Effect of lisinopril on progression of retinopathy in normotensive people with type I diabetes. The EUCLID Study Group. EURODIAB Controlled Trial of Lisinopril in Insulin-Dependent Diabetes Mellitus. Lancet. 1998;351:28–31. doi: 10.1016/s0140-6736(97)06209-0. [DOI] [PubMed] [Google Scholar]
- Chung O, Kuhl H, Stoll M, et al. Physiological and pharmacological implications of AT1 versus AT2 receptors. Kidney Int Suppl. 1998;67:S95–9. doi: 10.1046/j.1523-1755.1998.06719.x. [DOI] [PubMed] [Google Scholar]
- Collaborative Normal-Tension Glaucoma Study Group Comparison of glaucomatous progression between untreated patients with normal-tension glaucoma and patients with therapeutically reduced intraocular pressures. Am J Ophthalmol. 1998a;126:487–97. doi: 10.1016/s0002-9394(98)00223-2. [DOI] [PubMed] [Google Scholar]
- Collaborative Normal-Tension Glaucoma Study Group The effectiveness of intraocular pressure reduction in the treatment of normal-tension glaucoma. Am J Ophthalmol. 1998b;126:498–505. doi: 10.1016/s0002-9394(98)00272-4. [DOI] [PubMed] [Google Scholar]
- Constad WH, Fiore P, Samson C, et al. Use of an angiotensin converting enzyme inhibitor in ocular hypertension and primary open-angle glaucoma. Am J Ophthalmol. 1988;105:674–7. doi: 10.1016/0002-9394(88)90063-3. [DOI] [PubMed] [Google Scholar]
- Crawford K, Kaufman PL. Pilocarpine antagonizes prostaglandin F2α-induced ocular hypotension in monkeys. Evidence for enhancement of Uveoscleral outflow by prostaglandin F2α. Arch Ophthalmol. 1987;105:1112–16. doi: 10.1001/archopht.1987.01060080114039. [DOI] [PubMed] [Google Scholar]
- Cugno M, Agostoni P, Mari D, et al. Impaired bradykinin response to ischaemia and exercise in patients with mild congestive heart failure during angiotensin-converting enzyme treatment. Relationships with endothelial function, coagulation and inflammation. Br J Haematol. 2005;130:113–20. doi: 10.1111/j.1365-2141.2005.05569.x. [DOI] [PubMed] [Google Scholar]
- Culman J, Hohle S, Qadri F, et al. Angiotensin as neuromodulator/neurotransmitter in central control of body fluid and electrolyte homeostasis. Clin Exp Hypertens. 1995;17:281–93. doi: 10.3109/10641969509087071. [DOI] [PubMed] [Google Scholar]
- Danser AH, Derkx FH, Admiraal PJ, et al. Angiotensin levels in the eye. Invest Ophthalmol Vis Sci. 1994;35:1008–18. [PubMed] [Google Scholar]
- Drance S, Anderson DR, Schulzer M, Collaborative Normal-Tension Glaucoma Study Group Risk factors for progression of visual field abnormalities in normal-tension glaucoma. Am J Ophthalmol. 2001;131:699–708. doi: 10.1016/s0002-9394(01)00964-3. [DOI] [PubMed] [Google Scholar]
- Dubey RK. Vasodilator-derived nitric oxide inhibits fetal calf serum- and angiotensin-II-induced growth of renal anterior smooth muscle cells. J Pharm Exp Ther. 1994;269:402–8. [PubMed] [Google Scholar]
- Ehring T, Baumgart D, Krajcar M, et al. Attenuation of myocardial stunning by the ACE inhibitor ramiprilat through a signal cascade of bradykinin and prostaglandins but not nitric oxide. Circulation. 1994;90:1368–85. doi: 10.1161/01.cir.90.3.1368. [DOI] [PubMed] [Google Scholar]
- Flammer J, Haefliger IO, Orgul S, et al. Vascular dysregulation: a principal risk factor for glaucomatous damage? J Glaucoma. 1999;8:212–9. [PubMed] [Google Scholar]
- Forette F, Seux ML, Staessen JA, et al. The prevention of dementia with antihypertensive treatment: new evidence from the Systolic Hypertension in Europe (Syst-Eur) study. Arch Inter Med. 2002;162:2046–52. doi: 10.1001/archinte.162.18.2046. [DOI] [PubMed] [Google Scholar]
- Funatsu H, Yamashita H, Nakanishi Y, et al. Angiotensin II and vascular endothelial growth factor in the vitreous fluid of patients with proliferative diabetic retinopathy. Br J Ophthalmol. 2002;86:311–5. doi: 10.1136/bjo.86.3.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garg UC, Hassid A. Nitric oxide-generating vasodilators and 8-bromo-cyclic guanosine monophosphate inhibit mitogenesis and proliferation of cultured rat vascular smooth muscle cells. J Clin Invest. 1989;83:1774–7. doi: 10.1172/JCI114081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert RE, Krum H, Wilkinson-Berka J, et al. The rennin-angiotensin system and the long-term complications of diabetes: pathophysiological and therapeutic consideration. Diabet Med. 2003;20:607–21. doi: 10.1046/j.1464-5491.2003.00979.x. [DOI] [PubMed] [Google Scholar]
- Gilbert RE, Vranes D, Berka JL, et al. Vascular endothelial growth factor and its receptors in control and diabetic rat eyes. Lab Invest. 1998;78:1017–27. [PubMed] [Google Scholar]
- Goldstein DE, Blinder KJ, Ide CH, et al. Glycemic control and development of retinopathy in youth-onset insulin-dependent diabetes mellitus. Results of a 12-year longitudinal study. Ophthalmology. 1993;100:1125–31. doi: 10.1016/s0161-6420(93)31516-2. [DOI] [PubMed] [Google Scholar]
- Gottfredsdottir MS, Allingham RR, Shields MB. Physicians’ guide to interactions between glaucoma and systemic medications. J Glaucoma. 1997;6:377–83. [PubMed] [Google Scholar]
- Haefliger IO, Flammer J, Lüscher TF. Nitric oxide and endothelium-1 are important regulators of human ophthalmic artery. Invest Ophthalmol Vis Sci. 1992;33:2340–3. [PubMed] [Google Scholar]
- Haefliger IO, Meyer P, Flammer J, et al. The vascular endothelium as a regulator of the ocular circulation: A new concept in ophthalmology? Surv Ophthalmol. 1994;39:123–32. doi: 10.1016/0039-6257(94)90157-0. [DOI] [PubMed] [Google Scholar]
- Hayreh SS. The role of age and cardiovascular diseases in glaucomatous optic neuropathy. Surv Ophthalmol. 1999;43:S27–42. doi: 10.1016/s0039-6257(99)00018-1. [DOI] [PubMed] [Google Scholar]
- Higgins RD, Yan Y, Geng Y, et al. Captopril and vascular endothelial growth factor in a mouse model of retinopathy. Curr Eye Res. 2003;27:123–9. doi: 10.1076/ceyr.27.2.123.15955. [DOI] [PubMed] [Google Scholar]
- Hirooka K, Shiraga F, Hasegawa E. Bradykinin sensitivity in primary open-angle glaucoma and normal-tension glaucoma patients. Am J Ophthalmol. 2002;134:922–4. doi: 10.1016/s0002-9394(02)01703-8. [DOI] [PubMed] [Google Scholar]
- Hirooka K, Baba T, Fujimura T, et al. Prevension of visual field defect progression with angiotensin-converting enzyme inhibitor in eyes with normal-tension glaucoma. Am J Ophthalmol. 2006;142:523–5. doi: 10.1016/j.ajo.2006.04.020. [DOI] [PubMed] [Google Scholar]
- Hogeboom van Buggenum IM, Polak BC, Reichert-Thoen JW, et al. Angiotensin converting enzyme inhibiting therapy is associated with lower vitreous vascular endothelial growth factor concentrations in patients with proliferative diabetic retinopathy. Diabetologia. 2002;45:203–9. doi: 10.1007/s00125-001-0747-8. [DOI] [PubMed] [Google Scholar]
- Hornig B, Drexler H. Endothelial function and bradykinin in humans. Drugs. 1997;54:42–7. doi: 10.2165/00003495-199700545-00007. [DOI] [PubMed] [Google Scholar]
- Igic R. Kallikrein and kininases in ocular tissues. Exp Eye Res. 1985;41:117–20. doi: 10.1016/0014-4835(85)90100-9. [DOI] [PubMed] [Google Scholar]
- Ito M, Oliverio MI, Mannon PJ, et al. Regulation of blood pressure by the type 1A angiotensin II receptor gene. Proc Natl Acad Sci USA. 1995;92:3521–5. doi: 10.1073/pnas.92.8.3521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue T, Yokoyama T, Koike H. CS-088, an angiotensin type 1 receptor antagonist, ameliorated the impaired blood flow in the optic nerve head of rabbit. Ophthalmic Res. 2003;35:351–4. doi: 10.1159/000074076. [DOI] [PubMed] [Google Scholar]
- Kanellopoulos AJ, Erickson KA, Netland PA. Systemic calcium channel blockers and glaucoma. J Glaucoma. 1996;5:357–62. [PubMed] [Google Scholar]
- Kiel JW. Choroidal myogenic autoregulation and intraocular pressure. Exp Eye Res. 1994;58:529–43. doi: 10.1006/exer.1994.1047. [DOI] [PubMed] [Google Scholar]
- Koh KK, Ahn JY, Hen SH, et al. Pleiotropic effects of angiotensin II receptor blocker in hypertensive patients. J Am Coll Cardiol. 2003;42:905–10. doi: 10.1016/s0735-1097(03)00846-5. [DOI] [PubMed] [Google Scholar]
- Levy BI, Benessiano J, Henrion D, et al. Chronic blockade of AT2-subtype receptors prevents the effect of angiotensin II on the rat vascular structure. J Clin Invest. 1996;98:418–25. doi: 10.1172/JCI118807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Araujo SV, Spaeth GL, et al. Lack of effect of calcium channel blockers on open-angle glaucoma. J Glaucoma. 1996;5:187–90. [PubMed] [Google Scholar]
- Lotti VJ, Pawlowski N. Prostaglandins mediate the ocular hypotensive action of the angiotensin converting enzyme inhibitor MK-422 (enalaprilat) in African green monkeys. J Ocul Phalmacol. 1990;6:1–7. doi: 10.1089/jop.1990.6.1. [DOI] [PubMed] [Google Scholar]
- Ma JX, Song Q, Hatcher HC, et al. Expression and cellular localization of the kallikrein-kinin system in human ocular tissues. Exp Eye Res. 1996;63:19–26. doi: 10.1006/exer.1996.0087. [DOI] [PubMed] [Google Scholar]
- Mancini GB, Henry GC, Macaya C, et al. Angiotensin-converting enzyme inhibitor with quinapril improves endothelial vasomotor dysfunction in patients with coronary artery disease. The TREND (Trial on Reversing ENdothelial Dysfunction) Study. Circulation. 1996;94:258–65. doi: 10.1161/01.cir.94.3.258. [DOI] [PubMed] [Google Scholar]
- Mao LK, Stewart WC, Shields MB. Correlation between intraocular pressure control and progressive glaucomatous damage in primary open-angle glaucoma. Am J Ophthalmol. 1991;111:51–5. doi: 10.1016/s0002-9394(14)76896-5. [DOI] [PubMed] [Google Scholar]
- Meyer P, Flammer J, Lüscher TF. Endothelium-dependent regulation of the ophthalmic microcirculation in the perfused porcine eye: Role of nitric oxide and endothelins. Invest Ophthalmol Vis Sci. 1993;34:3614–21. [PubMed] [Google Scholar]
- Meyer P, Flammer J, Lüscher TF. Local action of the renin angiotensin system in the porcine ophthalmic circulation: effects of ACE-inhibitors and angiotensin receptor antagonists. Invest Ophthalmol Vis Sci. 1995;36:555–62. [PubMed] [Google Scholar]
- Mombouli JV, Vanhoutte PM. Kinins and endothelial control of vascular smooth muscle. Annu Rev Pharmacol Toxicol. 1995;35:679–05. doi: 10.1146/annurev.pa.35.040195.003335. [DOI] [PubMed] [Google Scholar]
- Moravski CJ, Kelly DJ, Cooper ME, et al. Retinal neovascularization is prevented by blockade of the renin-angiotensin system. Hypertension. 2000;36:1099–104. doi: 10.1161/01.hyp.36.6.1099. [DOI] [PubMed] [Google Scholar]
- Morbidekki L, Parenti A, Giovannelli L, et al. B1 receptor involvement in the effect of bradykinin on venular endothelial cell proliferation and potentiation of FGF-2 effects. Br J Pharmacol. 1998;124:1286–92. doi: 10.1038/sj.bjp.0701943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller DN, Fiebeler A, Park JK, et al. Angiotensin II and endothelin induce inflammation and thereby promote hypertension-induced end-organ damage. Clin Nephrol. 2003;60(Suppl 1):S2–12. [PubMed] [Google Scholar]
- Murata M, Nakagawa M, Takahashi S. Expression and lacalization of angiotensin II type I receptor mRNA in rat ocular tissues. Ophthalmologica. 1997;211:384–6. doi: 10.1159/000310835. [DOI] [PubMed] [Google Scholar]
- Netland PA, Chaturvedi N, Dreyer EB. Calcium channel blockers in the management of low-tension and open-angle glaucoma. Am J Ophthalmol. 1993;115:608–13. doi: 10.1016/s0002-9394(14)71458-8. [DOI] [PubMed] [Google Scholar]
- Nouri-Mahdavi K, Brigatti L, Weitzman M, et al. Outcomes of trabeculectomy for primary-open angle glaucoma. Ophthalmology. 1995;102:1760–9. doi: 10.1016/s0161-6420(95)30796-8. [DOI] [PubMed] [Google Scholar]
- Nyborg NC, Nielsen PJ, Prieto D, et al. Angiotensin-II does not contract bovine retinal resistance arteries in vitro. Exp Eye Res. 1990;50:469–74. doi: 10.1016/0014-4835(90)90034-r. [DOI] [PubMed] [Google Scholar]
- Ohrui T, Matsui T, Yamaya M, et al. Angiotensin-converting enzyme inhibitors and incidence of Alzheimer’s disease in Japan. J Am Geriatr Soc. 2004;52:649–50. doi: 10.1111/j.1532-5415.2004.52178_7.x. [DOI] [PubMed] [Google Scholar]
- Okada Y, Yamanaka I, Sakamoto T, et al. Increased expression of angiotensin-converting enzyme in retinas of diabetic rats. Jpn J Ophthalmol. 2001;45:585–91. doi: 10.1016/s0021-5155(01)00412-9. [DOI] [PubMed] [Google Scholar]
- Otani A, Takagi H, Oh H, et al. Angiotensin II-stimulated vascular endothelial growth factor expression in bovine retinal pericytes. Invest Ophthalmol Vis Sci. 2000;41:1192–9. [PubMed] [Google Scholar]
- Quigley HA, Nickells RW, Kerrigan LA, et al. Retinal ganglion cell death in experimental glaucoma and after axotomy occurs by apoptosis. Invest Ophthalmol Vis Sci. 1995;36:774–86. [PubMed] [Google Scholar]
- Raidoo DM, Ramchurren N, Naidoo Y, et al. Visualisation of bradykinin B2 receptors on human brain neurons. Immunopharmacology. 1996;33:104–7. doi: 10.1016/0162-3109(96)00021-5. [DOI] [PubMed] [Google Scholar]
- Rajagopalan S, Harrison DG. Reversing endothelial dysfunction with ACE inhibitors. A new trend. Circulation. 1996;94:240–3. doi: 10.1161/01.cir.94.3.240. [DOI] [PubMed] [Google Scholar]
- Regoli D, Barabe J. Pharmacology of bradykinin and related kinins. Pharmacol Rev. 1980;32:1–46. [PubMed] [Google Scholar]
- Sarlos S, Rizkalla B, Moravski CJ, et al. Retinal angiogenesis is mediated by an interaction between the angiotensin type 2 receptor, VEGF, and angiopoietin. Am J Pathol. 2003;163:879–87. doi: 10.1016/S0002-9440(10)63448-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmechel DE. Apoptosis in neurodegenerative diseases. In: Hannun YA, Boustany RM, editors. Apoptosis in neurobiology. Boca Raton, FL: CRC Press; 1999. pp. 23–48. [Google Scholar]
- Schrier RW, Estacio RO, Ealer A, et al. Effect of aggressive blood pressure control in normotensive type 2 diabetic patients on albuminuria, retinopathy and strokes. Kidney Int. 2002;61:1086–97. doi: 10.1046/j.1523-1755.2002.00213.x. [DOI] [PubMed] [Google Scholar]
- Silvestre JS, Bergaya S, Tamarat R, et al. Proangiogenic effect of angiotensin-converting enzyme inhibition is mediated by the bradykinin B2 receptor pathway. Circ Res. 2001;89:678–83. doi: 10.1161/hh2001.097691. [DOI] [PubMed] [Google Scholar]
- Steigerwalt RD, Jr, Belcaro GV, Laurora G, et al. Ocular and orbital blood flow in patients with essential hypertension treated with trandolapril. Retina. 1998;18:539–45. doi: 10.1097/00006982-199806000-00008. [DOI] [PubMed] [Google Scholar]
- Strandgaard S, Paulson OB. Regulation of cerebral blood flow in health and diseases. J Cardiovasc Pharmacol. 1992;19:S89–93. doi: 10.1097/00005344-199219006-00014. [DOI] [PubMed] [Google Scholar]
- Suzuma I, Hata Y, Clermont A, et al. Cyclic stretch and hypertension induce retinal expression of vascular endothelial growth factor and vascular endothelial growth factor receptor-2: potential mechanisms for exacerbation of diabetic retinopathy by hypertension. Diabetes. 2001;50:444–54. doi: 10.2337/diabetes.50.2.444. [DOI] [PubMed] [Google Scholar]
- Takeda H, Kimura Y, Higashida H, et al. Localization of B2 bradykinin receptor mRNA in the rat retina and sclerocornea. Immunophalmacology. 1999;45:51–5. doi: 10.1016/s0162-3109(99)00057-0. [DOI] [PubMed] [Google Scholar]
- The EUCLID Study Group Randomized placebo-controlled trial of lisinopril in normotensive patients with insulin-dependent diabetes and normoalbuminuria or microalbuminuria. Lancet. 1997;349:1787–92. [PubMed] [Google Scholar]
- Tomita K, Araie M, Tamaki Y, et al. Effects of nilvadipine, a calcium antagonist, on rabbit ocular circulation and optic nerve head circulation in NTG subjects. Invest Ophthalmol Vis Sci. 1999;40:1144–51. [PubMed] [Google Scholar]
- Tzourio C, Anderson C, Chapman N, et al. Effects of blood pressure lowering with perindopril and indapamide therapy on dementia and cognitive decline in patients with cerebrovascular disease. Arch Inter Med. 2003;163:1069–75. doi: 10.1001/archinte.163.9.1069. [DOI] [PubMed] [Google Scholar]
- Wagner J, Jan Danser AH, Derkx FH, et al. Demonstration of rennin mRNA, angiotensinogen mRNA, and angiotensin converting enzyme mRNA expression in the human eye: evidence for an intraocular rennin-angiotensin system. Br J Ophthalmol. 1996;80:159–63. doi: 10.1136/bjo.80.2.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler-Schilling TH, Kohler K, Sautter M, et al. Angiotensin II receptor subtype gene expression and cellular localization in the retina and non-neuronal ocular tissues of the rat. Eur J Neurosci. 1999;11:3387–94. doi: 10.1046/j.1460-9568.1999.00787.x. [DOI] [PubMed] [Google Scholar]
- Yamauchi T, Kashii S, Yasuyoshi H, et al. Mitochondrial ATP-sensitive potassium channel: a novel site for neuroprotection. Invest Ophthalmol Vis Sci. 2003;44:2750–6. doi: 10.1167/iovs.02-0815. [DOI] [PubMed] [Google Scholar]
- Yao K, Tschudi M, Flammer J, et al. Endothelium-dependent regulation of vascular tone of the porcine ophthalmic artery. Invest Ophthalmol Vis Sci. 1991;32:1791–8. [PubMed] [Google Scholar]
- Yasuyoshi H, Kashii S, Zhang S, et al. Protective effect of bradykinin against glutamate neurotoxicity in cultured rat retinal neurons. Invest Ophthalmol Vis Sci. 2000;41:2273–8. [PubMed] [Google Scholar]
- Yoles E, Schwartz M. Potential neuroprotective therapy for glaucomatous optic neuropathy. Surv Ophthalmol. 1998;42:367–72. doi: 10.1016/s0039-6257(97)00123-9. [DOI] [PubMed] [Google Scholar]
- Yoshiji H, Kuriyama S, Fukui H. Angiotensin-I-converting enzyme inhibitors may be an alternative anti-angiogenic atrategy in the treatment of liver fibrosis and hepatocellular carcinoma. Possible role of vascular endothelial growth factor. Tumor Biol. 2002;23:348–56. doi: 10.1159/000069792. [DOI] [PubMed] [Google Scholar]
- Yoshiji H, Kuriyama S, Kawata M, et al. The angiotensin-I-converting enzyme inhibitor perindopril suppresses tumor growth and angiogenesis: possible role of the vascular endothelial growth factor. Clin Cancer Res. 2001;7:1073–8. [PubMed] [Google Scholar]
- Yusuf S, Sleight P, Pogue J, et al. Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. The Heart Outcomes Prevention Evaluation Study Investigators. N Engl J Med. 2000;342:145–53. doi: 10.1056/NEJM200001203420301. [DOI] [PubMed] [Google Scholar]
- Zou Y, Akazawa H, Qin Y, et al. Mechanical stress activates angiotensin II type 1 receptor wiithout the involvement of angiotensin II. Nat Cell Biol. 2004;6:499–506. doi: 10.1038/ncb1137. [DOI] [PubMed] [Google Scholar]