Abstract
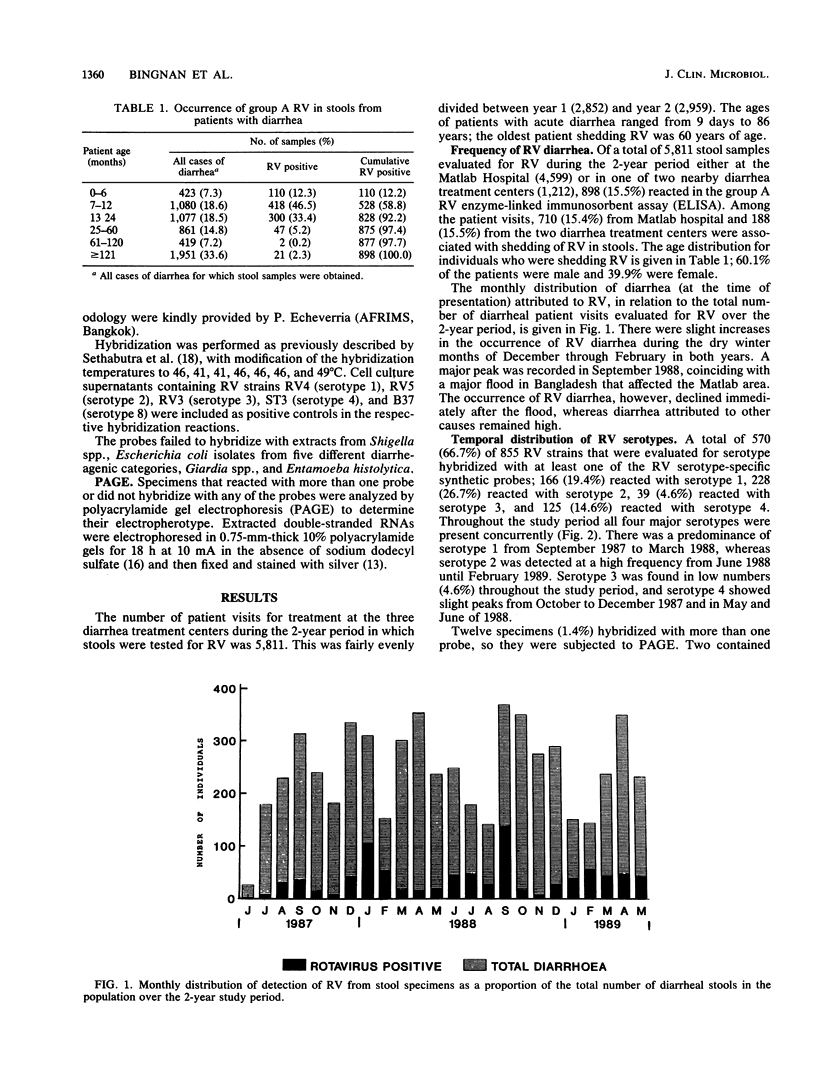
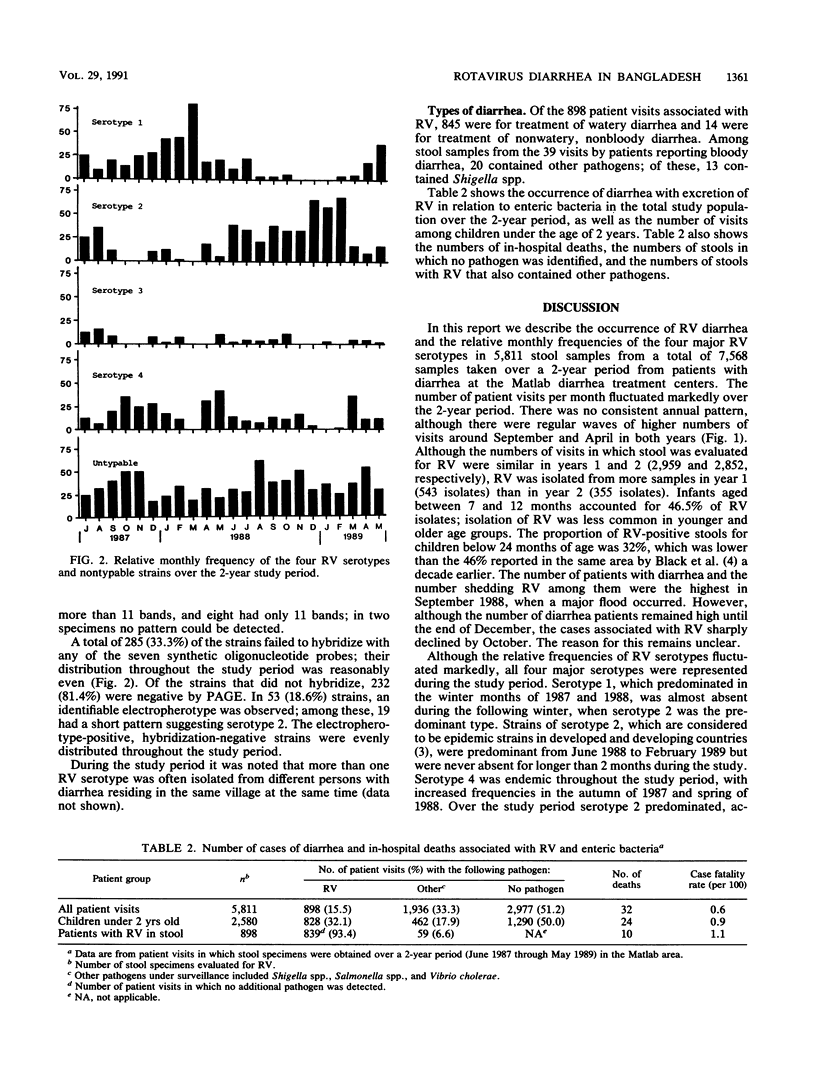
Stools were evaluated from 5,811 patient visits for treatment of diarrhea in Matlab, Bangladesh, between June 1987 and May 1989. The stools were analyzed to determine the distribution of serotypes of group A rotaviruses (RV). A total of 898 stool samples (15.5%) contained RV, as determined by using an enzyme-linked immunosorbent assay. RV isolates from 855 of these samples were serotyped by using serotype-specific synthetic oligonucleotide probes. A total of 558 (65.3%) could be assigned to specific serotypes: 166 (19.4%), 228 (26.7%), 39 (4.6%), and 125 (14.6%) belonged to serotypes 1 through 4, respectively; 12 (1.4%) hybridized with more than one serotype; and 285 (33.3%) failed to hybridize. RV diarrhea was evident throughout the year, with peaks in the dry winter months and in September 1988, coinciding with a major flood. RV was isolated from 46.6% of patients between 7 and 12 months old. Among children under 24 months of age with RV diarrhea, 1.2% (10 of 828) died. The corresponding percentage for children with diarrhea from all causes is 0.9% (29 of 3,301).
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmed M. U., Taniguchi K., Kobayashi N., Urasawa T., Wakasugi F., Islam M., Shaikh H., Urasawa S. Characterization by enzyme-linked immunosorbent assay using subgroup- and serotype-specific monoclonal antibodies of human rotavirus obtained from diarrheic patients in Bangladesh. J Clin Microbiol. 1989 Jul;27(7):1678–1681. doi: 10.1128/jcm.27.7.1678-1681.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert M. J., Unicomb L. E., Bishop R. F. Cultivation and characterization of human rotaviruses with "super short" RNA patterns. J Clin Microbiol. 1987 Jan;25(1):183–185. doi: 10.1128/jcm.25.1.183-185.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black R. E., Merson M. H., Rahman A. S., Yunus M., Alim A. R., Huq I., Yolken R. H., Curlin G. T. A two-year study of bacterial, viral, and parasitic agents associated with diarrhea in rural Bangladesh. J Infect Dis. 1980 Nov;142(5):660–664. doi: 10.1093/infdis/142.5.660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D. W., Mathan M. M., Mathew M., Martin R., Beards G. M., Mathan V. I. Rotavirus epidemiology in Vellore, south India: group, subgroup, serotype, and electrophoretype. J Clin Microbiol. 1988 Nov;26(11):2410–2414. doi: 10.1128/jcm.26.11.2410-2414.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemens J. D., Harris J. R., Sack D. A., Chakraborty J., Ahmed F., Stanton B. F., Khan M. U., Kay B. A., Huda N., Khan M. R. Field trial of oral cholera vaccines in Bangladesh: results of one year of follow-up. J Infect Dis. 1988 Jul;158(1):60–69. doi: 10.1093/infdis/158.1.60. [DOI] [PubMed] [Google Scholar]
- Clemens J. D., Sack D. A., Harris J. R., Chakraborty J., Khan M. R., Stanton B. F., Ali M., Ahmed F., Yunus M., Kay B. A. Impact of B subunit killed whole-cell and killed whole-cell-only oral vaccines against cholera upon treated diarrhoeal illness and mortality in an area endemic for cholera. Lancet. 1988 Jun 18;1(8599):1375–1379. doi: 10.1016/s0140-6736(88)92189-7. [DOI] [PubMed] [Google Scholar]
- Clemens J. D., Sack D. A., Harris J. R., Chakraborty J., Khan M. R., Stanton B. F., Ali M., Ahmed F., Yunus M., Kay B. A. Impact of B subunit killed whole-cell and killed whole-cell-only oral vaccines against cholera upon treated diarrhoeal illness and mortality in an area endemic for cholera. Lancet. 1988 Jun 18;1(8599):1375–1379. doi: 10.1016/s0140-6736(88)92189-7. [DOI] [PubMed] [Google Scholar]
- Clemens J. D., Sack D. A., Harris J. R., Chakraborty J., Khan M. R., Stanton B. F., Kay B. A., Khan M. U., Yunus M., Atkinson W. Field trial of oral cholera vaccines in Bangladesh. Lancet. 1986 Jul 19;2(8499):124–127. doi: 10.1016/s0140-6736(86)91944-6. [DOI] [PubMed] [Google Scholar]
- Clemens J. D., Sack D. A., Harris J. R., Chakraborty J., Neogy P. K., Stanton B., Huda N., Khan M. U., Kay B. A., Khan M. R. Cross-protection by B subunit-whole cell cholera vaccine against diarrhea associated with heat-labile toxin-producing enterotoxigenic Escherichia coli: results of a large-scale field trial. J Infect Dis. 1988 Aug;158(2):372–377. doi: 10.1093/infdis/158.2.372. [DOI] [PubMed] [Google Scholar]
- Coulson B. S., Unicomb L. E., Pitson G. A., Bishop R. F. Simple and specific enzyme immunoassay using monoclonal antibodies for serotyping human rotaviruses. J Clin Microbiol. 1987 Mar;25(3):509–515. doi: 10.1128/jcm.25.3.509-515.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyall-Smith M. L., Holmes I. H. Sequence homology between human and animal rotavirus serotype-specific glycoproteins. Nucleic Acids Res. 1984 May 11;12(9):3973–3982. doi: 10.1093/nar/12.9.3973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores J., Taniguchi K., Green K., Perez-Schael I., Garcia D., Sears J., Urasawa S., Kapikian A. Z. Relative frequencies of rotavirus serotypes 1, 2, 3, and 4 in Venezuelan infants with gastroenteritis. J Clin Microbiol. 1988 Oct;26(10):2092–2095. doi: 10.1128/jcm.26.10.2092-2095.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herring A. J., Inglis N. F., Ojeh C. K., Snodgrass D. R., Menzies J. D. Rapid diagnosis of rotavirus infection by direct detection of viral nucleic acid in silver-stained polyacrylamide gels. J Clin Microbiol. 1982 Sep;16(3):473–477. doi: 10.1128/jcm.16.3.473-477.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Matsuno S., Hasegawa A., Mukoyama A., Inouye S. A candidate for a new serotype of human rotavirus. J Virol. 1985 May;54(2):623–624. doi: 10.1128/jvi.54.2.623-624.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sethabutr O., Unicomb L. E., Holmes I. H., Taylor D. N., Bishop R. F., Echeverria P. Serotyping of human group A rotavirus with oligonucleotide probes. J Infect Dis. 1990 Aug;162(2):368–372. doi: 10.1093/infdis/162.2.368. [DOI] [PubMed] [Google Scholar]
- Taniguchi K., Urasawa T., Morita Y., Greenberg H. B., Urasawa S. Direct serotyping of human rotavirus in stools by an enzyme-linked immunosorbent assay using serotype 1-, 2-, 3-, and 4-specific monoclonal antibodies to VP7. J Infect Dis. 1987 Jun;155(6):1159–1166. doi: 10.1093/infdis/155.6.1159. [DOI] [PubMed] [Google Scholar]
- Unicomb L. E., Bishop R. F. Epidemiology of rotavirus strains infecting children throughout Australia during 1986-1987. A study of serotype and RNA electropherotype. Arch Virol. 1989;106(1-2):23–34. doi: 10.1007/BF01311035. [DOI] [PubMed] [Google Scholar]
- Unicomb L. E., Coulson B. S., Bishop R. F. Experience with an enzyme immunoassay for serotyping human group A rotaviruses. J Clin Microbiol. 1989 Mar;27(3):586–588. doi: 10.1128/jcm.27.3.586-588.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urasawa S., Urasawa T., Taniguchi K., Wakasugi F., Kobayashi N., Chiba S., Sakurada N., Morita M., Morita O., Tokieda M. Survey of human rotavirus serotypes in different locales in Japan by enzyme-linked immunosorbent assay with monoclonal antibodies. J Infect Dis. 1989 Jul;160(1):44–51. doi: 10.1093/infdis/160.1.44. [DOI] [PubMed] [Google Scholar]
- de Zoysa I., Feachem R. G. Interventions for the control of diarrhoeal diseases among young children: rotavirus and cholera immunization. Bull World Health Organ. 1985;63(3):569–583. [PMC free article] [PubMed] [Google Scholar]


