Abstract
Consumption of food or feed contaminated with fumonisin B1 (FB1), a mycotoxin produced by Fusarium verticillioides, can lead to disease in humans and animals. The present study was conducted to examine the effect of FB1 intake on the intestinal immune system. Piglets were used as a target and as a model species for humans since their gastro-intestinal tract is very similar. The animals were orally exposed to a low dose of FB1 (1 mg/kg body weight FB1) for 10 days which did not result in clinical signs. However, when compared to non-exposed animals, FB1-exposed animals showed a longer shedding of F4+ enterotoxigenic Escherichia coli (ETEC) following infection and a lower induction of the antigen-specific immune response following oral immunization. Further analyses to elucidate the mechanisms behind these observations revealed a reduced intestinal expression of IL-12p40, an impaired function of intestinal antigen presenting cells (APC), with decreased upregulation of Major Histocompatibility Complex Class II molecule (MHC-II) and reduced T cell stimulatory capacity upon stimulation. Taken together, these results indicate an FB1-mediated reduction of in vivo APC maturation.
Keywords: fumonisin B1, intestinal APC, F4(K88)+ enterotoxigenic Escherichia coli, mucosal immunization
1. INTRODUCTION
Mycotoxins are secondary fungal metabolites that can be produced in crops and other food commodities both pre- and post-harvest. The consumption of contaminated food/feed can be responsible for diseases in humans and animals [26, 30]. The fumonisins, which are produced by Fusarium verticillioides and related fungi, are among the most widespread mycotoxins [23]. Different types of fumonisins have been structurally identified. The ‘B’ series include the most prevalent type, the fumonisin B1 (FB1), which is estimated to contaminate 59% of the corn and corn-based products worldwide [14]. Consumption of FB1-contaminated moldy sorghum and maize can result in several human gastro-intestinal disorders [3]. An increased prevalence of neural tube defects among Mexican-American women is also attributed to FB1 contamination of the crop harvest. Furthermore, fumonisins can cause liver and kidney toxicity and carcinogenicity, pulmonary edema and immunosuppression [24]. Their mode of action is not completely understood but they are known to influence the sphingolipid metabolism through the inhibition of ceramide synthase [11]. Sphingolipids are a class of membrane lipids which play an important role in several cellular mechanisms such as signal transduction pathways, cell contact, growth, differentiation and death, and immunity.
As fumonisins enter the body following consumption of contaminated food/feed, the intestine will be the first organ exposed to these toxins and negative effects of these toxins on the gastro-intestinal tract have been reported [9]. In pig, FB1 increases the intestinal colonization by pathogenic Escherichia coli [27]. This could be attributed to the negative impact of FB1 on the intestinal barrier. Indeed, FB1 alters the intestinal barrier function by influencing the sphingolipid metabolism, as demonstrated by an increase in the amount of free sphingoid bases, a depletion of glycolipids in the plasma membrane and an increase in trans-epithelial flux [6, 18]. However, an effect of FB1 on the mucosal immune system could also be involved in the altered interaction between FB1-exposed animals and intestinal pathogens. Several studies demonstrated that FB1 affects the innate immunity, as well as humoral and cellular responses from the acquired immunity [2, 7, 21, 24, 38]. It is shown that FB1 decreases IL-8 expression both in intestinal tissue and in an intestinal epithelial cell line [8]. Further information concerning the mode of action of FB1 on the intestinal immune system is however needed.
F4+ enterotoxigenic E. coli (ETEC) adhere with their F4 fimbriae to F4-specific receptors (F4R) on the intestinal epithelium of piglets, then colonize the small intestine and induce diarrhea. Following infection, an F4-specific immune response is induced within a week which terminates the F4+ ETEC excretion [44]. F4 fimbriae induce a strong mucosal immunity in piglets. Mucosal immunization of weaned piglets with purified F4 fimbriae induces a potent F4-specific intestinal immune response protecting these animals against a subsequent oral challenge with F4+ ETEC [41]. The F4-mediated adhesion to specific F4R is required for this protective F4-specific immune response as an oral immunization of F4R-negative animals fails to induce such an immune response [42]. In the present study, the F4 model is used to analyze the influence of FB1 feeding on the porcine intestinal immune system.
2. MATERIALS AND METHODS
2.1. Experimental design
2.1.1. Animals and housing
Twenty five conventionally bred pigs (Belgian Landrace × Pietrain) weaned between 3 and 4 weeks of age were housed in isolation units. They were fed ad libitum with a commercial starter diet and treated orally with colistine, an antibiotic against Gr- bacteria, for three consecutive days following weaning (Promycine pulvis, VMD, Berendonk, Belgium, 150 000 U/kg body weight/day) to prevent E. coli infections which could arise due to handling and transport of the animals. The piglets were screened to be F4-seronegative using the F4-specific ELISA [41] as well as F4R+ using the PCR method described by Rasschaert et al. [33]. The presence of the F4R was confirmed post-mortem using the in vitro villous adhesion assay. Adhesion of > 5 bacteria per 250 μm villous length was considered as positive [10]. Males and females were equally divided in the groups. All animal procedures were approved by a local ethical committee.
2.1.2. FB1 feeding and allotment
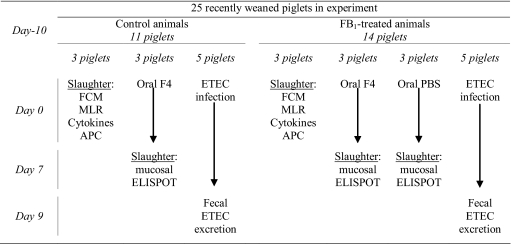
Piglets were randomly divided into a group that received a FB1-containing fungal extract (n = 11) and a group that received PBS (n = 14) (Tab. I). FB1 was extracted from an in vitro culture of the Fusarium verticilloides strain NRRL 34281 as previously described [27]. This crude extract contained 54% FB1, 8% FB2 and 9% FB3 and no detectable amounts of zearalenone, fusarochromanone, deoxynivalenol or other thrichothecenes. One week after weaning, FB1 (or PBS) was orally provided at a dose of 1 mg/kg of body weight/day in 10 mL PBS for 10 days (day -10 until day 0). All animals were weighed at the beginning and at the end of the administration period. On day 0, animals were either challenged with an F4+ ETEC strain (n = 10), orally immunized with purified F4 fimbriae (n = 9) or euthanized to analyze the effects of FB1 on the mucosal immune system (n = 6).
Table I.
Overview of the experimental design and analyses.
FCM: Flow CytoMetry; MLR: Mixed Lymphocyte Reaction; APC: antigen presenting cells.
2.2. Experimental infection and immunization
2.2.1. F4+ ETEC infection
Five piglets of both the FB1 and PBS group were orally infected with the F4+ ETEC strain GIS26 (Tab. I). Piglets were sedated with Stresnil (40 mg/mL; Janssen-Cilag, Berchem, Belgium), after which the gastric pH was neutralized by intragastric administration of 62 mL NaHCO3 (1.4% (w/v) in distilled water). Next, 1010 F4+ ETEC in 10 mL PBS were intragastrically administered. From day 0 till day 9, the fecal F4+ E. coli excretion was daily quantified by dot blotting, using the F4-specific monoclonal antibody (mAb) IMM01 as previously described [41]. The prevalence and the severity of diarrhea were scored blindly according to the visual aspect of the feces. All piglets were weighed every 3 days from the start of the FB1 administration till the end of the F4+ E. coli excretion.
2.2.2. Oral F4 E. coli immunization
Three animals of both the FB1 and the PBS group were orally immunized with 2 mg purified F4 fimbriae in 10 mL PBS (FB1+F4 and F4 group respectively) on three subsequent days (day 0–2) (Tab. I). Three non-exposed animals receiving only PBS served as negative controls (PBS group). F4 fimbriae were purified from strain IMM01 as previously described [41]. From 3 h before till 2 h after immunization, piglets were deprived of food and water. One week later (day 7–8), the animals were euthanized by intracardial injection of pentobarbital (24 mg/kg; Nembutal, Sanofi Santé Animale, Brussels, Belgium) followed by exsanguination. Subsequently, monomorphonuclear cells (MC) were isolated from the jejunal lamina propria (LP) as described in Section 2.4.1 and from the mesenteric lymph node (MLN) and jejunal and ileal Peyer’s patches (JPP and IPP) as previously described [44]. For each MC suspension, the number of F4-specific IgM, IgA and IgG antibody secreting cells (ASC) were determined as described by Van den Broeck et al. [42]. Spots in 5 wells (106 MC/well) were counted with an ELIspot reader (Immunospot, CTL) to obtain the number of isotype-specific ASC per 5 × 106 MC.
2.3. FB1 and the mucosal immune system
Three animals from both the FB1 and the PBS group were euthanized at day 0 to analyze the influence of FB1 exposure on the intestinal immune system (Tab. I). MC were isolated from MLN, LP, IPP and JPP (Section 2.2.2).
2.3.1. Intestinal immune cell subpopulations
To analyze whether FB1 feeding influenced the number of immune cells in the intestinal mucosa (T cell populations: CD2+CD3+, CD3+CD4+, CD3+CD8+, CD4+CD8+; myeloid cells: CD172+; B cells: IgM+MHC-II+; antigen presenting cell (APC) and endothelial cells: IgM-MHC-II+), 2.5 × 105 cells were incubated with optimal concentrations of mAbs (Tab. II), washed and incubated with the appropriate isotype-specific FITC- or PE-conjugated antibodies (Southern Biotech, Birmingham, AL, USA). After a final wash step with ice-cold PBS, 5 nM Sytox Red (Invitrogen, Merelbeke, Belgium) was added to allow gating of living cells. Data were acquired on a FACSCanto flow cytometer (Beckton Dickinson, Erebodegem, Belgium) with a minimum event count of 30 000 and analyzed with FACSDiva® software.
Table II.
Overview of the antibodies used in flow cytometry.
| Specificity | Name | Isotype | Reference |
|---|---|---|---|
| CD2 | MSA4 | IgG2a | [29] |
| CD3 | PTT3 | IgG1 | [49] |
| CD4 | 74-12-4 | IgG2b | [29] |
| CD8 | 11/295/33 | IgG2a | [35] |
| CD11R1 | TMG6-5 | IgG1 | [13] |
| CD25 | K231.3B2 | IgGI | [1] |
| CD80/86 | huCTLA-4 muIg fusion protein (Ancell, Bayport, MN, USA) | IgG2a | |
| CD172 | 74-22-15 | IgG2b | [29] |
| MHC-II | MSA3 | IgG2a | [19] |
| IgM | 28.4.1 | IgG1 | [43] |
hu: human; mu: murine.
2.3.2. MC proliferative activity
The MC were also functionally characterized by analyzing their proliferative activity. The cells were cultured at 5 × 105 cells/well in a 96-well culture plate in leukocyte medium (RPMI-1640, 5% FCS, 1 mM sodium pyruvate (Gibco, Merelbeke, Belgium), 2 mM L-glutamine (Gibco), 100 μg/mL kanamycin (Gibco), 50 μg/mL gentamycin (Gibco), 50 μM 2-mercaptoethanol) and subsequently treated with F4 (20 μg), flagellin (20 μg) or 5 μg of one of the mitogens concanavalin A (ConA) (Sigma, Bornem, Belgium), pokeweed mitogen (PWM) (Sigma) or phytohemagglutinin (PHA) (Sigma) or left untreated. Flagellin was isolated from the strain GIS26ΔFaeG using the same protocol as to purify F4 fimbriae [46]. Cultures were maintained at 37 °C in a humidified atmosphere at 5% CO2. After 72 h the cells were pulse-labeled with 1 μCi [3H]-thymidine (Amersham ICN, Bucks, UK) per well and 18 h later harvested onto glass fiber filters (Perkin-Elmer, Life Science, Brussels, Belgium). The incorporated radioactivity was measured using a β-scintillation counter (Perkin-Elmer).
2.3.3. Cytokine expression by MC
To analyze the influence of FB1 feeding on cytokine expression by intestinal MC, 107 cells per sample were stored at –80 °C in TRIzol Reagent (Invitrogen) until cDNA synthesis. The amount of mRNA transcripts from genes encoding cytokines was measured in triplicates by real-time quantitative reverse transcriptase-polymerase chain reaction (qRT-PCR). Briefly, 8 ng cDNA was mixed with the Power SYBR® Green PCR Master Mix (Applied Biosystems, Foster City, CA, USA) and porcine-specific primers for housekeeping genes (cyclophilin A, β2-microglobulin, β-actin) and genes encoding cytokines (IL-1β, IL-6, IL-12p40, IL-18, TNF-α) (Tab. III). The reactions were denatured at 95 °C for 10 min followed by 40 cycles of 95 °C for 15 s and 60 °C for 1 min in the ABI Prism 7000 Sequence Detection System. Data were analyzed using the method of Peirson et al. [28] and individual values for cytokine expression were obtained after normalization to the 3 housekeeping genes (relative quantification).
Table III.
Nucleotide sequences of primers for real-time PCR.
| Gene symbol (gene bank accession number) | Primer sequence | Amplicon size |
|---|---|---|
| Cyclophilin Aa | F (600 nM) CCCACCGTCTTCTTCGACAT | 92 |
| (NM_214353) | R (600 nM) TCTGCTGTCTTTGGAACTTTGTCT | |
| β2-microglobulina | F (900 nM) TTCTACCTTCTGGTCCACACTGA | 162 |
| (NM_213978) | R (300 nM) TCATCCAACCCAGATGCA | |
| β-actin, [47] | F (300 nM) TCATCACCATCGGCAACG | 133 |
| (AY550069) | R (300 nM) TTCCTGATGTCCACGTCGC | |
| IL-1βa | F (300 nM) GAGCTGAAGGCTCTCCACCTC | 87 |
| (NM_001005149) | R (300 nM) ATCGCTGTCATCTCCTTGCAC | |
| IL-6a | F (900 nM) TTCACCTCTCCGGACAAAACTG | 122 |
| (NM_214399) | R (50 nM) TCTGCCAGTACCTCCTTGCTGT | |
| IL-12p40a | F (300 nM) GGTTTCAGACCCGACGAACTCT | 112 |
| (NM_214399) | R (900 nM) CATATGGCCACAATGGGAGATG | |
| IL-18, [34] | F (300 nM) CGTGTTTGAGGATATGCCTGATT | 107 |
| (NM_213997) | R (300 nM) TGGTTACTGCCAGACCTCTAGTGA | |
| TNF-α, [22] | F (300 nM) ACTGCACTTCGAGGTTATCGG | 118 |
| (NM_214022) | R (300 nM) GGCGACGGGCTTATCTGA |
Present study for reference.
2.4. Activity of the small intestinal lamina propria CD11R1+ cell population
2.4.1. Enrichment of CD11R1+ cells from jejunal lamina propia MC
MC were isolated from the LP by collagenase digestion as previously described [46]. The peritoneum and muscle layers were stripped off, after which the intestine was opened along the mesenteric side. Subsequently, the remaining tissue was cut into pieces (1 cm2) and incubated in a shaking incubator for 3 × 40 min at 37 °C, 150 rpm, in HBSS-EDTA solution (Ca2+/Mg2+-free HBSS pH 7.2, 0.94 mM DTT (Sigma), 2.52 mM kestranal (EDTA), 100 U/mL penicillin, 100 μg/mL streptomycin) to remove the epithelium. Next, the tissue was incubated in a shaking incubator for 30 min at 37 °C, 150 rpm in complete medium (RPMI 1640, 5% FCS, 20 mM HEPES (Gibco), 0.1 mg/mL DNaseI (Roche), penicillin/streptomycin (P/S)) and subsequently into a collagenase solution (complete medium, 0.18 U/mL Collagenase A (Sigma)) for 1 h at 37 °C, 120 rpm. The cell suspension was then filtered through a wire mesh, followed by a 70 μm cell strainer (Beckton Dickinson), pelleted (400 g, 10 min, 4 °C) and resuspended in complete medium for 10 min at 37 °C. Subsequently, the low density LP MC were enriched by lymphoprep density gradient centrifugation (Axis-Shield, Oslo, Norway) at 800 g and 18 °C for 30 min.
APC were further enriched from the MC fraction by immunomagnetic separation with the anti-CD11R1 mAb (Tab. II) and the EasySep® FITC selection kit (StemCell Technologies, Grenoble, France) according to the manufacturer’s instructions. CD11R1 is recognized by the anti-human CD11b mAb TMG6-5, which binds the same epitope on human CD11b and porcine CD11R1. However, the cellular expression pattern of both molecules differs greatly, implying a different function of CD11R1 in pigs [13]. Nonetheless, CD11R1 has previously been used to characterize porcine APC [4, 50]. The isolated cells were labelled with anti-CD11R1 and FITC-conjugated anti-mouse IgG1 (BD). Subsequently, these cells were linked to magnetic nanoparticles using the FITC selection cocktail. To remove unlabelled cells, the cell suspension was placed in a EasySep® magnet for 5 min, after which the supernatans was poured off. The retained cells were resuspended in PBS/1mM EDTA and the procedure was repeated to achieve a 90–95% purity of CD11R1+ LP cells.
2.4.2. Phenotypic analysis and Mixed Lymphocyte Reaction (MLR)
The enriched CD11R1+ LP cells were cultured in a 24-well plate at 5 × 105 cells/well in DMEM (Gibco), supplemented with 10% FCS, P/S and 20 μg/mL gentamycin. The cells were stimulated overnight with 50 μg purified endotoxin-free (AffinityPak Detoxi-gel; Pierce) F4 or flagellin at 37 °C in a humidified atmosphere at 5% CO2. Following stimulation, cells were analyzed for surface expression of MHC-II and CD80/86 by flow cytometry as described above. Gates were set on the CD11R1+ LP APC population.
Stimulated CD11R1+ LP cells were also used as stimulator cells in a MLR to analyze their allogeneic T cell stimulatory capacity as previously described [5]. Briefly, PBMC were purified from heparinized blood samples from an unrelated pig by lymphoprep density gradient centrifugation. Lymphocytes were further enriched from the PBMC cell fraction by depletion of CD172+ monocytic cells (Tab. II) using magnetic cell sorting (MACS system; Miltenyi Biotec GmbH). CD11R1+ LP cells were added to 2 × 105 lymphocytes at different ratios per well of a 96-well plate. After 3 days of allogeneic responses, cocultures were pulsed, harvested and the proliferation was measured as described above.
2.5. Statistics
Statistical analyses were performed using SPSS 16. Normal distribution of all the data was analyzed using the Kolmogorov-Smirnov test and tested for variance homogeneity with the Levine test. The number of F4+ E. coli excreted per day, average daily weight gain, the FCM data and cytokine results were compared with one-way ANOVA and Bonferroni adjustment. The ELIspot and the MLR data were first logarithmically transformed to homogenize variances and then analyzed with a one-way ANOVA and LSD adjustment to test the effect of FB1 exposure. A two-way ANOVA (FB1 exposure, day of the experiment) was used to analyze the effect of the FB1 treatment on the whole F4+ E. coli excretion period. A value of p < 0.05 was considered statistically significant.
3. RESULTS
3.1. Oral FB1 intake prolongs intestinal infection
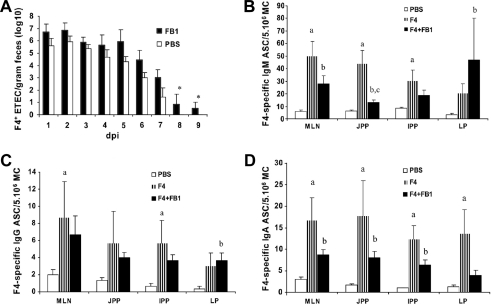
F4-seronegative and F4R+ piglets were infected with F4+ ETEC to analyze the influence of FB1 exposure on an intestinal infection. The overall shedding of F4+ E. coli was higher in the FB1 group (p = 0.040) and the excretion lasted 2 more days than in the PBS group (9 versus 7 days) (Fig. 1A). To examine if this longer shedding could be attributed to a different F4R expression level, a villus adhesion test was performed. There was no difference between FB1-exposed and control animals in adherence of F4+ ETEC to the villi (30 to 89 bacteria per 250 μm villous length for all piglets). The mean consistency index of the feces was similar for both groups and the average daily weight gain was not affected by FB1 before (FB1 group 0.189 ± 0.052 kg/day; PBS group 0.205 ± 0.028 kg/day) or after the F4+ E. coli infection (FB1 group 0.275 ± 0.063 kg/day; PBS group 0.297 ± 0.053 kg/day). These results show that consumption of FB1-contaminated feed could lead to a prolonged intestinal infection.
Figure 1.
(A) Mean fecal F4+ E. coli (log 10) per gram feces (± SEM) following F4+ ETEC infection of FB1-exposed (FB1, n = 5) and non-exposed animals (PBS, n = 5) at 1 to 9 days post infection (dpi). The asterisk indicates a significant difference (p < 0.05) between both groups. (B) Mean F4-specific IgM, (C) IgG and (D) IgA ASCs (± SEM) per 5 × 106 MC in lamina propria (LP), mesenteric lymph nodes (MLN), jejunal (JPP) and ileal Peyer’s patches (IPP) of control animals (PBS, n = 3), F4-immunized animals (F4, n = 3) and FB1-exposed and F4-immunized animals (F4+FB1, n = 3) at one week following immunization. Different letters indicate a significant difference (p < 0.05) between groups: (a) PBS-F4, (b) PBS-F4+FB1, (c) F4-F4+FB1.
3.2. Oral FB1 intake reduces the mucosal immune response
The effects of FB1 exposure on the immune response of piglets to an F4 mucosal immunization were analyzed by enumerating the F4-specific ASC (Figs. 1B–1D). One week after the immunization, significant higher numbers of F4-specific ASC were found in the F4 group compared to the PBS group (IgM: MLN (p = 0.001), JPP (0.001) and the IPP (0.014); IgA: MLN (0.005), JPP (0.044), IPP (0.001) and the LP, (0.013); IgG: MLN (0.040) and IPP (0.023)). Furthermore, immunization of FB1-exposed animals resulted in significant more F4-specific ASC compared to the PBS group (IgM: MLN (p = 0.002), JPP (0.021) and LP (0.043); IgA: MLN (0.026), JPP (0.03) and IPP (0.001); IgG: LP (0.043)). These results indicate that both in the F4 and the F4+FB1 group an F4-specific immune response was mounted. When the F4+FB1 group was compared to the F4 group, lower numbers of F4-specific IgM and IgA ASC were observed in most tissues. This difference is only significant for IgM ASC in the JPP (p = 0.003). Nonetheless, the overall mucosal immune response (total number of IgA ASC, independent from intestinal location) was significant lower in the F4+FB1 group compared to the F4 group (p = 0.02), which seems to indicate that FB1-contaminated feed could have a negative influence on the induction of antigen-specific immune responses following mucosal vaccination.
3.3. Modulation of the mucosal immune system by oral FB1 exposure
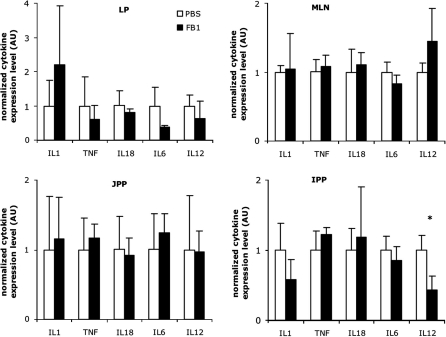
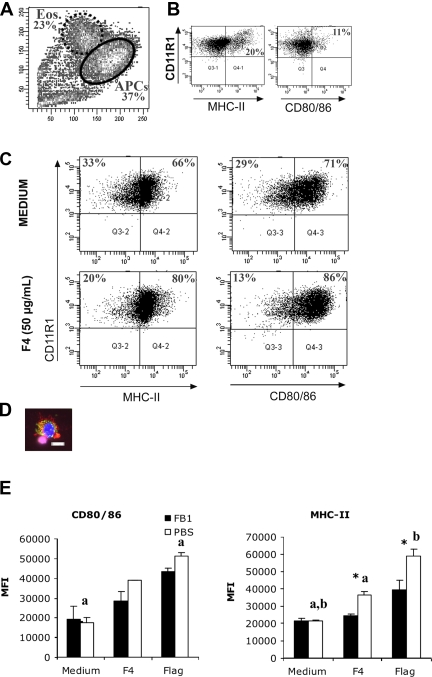
To analyze the influence of FB1 exposure on the mucosal immune system, MC were isolated from MLN, LP, IPP and JPP of FB1-exposed (FB1 group) and non-exposed (PBS group) animals. The number of B cells, myeloid cells and specific T cell populations were identical in both groups (data not shown). Moreover, the number of MHC-II+IgM- LP MC was not influenced by FB1 treatment (FB1: 18.9% ± 8.5; PBS: 16.3% ± 6.3). Similarly, their proliferation following incubation with different mitogens, F4 fimbriae or flagellin, was not influenced by FB1 (data not shown). However, the analysis of cytokine mRNA by qRT-PCR revealed a significant reduction of IL-12p40 expression in the IPP of FB1-exposed animals (p = 0.027) (Fig. 2). Furthermore, in the LP both IL-6 and IL-12p40 mRNA expression were reduced. The IL-12 cytokine is mainly produced by APC. Therefore, further experiments were performed to analyze whether FB1 exposure could influence intestinal LP APC. Immunomagnetic enrichment of LP cells routinely yielded a 90–95% pure population of CD11R1+ cells, consisting of MHC-II+ APC with contaminating MHC-II- eosinophils (Figs. 3Aand 3B). The CD11R1+MHC-II+ LP APC appear to have a typical dendritic cell morphology (Fig. 3D). Interestingly, in vivo FB1 exposure had no influence on the relative CD11R1+ eosinophil or APC percentages in FCM analyses (data not shown).
Figure 2.
The mean fold change of expressed cytokines to a combination of the housekeeping genes cyclophilin A, β2-microglobulin and β-actin (± SD) in the lamina propria (LP), mesenteric lymph nodes (MLN), jejunal (JPP) and ileal Peyer’s patches (IPP) of non-exposed animals (PBS, n = 3) or FB1-exposed animals (FB1, n = 3) at the end of the FB1 administration period. The cytokine expression level is represented in fold change compared to the PBS group (mean value of 1). The asterisk indicates a significant difference (p < 0.05) between both groups.
Figure 3.
Increased expression of APC maturation markers MHC-II and CD80/86 on CD11R1+ LP APC in response to stimulation with 50 μg F4. (A) Forward and side scatter characteristics of enriched CD11R1+ cells, consisting of APC and contaminating eosinophils. (B) Eosinophils do not express APC maturation markers. (C) Purified CD11R1+ cells, gated on the APC population. One representative experiment out of 3 independent experiments is shown. (D) An isolated LP APC stained with anti-CD11R1 TexasRed, anti-MHC-II FITC and DAPI, bar = 10 μm (E) Mean fluorescence intensity (MFI (± SD)) of isolated CD11R1+ LP cells stained for CD80/86 and MHC-II expression and gated on the APC population from non-exposed (PBS, n = 3) or FB1-exposed animals (FB1, n = 3) following overnight stimulation with 50 μg F4 (F4) or flagellin (Flag). The asterisk indicates a significant difference (p < 0.05) between both groups; the letters indicate a significant difference between unstimulated (medium) and stimulated cells within groups.
In vitro stimulation of CD11R1+ LP cells with F4 and the TLR5 ligand flagellin resulted in a significantly increased expression of the APC maturation markers MHC-II (F4: p = 0.003 and Flag: 0.001) and CD80/86 (Flag: p = 0.001) in comparison to unstimulated cells, implicating CD11R1+ LP APC in the mucosal immune response to F4+ ETEC (Figs. 3C and 3E). Importantly, previous oral exposure of piglets to FB1 impaired the ability of these CD11R1+ LP APC to mature in response to the mucosal antigens F4 and flagellin. Upregulation of MHC-II in cells from FB1-exposed animals was reduced in comparison to APC from the control animals (p = 0.02 and 0.043 respectively), whereas the difference in CD80/86 expression was not significantly, it was however numerically lower, in the FB1 group (Fig. 3E). To address a possible influence of FB1 on APC function, isolated CD11R1+ LP cells were analyzed for their T cell stimulatory capacity in an allogeneic MLR. The cells were stimulated overnight with F4 or flagellin and used as stimulators. Stimulated CD11R1+ cells from control animals induced potent T cell proliferation, whereas previous FB1 exposure of piglets significantly reduced the T cell stimulatory capacity of these cells, whatever the stimulator or the APC/T cell ratio (Tab. IV). These data seem to indicate that oral FB1 exposure reduces the maturation of intestinal CD11R1+ LP APC.
Table IV.
The stimulation index of proliferated allogeneic lymphocytes after coculture with graded numbers of CD11R1+ LP cells, consisting of APC and eosinophils, isolated from FB1-exposed and non-exposed animals.
| APC stimulation | Mean stimulation index |
p-value | |
|---|---|---|---|
| APC/T cell ratio | Control animals | FB1-treated animals | |
| F4 stimulation | |||
| 1/10 | 38.60 ± 6.18 | 1.33 ± 0.75 | < 0.05 |
| 1/30 | 13.12 ± 1.16 | 1.10 ± 0.07 | < 0.001 |
| 1/90 | 17.92 ± 2.20 | 0.97 ± 0.09 | < 0.01 |
| 1/270 | 6.63 ± 1.41 | 0.95 ± 0.71 | 0.08 |
| Flagellin stimulation | |||
| 1/10 | 71.92 ± 15.94 | 2.84 ± 1.64 | < 0.05 |
| 1/30 | 30.30 ± 5.54 | 1.65 ± 0.20 | < 0.01 |
| 1/90 | 41.02 ± 8.28 | 1.13 ± 0.46 | < 0.01 |
| 1/270 | 9.84 ± 2.99 | 0.86 ± 0.33 | < 0.05 |
Mean ± SD (n = 3). CD11R1+ cells were incubated overnight with either F4 or flagellin to induce activation. The SI was calculated by dividing the individual cpm values of every condition by the mean cpm value of the control coculture (untreated CD11R1+ cells and lymphocytes). SI from both negative controls, CD11R1+ cells and lymphocytes alone, were lower than 1.5.
4. DISCUSSION
The present study shows that despite the short administration period and the low dose (1 mg/kg BW/day), which did not result in any clinical signs or reduced weight gain, oral FB1 intake prolongs an intestinal ETEC infection. This appears to agree with previous reports wherein piglets receiving FB1 orally had an increased intestinal colonization 48 h following infection with pathogenic E. coli [27]. We did not analyze ETEC colonization, but FB1 had probably no influence on the intestinal colonization since the F4R expression level was similar on villi of FB1-exposed and non-exposed animals. Furthermore, based on results of previous F4+ ETEC infection experiments, large differences in in vivo F4R expression are required to see differences in F4+ ETEC colonization between animals [41, 45]. On the other hand, FB1 is known to affect the intestinal barrier [6], cytokine production by enterocytes [8] and other components of the innate immune response that are first line defense mechanisms [7]. The latter are involved in the initiation of the adaptive immunity which seems to be delayed since the excretion lasted two days longer in the FB1-exposed piglets. Therefore, an effect of FB1 on the acquired immune system could be involved.
It has been shown that FB1 reduces the efficiency of intramuscular vaccination [37]. Accordingly, the present study shows for the first time that a mycotoxin could reduce the induction of an antigen-specific intestinal immune response. In a model with oral F4 immunisation, significant lower numbers of antigen-specific IgM ASC were detected in the JPP of FB1-exposed piglets. Furthermore, both the F4-specific IgM and IgA ASC were reduced after FB1-exposure. This result suggests that FB1 could interfere with the induction phase of the immune response, especially since the overall mucosal IgA immune response was significantly reduced in FB1-exposed piglets. The adaptive immune system needs around 3 to 5 days to induce an F4-specific immune response and the first F4-specific ASC that appear in the intestine are mainly F4-specific IgM ASC that subsequently switch to IgA [42, 44]. There are no indications that FB1 influences IgA synthesis, in contrast to the mycotoxins deoxynivalenol (DON) and nivalenol which selectively stimulate IgA production in porcine Peyer’s patches [31].
Dendritic cells (DC) have a central position in the mucosal immune system by connecting innate and acquired immune responses. In the LP, DC take up antigens and migrate to the MLN where they interact with T cells. Binding of pathogen-associated molecular patterns to pathogen recognition receptors triggers DC maturation and enables them to induce effector T cell responses. DC maturation is characterized by an increased expression of MHC-II and co-stimulatory molecules like CD40, CD80 and CD86 and the secretion of polarizing cytokines like IL-10 and IL-12 [16]. After in vivo FB1-exposure, we observed a reduced transcription of IL-12p40 by MC, although restricted to the intestinal IPP, which suggests an effect of FB1 on APC since these cells are the main producers of this cytokine [39]. Here we found that CD11R1+ LP APC from control animals upregulated MHC-II and CD80/86 expression in response to F4 and Flag stimulation. On the contrary, CD11R1+ LP APC from FB1-exposed animals had a reduced upregulation of MHC-II expression. We also observed a tendency of FB1 to reduce the upregulation of CD80/86 expression. Both findings indicate that intestinal CD11R1+ APC from FB1-exposed piglets are less responsive to F4 and Flag stimulation. This contradicts results obtained with human MoDC, where the expression of CD86 was not modulated by FB1 treatment [36].
These data suggest that FB1 could affect the maturation of LP APC. A hallmark of mature DC is their ability to induce T cell proliferation due to increased expression of MHC-II and costimulatory molecules, such as CD80/86 [40]. Although a mixed population of CD11R1+ APC and eosinophils was used to investigate the T cell stimulatory capacity, the obtained results were consistent with the MHC-II and CD80/86 expression. We show for the first time that F4 and flagellin enhance the T cell stimulatory capacity of porcine intestinal CD11R1+ APC. Moreover, we demonstrate that in vivo FB1 exposure of piglets resulted in an impaired T cell stimulatory capacity of intestinal CD11R1+ LP APC. To our knowledge, this has not been previously demonstrated. On the contrary, Bimczok et al. [5] did not observe a reduced ability of porcine MoDC to induce T cell proliferation after treatment with DON, despite the reduced expression of MHCII, CD40 and CD80/86. This discrepancy could be explained by the type of mycotoxin itself or the cell type used as APC model. The possibility that the observed reduction in functional APC maturation is due to an effect of FB1 on CD11R1+ APC or eosinophil migration seems unlikely since we observed no difference between FB1-exposed and control animals in the number of isolated CD11R1+ cells, in the relative percentage of CD11R1+ APC or eosinophils or in the percentage of MHC-II+IgM- LP APC. The small intestinal LP MC contains mainly 2 MHC-II+ cell populations, APC and endothelial cells [15]. Although we cannot discriminate between these populations in this study, the number of endothelial cells should remain constant irrespective of FB1 exposure. Although APC migration does not seem to be affected by FB1, the latter could still influence the function of CD11R1- LP APC. However, it has been demonstrated that only the CD11R1+ LP APC migrate from the LP to the MLN [4], suggesting that these cells are functionally the most relevant APC with respect to the induction of a primary immune response.
Despite the small numbers of animals, we report for the first time an effect of a mycotoxin on intestinal APC maturation. The reduced maturation of FB1-exposed intestinal APC could explain the longer F4+ ETEC excretion and the lower F4-specific immune response to oral F4 immunization in FB1-exposed animals. Other effects of FB1 on the acquired immune system are reported like influx of immune cells [32, 38] or altered T cell proliferation following mitogen stimulation [17, 21]. In the present study FB1 exposure did not alter these parameters in the porcine intestine.
The observed negative impact of FB1 on the immune system could be a threat for the health status of humans and animals. The results of this study could be extrapolated to man since the gastro-intestinal tract of both species share a lot of similarities [12]. This stresses the need to further develop methods to reduce the consumption of FB1-contaminated food [48], which is common in developing countries where the prevalence of several infectious diseases is high [20, 25]. The negative impact of FB1 on the mucosal immune system can support the emergence of infectious diseases and suggests that the efficiency of vaccination programs would be increased with a concomitant reduction of FB1 intake by the local population.
Acknowledgments
We thank G. De Smet, D. Slos and R. Cooman for their technical assistance. B. Devriendt has a Ph.D. grant of the Institute for the Promotion of Innovation through Science and Technology in Flanders (IWT-Vlaanderen). F. Verdonck had a post-doctoral grant from the FWO-Vlaanderen, which also provided financial support (projects 1.5.213.07 and G.0013.05). This study was part of the bilateral project Tournesol funded by the Ministerie van de Vlaamse Gemeenschap (Belgium) and the Ministère des Affaires étrangères (France).
References
- 1.Bailey M., Stevens K., Bland P.W., Stokes C.R.. A monoclonal antibody recognising an epitope associated with pig interleukin-2 receptors. J. Immunol. Methods. 1992;153:85–91. doi: 10.1016/0022-1759(92)90309-h. [DOI] [PubMed] [Google Scholar]
- 2.Bhandari N., Sharma R.P.. Fumonisin B(1)-induced alterations in cytokine expression and apoptosis signaling genes in mouse liver and kidney after an acute exposure. Toxicology. 2002;172:81–92. doi: 10.1016/s0300-483x(02)00007-0. [DOI] [PubMed] [Google Scholar]
- 3.Bhat R.V., Shetty P.H., Amruth R.P., Sudershan R.V.. A foodborne disease outbreak due to the consumption of moldy sorghum and maize containing fumonisin mycotoxins. J. Toxicol. Clin. Toxicol. 1997;35:249–255. doi: 10.3109/15563659709001208. [DOI] [PubMed] [Google Scholar]
- 4.Bimczok D., Sowa E.N., Faber-Zuschratter H., Pabst R., Rothkötter H.J.. Site-specific expression of CD11b and SIRPalpha (CD172a) on dendritic cells: implications for their migration patterns in the gut immune system. Eur. J. Immunol. 2005;35:1418–1427. doi: 10.1002/eji.200425726. [DOI] [PubMed] [Google Scholar]
- 5.Bimczok D., Doll S., Rau H., Goyarts T., Wundrack N., Naumann M.. et al. The Fusarium toxin deoxynivalenol disrupts phenotype and function of monocyte-derived dendritic cells in vivo and in vitro. Immunobiology. 2007;212:655–666. doi: 10.1016/j.imbio.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 6.Bouhet S., Hourcade E., Loiseau N., Fikry A., Martinez S., Roselli M.. et al. The mycotoxin fumonisin B1 alters the proliferation and the barrier function of porcine intestinal epithelial cells. Toxicol. Sci. 2004;77:165–171. doi: 10.1093/toxsci/kfh006. [DOI] [PubMed] [Google Scholar]
- 7.Bouhet S., Oswald I.P.. The effects of mycotoxins fungal food contaminants, on the intestinal epithelial cell derived innate immune response. Vet. Immunol. Immunopathol. 2005;108:199–209. doi: 10.1016/j.vetimm.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 8.Bouhet S., Le Dorze E., Pérès S.Y., Fairbrother J.M., Oswald I.P.. Mycotoxin fumonisin B1 selectively down-regulates basal IL-8 expression in pig intestine: in vivo and in vitro studies. Food Chem. Toxicol. 2006;44:1768–1773. doi: 10.1016/j.fct.2006.05.018. [DOI] [PubMed] [Google Scholar]
- 9.Bouhet S., Oswald I.P.. The intestine as a possible target for fumonisin toxicity. Mol. Nutr. Food. Res. 2007;51:925–931. doi: 10.1002/mnfr.200600266. [DOI] [PubMed] [Google Scholar]
- 10.Cox E., Houvenaghel A.. Comparison of the in vitro adhesion of K88, K99, F41 and P987 positive Escherichia coli to intestinal villi of 4- to 5-week-old pigs. Vet. Microbiol. 1993;34:7–18. doi: 10.1016/0378-1135(93)90003-p. [DOI] [PubMed] [Google Scholar]
- 11.Desai K., Sullards M.C., Allegood J., Wang E., Schmelz E.M., Hartl M.. et al. Fumonisins and fumonisin analogs as inhibitors of ceramide synthase and inducers of apoptosis. Biochim. Biophys. Acta. 2002;1585:188–189. doi: 10.1016/s1388-1981(02)00340-2. [DOI] [PubMed] [Google Scholar]
- 12.Domeneghini C., Di Giancamillo A., Arrighi S., Bosi G.. Gut-trophic feed additives and their effects upon the gut structure and intestinal metabolism State of the art in the pig, and perspectives towards humans. Histol. Histopathol. 2006;21:273–283. doi: 10.14670/HH-21.273. [DOI] [PubMed] [Google Scholar]
- 13.Domínguez J., Alvarez B., Alonso F., Thacker E., Haverson K., McCullough K.. et al. Workshop studies on monoclonal antibodies in the myeloid panel with CD11 specificity. Vet. Immunol. Immunopathol. 2001;80:111–119. doi: 10.1016/s0165-2427(01)00286-0. [DOI] [PubMed] [Google Scholar]
- 14.Haschek W.M., Gumprecht L.A., Smith G., Tumbleson M.E., Constable P.D.. Fumonisin toxicosis in swine: an overview of porcine pulmonary edema and current perspectives. Environ. Health Perspect. 2001;109:251–257. doi: 10.1289/ehp.01109s2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haverson K., Singha S., Stokes C.R., Bailey M.. Professional and non-professional antigen-presenting cells in the porcine small intestine. Immunology. 2000;101:492–500. doi: 10.1046/j.1365-2567.2000.00128.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iwasaki A.. Mucosal Dendritic Cells. Annu. Rev. Immunol. 2007;25:381–418. doi: 10.1146/annurev.immunol.25.022106.141634. [DOI] [PubMed] [Google Scholar]
- 17.Johnson V.J., Sharma R.P.. Gender-dependent immunosuppression following subacute exposure to fumonisin B1. Int. Immunopharmacol. 2001;1:2023–2034. doi: 10.1016/s1567-5769(01)00131-x. [DOI] [PubMed] [Google Scholar]
- 18.Loiseau N., Debrauwer L., Sambou T., Bouhet S., Miller J.D., Martin P.G.. et al. Fumonisin B1 exposure and its selective effect on porcine jejunal segment: sphingolipids glycolipids and trans-epithelial passage disturbance. Biochem. Pharmacol. 2007;74:144–152. doi: 10.1016/j.bcp.2007.03.031. [DOI] [PubMed] [Google Scholar]
- 19.Lunney J.K., Walker K., Goldman T., Aasted B., Bianchi A., Binns R.. et al. Overview of the first international workshop to define swine leukocyte cluster of differentiation (CD) antigens. Vet. Immunol. Immunopathol. 1994;43:193–206. doi: 10.1016/0165-2427(94)90136-8. [DOI] [PubMed] [Google Scholar]
- 20.Maenetje P.W., Dutton M.F.. The incidence of fungi and mycotoxins in South African barley and barley products. J. Environ. Sci. Health B. 2007;42:229–236. doi: 10.1080/03601230601125644. [DOI] [PubMed] [Google Scholar]
- 21.Marin D.E., Gouze M.E., Taranu I., Oswald I.P.. Fumonisin B1 alters cell cycle progression and interleukin-2 synthesis in swine peripheral blood mononuclear cells. Mol. Nutr. Food Res. 2007;51:1406–1412. doi: 10.1002/mnfr.200700131. [DOI] [PubMed] [Google Scholar]
- 22.Meissonnier G.M., Pinton P., Laffitte J., Cossalter A.M., Gong Y.Y., Wild C.P.. et al. Immunotoxicity of aflatoxin B1: Impairment of cell-mediated response to vaccine antigen and modulation of cytokine expression. Toxicol. Appl. Pharmacol. 2008;231:142–149. doi: 10.1016/j.taap.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 23.Miller J.D.. Aspects of the ecology of Fusarium toxins in cereals. Adv. Exp. Med. Biol. 2002;504:19–27. doi: 10.1007/978-1-4615-0629-4_3. [DOI] [PubMed] [Google Scholar]
- 24.Missmer S.A., Suarez L., Felkner M., Wang E., Merrill A.H. Jr., Rothman K.J., Hendricks K.A.. Exposure to fumonisins and the occurrence of neural tube defects along the Texas-Mexico border. Environ. Health Perspect. 2006;114:237–241. doi: 10.1289/ehp.8221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nikiema P.N., Worrillow L., Traore A.S., Wild C.P., Turner P.C.. Fumonisin contamination of maize in Burkina Faso. West Africa, Food Addit. Contam. 2004;21:865–870. doi: 10.1080/02652030400004242. [DOI] [PubMed] [Google Scholar]
- 26.Oswald I., Comera C.. Immunotoxicity of mycotoxins. Rev. Med. Vet. 1998;149:585–590. [Google Scholar]
- 27.Oswald I.P., Desautels C., Laffitte J., Fournout S., Peres S.Y., Odin M.. et al. Mycotoxin fumonisin B1 increases intestinal colonization by pathogenic Escherichia coli in pigs. Appl. Environ. Microbiol. 2003;69:5870–5874. doi: 10.1128/AEM.69.10.5870-5874.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peirson S.N., Butler J.N., Foster R.. Experimental validation of novel and conventional approaches to quantitative real-time PCR data analysis. Nucleic Acids Res. 2003;31:e73. doi: 10.1093/nar/gng073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pescovitz M.D., Lunney J.K., Sachs D.H.. Preparation and characterization of monoclonal antibodies reactive with porcine PBL. J. Immunol. 1984;133:368–375. [PubMed] [Google Scholar]
- 30.Pestka J.J., Smolinski A.T.. Deoxynivalenol: toxicology and potential effects on humans. J. Toxicol. Environ. Health B Crit. Rev. 2005;8:39–69. doi: 10.1080/10937400590889458. [DOI] [PubMed] [Google Scholar]
- 31.Pinton P., Accensi F., Beauchamp E., Cossalter A.M., Callu P., Grosjean F., Oswald I.P.. Ingestion of deoxynivalenol (DON) contaminated feed alters the pig vaccinal immune responses. Toxicol. Lett. 2008;177:215–222. doi: 10.1016/j.toxlet.2008.01.015. [DOI] [PubMed] [Google Scholar]
- 32.Piva A., Casadei G., Pagliuca G., Cabassi E., Galvano F., Solfrizzo M.. et al. Activated carbon does not prevent the toxicity of culture material containing fumonisin B1 when fed to weanling piglets. J. Anim. Sci. 2005;83:1939–1947. doi: 10.2527/2005.8381939x. [DOI] [PubMed] [Google Scholar]
- 33.Rasschaert K., Verdonck F., Goddeeris B.M., Duchateau L., Cox E.. Screening of pigs resistant to F4 enterotoxigenic Escherichia coli (ETEC) infection. Vet. Microbiol. 2007;123:249–253. doi: 10.1016/j.vetmic.2007.02.017. [DOI] [PubMed] [Google Scholar]
- 34.Royaee A.R., Husmann R.J., Dawson H.D., Calzada-Nova G., Schnitzlein W.M., Zuckermann F.A., Lunney J.K.. Deciphering the involvement of innate immune factors in the development of the host response to PRRSV vaccination. Vet. Immunol. Immunopathol. 2004;102:199–216. doi: 10.1016/j.vetimm.2004.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Saalmüller A., Aasted B., Canals A., Dominguez J., Goldman T., Lunney J.K.. et al. Analyses of mAb reactive with porcine CD8. Vet. Immunol. Immunopathol. 1994;43:249–254. doi: 10.1016/0165-2427(94)90144-9. [DOI] [PubMed] [Google Scholar]
- 36.Stockmann-Juvala H., Alenius H., Savolainen K.. Effects of fumonisin B(1) on the expression of cytokines and chemokines in human dendritic cells. Food Chem. Toxicol. 2008;46:1444–1451. doi: 10.1016/j.fct.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 37.Taranu I., Marin D.E., Bouhet S., Pascale F., Bailly J.D., Miller J.D.. et al. Mycotoxin fumonisin B1 alters the cytokine profile and decreases the vaccinal antibody titer in pigs. Toxicol. Sci. 2005;84:301–307. doi: 10.1093/toxsci/kfi086. [DOI] [PubMed] [Google Scholar]
- 38.Theumer M.G., Lopez A.G., Masih D.T., Chulze S.N., Rubinstein H.R.. Immunobiological effects of fumonisin B1 in experimental subchronic mycotoxicoses in rats. Clin. Diagn. Lab. Immunol. 2002;9:149–155. doi: 10.1128/CDLI.9.1.149-155.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Trinchieri G.. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat. Rev. Immunol. 2003;3:133–146. doi: 10.1038/nri1001. [DOI] [PubMed] [Google Scholar]
- 40.Turley S.J., Inaba K., Garrett W.S., Ebersold M., Unternaehrer J., Steinman R.M., Mellman I.. Transport of peptide-MHC class II complexes in developing dendritic cells. Science. 2000;288:522–527. doi: 10.1126/science.288.5465.522. [DOI] [PubMed] [Google Scholar]
- 41.Van den Broeck W., Cox E., Goddeeris B.M.. Receptor-dependent immune responses in pigs after oral immunization with F4 fimbriae. Infect. Immun. 1999;67:520–526. doi: 10.1128/iai.67.2.520-526.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Van den Broeck W., Cox E., Goddeeris B.M.. Induction of immune responses in pigs following oral administration of purified F4 fimbriae. Vaccine. 1999;17:2020–2029. doi: 10.1016/s0264-410x(98)00406-x. [DOI] [PubMed] [Google Scholar]
- 43.Van Zaane D., Hulst M.M.. Monoclonal antibodies against porcine immunoglobulin isotypes. Vet. Immunol. Immunopathol. 1987;16:23–36. doi: 10.1016/0165-2427(87)90171-1. [DOI] [PubMed] [Google Scholar]
- 44.Verdonck F., Cox E., van Gog K., Van der Stede Y., Duchateau L., Deprez P., Goddeeris B.M.. Different kinetic of antibody responses following infection of newly weaned pigs with an F4 enterotoxigenic Escherichia coli strain or an F18 verotoxigenic Escherichia coli strain. Vaccine. 2002;20:2995–3004. doi: 10.1016/s0264-410x(02)00220-7. [DOI] [PubMed] [Google Scholar]
- 45.Verdonck F., Cox E., Van der Stede Y., Goddeeris B.M.. Oral immunization of piglets with recombinant F4 fimbrial adhesin FaeG monomers induces a mucosal and systemic F4-specific immune response. Vaccine. 2004;22:4291–4299. doi: 10.1016/j.vaccine.2004.04.016. [DOI] [PubMed] [Google Scholar]
- 46.Verdonck F., Joensuu J.J., Stuyven E., De Meyer J., Muilu M., Pirhonen M.. et al. The polymeric stability of the Escherichia coli F4 (K88) fimbriae enhances its mucosal immunogenicity following oral immunization. Vaccine. 2008;26:5728–5735. doi: 10.1016/j.vaccine.2008.08.017. [DOI] [PubMed] [Google Scholar]
- 47.Von der Hardt K., Kandler M.A., Fink L., Schoof E., Dotsch J., Brandenstein O.. et al. High frequency oscillatory ventilation suppresses inflammatory response in lung tissue and microdissected alveolar macrophages in surfactant depleted piglets. Pediatr. Res. 2004;55:339–346. doi: 10.1203/01.PDR.0000106802.55721.8A. [DOI] [PubMed] [Google Scholar]
- 48.Whitaker T.B., Doko M.B., Maestroni B.M., Slate A.B., Ogunbanwo B.F.. Evaluating the performance of sampling plans to detect fumonisin B1 in maize lots marketed in Nigeria. J. AOAC Int. 2007;90:1050–1059. [PubMed] [Google Scholar]
- 49.Yang H., Oura C.A., Kirkham P.A., Parkhouse R.M.. Preparation of monoclonal anti-porcine CD3 antibodies and preliminary characterization of porcine T lymphocytes. Immunology. 1996;88:577–585. doi: 10.1046/j.1365-2567.1996.d01-682.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang W., Wen K., Azevedo M.S., Gonzalez A., Saif L.J., Li G.. et al. Lactic acid bacterial colonization and human rotavirus infection influence distribution and frequencies of monocytes/macrophages and dendritic cells in neonatal gnotobiotic pigs. Vet. Immunol. Immunopathol. 2008;121:222–231. doi: 10.1016/j.vetimm.2007.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]