Abstract
CART (Cocaine- Amphetamine-Regulated Transcript) has been shown to be regulated by corticosteroids in the hypothalamus, but its regulation by corticosteroids and stress has not been well examined in the hippocampus or the amygdala. Further, CART has been implicated in the transition to puberty. In this study we examine the effects of acute (30 minute) on CART mRNA in prepubescent and adult rats. In addition, we examined chronic (21 day × 6 hours) restraint stress upon the expression of CART mRNA in the hippocampus and the amygdala and the effects of 7 days of adrenalectomy and corticosteroid replacement upon CART expression in these regions of the adult rat brain. We found an up-regulation of CART mRNA in the central amygdala induced by acute but not chronic stress and an up-regulation in the dentate gyrus induced by chronic but not acute stress. Adrenalectomy reduced CART expression in the dentate gyrus but not the amygdala and this effect was blocked by corticosterone but not RU28,362 or aldosterone replacement, suggesting a synergism of mineralocorticoid and glucocorticoid receptors. Our data establish that CART expression is regulated by stress in a regionally and time specific manner and that CART is regulated by corticosteroid actions in the hippocampus.
Keywords: HPA axis, anxiety, depression
Introduction
Stress and corticosteroids play a role in a number of neuropsychiatric disorders, particularly major depressive disorder. Both stress and corticosteroids have been linked to the structural changes seen in these disorders and in animal models such as chronic restraint stress (McEwen and Olie, 2005). CART (Cocaine- Amphetamine-Regulated Transcript) plays a role in a number of different behaviors, such as feeding and drug use (Hunter and Kuhar, 2003), and a number of studies have suggested that CART may have a role in the central response to stressful stimuli. Early anatomical work demonstrated its localization in a number of regions associated with stress and anxiety (Douglass et al., 1995; Couceyro et al., 1997; Koylu et al., 1997; Koylu et al., 1998) which led to several studies demonstrating the anxiogenic effects of centrally injected CART peptides in the elevated plus maze and social interaction (Kask et al., 2000; Asakawa et al., 2001; Chaki et al., 2003; Stanek, 2006). More recently, it has been shown that human adolescents carrying the Leu34Phe substitution in the proCART peptide, had a higher level of anxiety and depression than control subjects (Miraglia Del Giudice et al., 2006). It seems likely that CART may have a role in anxiety and depression that is only beginning to be understood.
In parallel to the studies of CART’s influence on anxious behaviors, a more substantial literature has demonstrated a number of interactions between CART and the HPA axis, especially in regard to corticosteroids. Central CART injection produces increased plasma corticosterone and ACTH (Vrang et al., 2000; Stanley et al., 2001) and, reciprocally, CART expression in the brain and blood is subject to regulation by corticosteroids (Balkan et al., 2001; Vrang et al., 2003; Vicentic et al., 2004; Hunter et al., 2005; Koylu et al., 2006). More recently Balkan has shown that a forced swim stress increases CART peptide levels in the amygdala of male rats while lowering it in females (Balkan et al., 2006). Other evidence implicates CART in the HPG axis as well as the HPA, particularly in the regulation of GnRH secretion (Lebrethon et al., 2000a; Lebrethon et al., 2000b; Parent et al., 2000) and, controversially, the onset of puberty (Adam et al., 2000; Lebrethon et al., 2000b; Brann et al., 2002). On this basis we decided to examine changes in the levels of CART mRNA in the hippocampus and amygdala following acute stress in adolescent and adult animals and the effects of chronic stress and corticosteroid manipulations in adults.
Results
Acute stress
Based upon the aforementioned findings that CART may have a role in the onset of puberty in rodents we chose to analyze the effect of acute stress upon both adult and prepubertal animals. Analysis of CART mRNA expression in the dentate gyrus showed a significant effect of age (n=6, F (1,18)=10.66) but not stress with higher levels in unstressed prepubertal animals than adults in either condition (see Figure 1) and a trend towards higher levels in the stressed condition.
Figure 1.
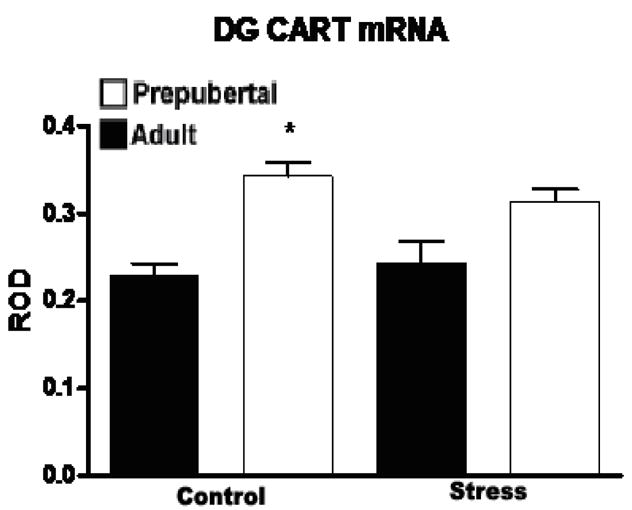
Relative optical density (ROD) of CART mRNA expression (+/− SEM) in the dentate gyrus of adult and prepubertal rats at baseline and 2 hours after a 30 minute restraint stress. Prepubertal rats showed significantly (F (1,18)=10.66) higher levels of CART mRNA expression than adults, though no effect of stress was observed at either age. Asterix indicates a significant difference between unstressed adult and prepubertal rats (n=6, p<0.05).
In the central amygdala, a similar pattern emerged with regard to age, though not to stress. There was a significant main effect of age (F (1,16)=59.93) such that both stressed and unstressed prepubertal animals showed substantially higher CART mRNA expression than unstressed adults. There was also a significant main effect of stress (F (1,16)=9.20) which was observed in adult animals but not the prepubertal rats. This increase did not produce CART mRNA levels comparable to those of prepubertal animals (see Figure 2), suggesting the possibility that the younger animals may be subject to a ceiling effect. Corticosterone levels for these animals was reported in (Romeo et al., 2004a) where it was shown that peak levels of corticosterone did not differ between adults and prepubescent rats, though the younger animals showed a longer elevation in corticosterone levels than the adults.
Figure 2.
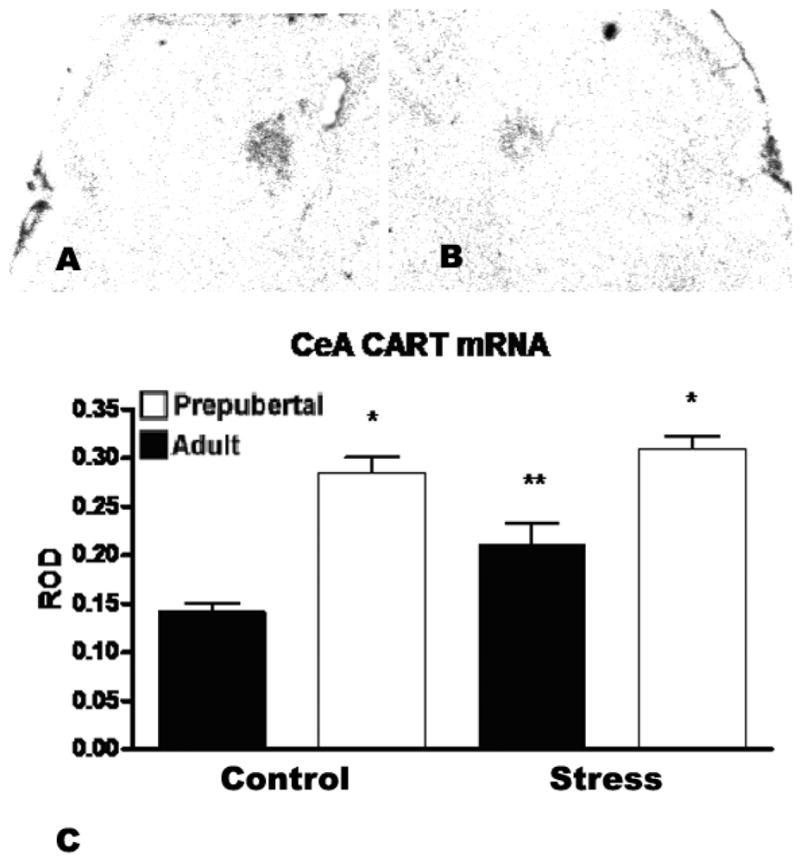
A) shows an autoradiogram of the region of the central amygala in an adult male rat acutely restrained for 30 minutes prior to sacrifice two hours later. B) shows the central amygdala of an unrestrained control. C) ROD of CART mRNA expression (+/− SEM) in the central amygdala of adult and prepubertal rats at baseline and 2 hours after a 30 minute restraint stress. Prepubertal rats showed significantly higher CART mRNA levels than adults (F (1,16)=59.93, n=6, p<0.001 vs. unstressed adults, p<0.05 vs. stressed adults), though no effect of stress. Adult animals showed a significant (F 1,16)=9.20, n=6, p<0.05 vs. unstressed adults and prepubertal rats) post-stress elevation of CART expression. Asterix indicates a significant difference between adult and prepubertal rats and a double asterix indicates a significant difference from both unstressed adults and prepubertal animals.
Chronic Stress
We followed up our acute stress studies with an examination of the effects of chronic restraint stress on adult male rats. Prepubertal animals were not included due to the absence of an effect in the acute stress study and due to the fact that the chronic restraint stress paradigm would include most if not all of the period of puberty.
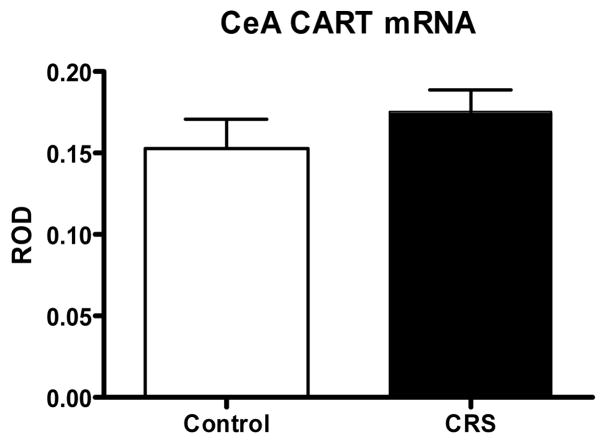
In contrast to the acute stress study, we found that chronic restraint stress increased the expression of CART mRNA in the dentate gyrus by more than 85% (±20%, p<0.05, see Figure 3.) and had no impact on CART mRNA levels in the central amygdala (Figure 4).
Figure 3.
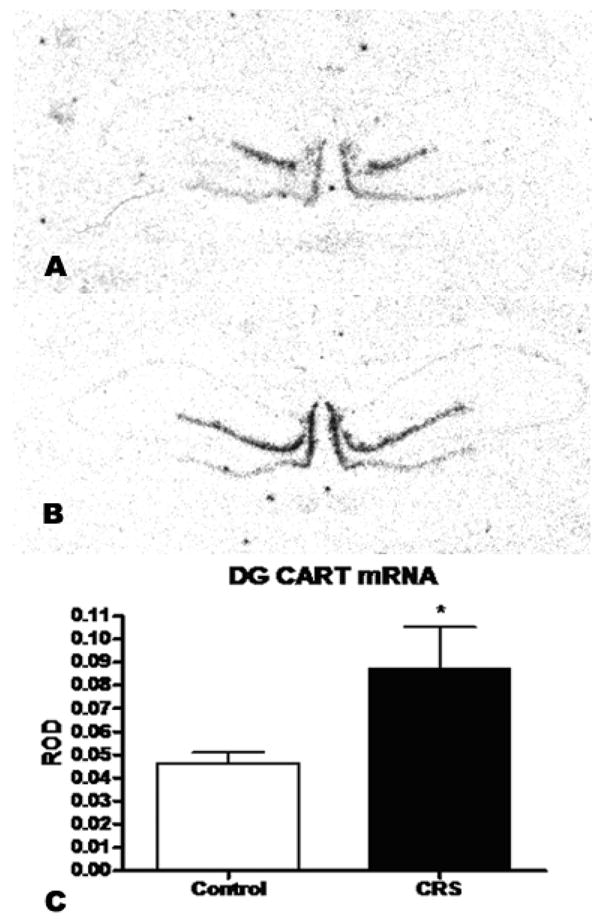
A) Shows an autoradiogram of CART mRNA hybridization signal in the DG of an unrestrained control rat. B) shows the CART hybridization signal in a chronically restrained animal. C) ROD of CART mRNA expression (+/− SEM) in the dentate gyrus of adult male rats after 21 days of chronic restraint stress. CRS significantly elevated CART expression in the dentate (p<0.05, n=8).
Figure 4.
ROD of CART mRNA expression (+/− SEM) in the central amygdala of adult rats after 21 day CRS. No effect of chronic stress was observed in this region (n=8).
Adrenalectomy and Corticosteroid Treatment
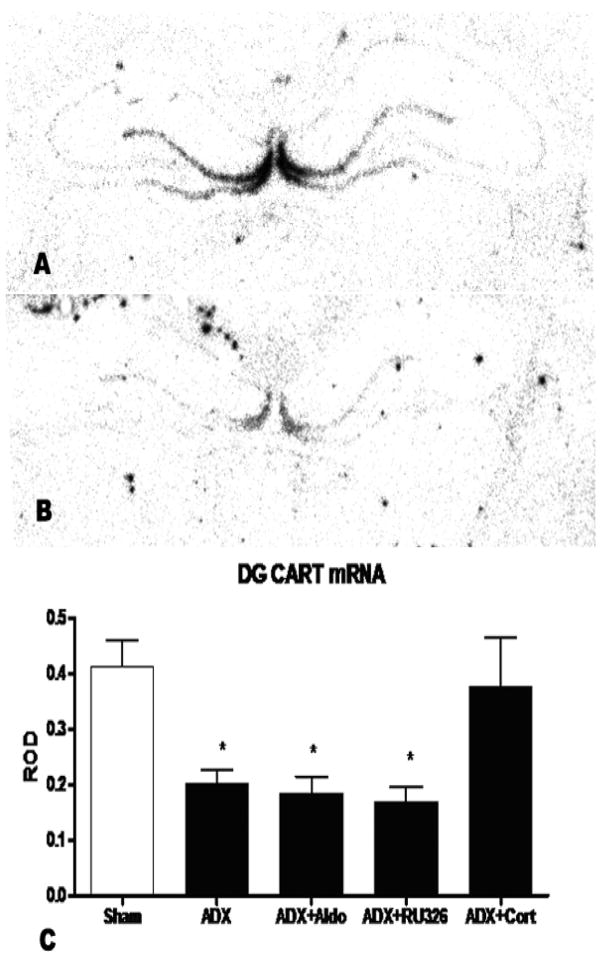
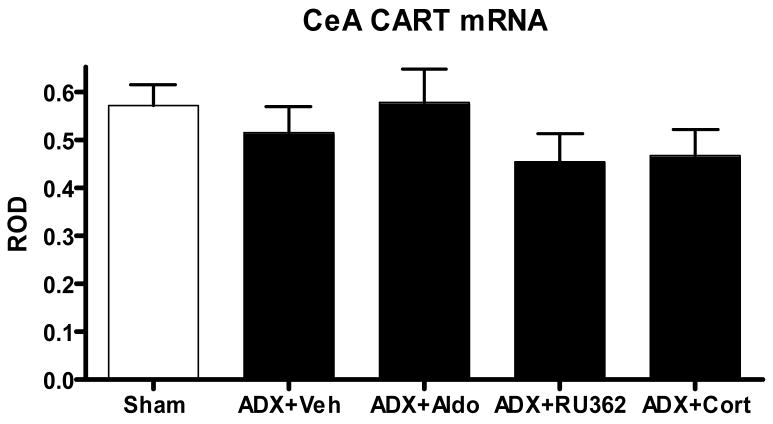
There was a significant main effect of treatment (F (4,30)=5.62) in the dentate (Figure 5.) but no effect in the central amygdala (Figure 6). Adrenalectomy reduced CART message by more than 50% (±12%, p<0.05). Neither the selective MR agonist aldosterone nor the selective GR agonist RU28,362, given alone, reversed this effect, but treatment with corticosterone, which occupies both MR and GR, did restore CART mRNA expression in the dentate.
Figure 5.
A) Shows an autoradiogram of CART mRNA hybridization signal in the DG of a control rat subjected to sham surgery. B) shows the CART hybridization signal in an adrenalectomized animal. C) ROD of CART mRNA expression (+/− SEM) in the dentate gyrus after either sham surgery, or adrenalectomy and 7 days of either vehicle, 10mg/hour aldosterone, 10 μg/hour RU28,362 or vehicle plus corticosterone in the drinking water. Adrenalectomy produced a significant reduction in dentate CART mRNA (F (4, 30)=5.62, p<0.05 sham versus ADX, n=7). This effect was blocked by corticosterone in the drinking water.
Figure 6.
ROD of CART mRNA expression (+/− SEM) in the central amygdala after either sham surgery, or adrenalectomy and 7 days of either vehicle, 10mg/hour aldosterone, 10 μg/hour RU28,362 or vehicle plus corticosterone in the drinking water. There was no effect of any of the treatments on CART mRNA levels in this region.
Discussion
Our results demonstrate that stress alters CART mRNA expression in a regionally specific manner in response to stress duration. In addition we show that acute stress has no impact on CART expression in adolescent animals relative to adults, though this may be a ceiling effect. We also show that CART is regulated by chronic corticosterone and adrenalectomy in the hippocampus but not in the amygdala by mechanisms potentially involving both MR and GR.
We first examined the effect of an acute restraint stress on CART mRNA expression in the prepubertal and adult rat brain. We found that acute stress elevates CART mRNA expression in the central amygdala of adults but not prepubertal animals. The change observed in the adults is in agreement with Balkan’s observations of CART peptide levels after an acute swim stress (Balkan et al., 2006). The elevated levels of CART mRNA in the prepubertal animals, which did not respond to stress, suggest that CART may contribute to the differences in stress response between this age group and adults (Romeo et al., 2004a; Romeo et al., 2004b; Romeo et al., 2006), though only further study will establish the actual relationship between CART and the juvenile stress response.
Our confirmation of Balkan’s result led us to examine whether chronic stress had the same impact on CART expression. The effect of chronic stress was in fact quite different from acute stress. CRS increased CART expression in the dentate gyrus and had no impact on CART mRNA levels in the central amygdala. Further, adrenalectomy reduced CART levels in the dentate gyrus, an effect which was reversed by corticosterone replacement. Adrenalectomy had no effect on CART expression in the amygdala. These data suggest that the regulation of CART in the amygdala is largely independent of corticosteroids and may depend on other elements of the stress response, such as catecholamines or endogenous neural activity.
In the hippocampus it is clear that corticosterone plays a role in the chronic regulation of CART expression. Adrenalectomy has been shown to down regulate CART expression in the brain and blood (Balkan et al., 2001; Vicentic et al., 2004) and glucocorticoid administration has been shown to elevate it in the hypothalamus and nucleus accumbens (Vrang et al., 2003; Hunter et al., 2005). It would appear that corticosterone acting in a non-classical fashion with regard to CART as neither activation of GR with the pure agonist RU28362 nor activation of the MR with the specific MR agonist, aldosterone, appears to have an effect upon CART expression after adrenalectomy. Given that the drugs in this study were systemically administered it is of course impossible be certain, based on the present data, of the mechanism by which corticosteroids or stress are altering CART expression. It is possible, however, that it may be due to interaction with CREB, as CREB has been shown to regulate CART expression in other systems (Dominguez et al., 2002; Lakatos et al., 2002; Dominguez and Kuhar, 2004; Jones and Kuhar, 2006) and the CART promoter is not known to possess a GRE site (see (Dominguez, 2006) for a review on CART promoter structure). Alternatively, CART may be regulated by the action of MR/GR heterodimers (Trapp et al., 1994; Trapp and Holsboer, 1996). Corticosterone binds to both receptors with high affinity and both are present within the dentate gyrus (Reul and de Kloet, 1985; Reul and de Kloet, 1986). In fact, the both adrenalectomy (Sloviter et al., 1989) and the GR selective agonist dexamethasone (Hassan et al., 1996) can produce cell death in the dentate, whereas corticosterone prevents these effects and there is evidence CART is neuroprotective (Louis, 1996; Wu et al., 2006; Xu et al., 2006). At present the mechanism by which CART is regulated by corticosterone is unclear.
It is also clear, given that adrenalectomy does not abolish CART expression in the hippocampus, that corticosteroids are not the only regulators of CART in that region. Interestingly, the hippocampus also expresses leptin receptors (Huang et al., 1996; Mercer et al., 1996; Shioda et al., 1998) and leptin is a regulator of CART expression in the hypothalamus (Kristensen et al., 1998). It is possible, therefore, that CART is also subject to regulation by leptin in the hippocampus as well.
Given that CART is known to be anxiogenic (Stanek, 2006), it is possible that the changes in CART expression observed here relate to the increases in anxiety levels produced by stress (Adamec and Shallow, 1993; Vyas et al., 2002; Gameiro et al., 2005). The up regulation of CART we observed in the hippocampus may also be neuroprotective, and there is evidence that the effects of chronic stress on the hippocampus may be reversible (Conrad et al., 1999; Sousa et al., 2000) and do not of themselves lead to neuron loss. In fact it has recently been shown that CART promotes the survival of cultured hippocampal neurons (Wu et al., 2006) and is also a mediator of the neuroprotective properties of estradiol (Xu et al., 2006).
In conclusion, evidence in this paper indicates that CART is robustly regulated by stressors in a regionally and temporally specific manner. Our findings extend and expand the emerging body of evidence that CART is a potentially important element of the stress response.
Experimental Procedures
Animals
Male Sprague Dawley rats were obtained from Charles River (Kingston, NY) at either 21 or 70 days of age. Animals were housed 2–3 per cage (same age cage mates) in clear polycarbonate cages with wood chip bedding. All animals were maintained on a 12 h light-dark schedule (lights on at 0800 h) and the temperature was kept at 21±2°C. All animals had ad libitum access to food and water. All procedures were carried out in accordance with the guidelines established by the NIH Guide for the Care and Use of Laboratory Animals.
Acute and chronic restraint stress
Animals were left undisturbed after arrival for one week (i.e., until either 28 or 77 days of age). For acute stress, animals were weighed and rapidly decapitated by a guillotine either immediately without being stressed (basal) or two hours after a 30 min session of restraint stress. CART was examined at the two hour time point based on previous studies of the time course of changes in CART expression (Hunter et al., 2006). Animals were restrained in wire mesh restrainers, secured at the head and tail ends with clips. Chronic stress experiments used the same restraint methodology as the acute stress, but the restraint was administered for 6 hours daily for 21 days. These animals were sacrificed 18 hours after the last stress. Further we chose not to examine prepubescent animals in the CRS and corticosteroid treatment experiments due to the absence of an effect in the acute stress experiment. Stressed animals were returned to their home cages immediately after termination of the stressor, until sacrifice. At the time of sacrifice, trunk blood samples were collected and hormone levels for these animals can be found in (Romeo et al., 2004a). Brains were removed and flash frozen on dry ice then stored at −80°C until processing. All animals were killed between 1300 and 1700 h.
Steroid Treatments
Doses were chosen based on those shown to have an effect upon a number of neurotransmitters and receptors in previous studies (Albeck et al., 1994; Kuroda et al., 1994; Watanabe et al., 1995) and these treatments follow those administered by Watanabe (Watanabe et al., 1995) with some modification. Animals were anesthetized using ketamine and xylazine and the adrenal glands removed, save for one group which received a sham surgery. During the same surgery, osmotic mini-pumps (Alzet, Cupertino, CA) were implanted s.c. between the scapula of all groups, including sham adrenalectomized animals. These pumps delivered either vehicle (propylene glycol), the mineralocorticoid receptor agonist aldosterone at 10mg/hour or the glucocorticoid receptor agonist RU28,362 at 10μg/hour. Animals who underwent ADX received 0.9% saline in their drinking water and one group received 400μg/ml corticosterone in addition to the saline, sham animals received tap water. Seven days after the completion of the surgeries the animals were sacrificed and their brains frozen as described above.
In Situ Hybridization
Brain sections were cut at 14 μm on a cryostat and placed on Fisher Biotech ProbeOn Plus slides (Fisher, Pittsburgh, PA). In situ hybridization began with a tailing reaction to radioactively label the oligonucleotide probes with 35S. The CART probe was 5′-ATC GGA ATG CGT TTA CTC TTG AGC TTC TTC AGG-3′. Processing of the slides followed methods as previously described (Couceyro et al., 1997) with some modification as described in (Hunter et al., 2006). Anatomical locations were determined with the assistance of the atlas of Paxinos and Watson (Paxinos and Watson, 1986), as well as previous studies of CART distribution in the hippocampus and amygdala (Douglass et al., 1995; Koylu et al., 1998; Hurd et al., 1999) and light microscopic examination of the relevant sections. Optical density was determined using MCID 5.0 (Imaging Research, St. Catharine’s, OT, Canada).
Statistics
Optical density measurements were analyzed by a two way ANOVA (stress versus age for the acute stress study), one way ANOVA for the chronic steroid study and Student’s t-test for the chronic stress study. Significant main effects and interactions in ANOVA were further analyzed using Fisher’s protected least significant difference test and Tukey’s test, respectively. Differences are considered significant at p<0.05. All data are presented as mean ±SEM.
Acknowledgments
This work was supported by NIH Training grant MH 15125, Bruce McEwen MH41256 and Russ Romeo MH065749.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adam CL, Moar KM, et al. Photoperiod regulates growth, puberty and hypothalamic neuropeptide and receptor gene expression in female Siberian hamsters. Endocrinology. 2000;141(12):4349–56. doi: 10.1210/endo.141.12.7807. [DOI] [PubMed] [Google Scholar]
- Adamec RE, Shallow T. Lasting effects on rodent anxiety of a single exposure to a cat. Physiol Behav. 1993;54(1):101–9. doi: 10.1016/0031-9384(93)90050-p. [DOI] [PubMed] [Google Scholar]
- Albeck DS, Hastings NB, et al. Effects of adrenalectomy and type I or type II glucocorticoid receptor activation on AVP and CRH mRNA in the rat hypothalamus. Brain Res Mol Brain Res. 1994;26(1–2):129–34. doi: 10.1016/0169-328x(94)90083-3. [DOI] [PubMed] [Google Scholar]
- Asakawa A, Inui A, et al. Cocaine-amphetamine-regulated transcript influences energy metabolism, anxiety and gastric emptying in mice. Horm Metab Res. 2001;33(9):554–8. doi: 10.1055/s-2001-17205. [DOI] [PubMed] [Google Scholar]
- Balkan B, Gozen O, et al. CART expression in limbic regions of rat brain following forced swim stress: sex differences. Neuropeptides. 2006;40(3):185–93. doi: 10.1016/j.npep.2006.02.001. [DOI] [PubMed] [Google Scholar]
- Balkan B, Koylu EO, et al. The effect of adrenalectomy on cocaine and amphetamine-regulated transcript (CART) expression in the hypothalamic nuclei of the rat. Brain Res. 2001;917(1):15–20. doi: 10.1016/s0006-8993(01)02899-2. [DOI] [PubMed] [Google Scholar]
- Brann DW, Wade MF, et al. Leptin and reproduction. Steroids. 2002;67(2):95–104. doi: 10.1016/s0039-128x(01)00138-6. [DOI] [PubMed] [Google Scholar]
- Chaki S, Kawashima N, et al. Cocaine- and amphetamine-regulated transcript peptide produces anxiety-like behavior in rodents. Eur J Pharmacol. 2003;464(1):49–54. doi: 10.1016/s0014-2999(03)01368-2. [DOI] [PubMed] [Google Scholar]
- Conrad CD, LeDoux JE, et al. Repeated restraint stress facilitates fear conditioning independently of causing hippocampal CA3 dendritic atrophy. Behav Neurosci. 1999;113(5):902–13. doi: 10.1037//0735-7044.113.5.902. [DOI] [PubMed] [Google Scholar]
- Couceyro P, Koylu E, et al. Further studies on the anatomical distribution of CART by in situ hybridization. J Chem Neuroanat. 1997;12:229–241. doi: 10.1016/s0891-0618(97)00212-3. [DOI] [PubMed] [Google Scholar]
- Dominguez G. The CART gene: structure and regulation. Peptides. 2006;27(8):1913–8. doi: 10.1016/j.peptides.2006.01.025. [DOI] [PubMed] [Google Scholar]
- Dominguez G, Kuhar MJ. Transcriptional regulation of the CART promoter in CATH. a cells. Brain Res Mol Brain Res. 2004;126(1):22–9. doi: 10.1016/j.molbrainres.2004.02.027. [DOI] [PubMed] [Google Scholar]
- Dominguez G, Lakatos A, et al. Characterization of the cocaine- and amphetamine-regulated transcript (CART) peptide gene promoter and its activation by a cyclic AMP-dependent signaling pathway in GH3 cells. J Neurochem. 2002;80(5):885–93. doi: 10.1046/j.0022-3042.2002.00775.x. [DOI] [PubMed] [Google Scholar]
- Douglass J, McKinzie AA, et al. PCR differential display identifies a rat brain mRNA that is transcriptionally regulated by cocaine and amphetamine. J Neurosci. 1995;15(3 Pt 2):2471–81. doi: 10.1523/JNEUROSCI.15-03-02471.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gameiro GH, Andrade Ada S, et al. The effects of restraint stress on nociceptive responses induced by formalin injected in rat’s TMJ. Pharmacol Biochem Behav. 2005;82(2):338–44. doi: 10.1016/j.pbb.2005.09.003. [DOI] [PubMed] [Google Scholar]
- Hassan AH, von Rosenstiel P, et al. Exacerbation of apoptosis in the dentate gyrus of the aged rat by dexamethasone and the protective role of corticosterone. Exp Neurol. 1996;140(1):43–52. doi: 10.1006/exnr.1996.0113. [DOI] [PubMed] [Google Scholar]
- Huang XF, Koutcherov I, et al. Localization of leptin receptor mRNA expression in mouse brain. Neuroreport. 1996;7(15–17):2635–8. doi: 10.1097/00001756-199611040-00045. [DOI] [PubMed] [Google Scholar]
- Hunter RG, Jones D, et al. Regulation of CART mRNA in the rat nucleus accumbens via D3 dopamine receptors. Neuropharmacology. 2006 doi: 10.1016/j.neuropharm.2005.12.007. [DOI] [PubMed] [Google Scholar]
- Hunter RG, Kuhar MJ. CART peptides as targets for CNS drug development. Curr Drug Target CNS Neurol Disord. 2003;2(3):201–5. doi: 10.2174/1568007033482896. [DOI] [PubMed] [Google Scholar]
- Hunter RG, Vicentic A, et al. The effects of cocaine on CART expression in the rat nucleus accumbens: a possible role for corticosterone. Eur J Pharmacol. 2005;517(1–2):45–50. doi: 10.1016/j.ejphar.2005.05.025. [DOI] [PubMed] [Google Scholar]
- Hurd YL, Svensson P, et al. The role of dopamine, dynorphin, and CART systems in the ventral striatum and amygdala in cocaine abuse. Ann N Y Acad Sci. 1999;877:499–506. doi: 10.1111/j.1749-6632.1999.tb09285.x. [DOI] [PubMed] [Google Scholar]
- Jones DC, Kuhar MJ. Cocaine-amphetamine-regulated transcript expression in the rat nucleus accumbens is regulated by adenylyl cyclase and the cyclic adenosine 5′-monophosphate/protein kinase a second messenger system. J Pharmacol Exp Ther. 2006;317(1):454–61. doi: 10.1124/jpet.105.096123. [DOI] [PubMed] [Google Scholar]
- Kask A, Schioth HB, et al. Anorexigenic cocaine- and amphetamine-regulated transcript peptide intensifies fear reactions in rats. Brain Res. 2000;857(1–2):283–5. doi: 10.1016/s0006-8993(99)02383-5. [DOI] [PubMed] [Google Scholar]
- Koylu EO, Balkan B, et al. Cocaine and amphetamine regulated transcript (CART) and the stress response. Peptides. 2006;27(8):1956–69. doi: 10.1016/j.peptides.2006.03.032. [DOI] [PubMed] [Google Scholar]
- Koylu EO, Couceyro PR, et al. Cocaine- and amphetamine-regulated transcript peptide immunohistochemical localization in the rat brain. J Comp Neurol. 1998;391(1):115–32. [PubMed] [Google Scholar]
- Koylu EO, Couceyro PR, et al. Immunohistochemical localization of novel CART peptides in rat hypothalamus, pituitary and adrenal gland. J Neuroendocrinol. 1997;9(11):823–33. doi: 10.1046/j.1365-2826.1997.00651.x. [DOI] [PubMed] [Google Scholar]
- Kristensen P, Judge ME, et al. Hypothalamic CART is a new anorectic peptide regulated by leptin. Nature. 1998;393(6680):72–6. doi: 10.1038/29993. [DOI] [PubMed] [Google Scholar]
- Kuroda Y, Watanabe Y, et al. Effects of adrenalectomy and type I or type II glucocorticoid receptor activation on 5-HT1A and 5-HT2 receptor binding and 5-HT transporter mRNA expression in rat brain. Brain Res. 1994;648(1):157–61. doi: 10.1016/0006-8993(94)91916-x. [DOI] [PubMed] [Google Scholar]
- Lakatos A, Dominguez G, et al. CART promoter CRE site binds phosphorylated CREB. Brain Res Mol Brain Res. 2002;104(1):81–5. doi: 10.1016/s0169-328x(02)00321-2. [DOI] [PubMed] [Google Scholar]
- Lebrethon MC, Vandersmissen E, et al. Cocaine and amphetamine-regulated-transcript peptide mediation of leptin stimulatory effect on the rat gonadotropin-releasing hormone pulse generator in vitro. J Neuroendocrinol. 2000a;12(5):383–5. doi: 10.1046/j.1365-2826.2000.00497.x. [DOI] [PubMed] [Google Scholar]
- Lebrethon MC, Vandersmissen E, et al. In vitro stimulation of the prepubertal rat gonadotropin-releasing hormone pulse generator by leptin and neuropeptide Y through distinct mechanisms. Endocrinology. 2000b;141(4):1464–9. doi: 10.1210/endo.141.4.7432. [DOI] [PubMed] [Google Scholar]
- Louis JCM. Methods of preventing neuron degeneration and promoting neuron regeneration. Amgen, International Patent Application Publication. 1996 #WO96/34619. [Google Scholar]
- McEwen BS, Olie JP. Neurobiology of mood, anxiety, and emotions as revealed by studies of a unique antidepressant: tianeptine. Mol Psychiatry. 2005;10(6):525–37. doi: 10.1038/sj.mp.4001648. [DOI] [PubMed] [Google Scholar]
- Mercer JG, Hoggard N, et al. Localization of leptin receptor mRNA and the long form splice variant (Ob-Rb) in mouse hypothalamus and adjacent brain regions by in situ hybridization. FEBS Lett. 1996;387(2–3):113–6. doi: 10.1016/0014-5793(96)00473-5. [DOI] [PubMed] [Google Scholar]
- Miraglia Del Giudice E, Santoro N, et al. Adolescents carrying a missense mutation in the CART gene exhibit increased anxiety and depression. Depress Anxiety. 2006 doi: 10.1002/da.20156. [DOI] [PubMed] [Google Scholar]
- Parent AS, Lebrethon MC, et al. Leptin effects on pulsatile gonadotropin releasing hormone secretion from the adult rat hypothalamus and interaction with cocaine and amphetamine regulated transcript peptide and neuropeptide Y. Regul Pept. 2000;92(1–3):17–24. doi: 10.1016/s0167-0115(00)00144-0. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. New York: Academic Press; 1986. [Google Scholar]
- Reul JM, de Kloet ER. Two receptor systems for corticosterone in rat brain: microdistribution and differential occupation. Endocrinology. 1985;117(6):2505–11. doi: 10.1210/endo-117-6-2505. [DOI] [PubMed] [Google Scholar]
- Reul JM, de Kloet ER. Anatomical resolution of two types of corticosterone receptor sites in rat brain with in vitro autoradiography and computerized image analysis. J Steroid Biochem. 1986;24(1):269–72. doi: 10.1016/0022-4731(86)90063-4. [DOI] [PubMed] [Google Scholar]
- Romeo RD, Bellani R, et al. Stress history and pubertal development interact to shape hypothalamic-pituitary-adrenal axis plasticity. Endocrinology. 2006 doi: 10.1210/en.2005-1432. [DOI] [PubMed] [Google Scholar]
- Romeo RD, Lee SJ, et al. Testosterone cannot activate an adult-like stress response in prepubertal male rats. Neuroendocrinology. 2004a;79(3):125–32. doi: 10.1159/000077270. [DOI] [PubMed] [Google Scholar]
- Romeo RD, Lee SJ, et al. Differential stress reactivity in intact and ovariectomized prepubertal and adult female rats. Neuroendocrinology. 2004b;80(6):387–93. doi: 10.1159/000084203. [DOI] [PubMed] [Google Scholar]
- Shioda S, Funahashi H, et al. Immunohistochemical localization of leptin receptor in the rat brain. Neurosci Lett. 1998;243(1–3):41–4. doi: 10.1016/s0304-3940(98)00082-2. [DOI] [PubMed] [Google Scholar]
- Sloviter RS, Valiquette G, et al. Selective loss of hippocampal granule cells in the mature rat brain after adrenalectomy. Science. 1989;243(4890):535–8. doi: 10.1126/science.2911756. [DOI] [PubMed] [Google Scholar]
- Sousa N, Lukoyanov NV, et al. Reorganization of the morphology of hippocampal neurites and synapses after stress-induced damage correlates with behavioral improvement. Neuroscience. 2000;97(2):253–66. doi: 10.1016/s0306-4522(00)00050-6. [DOI] [PubMed] [Google Scholar]
- Stanek LM. Cocaine- and amphetamine related transcript (CART) and anxiety. Peptides. 2006;27(8):2005–11. doi: 10.1016/j.peptides.2006.01.027. [DOI] [PubMed] [Google Scholar]
- Stanley SA, Small CJ, et al. Actions of cocaine- and amphetamine-regulated transcript (CART) peptide on regulation of appetite and hypothalamo-pituitary axes in vitro and in vivo in male rats. Brain Res. 2001;893(1–2):186–94. doi: 10.1016/s0006-8993(00)03312-6. [DOI] [PubMed] [Google Scholar]
- Trapp T, Holsboer F. Heterodimerization between mineralocorticoid and glucocorticoid receptors increases the functional diversity of corticosteroid action. Trends Pharmacol Sci. 1996;17(4):145–9. doi: 10.1016/0165-6147(96)81590-2. [DOI] [PubMed] [Google Scholar]
- Trapp T, Rupprecht R, et al. Heterodimerization between mineralocorticoid and glucocorticoid receptor: a new principle of glucocorticoid action in the CNS. Neuron. 1994;13(6):1457–62. doi: 10.1016/0896-6273(94)90431-6. [DOI] [PubMed] [Google Scholar]
- Vicentic A, Dominguez G, et al. CART Peptide Levels in Blood Exhibit a Diurnal Rhythm: Regulation by Glucocorticoids. Endocrinology. 2004 doi: 10.1210/en.2003-1648. [DOI] [PubMed] [Google Scholar]
- Vrang N, Larsen PJ, et al. Central administration of cocaine-amphetamine-regulated transcript activates hypothalamic neuroendocrine neurons in the rat. Endocrinology. 2000;141(2):794–801. doi: 10.1210/endo.141.2.7295. [DOI] [PubMed] [Google Scholar]
- Vrang N, Larsen PJ, et al. Hypothalamic cocaine-amphetamine regulated transcript (CART) is regulated by glucocorticoids. Brain Res. 2003;965(1–2):45–50. doi: 10.1016/s0006-8993(02)04064-7. [DOI] [PubMed] [Google Scholar]
- Vyas A, Mitra R, et al. Chronic stress induces contrasting patterns of dendritic remodeling in hippocampal and amygdaloid neurons. J Neurosci. 2002;22(15):6810–8. doi: 10.1523/JNEUROSCI.22-15-06810.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe Y, Weiland NG, et al. Effects of adrenal steroid manipulations and repeated restraint stress on dynorphin mRNA levels and excitatory amino acid receptor binding in hippocampus. Brain Res. 1995;680(1–2):217–25. doi: 10.1016/0006-8993(95)00235-i. [DOI] [PubMed] [Google Scholar]
- Wu B, Hu S, et al. CART peptide promotes the survival of hippocampal neurons by upregulating brain-derived neurotrophic factor. Biochem Biophys Res Commun. 2006;347(3):656–61. doi: 10.1016/j.bbrc.2006.06.117. [DOI] [PubMed] [Google Scholar]
- Xu Y, Zhang W, et al. Role of cocaine- and amphetamine-regulated transcript in estradiol-mediated neuroprotection. Proc Natl Acad Sci U S A. 2006;103(39):14489–94. doi: 10.1073/pnas.0602932103. [DOI] [PMC free article] [PubMed] [Google Scholar]