Abstract
Objective
We examined whether all-cause mortality was predicted by physical activity level in peripheral arterial disease (PAD) patients limited by intermittent claudication.
Design of Study
Retrospective, natural history follow-up.
Setting
The Geriatrics, Research, Education, and Clinical Center at the Maryland Veterans Affairs Health Care System (MVAHCS) at Baltimore
Subjects
Patients with stable symptoms of intermittent claudication were evaluated at baseline between 1994 and 2002, and were classified into a physically sedentary group (n = 299) or a physically active group (n =135), and followed in 2004 using the Social Security Death Index.
Main Outcome Measure
Survival status of each patient in 2004.
Results
Median follow-up was 5.33 years (range = 0.25 to 8.33 years) for the physically active group, and 5.0 years (range = 0.17 to 8.5 years) for the sedentary group. At follow-up, 108 patients (24.9%) had died, consisting of 86 (28.8%) in the sedentary group and 22 (16.3%) in the active group. Unadjusted risk of mortality was lower (p = 0.005) in the physically active group (hazard ratio [HR] = 0.510, 95% CI = 0.319 to 0.816). In multivariate Cox Proportional Hazards analysis, age (HR = 1.045, 95% CI = 1.019 to 1.072, p< 0.001), BMI (HR = 0.943, 95% CI = 0.902 to 0.986, p = 0.009), ABI (HR = 0.202, 95% CI = 0.064 to 0.632, p = 0.006), and physical activity status (HR = 0.595, 95% CI = 0.370 to 0.955, p = 0.031) were predictors of mortality.
Conclusion
Patients limited by intermittent claudication who engage in any amount of weekly physical activity beyond light intensity at baseline have a lower mortality rate than their sedentary counterparts who perform either no physical activity or only light-intensity activities. The protective effect of physical activity persists even after adjusting for other predictors of mortality, which include age, ABI, and BMI.
INTRODUCTION
Peripheral arterial disease (PAD) is a strong prognostic indicator of poor long-term survival,1–14 as the relative risk for all-cause mortality associated with PAD ranges between 1.4 and 3.8.1,4,5,7,8,10,11,14 The prognosis worsens with more severe PAD, as the relative risk for mortality progressively increases with a decrease in the ankle/brachial index (ABI).3,6,7,10,11 Intermittent claudication afflicts 5% of the US population older than 55 years of age,15 and occurs during ambulation when the peripheral circulation is inadequate to meet the metabolic requirement of the active leg musculature. Consequently, patients with intermittent claudication have ambulatory dysfunction, thereby limiting daily physical activities16 and negatively affecting health-related quality of life.17 Because ambulation is one of the primary physical activities performed by the elderly,18 it is not surprising that PAD subjects adopt a sedentary lifestyle.19
The poor prognosis of patients with PAD is further compounded by sedentary living.20–22 The mortality risk of sedentary PAD patients is higher than their more physically active counterparts.23 Although physical activity is associated with survival in PAD patients,23 it is uncertain whether this relationship exists in symptomatic patients who are functionally limited by intermittent claudication. It is unclear whether PAD patients with intermittent claudication are capable of performing an adequate volume of physical activity to elicit health benefits that may lower their mortality risk.
The aim of this investigation was to determine whether all-cause mortality was predicted by physical activity level in PAD patients limited by intermittent claudication. It was hypothesized that (1) a higher level of physical activity would be predictive of a lower rate of all-cause mortality, and (2) physical activity level would remain predictive of all-cause mortality after adjusting for ABI and co-morbid conditions in PAD patients limited by intermittent claudication.
METHODS
PATIENTS
Screening
A total of 434 PAD patients with stable symptoms of intermittent claudication were evaluated in the Geriatrics, Research, Education, and Clinical Center at the Maryland Veterans Affairs Health Care System (MVAHCS) at Baltimore between 1994 and 2002. None of these patients were enrolled in subsequent exercise programs offered at the MVAHCS. They participated in this study to receive information regarding their physical function, physical activity, peripheral circulation, co-morbid conditions, and cardiovascular risk factors. The patients were recruited from the Vascular Clinic at the site of the Baltimore MVAHCS and from local newspaper and radio advertisements. All patients were classified as having Fontaine stage II PAD24 defined by the following inclusion criteria: (a) a history of intermittent claudication, (b) an ankle/brachial index (ABI) at rest < 0.90,15 and (c) the occurrence of intermittent claudication during a 6-minute walk test. Patients were excluded from this study for the following conditions: (a) absence of PAD, (b) inability to obtain an ABI measure due to non-compressible vessels, (c) asymptomatic PAD (Fontaine stage I), (d) rest pain PAD (Fontaine stage III), (e) use of medications indicated for the treatment of intermittent claudication (cilostazol and pentoxifylline) within three months prior to investigation, (f) exercise tolerance limited by factors other than claudication (e.g., severe coronary artery disease, dyspnea, poorly controlled blood pressure), and (g) active cancer, renal disease (serum creatinine value greater than 1.2 mg/dl), or liver disease. All patients lived independently at home. The Institutional Review Boards at the University of Maryland and the MVAHCS at Baltimore approved the procedures used in this study. Written informed consent was obtained from each patient prior to investigation.
Physical Activity Status
The Johnson Space Center (JSC) physical activity scale was used to assess the activity level of the participants over the preceding month.25 This 8-point Likert scale consists of the following score choices: 0 = avoid physical activities whenever possible, 1 = light physical activities done occasionally, 2 = moderate physical activities done regularly for less than 1 hour per week, 3 = moderate physical activities done regularly for more than 1 hour per week, 4 = heavy physical activities done regularly for less than 30 minutes per week, 5 = heavy physical activities done regularly between 30 and 60 minutes per week, 6 = heavy physical activities done regularly between 1 and 3 hours per week, and 7 = heavy physical activities done regularly for more than 3 hours per week. The patients were asked to select the appropriate score (0 to 7) which best described their general level of physical activity for the previous month. The distribution of scores from this scale was as follows: “0” n = 57, “1” n = 241, “2” n = 57, “3” n = 73, “4” n = 2, “5” n = 1, “6” n = 0, and “7” n = 3.
Patients who selected a score of either 0 or 1 were classified as physically sedentary (n = 299) because these activity values represent either no physical activity performed, or an insufficient and inconsistent amount of physical activity that was far below the minimum recommendations.26 Those patients who selected a score of 2 or higher were classified as physically active (n = 135) because these activity levels either approach or exceed the recommendations. The JSC physical activity scale has a strong, independent relationship with maximal oxygen uptake in men and women between the ages of 20 and 79 years.25,27
BASELINE MEASUREMENTS
Medical History and Physical Examination
Demographic information, cardiovascular risk factors, co-morbid conditions, claudication history, a complete metabolic panel blood draw, and a list of current medications were obtained during a medical history interview and a physical examination.
ABI
After 10 minutes of supine rest, the ankle and brachial systolic blood pressures were obtained as previously described.28,29 The ABI was calculated as ankle systolic pressure/brachial systolic pressure.
6-Minute Walk Test
Patients performed a 6-minute walk test in a hallway supervised by trained exercise technicians as previously described.30 The pain-free and total distance walked during the test were recorded.
Walking Impairment Questionnaire (WIQ)
Self-reported ambulatory ability was assessed using a validated questionnaire for PAD patients that assesses ability to walk at various speeds and distances, and to climb stairs.31
Anthropometry
Height was recorded from a stadiometer (SECA, Germany) and body weight was recorded from a balance beam scale (Health-O-Meter Inc., Bridgeview, IL) without shoes. From these measurements, body mass index was calculated as follows: weight (kg)/height (m)2. The waist/hip ratio was obtained from the minimal waist and maximal hip circumferences with a steel measuring tape according to recent recommendations.32
SURVIVAL STATUS AT FOLLOW-UP
This retrospective, natural history follow-up study determined survival status of each patient in 2004 using the Social Security Death Index from the Ancestry.com website. One investigator (PSM), who was blinded to the group assignment, entered each patient’s first name, last name, social security number, and date of birth to determine whether the patient was deceased, and if so, the date of death. The search results listed at least one potential match for each patient, and the entered information in addition to the last known residence was used to confirm a match. Survival status information was obtained on all of the patients in the study.
STATISTICAL ANALYSES
Independent t-tests and chi-square tests were used to examine baseline differences in the measurements between the sedentary and physically active patients. The effect of the baseline physical activity status on survival without adjustment for other variables was examined using Kaplan-Meier survival curves with logrank test. A hierarchical forward selection with switching method was used to obtain a multivariate Cox proportional hazards prediction model for mortality. Included as possible selection variables were the continuous variables age, ABI, BMI, and categorical variables that included baseline physical activity status, each disease status, smoking status, gender, and race. Furthermore, a backward stepwise procedure was used to reduce the full model with all variables to a model with only variables that remained significant after adjustment for others in the model. All analyses were performed using the NCSS statistical package. Statistical significance was set at p < 0.05. Measurements are presented as means ± standard deviations.
RESULTS
The physically active patients were younger (p = 0.015) and had a higher ABI (p = 0.001) than the sedentary patients (Table 1). The physically active group had higher levels of activity (p < 0.001), as well as higher ambulatory function determined from the 6-minute walk and WIQ measurements (p < 0.001) (Table 2).
Table I.
Baseline characteristics of sedentary and active patients with intermittent claudication. Values are means (SD) and percentages.
| Variables | Sedentary Group (n = 299) |
Active Group (n = 135) |
P Value |
|---|---|---|---|
| Age (years) | 69 (8) | 66 (8) | 0.015 |
| Weight (kg) | 80.5 (15.7) | 81.2 (14.0) | 0.677 |
| Body Mass Index (kg)/(meter)2 | 27.7 (4.8) | 27.9 (4.4) | 0.760 |
| Waist/Hip Ratio | 0.94 (0.08) | 0.94 (0.07) | 0.655 |
| Ankle/Brachial Index | 0.59 (0.17) | 0.65 (0.16) | 0.001 |
| Sex (% Men) | 87 | 86 | 0.844 |
| Race (% Caucasian) | 58 | 57 | 0.329 |
| Myocardial Infarction (%) | 30 | 26 | 0.420 |
| Current Smoking (%) | 41 | 42 | 0.894 |
| Diabetes (%) | 33 | 31 | 0.829 |
| Hypertension (%) | 64 | 59 | 0.557 |
| Hyperlipidemia (%) | 49 | 56 | 0.253 |
| COPD (%) | 15 | 8 | 0.061 |
IC = intermittent claudication.
Table II.
Baseline physical activity level and ambulatory function of sedentary and active patients with intermittent claudication. Values are means (SD).
| Variables | Sedentary Group (n = 299) |
Active Group (n = 135) |
P Value |
|---|---|---|---|
| Physical Activity Scale (activity score) | 0.8 (0.4) | 2.7 (0.9) | < 0.001 |
| 6-Minute Walk Distance (meters) | 341 (86) | 400 (92) | < 0.001 |
| WIQ Distance Score (%) | 26 (28) | 40 (32) | < 0.001 |
| WIQ Speed Score (%) | 27 (24) | 43 (27) | < 0.001 |
| WIQ Stair Climbing Score (%) | 32 (30) | 54 (34) | < 0.001 |
WIQ = walking impairment questionnaire.
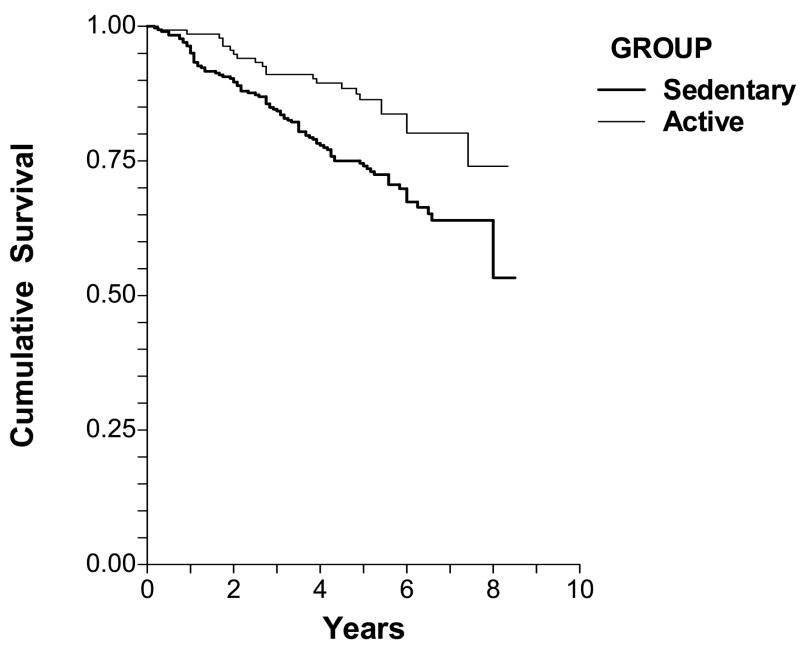
The median follow-up period was 5.33 years (range = 0.25 to 8.33 years) for the physically active group, and 5.0 years (range = 0.17 to 8.5 years) for the sedentary group. At follow-up, 108 patients (24.9%) of the entire cohort had died, consisting of 86 patients (28.8%) who were classified in the sedentary group at baseline and 22 patients (16.3%) who were in the active group. The risk of mortality, unadjusted for other variables, was lower (p = 0.005) in the physically active group (hazard ratio = 0.510, 95% CI = 0.319 to 0.816) than the sedentary group. Figure 1 depicts the Kaplan-Meier cumulative survival probability for both groups throughout the follow-up period. Subsequently, we performed similar analyses using different cut points to define sedentary and active groups to determine if a threshold value in physical activity was related to mortality. Similar to the above results when a score of 2 or greater was used to define the active group, a significant difference in mortality existed between sedentary and active groups when a score of 1 or greater was used to define the active group (p = 0.040), and when a score of 3 or greater was used to separate the groups (p = 0.001). Using a cut point score of 4 or above to separate groups was not possible because very few patients (n = 6) had scores that exceeded a physical activity value of 3. Finally, when the physical activity data was analyzed as a continuous variable with values ranging 0 and 7, physical activity remained highly correlated with mortality (hazard ratio = 0.677, 95% CI = 0.542 to 0.847, p = 0.001).
Figure 1.
Cumulative survival of sedentary and physically active patients limited by intermittent claudication. The survival curves were different between the two groups (log likelihood = −623.7, chi-square = 8.71, p = 0.003).
Proportional Hazards analyses relating baseline clinical characteristics to mortality in patients with intermittent claudication are shown in Table 3. Unadjusted values for age (p < 0.001), BMI (p = 0.002), ABI (p < 0.001), history of myocardial infarction (p = 0.045), baseline physical activity (p = 0.001), and the WIQ stair climbing score (p = 0.023) were significantly related to mortality. Furthermore, sex (p = 0.065), current smoking status (p = 0.069), 6-minute walk distance (p = 0.077), and the WIQ distance score (p = 0.055) approached significance. Using a forward selection multivariate approach (Table 4), age at baseline was the first predictor of mortality entered into the model (hazard ratio = 1.045, 95% CI = 1.019 to 1.072, p < 0.001), followed by BMI (hazard ratio = 0.943, 95% CI = 0.902 to 0.986, p = 0.009), ABI (hazard ratio = 0.202, 95% CI = 0.064 to 0.632, p = 0.006), and physical activity status (hazard ratio = 0.595, 95% CI = 0.370 to 0.955, p = 0.031). After adjustment for these predictors, none of the remaining variables obtained at baseline were significant predictors of mortality (p > 0.05). As a final analysis, when adjusting for all other variables, physical activity status remained a predictor of mortality (hazard ratio = 0.693, 95% CI = 0.521 to 0.922, p = 0.012).
Table III.
Proportional Hazards analyses relating baseline clinical characteristics to mortality in patients with intermittent claudication.
| Variables | Relative Risk | 95% Confidence Interval | P Value |
|---|---|---|---|
| Age (years) | 1.052 | 1.027 to 1.078 | < 0.001 |
| Body Mass Index (kg)/(meter)2 | 0.936 | 0.897 to 0.977 | 0.002 |
| Ankle/Brachial Index | 0.115 | 0.037 to 0.356 | < 0.001 |
| Male Sex | 1.972 | 0.960 to 4.065 | 0.065 |
| Caucasian Race | 1.117 | 0.764 to 1.633 | 0.569 |
| Myocardial Infarction | 1.592 | 1.010 to 2.513 | 0.045 |
| Current Smoking | 1.418 | 0.973 to 2.066 | 0.069 |
| Diabetes | 1.195 | 0.754 to 1.890 | 0.449 |
| Hypertension | 0.933 | 0.440 to 1.976 | 0.856 |
| Hyperlipidemia | 0.700 | 0.449 to 1.091 | 0.114 |
| COPD | 1.479 | 0.816 to 2.681 | 0.197 |
| Physical Activity Scale (activity score) | 0.677 | 0.542 to 0.847 | 0.001 |
| 6-Minute Walk Distance (meters) | 0.998 | 0.995 to 1.000 | 0.077 |
| WIQ Distance Score (%) | 0.993 | 0.985 to 1.000 | 0.055 |
| WIQ Speed Score (%) | 0.994 | 0.985 to 1.002 | 0.132 |
| WIQ Stair Climbing Score (%) | 0.992 | 0.986 to 0.999 | 0.023 |
Table IV.
Multivariate Cox Proportional Hazards model to predict mortality from baseline measures in patients with intermittent claudication.
| Step: Predictor Variables | Relative Risk | 95% Confidence Interval | P Value |
|---|---|---|---|
| Step 1: Age | 1.045 | 1.019 to 1.072 | < 0.001 |
| Step 2: BMI | 0.943 | 0.902 to 0.986 | 0.009 |
| Step 3: ABI | 0.202 | 0.064 to 0.632 | 0.006 |
| Step 4: Activity Group | 0.595 | 0.370 to 0.955 | 0.031 |
ABI = ankle/brachial index, BMI = body mass index.
DISCUSSION
This investigation examined the impact of physical activity level on all-cause mortality in PAD patients who are functionally limited by intermittent claudication. Patients who perform less than one hour of physical activity each week that exceeds light intensity at baseline have a lower unadjusted risk of mortality than their sedentary counterparts, suggesting that even minimal amounts of physical activity performed at moderate intensity have protective effects in PAD patients limited by intermittent claudication. Furthermore, the association between physical activity and all-cause mortality was robust, as it remained significant even after adjusting for other predictors of mortality such as age, ABI, and BMI.
Our results support a recent study that found PAD patients in the highest quartile of physical activity had lower rates of all-cause mortality, cardiovascular disease events, and cardiovascular mortality than those in the lowest quartile.23 Since only a minority of these patients had intermittent claudication, it was not clear whether the association between physical activity and survival was present in this subgroup. The current investigation confirms this notion by demonstrating the beneficial effect of physical activity on survival in PAD patients with ambulatory limitations due to intermittent claudication. These findings also agree with previous studies on relatively healthy middle-age and older men. Harvard alumni who engaged in a modest amount of weekly physical activity that exceeded 500 kcal/week had a lower risk of mortality than those who had activity values below this cut point.22 Similarly, men who were above the lowest tertile for leisure-time physical activity have lower rates of all-cause and cardiovascular mortality.20 Thus, the results from the present investigation indicate that physical activity level in PAD patients with intermittent claudication has a protective effect on all-cause mortality similar to that seen in middle-age and older men.
The intensity of physical activity necessary for patients with intermittent claudication to perceive their physical activity as being moderate in nature is easily attainable. The JSC physical activity scale listed “fast walking” as an example of an activity included in the moderate category.
Since very few PAD patients limited by intermittent claudication typically walk fast, the majority of these patients may perceive that they perform activities at a moderate level of intensity simply by walking for any duration at a pace faster than normal. All of the patients classified as being physically active perceived that they did at least some of their activities at an intensity equivalent to fast walking, whereas the sedentary group consisted of patients who either reported that they avoid doing physical activities altogether, or that they never exceed a light intensity of activity such as walking at a normal pace.
Baseline ABI in this investigation was an independent predictor of mortality, as lower ABI was associated with greater relative risk of death. This observation supports previous studies which found that the presence of PAD is associated with a poor prognosis of long-term survival.1–14 Compared to subjects without PAD, the relative risk for all-cause mortality associated with PAD ranges between 1.4 and 3.8.1,4,5,7,8,10,11,14 The 5-year survival estimate for PAD patients and non-PAD controls is 63% and 90%, respectively, while the 10-year survival is 46% and 77%.7 This supports the current study, which found that the 5-year survival rate was approximately 75%. A subsequent study found an even lower 10-year survival estimate of 39% and 35% in older men and women with PAD, respectively,11 whereas the 10-year survival estimate in patients with severe symptomatic large-vessel PAD is even lower at approximately 25%.1 Collectively, these natural history examinations have much higher mortality rates than compared to randomized controlled clinical trial studies assessing claudication drug therapies,33 likely because the screening procedures and enrollment criteria in the clinical trials are more rigorous.
The prognosis of PAD patients worsens as the severity of PAD increases.3,6,7,10,11,14 For example, compared to controls with ABI values greater than 0.85, patients with ABI values between 0.40 and 0.85 have an adjusted relative risk of mortality of 2.02, whereas patients with ABI values below 0.40 have an adjusted relative risk of 3.35.7 This gradient of higher relative risk of death with lower ABI is also reported in a later study, in which patients with ABI values between 0.31 and 0.49 have a relative risk of death of 1.17 compared to the reference group of patients with ABI values between 0.50 to 0.91, whereas patients with ABI values below or equal to 0.30 have a relative risk of 1.84.6 These data suggest that ABI is an important predictor of the relative risk of all-cause mortality in PAD patients. The association between all-cause mortality and PAD is also evident when symptomatology is used to quantify disease. Patients with intermittent claudication have a relative risk of mortality that is about twofold higher than those without PAD,34–37 and patients with atypical claudication have similar risk as those with classic symptoms of intermittent claudication.38,39 Furthermore, a gradient exists between the relative risk of all-cause mortality and the severity of symptoms, as asymptomatic patients have about half the risk as those with intermittent claudication.1 This increment in risk between asymptomatic and symptomatic patients is also evident for cardiovascular disease mortality, and coronary heart disease mortality.1
Baseline BMI, adjusted for age, was another predictor of mortality in this investigation, as lower BMI was associated with greater relative risk of death. This may be due to the influential effect that cigarette smoking has on BMI, the prevalence of PAD, and mortality. In a previous report from our laboratory, PAD patients who were current smokers had a lower BMI than former smokers who had quit smoking for an average of 12 years prior to investigation.40 Cigarette smoking also predicts those who have PAD, as the relative risk associated with having an ABI value below 0.90 is 2.55 in current smokers.9 Furthermore, cigarette smoking is associated with a higher mortality rate across the entire spectrum of relative body weight, indicating that although smoking and BMI are interrelated, they each exert independent effects on mortality. It is interesting that in the present investigation BMI is more predictive of mortality than cigarette smoking in PAD patients limited by intermittent claudication. This suggests that additional factors besides smoking may alter BMI, such as diet and physical activity, thereby making it a stronger age-adjusted predictor of mortality than smoking in PAD patients.
The physically active and sedentary groups were similar in many baseline characteristics, except that the active patients were younger, had higher ABI values, and had higher ambulatory function. Of these measures, baseline age and ABI were also significant predictors of mortality. Baseline BMI was not different between the active and sedentary groups, but it was a predictor of mortality. After adjusting for age, BMI, and ABI, physical activity status was a significant predictor of all-cause mortality in patients with varying levels of ambulatory function. This finding suggests that physical activity is beneficial regardless of ambulatory ability of patients limited by intermittent claudication. Thus, the recommendation of incorporating less than one hour of walking each week at a speed faster than normal pace to reduce risk of death may apply equally well to patients who have severe intermittent claudication and to those who have milder symptoms. It is not clear whether ambulating faster than normal represents a threshold of intensity that must be attained to impact all-cause mortality, or whether this pace is beneficial simply because it results in a greater volume of physical activity accomplished. If the latter is true, it can be speculated that patients who walk at their usual pace, but for longer durations, may reduce their risk of death as well.
There are several limitations to this study. First, this study used a retrospective, natural history follow-up study design. As such, historical data such as change in physical activity status, change in medication therapy, development of co-morbid conditions, and the number and type of interventional procedures performed during the follow-up period that are typically recorded in a prospectively designed trial were not available for the current investigation. Second, the JSC physical activity scale is a self-reported value in which patients assessed their activity level over the preceding month. Self-report is prone to errors, and even if accurate, the baseline activity level over the preceding month may not reflect chronic activity level prior to the study or the activity status during the follow-up period. Third, the JSC physical activity scale was developed on relatively healthy men and women ranging from 20 and 79 years of age to predict maximal oxygen uptake, and was not specifically developed on patients with intermittent claudication. Fourth, during the search in the Social Security Death Index, we assumed that individuals were alive if they were not listed as deceased. Some time is required for deaths to be recorded in the registry, and it is therefore possible that the mortality data reported in the current investigation is a slight underestimate due to recent deaths not having been posted at the time of our search. However, it is unlikely that the potential underestimate in mortality was biased towards either the sedentary or physically active groups.
In conclusion, patients limited by intermittent claudication who engage in any amount of weekly physical activity beyond light intensity at baseline have a lower mortality rate than their sedentary counterparts who perform either no physical activity or only light-intensity activities. The protective effect of physical activity persists even after adjusting for other predictors of mortality, which include age, ABI, and BMI. The clinical implication of these findings is that PAD patients of varying ages, disease severity, and body composition may reduce their heightened risk of mortality by engaging in less than one hour of physical activity each week that exceeds light intensity, such as ambulating at speeds faster than their typical pace.
Acknowledgments
Andrew W. Gardner, Ph.D., was supported by grants from the National Institute on Aging (NIA) (R01-AG-16685, K01-00657), by a Claude D. Pepper Older American Independence Center grant from NIA (P60-AG12583), by a Geriatric, Research, Education, and Clinical Center grant (GRECC) from the Veterans Affairs administration, and by a National Institutes of Health, National Center for Research Resources, General Clinical Research Center grant (M01-RR-14467).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Criqui MH, Langer RD, Fronek A, et al. Mortality over a period of 10 years in patients with peripheral arterial disease. N Engl J Med. 1992;326:381–386. doi: 10.1056/NEJM199202063260605. [DOI] [PubMed] [Google Scholar]
- 2.Feringa HHH, Bax JJJ, van Waning VH, et al. The long-term prognostic value of the resting and postexercise ankle-brachial index. Arch Int Med. 2006;166:529–535. doi: 10.1001/archinte.166.5.529. [DOI] [PubMed] [Google Scholar]
- 3.Howell MA, Colgan MP, Seeger RW, Ramsey DE, Sumner DS. Relationship of severity of lower limb peripheral vascular disease to mortality and morbidity: A six-year follow-up study. J Vasc Surg. 1989;9:691–697. doi: 10.1067/mva.1989.vs0090691. [DOI] [PubMed] [Google Scholar]
- 4.Lee AJ, Price JF, Russell MJ, Smith FB, van Wijk MCW, Fowkes FGR. Improved prediction of fatal myocardial infarction using the ankle brachial index in addition to conventional risk factors: the Edinburgh Artery Study. Circulation. 2004;110:3075–3080. doi: 10.1161/01.CIR.0000143102.38256.DE. [DOI] [PubMed] [Google Scholar]
- 5.Leng GC, Fowkes FGR, Lee AJ, Dunbar J, Housley E, Ruckley CV. Use of the ankle brachial pressure index to predict cardiovascular events and death: a cohort study. Brit Med J. 1996;313:1440–1444. doi: 10.1136/bmj.313.7070.1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McDermott MM, Feinglass J, Slavensky R, Pearce WH. The ankle-brachial index as a predictor of survival in patients with peripheral vascular disease. J Gen Intern Med. 1994;9:445–449. doi: 10.1007/BF02599061. [DOI] [PubMed] [Google Scholar]
- 7.McKenna M, Wolfson S, Kuller L. The ratio of ankle and arm arterial pressure as an independent predictor of mortality. Atherosclerosis. 1991;87:119–128. doi: 10.1016/0021-9150(91)90014-t. [DOI] [PubMed] [Google Scholar]
- 8.Newman AB, Sutton-Tyrrell K, Vogt MT, Kuller LH. Morbidity and mortality in hypertensive adults with a low ankle/arm blood pressure index. JAMA. 1993;270:487–489. [PubMed] [Google Scholar]
- 9.Newman AB, Siscovick DS, Manolio TA, et al. Ankle-arm index as a marker of atherosclerosis in the cardiovascular health study. Circulation. 1993;88:837–845. doi: 10.1161/01.cir.88.3.837. [DOI] [PubMed] [Google Scholar]
- 10.Vogt MT, Cauley JA, Newman AB, Kuller LH, Hulley SB. Decreased ankle/arm blood pressure index and mortality in elderly women. J Am Med Assoc. 1993;270:465–469. [PubMed] [Google Scholar]
- 11.Vogt MT, McKenna M, Anderson SJ, Wolfson SK, Kuller LH. The relationship between ankle-arm index and mortality in older men and women. J Am Geriatr Soc. 1993;41:523–530. doi: 10.1111/j.1532-5415.1993.tb01889.x. [DOI] [PubMed] [Google Scholar]
- 12.Vogt MT, Wolfson SK, Kuller LH. Segmental arterial disease in the lower extremities: Correlates of disease and relationship to mortality. J Clin Epidemiol. 1993;46:1267–1276. doi: 10.1016/0895-4356(93)90091-e. [DOI] [PubMed] [Google Scholar]
- 13.Wilt TJ. Current strategies in the diagnosis and management of lower extremity peripheral vascular disease. J Gen Intern Med. 1992;7:87–101. doi: 10.1007/BF02599110. [DOI] [PubMed] [Google Scholar]
- 14.Mlacak B, Blinc A, Pohar M, Stare J. Peripheral arterial disease and ankle-brachial pressure index as predictors of mortality in residents of Metlika County, Slovenia. Croat Med J. 2006;47:327–334. [PMC free article] [PubMed] [Google Scholar]
- 15.Weitz JI, Byrne J, Clagett GP, et al. Diagnosis and treatment of chronic arterial insufficiency of the lower extremities: A critical review. Circulation. 1996;94:3026–3049. doi: 10.1161/01.cir.94.11.3026. [DOI] [PubMed] [Google Scholar]
- 16.Sieminski DJ, Gardner AW. The relationship between free-living daily physical activity and the severity of peripheral arterial occlusive disease. Vasc Med. 1997;2:286–291. doi: 10.1177/1358863X9700200402. [DOI] [PubMed] [Google Scholar]
- 17.Feinglass J, McCarthy WJ, Slavensky R, Manheim LM, Martin GJ. Effect of lower extremity blood pressure on physical functioning in patients who have intermittent claudication. J Vasc Surg. 1996;24:503–512. doi: 10.1016/s0741-5214(96)70066-6. [DOI] [PubMed] [Google Scholar]
- 18.US Department of Health and Human Services. Physical activity and health: A report of the surgeon general executive summary. 1996. pp. 1–14. [Google Scholar]
- 19.Sieminski DJ, Cowell LL, Montgomery PS, Pillai SB, Gardner AW. Physical activity monitoring in peripheral arterial occlusive disease patients. J Cardiopulmonary Rehabil. 1997;17:43–47. doi: 10.1097/00008483-199701000-00006. [DOI] [PubMed] [Google Scholar]
- 20.Leon AS, Connett J, Jacobs DR, Jr, Rauramaa R. Leisure-time physical activity levels and risk from coronary heart disease and death: The Minnesota Risk Factor Intervention. JAMA. 1987;258:2388–2395. [PubMed] [Google Scholar]
- 21.Manini TM, Everhart JE, Patel KV, et al. Daily activity energy expenditure and mortality among older adults. JAMA. 2006;296:171–179. doi: 10.1001/jama.296.2.171. [DOI] [PubMed] [Google Scholar]
- 22.Paffenbarger RS, Jr, Hyde RT, Wing AL, Hsieh C-C. Physical activity, all-cause mortality, and longevity of college alumni. N Engl J Med. 1986;314:605–613. doi: 10.1056/NEJM198603063141003. [DOI] [PubMed] [Google Scholar]
- 23.Garg PK, Tian L, Criqui MH, et al. Physical activity during daily life and mortality in patients with peripheral arterial disease. Circulation. 2006;114:242–248. doi: 10.1161/CIRCULATIONAHA.105.605246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pentecost MJ, Criqui MH, Dorros G, et al. Guidelines for peripheral percutaneous transluminal angioplasty of the abdominal aorta and lower extremity vessels. Circulation. 1994;89:511–531. doi: 10.1161/01.cir.89.1.511. [DOI] [PubMed] [Google Scholar]
- 25.Jackson AS, Blair SN, Mahar MT, Wier LT, Ross RM, Stuteville JE. Prediction of functional aerobic capacity without exercise testing. Med Sci Sports Exerc. 1990;22:863–70. doi: 10.1249/00005768-199012000-00021. [DOI] [PubMed] [Google Scholar]
- 26.Pate RR, Pratt M, Blair SN, et al. Physical activity and public health: A recommendation from the Centers for Disease Control and Prevention and the American College of Sports Medicine. JAMA. 1995;273:402–407. doi: 10.1001/jama.273.5.402. [DOI] [PubMed] [Google Scholar]
- 27.Heil DP, Freedson PS, Ahlquist LE, Price J, Rippe JM. Nonexercise regression models to estimate peak oxygen consumption. Med Sci Sports Exerc. 1995;27:599–606. [PubMed] [Google Scholar]
- 28.Gardner AW, Killewich LA, Katzel LI, et al. Relationship between free-living daily physical activity and peripheral circulation in patients with intermittent claudication. Angiology. 1999;50:289–297. doi: 10.1177/000331979905000404. [DOI] [PubMed] [Google Scholar]
- 29.Gardner AW, Skinner JS, Cantwell BW, Smith LK. Progressive vs single-stage treadmill tests for evaluation of claudication. Med Sci Sports Exerc. 1991;23:402–408. [PubMed] [Google Scholar]
- 30.Montgomery PS, Gardner AW. The clinical utility of a 6-minute walk test in peripheral arterial occlusive disease patients. J Am Geriatr Soc. 1998;46:706–711. doi: 10.1111/j.1532-5415.1998.tb03804.x. [DOI] [PubMed] [Google Scholar]
- 31.Regensteiner JG, Steiner JF, Panzer RL, Hiatt WR. Evaluation of walking impairment by questionnaire in patients with peripheral arterial disease. J Vasc Med Biol. 1990;2:142–152. [Google Scholar]
- 32.Lohman TG, Roche AF, Martorell R. Anthropometric standardization reference manual. Champaign, Illinois: Human Kinetics Books; 1988. [Google Scholar]
- 33.Brass EP, Hiatt WR. Review of mortality and cardiovascular event rates in patients enrolled in clinical trials for claudication therapies. Vasc Med. 2006;11:141–145. doi: 10.1177/1358863x06069513. [DOI] [PubMed] [Google Scholar]
- 34.Dormandy J, Mahir M, Ascady G, et al. Fate of the patient with chronic leg ischemia. J Cardiovasc Surg. 1989;30:50–57. [PubMed] [Google Scholar]
- 35.Hughson WG, Mann JI, Tibbs DJ, Woods HF, Walton I. Intermittent claudication: factors determing outcome. Brit Med J. 1978;1:1377–1379. doi: 10.1136/bmj.1.6124.1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jelnes R, Gaardsting O, Hougaard-Jensen K, Backgaard N, Tonnesen KH, Schroeder T. Fate in intermittent claudication: outcome and risk factors. Brit Med J. 1986;293:1137–1140. doi: 10.1136/bmj.293.6555.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reunanen A, Takkunen H, Aromaa A. Prevalence of intermittent claudication and its effect on mortality. Acta Med Scand. 1982;211:249–256. doi: 10.1111/j.0954-6820.1982.tb01939.x. [DOI] [PubMed] [Google Scholar]
- 38.Criqui MH, Coughlin SS, Fronek A. Noninvasively diagnosed peripheral arterial disease as a predictor of mortality: results from a prospective study. Circulation. 1985;72:768–773. doi: 10.1161/01.cir.72.4.768. [DOI] [PubMed] [Google Scholar]
- 39.Smith GD, Shipley MJ, Rose G. Intermittent claudication, heart disease risk factors, and mortality: the Whitehall Study. Circulation. 1990;82:1925–1931. doi: 10.1161/01.cir.82.6.1925. [DOI] [PubMed] [Google Scholar]
- 40.Gardner AW, Sieminski DJ, Killewich LA. The effect of cigarette smoking on free-living daily physical activity in older claudication patients. Angiology. 1997;48:947–955. doi: 10.1177/000331979704801103. [DOI] [PubMed] [Google Scholar]