Abstract
Objective
Alterations in reward-related brain function and phenomenological aspects of positive affect are increasingly examined in the development of major depressive disorder. The authors tested differences in reward-related brain function in healthy and depressed adolescents, and the authors examined direct links between reward-related brain function and positive mood that occurred in real-world contexts.
Method
Fifteen adolescents with major depressive disorder and 28 adolescents with no history of psychiatric disorder, ages 8–17 years, completed a functional magnetic resonance imaging guessing task involving monetary reward. Participants also reported their subjective positive affect in natural environments during a 4-day cell-phone-based ecological momentary assessment.
Results
Adolescents with major depressive disorder exhibited less striatal response than healthy comparison adolescents during reward anticipation and reward outcome, but more response in dorsolateral and medial prefrontal cortex. Diminished activation in a caudate region associated with this depression group difference was correlated with lower subjective positive affect in natural environments, particularly within the depressed group.
Conclusions
Results support models of altered reward processing and related positive affect in young people with major depressive disorder and indicate that depressed adolescents’ brain response to monetary reward is related to their affective experience in natural environments. Additionally, these results suggest that reward-processing paradigms capture brain function relevant to real-world positive affect.
Depression that begins in childhood or adolescence disrupts functioning in academic, family, peer, and affective contexts (1). A central issue in the pathophysiology of depression is how affective brain systems are disrupted in ways associated with mood correlates of the disorder. From a developmental affective neuroscience perspective, it is important to consider not only neural systems underpinning negative affect but also positive affect systems, because diminished pleasant mood, decreased motivation for rewarding experiences, and unusual dopamine system function may represent core aspects of depression, particularly early in its course (2, 3). Understanding early developmental changes in neural reward systems in depression could provide insights relevant to treatments while brain development is underway (4) because treatments provided early in development could have the potential for more enduring benefits. Because depressed children’s behavior and affect in laboratory studies may have little relevance to their behavior in real-world settings, establishing links between neural changes in controlled tasks and mood alterations in natural settings can provide particularly promising leads to advance etiologic understanding.
Key elements of positive affect include subjective experience of pleasant emotions and neural response to rewarding stimuli (2). Studies of both elements have found abnormalities in early-onset depression, including reduced pleasant mood (5) and reduced response in reward-related brain areas such as the striatum (6). However, no study to date has examined the relation of these two aspects of positive affect in young people with depression. Such validation is critical to efforts to examine the neural substrates of depression because it links brain function assessed in an artificial, laboratory environment to affective experience in natural environments. Thus, brain regions whose function in response to abstract reward stimuli distinguishes depressed from healthy young people are more meaningfully related to depression if they reflect real-life affect. Also, given that people are poor at reporting past moods or predicting future moods accurately (7), measuring current, momentary positive affect in natural settings through ecological momentary assessment can provide a more accurate index of true affective experience than can questionnaires administered in the laboratory.
Adult depression is associated with reduced activation in striatal areas (8, 9) and enhanced activation in medial prefrontal cortex areas related to sadness and social cognition (10) in response to pleasant stimuli. Reduced striatal activation might be a particularly meaningful difference in depression given that the striatum is critical to reward processing (11–13) and (inversely) implicated in anhedonia (14). In our previous studies of reward-related decision-making using different samples from the current study, young people with depression did not distinguish high- and low-magnitude likely rewards (15) and exhibited less brain activation in areas including dorsal striatum during reward processing (6).
The present study examined blood-oxygen-level-dependent (BOLD) response to anticipation and outcome of reward in young people with major depressive disorder and investigated the association of depression-related reward response with positive affect experienced in natural environments. Using a functional magnetic resonance imaging (fMRI) reward paradigm that reliably elicits striatal activation and a cell-phone ecological momentary assessment protocol, we examined group differences in reward processing and the relation of those group differences to positive affect. We hypothesized that major depressive disorder is associated with reduced striatal response and enhanced medial prefrontal cortex response and that areas reflecting depression-related differences in striatal function are related to real-life positive affect.
Method
Participants
The participants were 78 adolescents, ages 8–17 years, from a study of pediatric affective disorders. The final data set included 43 participants because three participants did not complete the guessing task and 32 had excessive movement. Final participants had major depressive disorder (N=15, 70% female) or were healthy comparison subjects with no history of psychiatric disorder (N=28, 75% female). (In previous reports, we have often separated comparison participants based on family history of affective disorder [e.g., high or low risk based on the number of family members with history of affective disorders]. However, in the current article, in order to maximize the sample size in the comparison group, we included all comparison subjects together. We conducted analyses with adjustment for family history and found that results did not differ from those reported here.)
Comorbidities among major depressive disorder participants included generalized anxiety disorder (N=8), social phobia (N=3), and panic disorder (N=1). The participants were free of psychotropic medications, nicotine, and illicit drugs. The participants were generally European American (87% of those with major depressive disorder; 93% of comparison subjects), and groups did not differ in age (mean=13.5 [SD=2.1] years for major depressive disorder; mean=13.1 [SD=2.6] for comparison subjects). The participants with major depressive disorder had higher depressive symptoms than did comparison subjects (mean=21.60 [SD= 11.99] versus 5.77 [SD=6.77], respectively; F=29.42, df=1, 38, p<0.001), higher self-reported anxiety symptoms (mean=26.42 [SD=17.80] versus 7.67 [SD=5.23]; F=15.15, df=1, 38, p<0.005), and lower subjective positive affect (mean=2.29 [SD=0.82] versus 3.10 [SD=0.62]; F=13.40, df=1, 42, p<0.005).
The participants with major depressive disorder were recruited from outpatient clinics at Western Psychiatric Institute and Clinic, Pittsburgh, Pa., and through advertisements. The comparison subjects were recruited through advertisements. All participants completed diagnostic assessment with the Schedule for Affective Disorders and Schizophrenia for School-Age Children—Present and Lifetime Version (16). Major depressive disorder diagnoses were confirmed by face-to-face interviews with a child psychiatrist. After complete description of the study, written informed consent was obtained from all parents and from participants age 14 and older; younger participants provided verbal assent.
Measures
Symptoms
The participants and their parents completed the Screen for Childhood Anxiety and Related Disorders (17) and the Mood and Feelings Questionnaire (18).
Reward processing
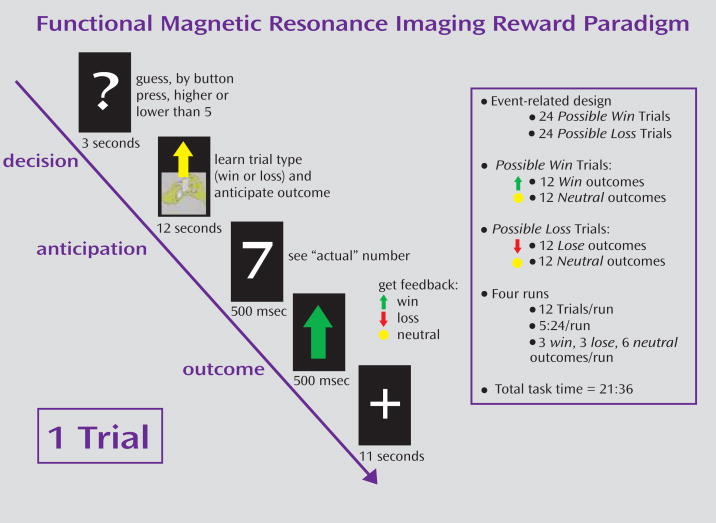
The fMRI paradigm (Figure 1) was an adaptation of a card-guessing paradigm developed by Delgado and colleagues (11) to probe striatal response to feedback associated with monetary reward. In our event-related paradigm, each trial included both an anticipation period and an outcome period, and participants received win, loss, or no-change feedback for each trial. The participants were told that their performance would determine a monetary reward to be received after the scan.
FIGURE 1.
Event-Related Guessing Task With Monetary Reward
Trials were presented in pseudorandom order with predetermined outcomes. During each 27-second trial, the participants had three seconds to guess, through button press, whether the value of a visually presented card with a possible value of 1–9 was higher or lower than 5 (index and middle finger, respectively). After a choice was made, the trial type (reward or loss) was presented visually for 12 seconds (anticipation). This was followed by the “actual” numerical value of the card (500 msec); outcome feedback (a green upward-facing arrow for win, a red downward-facing arrow for loss, or a yellow circle for neutral feedback; 500 msec); and a crosshair presented for 11 seconds (outcome). Trials were presented in 4 runs, with 12 trials per run, and a balanced number of trial types within runs.
The participants were told that they would receive $1 for each win, lose 50 cents for each loss, and experience no earnings change for neutral outcomes. The participants were unaware of the fixed outcome probabilities and were led to believe that performance would determine net monetary gain. The participants’ engagement and motivation to perform well were maintained by verbal encouragement during practice and between runs.
Subjective positive affect
The PANAS-C (19), a mood questionnaire with good psychometric properties, was used to assess positive affect by cell phone in natural settings. Mood is described by adjectives (e.g., happy) using a 5-point response scale (1=very slightly or not at all, 5=extremely). All 20 items were administered once per day, and a subset of eight items (four reflecting positive affect: happy, joyful, energetic, excited) was administered at all other calls. Because a priori hypotheses focused on positive affect and because mean positive affect score within the day was not excessively variable (SD=0.85–1.11), mean positive affect across the weekend was computed for analyses. Mean positive affect across the weekend was moderately negatively correlated with depressive symptoms (r=−0.58, p<0.001), indicating that the constructs of positive affect and depression were related but not completely overlapping.
Pubertal development
The participants underwent a physical examination by a nurse or pediatrician, their breast/genital development was rated (20), and they were classified as pre/early (Tanner=1–2) or mid/late (Tanner=3–5) adolescents.
Procedure
The local institutional review board approved the study. The participants’ assessment included diagnosis, symptom questionnaires, ecological momentary assessment, and an MRI scan. The ecological momentary assessment protocol was conducted over a weekend, and the scan was conducted on the following Thursday. Before the scan, the participants practiced the paradigm and experienced the scanning environment through a simulator to ensure familiarity with the environment and to gauge comfort. The participants received $60 for the scan, plus $15 for “playing the game.”
During the ecological momentary protocol, the participants reported on mood, activities, and companions “at the moment the phone rang.” PANAS-C items were used to assess mood. Calls were placed 12 times from Friday to Monday at specified windows of time distributed throughout each day (e.g., 4–7 p.m.), but only after school hours for calls on Friday and Monday. Ecological momentary assessment was conducted primarily during a weekend so that calls would not interfere with school participation and because participants would have more freedom to choose activities and companions. Data were missing or incomplete for 5% of calls. Calls were administered by research associates, who also ensured that participants understood the rating scales and vocabulary of the items. Based on responses about companions, the participants were in social contexts during 60% of the calls. One comparison participant declined the ecological moment assessment protocol.
BOLD fMRI Acquisition, Processing, and Analysis
Each participant was scanned using a Siemens 3T Allegra scanner (Malvern, Pa.). BOLD functional images were acquired with a gradient echo planar imaging sequence and covered 34 axial slices (3 mm thick) beginning at the cerebral vertex and encompassing the entire cerebrum and the majority of the cerebellum (TR/TE=2000/25 msec, field of view=20 cm, matrix=64×64). Scanning parameters were selected to optimize BOLD signal quality while maintaining a sufficient number of slices to acquire whole-brain data. Before the collection of fMRI data for each participant, a reference echoplanar imaging scan was acquired and visually inspected for artifacts (e.g., ghosting) and good signal across the entire volume. The data from all 43 participants were clear of such problems.
Whole-brain image analysis was conducted with SPM2 (http://www.fil.ion.ucl.ac.uk/spm). For each scan, images for each participant were realigned to the first volume in the time series to correct for head motion. The motion correction criterion was set at <4 mm, which is higher than that used in many fMRI studies, to maximize the size of this sample containing young people, many from a clinical population. (Only three participants had motion >2mm across runs 1 and 2 of the task [two with major depressive disorder, one from the comparison group; 2 male, and 1 female]. Because the results did not change when these three participants were excluded from analyses, and because motion over runs 1 and 2 was acceptably low for the sample overall [mean=1.05 mm, SD=0.74], we included all participants with movement <4 mm.)
Realigned images were spatially normalized into Montreal Neurological Institute stereotactic space using a 12-parameter affine model, then smoothed to minimize noise and residual difference in gyral anatomy with a Gaussian filter set at 6 mm full-width at half-maximum. Voxel-wise signal intensities were ratio-normalized to the whole-brain global mean.
Preprocessed data were analyzed using second-level random effects models that account for both scan-to-scan and participant-to-participant variability to determine task-specific regional responses. Analyses focused on data for the first two runs of the task so that fatigue, habituation, and frustration with task length would contribute less to responses. Analyses of the first run only, including two additional participants (Ns=16 with major depressive disorder, and 29 comparison subjects), yielded a pattern of results identical to those below. Given previous findings that the task half contributes to response in a similarly long reward task (6), focusing on the early trials allowed examination of relatively novel reward experiences. Analyses including participants who completed all four runs (N=12 with major depressive disorder, N=25 comparison) confirmed that the findings were largely unchanged when all trials were included: the major depressive disorder group exhibited less striatal reactivity during both anticipation and outcome and more prefrontal reactivity during anticipation than the comparison group.
Because a priori hypotheses concerned the role of positive affect in depression, analyses focused on reward rather than loss. For each participant and scan, predetermined condition effects at each voxel were calculated using a t statistic, producing a statistical image for two contrasts: reward anticipation > baseline and reward outcome > baseline. Individual contrast images were used to determine mean reward anticipation-related and outcome-related response using one-sample t tests. All tests were set to a threshold of p<0.05, required to have a minimum extent of 10 contiguous voxels, and corrected for multiple comparisons using false discovery rate across activation clusters of interest based on earlier region-of-interest analyses. Earlier analyses focused on two regions of interest: 1) a striatal region of interest, based on the typical pattern of response in similar reward tasks (sphere with 20 mm radius, centered on Talairach coordinates x=0, y=10, z=−10, encompassing the entire bilateral ventral striatum and adjacent regions of the caudate); and 2) a prefrontal region of interest that included areas associated with unusual response to pleasant stimuli in depression (Brodmann’s areas 9, 10, 11, 24, 25, and 32). The striatal region of interest was used for analyses within the comparison group and comparison >major depressive disorder contrasts. The prefrontal region of interest was used for analyses within the major depressive disorder group and major depressive disorder > comparison group contrasts. For testing associations between brain activation and real-life positive affect, the region of interest was defined as significant clusters from comparison subjects > major depressive disorder analyses.
Data Analyses
Within-group analyses were conducted to establish group-level response. Group differences were examined using one-way analyses of covariance (ANCOVAs) with major depressive disorder group as the independent variable and sex and age as covariates. BOLD signal values for striatal clusters reflecting a main effect of reward anticipation or outcome within the comparison group or clusters reflecting a main effect of reward anticipation or outcome within the major depressive disorder group were entered as dependent variables for ANCOVAs. Since the major depressive disorder group and the comparison group did not differ in reaction time, we did not account for performance. Results were largely unchanged when anxiety symptoms or family history were covaried. Associations between reward-related BOLD response and real-world positive affect were evaluated with regression analyses in SPM and also SPSS, with mean positive affect, sex, and age as independent variables.
Results
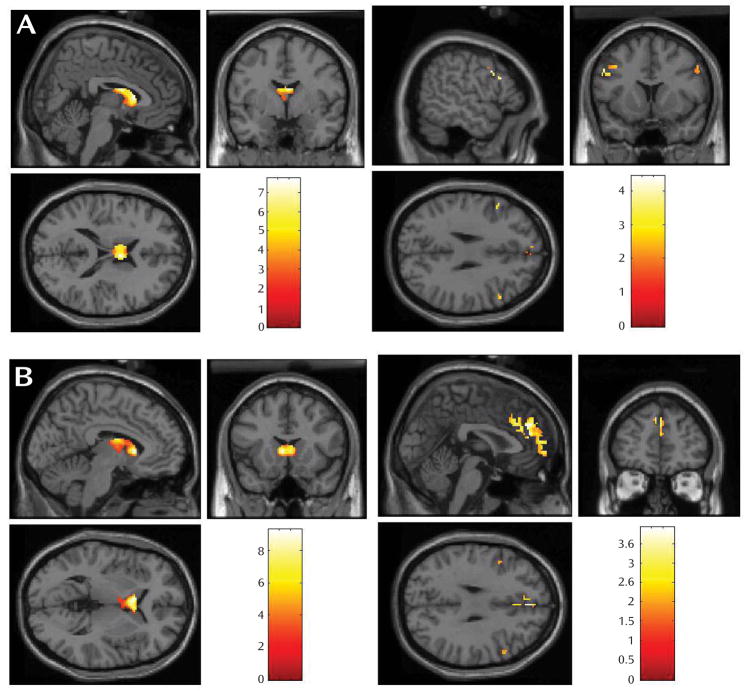
Task Effects
Within the comparison group, both reward anticipation and reward outcome elicited large-cluster activation in the caudate (Table 1, Figure 2). Within the major depressive disorder group, reward anticipation elicited activation in dorsolateral prefrontal cortex (Brodmann’s area 9) and Brodmann’s areas 6, 10, and 11; reward outcome elicited activation in Brodmann’s areas 8, 9, and 10.
TABLE 1.
Within-Group and Between-Group Effects on Reward-Related Blood-Oxygen-Level-Dependent Response, by Conditiona
| Talairach Coordinates of Maximum Voxel in Cluster |
||||||||
|---|---|---|---|---|---|---|---|---|
| Condition | Hemisphere | x | y | z | Cluster Size | t | df | p |
| Reward anticipation | ||||||||
| Within comparison group | ||||||||
| Caudate body | Right | 4 | 1 | 17 | 948 | 7.74 | 27 | <0.001 |
| Within major depressive disorder group | ||||||||
| DLPFC (Brodmann’s area 9) | Left | −54 | 21 | 33 | 45 | 4.44 | 14 | <0.001 |
| DLPFC (Brodmann’s area 9) | Right | 54 | 19 | 29 | 35 | 3.78 | 14 | <0.005 |
| DLPFC (Brodmann’s area 9) | Left | −4 | 50 | 31 | 40 | 3.65 | 14 | <0.005 |
| Medial frontal gyrus (Brodmann’s area 6) | Right | 2 | 48 | 33 | 27 | 3.65 | 14 | <0.005 |
| Orbital gyrus (Brodmann’s area 11) | Left | −40 | 34 | −12 | 17 | 3.49 | 14 | <0.005 |
| Middle frontal gyrus (Brodmann’s area 10) | Right | 44 | 52 | −9 | 43 | 3.18 | 14 | <0.005 |
| DLPFC (Brodmann’s area 9) | Right | 40 | 29 | 37 | 20 | 2.67 | 14 | <0.01 |
| DLPFC (Brodmann’s area 9) | Left | −40 | 13 | 36 | 31 | 2.64 | 14 | <0.05 |
| DLPFC (Brodmann’s area 9) | Left | −36 | 31 | 37 | 11 | 2.56 | 14 | <0.05 |
| Middle frontal gyrus (Brodmann’s area 10) | Left | −44 | 49 | 1 | 15 | 2.54 | 14 | <0.05 |
| Medial frontal gyrus (Brodmann’s area 10) | Right | 2 | 60 | −3 | 16 | 2.19 | 14 | <0.05 |
| Comparison > major depressive disorder | ||||||||
| Caudate head | Left | −6 | 18 | 3 | 270 | 4.49 | 39 | <0.001 |
| Major depressive disorder > comparison | ||||||||
| DLPFC (Brodmann’s area 9) | Right | 40 | 29 | 37 | 14 | 3.35 | 39 | <0.005 |
| DLPFC (Brodmann’s area 9) | Left | −4 | 48 | 33 | 14 | 2.67 | 39 | <0.01 |
| DLPFC (Brodmann’s area 9) | Left | −50 | 11 | 31 | 13 | 2.07 | 39 | <0.05 |
| Reward outcome | ||||||||
| Within comparison group | ||||||||
| Caudate head | Left | −6 | 16 | 3 | 1026 | 9.30 | 27 | <0.001 |
| Within major depressive disorder group | ||||||||
| DLPFC (Brodmann’s area 9) | Right | 2 | 44 | 16 | 198 | 3.92 | 14 | <0.005 |
| Medial frontal gyrus (Brodmann’s area 8) | Left | −4 | 48 | 31 | 36 | 3.69 | 14 | <0.005 |
| DLPFC (Brodmann’s area 9) | Left | −54 | 11 | 31 | 25 | 3.60 | 14 | <0.005 |
| Middle frontal gyrus (Brodmann’s area 10) | Right | 40 | 56 | −8 | 15 | 3.57 | 14 | <0.005 |
| DLPFC (Brodmann’s area 9) | Right | 40 | 29 | 37 | 41 | 3.47 | 14 | <0.005 |
| Superior frontal gyrus (Brodmann’s area 10) | Left | −4 | 60 | −1 | 60 | 3.18 | 14 | <0.005 |
| Superior frontal gyrus (Brodmann’s area 10) | Right | 28 | 62 | −3 | 13 | 3.12 | 14 | <0.005 |
| DLPFC (Brodmann’s area 9) | Left | −40 | 29 | 37 | 21 | 2.84 | 14 | <0.01 |
| DLPFC (Brodmann’s area 9) | Right | 54 | 19 | 29 | 19 | 2.51 | 14 | <0.05 |
| Comparison > major depressive disorder | ||||||||
| Caudate head | Left | −6 | 18 | 3 | 239 | 4.55 | 39 | <0.001 |
| Major depressive disorder > comparison | ||||||||
| Medial frontal gyrus (Brodmann’s area 10) | Left | −4 | 60 | 3 | 16 | 2.51 | 39 | <0.001 |
| DLPFC (Brodmann’s area 9) | Left | −4 | 48 | 33 | 11 | 2.44 | 39 | <0.01 |
| DLPFC (Brodmann’s area 9) | Right | 40 | 29 | 37 | 11 | 2.40 | 39 | <0.01 |
Significant clusters for each analysis are listed in descending order of t score. Comparison group > major depressive disorder indicates findings for greater activation in the comparison group relative to the depressed group, and major depressive disorder > comparison indicates the opposite. DLPFC: dorsolateral prefrontal cortex.
FIGURE 2.
Main Effects of Task Within Comparison Group (on left) and Major Depressive Disorder Group (on right) During A) Reward Anticipation and B) Reward Outcome (colored bars reflect t scores for each analysis)
Depression-Related Differences in Neural Response to Reward
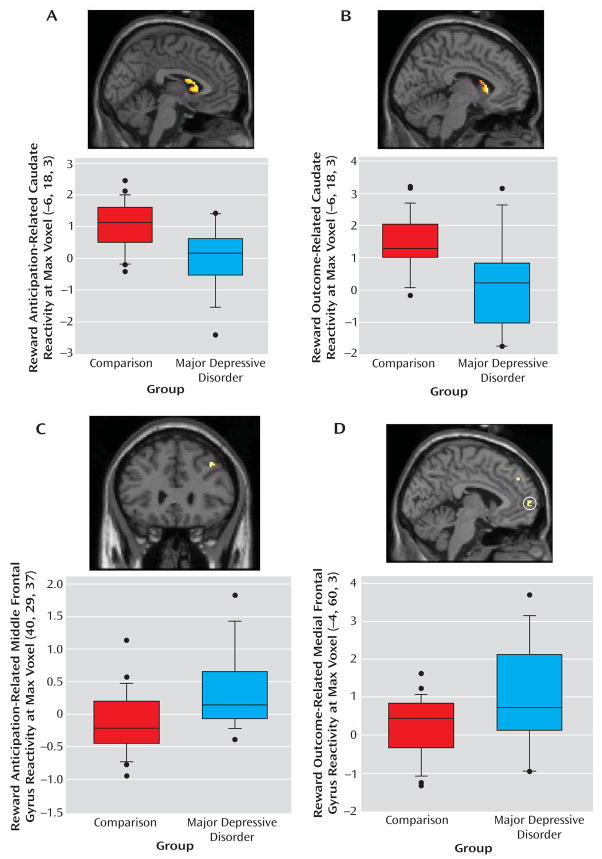
The participants with major depressive disorder displayed less BOLD response than the comparison subjects in the caudate during reward anticipation and outcome (Table 1, Figure 3). Participants with major depressive disorder displayed greater BOLD response than the comparison subjects in the dorsolateral prefrontal cortex during reward anticipation and in medial Brodmann’s area 10 and dorsolateral prefrontal cortex during reward outcome (Table 1, Figure 3).
FIGURE 3.
Differences in Reward-Related Brain Function Between Young People With Major Depressive Disorder and Those With No History of Psychiatric Disordera
a Images depict (A) striatal regions in which comparison subjects exhibited greater activation than subjects with major depressive disorder during reward anticipation; (b) striatal regions in which comparison > major depressive disorder during reward outcome; (C) prefrontal regions in which major depressive disorder > comparison during reward anticipation; and (D) prefrontal regions in which major depressive disorder > comparison during reward outcome. Boxplots in A–D depict BOLD signal change for evident or circled region by diagnostic group, in arbitrary units. Talaraich coordinates are indicated in y axis labels. Age and sex were included as covariates in all analyses. Results remained significant when outliers were excluded from analyses.
To examine possible developmental effects, we also conducted exploratory analyses of major depressive disorder effects within pre/early and mid/late adolescent groups. Findings of greater striatal reactivity in the comparison group remained evident in both developmental groups during both anticipation (pre/early: 303-voxels cluster at −6, 17, 1, t=3.29, df=1, 7, p<0.005; mid/late: 73 voxels, −6, 18, 3, t=2.58, df=1, 25, p<0.01) and outcome (pre/early: 349 voxels, −6, 12, 2, t=12.33, df=1, 7, p<0.001; mid/late: 68 voxels, 0, 16, 10, t=2.88, df=1, 25, p<0.005). Findings of greater activation in prefrontal areas in the major depressive disorder group during both conditions (e.g., Brodmann’s area 9 during outcome: 67 voxels, −2, 48, 31, t=3.42, df=1, 25, p<0.001) only remained evident within the mid/late developmental group.
Finally, we conducted analyses to further explore whether the major depressive disorder group exhibited different orbitofrontal or amygdala function than the comparison group. These analyses yielded no significant findings.
Depression-Related Differences in Neural Response to Reward and Real-World Positive Affect
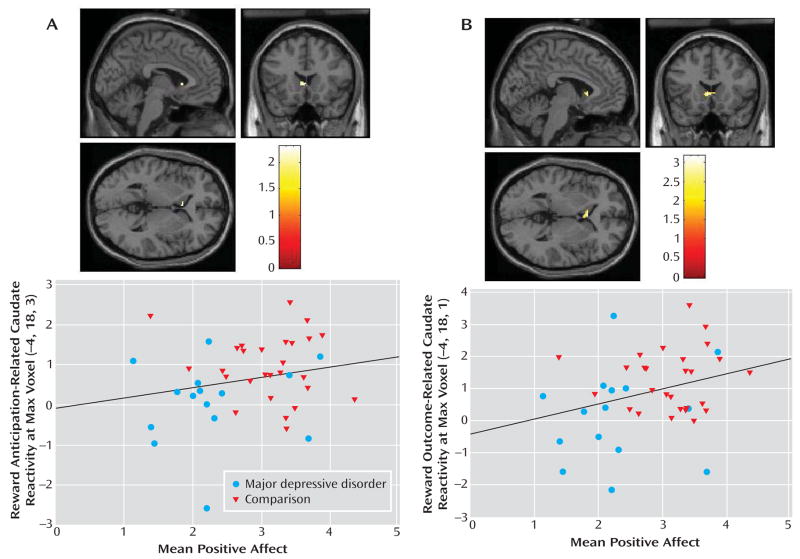
Activation in left caudate clusters that distinguished major depressive disorder and comparison groups during reward anticipation and outcome were significantly positively correlated with subjective positive affect (Figure 4). Thus, the participants who experienced higher positive affect in real-world settings exhibited greater activation in these striatal areas. Furthermore, this association between reward processing and positive affect was evident in the specific brain areas in which participants with major depressive disorder showed less reward-related activation than did comparison participants.
FIGURE 4.
Correlation of (A) Reward Anticipation-Related Striatal Reactivity (BOLD signal change) and (B) Reward Outcome-Related Striatal Reactivity With Mean Subjective Positive Affect Measured in Natural Environmentsa
a Results were masked for striatal regions in which the depression group exhibited less activation than the comparison group. Depicted are caudate clusters correlated with positive affect (for anticipation, 12 voxels; t=2.31, df=1, 38, p<0.02; for outcome, 37 voxels, t=3.18, df=1, 38, p<0.005) and scatterplots of caudate activation versus positive affect score, by group, with regression lines for the entire sample. Blood-oxygen-level-dependent signal change values in scatterplots are in arbitrary units. Colored bars reflect t scores for each analysis.
Traditional regression models yielded overall R2 values of 0.22 for reward anticipation and 0.26 for outcome. Positive affect and sex were significant predictors in each final model (positive affect: β=0.33, t=2.13, p<0.05 for anticipation, β=0.44, t=2.96, p<0.01 for outcome; sex: β=0.41, t= 2.83, p<0.01 for anticipation, β=0.39, t=2.75, p<0.01 for outcome), but age was not.
Finally, exploratory regression models predicting positive affect were computed within the major depressive disorder group. These yielded overall R2 values of 0.71 for reward anticipation and 0.75 for reward outcome, which indicate large effect sizes (Cohen’s f2=2.45 and 3.00, respectively). The reward-related brain function coefficient was marginally significant for reward outcome, also reflecting a large effect size (β=0.33, t=2.13, p=0.06; Cohen’s d=1.28). Similar models within the comparison group were nonsignificant.
Discussion
The current study’s findings are consistent with hypotheses of altered reward processing and reduced positive affect in young people with major depressive disorder (2). During the anticipation and receipt of monetary rewards, young people with diagnosed major depressive disorder exhibited reduced response in dorsal striatal reward areas. Activation in caudate regions in which the major depressive disorder group showed reduced response relative to the comparison group was positively correlated with subjective positive affect that participants experienced during a weekend in their natural settings. Within the major depressive disorder group, the combination of reward processing, sex, and age accounted for a considerable amount of variance in real-world positive affect. Thus, disruption of reward processing in adolescents with major depressive disorder in the context of a simple fMRI task appears to be predictive of their real-world affective experience.
Decreased striatal response in young people with major depressive disorder could indicate depression-related difficulties with several aspects of reward processing. Disruption of response in the caudate, for example, could suggest difficulties with the execution of action in response to rewarding cues (12). The caudate receives input from ventral tegmental dopamine neurons, and unusual function in young people with depression supports claims that depression involves disrupted dopamine signaling (3, 21). Whether such disruption represents a state-like response during a depressive episode or a stable, trait-like difference in reward processing—and whether it changes with development or clinical course—must be addressed by longitudinal, prospective studies.
In addition to exhibiting reduced responding in reward-related brain areas, the major depressive disorder group exhibited greater responding than comparison subjects in prefrontal cortical areas postulated to play a role in affect regulation (Brodmann’s area 9) and social cognition (Brodmann’s area 10) (22). Based on models of frontostriatal connectivity and the integrative function of dopamine systems (23, 24), greater activation in these regions in combination with less activation in reward-related areas may reflect overregulation of reward responding in young people with major depressive disorder. Perhaps when waiting for or obtaining a reward, young people with depression diminish the onset or maintenance of their response by engaging cognitive and regulatory circuits.
The presence of enhanced activation in medial Brodmann’s area 10 in the major depressive disorder group during rewarding outcomes is consistent with findings that depressed adults exhibit increased activation in this area during affective contexts intended to induce pleasant mood (10). In combination with the putative self-referential and social cognitive functions of medial Brodmann’s area 10 (22), this finding suggests that young people with depression might think about themselves or their status in relation to others (e.g., “everyone’s winning more than I am”) rather than enjoying a pleasant outcome. Given that medial prefrontal cortex is also active during so-called default states thought to involve self-reflection (25), another interpretation of this finding is that adolescents with depression find it difficult to disengage from their ongoing, baseline cognitive activity in the presence of reward.
Although sample size limited our power to detect some effects within developmental groups, analyses within the pre/early and mid/late adolescent groups revealed that youths with major depressive disorder in both developmental groups exhibited reduced striatal reactivity relative to comparison subjects. Thus, alterations to reward-related brain function in depression might occur in both children and adolescents. Of importance, the pattern we observed in the major depressive disorder group stands in contrast to developmental evidence that healthy adolescents exhibit enhanced striatal activation when responding to reward (26, 27). This difference from healthy adolescents raises questions about the consequences of disruptions to reward-related brain function during a developmental period in which this function is undergoing change. Therefore, it will be valuable for future studies to examine the separate and interacting influences of depression and development on reward processing in adolescents, as well as the importance of altered reward processing for long-term clinical course.
Our findings differ in some respects from those of other studies of reward-related brain function in depression. For example, others have reported decreased orbitofrontal reactivity in response to reward in young people with depression (6) or a negative relation between amygdala reactivity and anhedonia (28). While our hypotheses focused more on striatal reactivity, we found some orbitofrontal reactivity within the major depressive disorder group but no differences between the major depressive disorder and comparison groups in reactivity of the orbitofrontal cortex or amygdala. A possible reason for this discrepancy is that our reward task is similar to those that reliably engage striatal reward areas (11) and does not include other types of affective stimuli such as human faces.
Similarly, sample size and composition limited our ability to test whether depression-related differences were present within male and female subsamples. Girls were overrepresented in our sample, but gender was unlikely to serve as a confound because both the major depressive disorder and comparison groups had greater proportions of girls than boys. Anxiety symptoms, higher in the major depressive disorder group than the comparison group, were another possible confound. Although results did not change when we adjusted for anxiety symptoms, it is possible that the presence of anxiety influenced our results, especially since we found previously that anxiety symptoms appear to contribute uniquely to reward processing in young people (6). Our paradigm was a straightforward monetary reward task that allowed us to examine one class of natural rewards but precluded us from considering other aspects of reward, such as social context, subjective experience, and varying magnitude. We did not obtain mood ratings during the reward task, and unfortunately we were thus unable to test the hypothesis that group differences in brain function reflect differences in subjective experience of reward.
In sum, this study indicates that young people with depression appear to exhibit unusual patterns of neural response to two components of reward processing. Neural response was further related to subjective affect in natural settings, providing validation for the use of laboratory-based reward paradigms to measure aspects of positive affect. A greater understanding of the affective factors in depression will ideally lead not only to increased knowledge of the pathophysiology of depression but also to treatments that target the affective systems disrupted by the experience of this disorder.
Acknowledgments
Supported by an NIMH Program Project grant (P01 MH41712; Neal D. Ryan, principal investigator), an NIMH Research Network grant (R24 MH067346; Ronald E. Dahl, principal investigator), a Klingenstein Third Generation Foundation fellowship (Erika E. Forbes, principal investigator), two NARSAD Young Investigator Awards (Erika E. Forbes and Ahmad R. Hariri, principal investigators), and two NIMH Mentored Research Scientist Development Awards (K01 MH074769, Erika E. Forbes, principal investigator; K01 MH072837, Ahmad R. Hariri, principal investigator.)
The authors thank Greg Siegle and Mauricio Delgado for advice on task design, the staff of the Child and Adolescent Sleep and Neurobehavioral Laboratory, and the families who participated.
Footnotes
Dr. Birmaher has participated in forums sponsored by companies such as Solvay and Abcomm and has lectured at a Solvay-sponsored meeting and participated in the following forums: Jazz Pharmaceuticals, Solvay Pharmaceuticals, and Abcomm. All remaining authors report no competing interests.
References
- 1.Birmaher B, Ryan ND, Williamson DE, Brent DA, Kaufman J. Childhood and adolescent depression: a review of the past 10 years, part II. J Am Acad Child Adolesc Psychiatry. 1996;35:1575–1583. doi: 10.1097/00004583-199612000-00008. [DOI] [PubMed] [Google Scholar]
- 2.Forbes EE, Dahl RE. Neural systems of positive affect: relevance to understanding child and adolescent depression? Dev Psychopathol. 2005;17:827–850. doi: 10.1017/S095457940505039X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nestler EJ, Carlezon WA., Jr The mesolimbic dopamine reward circuit in depression. Biol Psychiatry. 2006;59:1151–1159. doi: 10.1016/j.biopsych.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 4.Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, Nugent TF, 3rd, Herman DH, Clasen LS, Toga AW, Rapoport JL, Thomson PM. Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci USA. 2004;101:8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lonigan CJ, Carey MP, Finch AJ., Jr Anxiety and depression in children and adolescents: negative affectivity and the utility of self-reports. J Cons Clin Psychol. 1994;62:1000–1008. doi: 10.1037//0022-006x.62.5.1000. [DOI] [PubMed] [Google Scholar]
- 6.Forbes EE, May JC, Siegle GJ, Ladouceur CD, Ryan ND, Carter CS, Birmaher B, Axelson DA, Dahl RE. Reward-related decision-making in pediatric major depressive disorder: an fMRI study. J Child Psychol Psychiatry. 2006;47:1031–1040. doi: 10.1111/j.1469-7610.2006.01673.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wirtz D, Kruger J, Napa Scollon C, Diener E. What to do on spring break? the role of predicted, on-line, and remembered experience in future choice. Psychological Science. 2003;5:520–524. doi: 10.1111/1467-9280.03455. [DOI] [PubMed] [Google Scholar]
- 8.Epstein J, Pan H, Kocsis JH, Yang Y, Butler T, Chusid J, Hochberg H, Murrough J, Strohmayer E, Stern E, Silbergweig DA. Lack of ventral striatal response to positive stimuli in depressed versus normal subjects. Am J Psychiatry. 2006;163:1784–1790. doi: 10.1176/ajp.2006.163.10.1784. [DOI] [PubMed] [Google Scholar]
- 9.Surguladze S, Brammer MJ, Keedwell P, Giampietro V, Young AW, Travis MJ, Williams SC, Phillips ML. A differential pattern of neural response toward sad versus happy facial expressions in major depressive disorder. Biol Psychiatry. 2005;57:201–209. doi: 10.1016/j.biopsych.2004.10.028. [DOI] [PubMed] [Google Scholar]
- 10.Keedwell PA, Andrew C, Williams SC, Brammer MJ, Phillips ML. A double dissociation of ventromedial prefrontal cortical responses to sad and happy stimuli in depressed and healthy individuals. Biol Psychiatry. 2005;58:495–503. doi: 10.1016/j.biopsych.2005.04.035. [DOI] [PubMed] [Google Scholar]
- 11.Delgado MR, Nystrom LE, Fissell C, Noll DC, Fiez JA. Tracking the hemodynamic responses to reward and punishment in the striatum. J Neurophysiol. 2000;84:3072–3077. doi: 10.1152/jn.2000.84.6.3072. [DOI] [PubMed] [Google Scholar]
- 12.Balleine BW, Delgado MR, Hikosaka O. The role of the dorsal striatum in reward and decision-making. J Neurosci. 2007;27:8161–8165. doi: 10.1523/JNEUROSCI.1554-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Knutson B, Adams CM, Fong GW, Hommer D. Anticipation of increasing monetary reward selectively recruits nucleus accumbens. J Neurosci. 2001;21:RC159. doi: 10.1523/JNEUROSCI.21-16-j0002.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Keedwell PA, Andrew C, Williams SC, Brammer MJ, Phillips ML. The neural correlates of anhedonia in major depressive disorder. Biol Psychiatry. 2005;58:843–853. doi: 10.1016/j.biopsych.2005.05.019. [DOI] [PubMed] [Google Scholar]
- 15.Forbes EE, Shaw DS, Dahl RE. Alterations in reward-related decision making in boys with recent and future depression. Biol Psychiatry. 2007;61:633–639. doi: 10.1016/j.biopsych.2006.05.026. [DOI] [PubMed] [Google Scholar]
- 16.Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Williamson D, Ryan N. Schedule for Affective Disorders and Schizophrenia for School-Age Children Present and Lifetime Version (K-SADS-PL); Initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- 17.Birmaher B, Khetarpal S, Brent D, Cully M, Balach L, Kaufman J, Neer SM. The Screen for Child Anxiety Related Emotional Disorders (SCARED): scale construction and psychometric characteristics. J Am Acad Child Adolesc Psychiatry. 1997;36:545–553. doi: 10.1097/00004583-199704000-00018. [DOI] [PubMed] [Google Scholar]
- 18.Angold A, Erkanli A, Silberg J, Eaves L, Costello EJ. Depression scale scores in 8–17-year-olds: effects of age and gender. J Child Psychol Psychiatry. 2002;43:1052–1063. doi: 10.1111/1469-7610.00232. [DOI] [PubMed] [Google Scholar]
- 19.Laurent J, Catanzaro SJ, Joiner TE, Jr, Rudolph KD, Potter KI, Lambert S, Osborne L, Gathright T. A measure of positive and negative affect for children: scale development and preliminary validation. Psychol Assessment. 1999;11:326–338. [Google Scholar]
- 20.Marshall WA, Tanner JM. Growth and physiological development during adolescence. Annu Rev Med. 1968;19:283–300. doi: 10.1146/annurev.me.19.020168.001435. [DOI] [PubMed] [Google Scholar]
- 21.Dunlop BW, Nemeroff CB. The role of dopamine in the pathophysiology of depression. Arch Gen Psychiatry. 2007;64:327–337. doi: 10.1001/archpsyc.64.3.327. [DOI] [PubMed] [Google Scholar]
- 22.Amodio DM, Frith CD. Meeting of the minds: the medial frontal cortex and social cognition. Nat Rev Neurosci. 2006;(4):268–277. doi: 10.1038/nrn1884. [DOI] [PubMed] [Google Scholar]
- 23.Haber SN, Fudge JL, McFarland NR. Striatonigrostriatal pathways in primates form an ascending spiral from the shell to the dorsolateral striatum. J Neurosci. 2000;20:2369–2382. doi: 10.1523/JNEUROSCI.20-06-02369.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tekin S, Cummings JL. Frontal-subcortical neuronal circuits and clinical neuropsychiatry: an update. J Psychosom Res. 2002;53:647–654. doi: 10.1016/s0022-3999(02)00428-2. [DOI] [PubMed] [Google Scholar]
- 25.Gusnard DA, Akbudak E, Shulman GL, Raichle ME. Medial pre-frontal cortex and self-referential mental activity: relation to a default mode of brain function. Proc Natl Acad Sci USA. 2001;98:4259–4264. doi: 10.1073/pnas.071043098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ernst M, Nelson EE, Jazbec S, McClure EB, Monk CS, Leibenluft E, Blair J, Pine DS. Amygdala and nucleus accumbens in responses to receipt and omission of gains in adults and adolescents. Neuroimage. 2005;25:1279–1291. doi: 10.1016/j.neuroimage.2004.12.038. [DOI] [PubMed] [Google Scholar]
- 27.Galvan A, Hare TA, Parra CE, Penn J, Voss H, Glover G, Casey BJ. Earlier development of the accumbens relative to orbitofrontal cortex might underlie risk-taking behavior in adolescents. J Neurosci. 2006;26:6885–6892. doi: 10.1523/JNEUROSCI.1062-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Keedwell PA, Andrew C, Williams SC, Brammer MJ, Phillips ML. The neural correlates of anhedonia in major depressive disorder. Biol Psychiatry. 2005;58:843–853. doi: 10.1016/j.biopsych.2005.05.019. [DOI] [PubMed] [Google Scholar]