Abstract
Neuroligin-3 is a member of the class of cell adhesion proteins that mediate synapse development and have been implicated in autism. Mice with the human R451C mutation (NL3), identical to the point mutation found in two brothers with autism spectrum disorders, were generated and phenotyped in multiple behavioral assays with face validity to the diagnostic symptoms of autism. No differences between NL3 and their wildtype littermate controls (WT) were detected on measures of juvenile reciprocal social interaction, adult social approach, cognitive abilities, and resistance to change in a spatial habit, findings which were replicated in several cohorts of males and females. Physical and procedural abilities were similar across genotypes on measures of general health, sensory abilities, sensorimotor gating, motor functions, and anxiety-related traits. Minor developmental differences were detected between NL3 and WT, including slightly different rates of somatic growth, slower righting reflexes at postnatal days 2−6, faster homing reflexes in females, and more vocalizations on postnatal day 8 in males. Significant differences in NL3 adults included somewhat longer latencies to fall from the rotarod, less vertical activity in the open field, and less acoustic startle to high decibel tones. The humanized R451C mutation in mice did not result in apparent autism-like phenotypes, but produced detectable functional consequences that may be interpreted in terms of physical development and/or reduced sensitivity to stimuli.
Keywords: autism, mouse model, neuroligin, behavior phenotyping, social behavior
Introduction
Neuroligins and neurexins are cell adhesion proteins that have been implicated in autism (Kim et al., 2008). Neurexins in axons bind to neuroligins in dendrites at synaptic junctions to trigger synaptic differentiation (Chubykin et al., 2005; Varoqueaux et al., 2006; Arac et al., 2007; Budreck and Scheiffele, 2007; Chubykin et al., 2007; Conroy et al., 2007; Craig and Kang, 2007). Mutations in neuroligin-3 (NL3) are reported to shift the balance between glutamatergic and GABAergic synapses (Chih et al., 2005; Chubykin et al., 2005; Levinson and El-Husseini, 2007; Sheng and Hoogenraad, 2007). An arginine to cysteine mutation at amino acid position 451 in NL3, a gene located on the X-chromosome, was discovered in two Swedish brothers, one with severe autism and one with Asperger's syndrome (Jamain et al., 2003). This point mutation causes defective neuroligin-3 protein trafficking, resulting in NL3 retention in the endoplasmic reticulum and decreased NL3 reaching the cell surface (Comoletti et al., 2004; Chubykin et al., 2005; De Jaco et al., 2006). In contrast, a large number of association studies have not detected NL3 mutations in autistic individuals, indicating that mutations in the neuroligin-3 gene are unlikely to account for a substantial number of cases of autism (Talebizadeh et al., 2004; Vincent et al., 2004; Gauthier et al., 2005; Yan et al., 2005; Ylisaukko-oja et al., 2005; Blasi et al., 2006; Talebizadeh et al., 2006; Wermter et al., 2008). However, multiple reports of mutations in several cell adhesion synaptic genes, including neurexins, neuroligins, SHANK3, and CNTNAP2, each in a small number of cases of autism, prompt the hypothesis that a shift in the interplay between synaptic genes during development contributes to autism spectrum disorders (Laumonnier et al., 2004; Jeffries et al., 2005; Autism Genome Project Consortium, 2007; Durand et al., 2007; Garber, 2007; Moessner et al., 2007; Alarcon et al., 2008; Arking et al., 2008; Jamain et al., 2008; Kim et al., 2008; Lawson-Yuen et al., 2008).
To begin to test the hypothesis that a mutation in NL3 contributes to the symptoms of autism, we generated knockin mice with the R451C NL3 mutation that was discovered in the two Swedish brothers (Jamain et al., 2003). A comprehensive analysis of the behavioral phenotype of 3 independent cohorts of NL3 and WT males and females from two different types of matings was conducted, employing multiple tests for traits relevant to juvenile and adult social interaction, developmental milestones including vocalizations, sensory abilities, motor functions, anxiety-related behaviors, habit reversal, and control measures of general health. Since synapse formation is essential to cognitive abilities, we also evaluated NL3 and WT on two learning and memory tasks. Results indicate that NL3 R451C mice do not display autism-like behavioral phenotypes or cognitive deficits. No genotype differences were detected on physical and procedural abilities, although unusual scores on a small number of behavioral measures indicate functional outcomes of the mutation.
As we were completing a comprehensive behavioral characterization at NIMH of the line of R451C NL3 knockin mice generated at Rockefeller University, a report was published of an independently generated line of R451C NL3 knockin mice (Tabuchi et al., 2007). Striking differences between our results and those described in Tabuchi et al., 2007 are addressed in the Discussion section below.
Methods
Mice
The humanized R451C mutation, in which the nucleotide sequence CGT in exon 6 for arginine 451 was modified to TGC for cysteine 451, was generated as described in Supplementary Methods. The targeting vector and confirmation of the conditional null allele by Southern blot analysis are shown in Figure 1, along with the initial breeding strategy.
Figure 1.
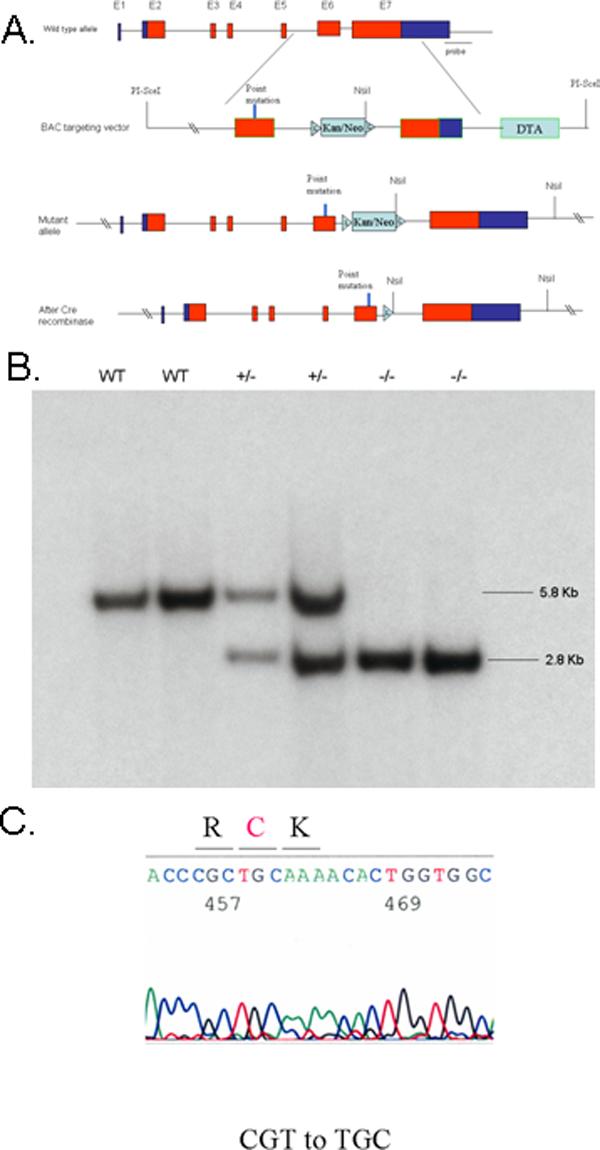
Gene targeting strategy and genotype analysis. (A) Diagram of the Nlgn3 locus with numbered exons, the BAC targeting vector with R451C mutation, the targeted Nlgn3 locus and the recombined locus after Cre-mediated excision of Kana/Neo marker cassette. PCR primers used for genotyping are noted by arrows. A floxed Kana/Neo cassette, a diphtheria toxin negative selection cassette and targeting arms are illustrated. The 3’ external probe used in the Southern blotting is indicated below the wild type allele. Diagram is not to scale. (B) Southern blot analysis of NsiI-digested tail DNA isolated from control wild type female, heterozygous female and homozygous female mice. (C) DNA sequence of RT-PCR product. The homozygous R451C (CGT to TGC) missense mutation is indicated.
Twenty mice (10 male and 10 female) homozygous for the neuroligin-3 R451C mutation were shipped from Rockefeller University in New York, NY to NIMH in Bethesda, MD for behavioral testing. Homozygotes were mated with C57BL/6J (The Jackson Laboratory, Bar Harbor, ME) to produce Cohort 1. Because the gene that codes for NL3 is located on the X chromosome, Cohort 1 contained litters in which all females were heterozygous (Het), and males were either full mutant (NL3) or full wildtype (WT), depending on the mother. Heterozygote females from Cohort 1 were mated with either C57BL/6J or NL3 knockin males to generate Cohorts 2 and 3, consisting of wildtype (WT) or NL3 males and wildtype (WT), heterozygous (Het), or homozygous (NL3) females. Identical behavioral tests were conducted with offspring from both breeding schemes. When tests were run with both types of cohorts, they were analyzed for genotype of the dam. Tailsnips were genotyped at Rockefeller University, as described in Supplementary Material. All mice were maintained in an NIH vivarium with a 12/12 light cycle (lights on at 7:00 am) under temperature (20° C) and humidity (∼ 48%) controlled conditions. Food and water were available ad libitum. Behavioral tests were conducted between 9:00 am and 6:00 pm. All procedures were conducted in accordance with the NIH Guide for the Care and Use of Laboratory Animals, and approved by the NIMH Animal Care and Use Committee.
Group sizes from each independent cohort used for behavioral testing were: Cohort 1: 17 male WT, 12 male NL3; Cohort 2: 19 male WT, 15 male NL3, 5 female WT, 19 female Het, 4 female NL3; Cohort 3: 14 male WT, 16 male NL3, 7 female WT, 16 female Het, and 13 female NL3. Results from WT were consistent across cohorts for the same sex on the same test.
Behavioral tests
Comprehensive behavioral phenotyping was conducted as described in Supplementary Materials. Pups were tested for developmental milestones, ultrasonic vocalizations during separation from the mother and siblings, and homing to familiar cage litter odors. Juveniles were tested for reciprocal social play and open field exploratory locomotion. Adults were tested for social approach in an automated three-chambered sociability apparatus, elevated plus maze and light↔dark anxiety-related behaviors, general health and home cage measures including nesting, neurological reflexes, open field locomotion, rotarod coordination and balance, forepaw reaching for vision, acoustic startle threshold for hearing, prepulse inhibition of acoustic startle for sensorimotor gating, hot plate and tail flick for pain sensitivity, contextual and cued fear conditioned learning and memory, and Morris water maze spatial learning and memory acquisition and reversal, as previously described (Crawley and Goodwin, 1980; Bailey et al., 2007; Crawley, 2007; Crawley et al., 2007; Moy et al., 2007; Yang et al., 2007; McFarlane et al., 2008; Scattoni et al., 2008) and described in detail in the Supplementary Methods.
Results
Juvenile social interaction
Figure 2A-D documents no significant differences between male genotypes for A) follow (F2,28 = 0.157, NS), B) nose-to-nose sniff (F2,28 = 0.087, NS), C) push-crawl-touch (F2,28 = 2.031, NS) or D) social groom (F2,28 = 0.337, NS). As shown in Supplementary Figure 1 (1S), there were also no significant differences between the genotypes for A) nose-to-anogenital sniff (F2,28 = 1.01, NS), B) self groom (F2,28 = 0.969, NS), C) jump (F2,28 = 3.127, NS), and D) exploration duration (F2,20 = 0.847, NS).
Figure 2.
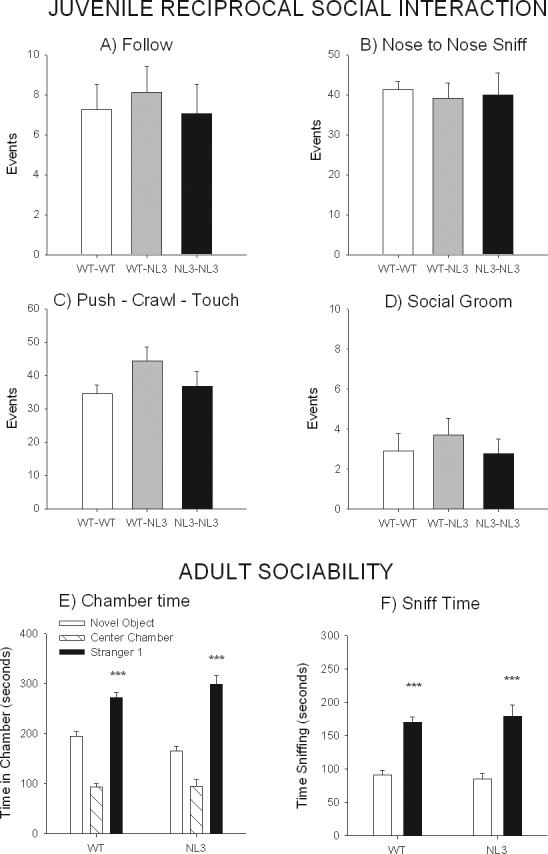
Juvenile social interaction and adult social approach in male neuroligin-3 (NL3) mutants and their wildtype (WT) littermate controls. No significant differences were detected between the genotype pairs on number of bouts (events) for any of the reciprocal social interaction parameters: A) Follow, B) Nose to Nose Sniff, C) Push-Crawl-Touch and D) Social Groom. N = 13 pairs of WT-WT, N=9 pairs of NL3-WT, and N=9 pairs of NL3-NL3. Both genotypes displayed sociability as measured by social approach parameters in the automated 3-chambered social approach test in adult males: E) Both the WT and NL3 mice spent more time in the chamber with the stranger mouse as compared to time in the chamber with the novel object. F) Both the WT and NL3 mice spent more time sniffing the stranger mouse as compared to the novel object. N = 34 WT, N =24 NL3. In Figures 1-5, all data are from male mice and shown as mean ± standard error of the mean, and * = p<.05, ** = p<.01, *** = p<.001, for the comparison of NL3 and WT. Additional behavioral parameters for male mice, results for female mice, complete methods, tables and graphs and procedures are located in Supplementary Material, as indicated in the text.
Adult Social Approach
Figure 2 panels E-F illustrates high levels of sociability for both NL3 and WT male mice. There was a significant effect of chamber time (F1,56 = 47.57, p<0.0001) but not of genotype (F1,56= 0.04, NS) or interaction of genotype and chamber time (F1,56= 3.28, NS). Both genotypes spent more time in the chamber with the stranger mouse than in the chamber with the novel object: WT (F33,1= 18.778, p<0.001) and NL3 (F23,1 = 26.061, p<0.0001). There was a significant effect of sniff time (F1,56 = 90.87, p<0.0001) but not of genotype (F1,56= 0.64, NS) or interaction of genotype and sniff time (F1,56= 3.34, NS). Both genotypes spent more time sniffing the stranger mouse than the novel object WT (F1,33= 56.55, p<0.0001) and NL3 (F1,23 = 36.10, p<0.0001). There were no significant differences in exploration during the 10 minute habituation period (Figure 2SA). There were no significant differences of entries (F1,56= 0.24, NS), genotype (F1,56= 0.90, NS) or interaction of genotype and sniff time (F1,56= 0.39, NS) for the number of entries into the chamber with the novel object versus entries into the chamber with the stranger mouse for either genotype (Figure 2SB): WT (F1,23 = 0.451, NS) and NL3 (F1,23 = 0.124, NS), suggesting that there were no locomotor impairments or differential levels of general exploration that could have affected the social approach scores. Results for the preference for social novelty phase, a control for social olfactory abilities, did not show any genotype differences and are presented in the Supplemental Materials Figure 2S panels C-E. Similar results obtained in the females are shown in Figure 3S panels A-G.
Morris water maze acquisition and reversal
Acquisition
Figure 3 panels A-D (left side of graphs) illustrate performance on acquisition in the Morris water maze spatial learning and memory task, and reversal of the original position habit in the Morris water maze. During acquisition, male WT and NL3 mice similarly improved across day of training for latency to reach the hidden platform (F4,216 =64.84, p<0.0001), with no main effect of genotype (F1,54 = 0.417, NS) and no interaction between genotype and day (F4,216 = 1.094, NS). Total distance traveled was significant for day of training (F4,216 =31.064, p<0.0001) and for genotype (F1,54 = 4.378, p <0.05) but not for the interaction of genotype × day of training (F4,216 = 0.939, NS). Swim speed showed a significant main effect of day of training (Figure 4S panel A): (F4,216 = 7.731, p<0.0001) but not of genotype (F1,54 = 2.24, NS) or interaction of genotype × day of training (F4,216 = 0.104, NS). However, as shown in Figure 4S panel B, a transient genotype difference was detected on thigmotaxis, a measure of distance from the perimeter wall during swimming. Thigmotaxis showed a significant main effect of day of training (F4,216 = 52.865, p<0.0001), and also of genotype (F1,54 = 4.291, p < 0.05) and the interaction of genotype × day of training (F4,216 = 5.707, p<0.001). Newman-Keuls post hoc analysis determined that thigmotaxis was significantly different between the WT and NL3 mice on day 1 (p<0.01). Probe trial results revealed that all genotypes spent significantly more time in the trained quadrant than in the other three quadrants (F3,165= 28.84, p <0.0001, post hoc all comparison p values < 0.05) and made significantly more platform crosses over the former location of the hidden platform than over the comparable locations in the other three quadrants (F3,165= 32.10, p <0.0001, post hoc all comparison p values < 0.05), demonstrating that both the WT and NL3 mice successfully learned the location of the hidden platform using distal spatial cues.
Figure 3.
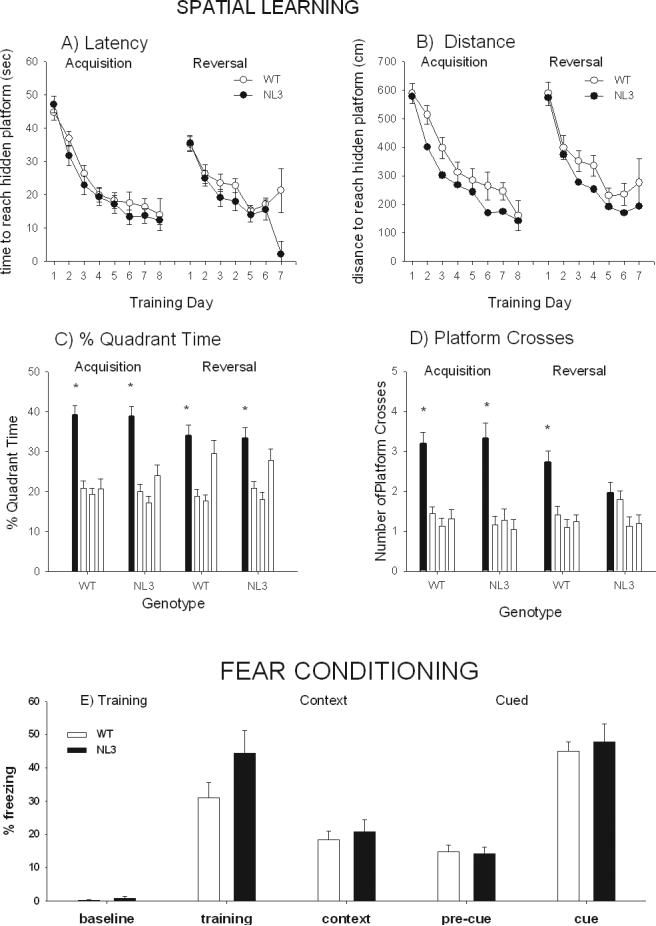
Morris water maze spatial learning and reversal, and contextual and cued fear conditioning measures of cognitive abilities in WT and NL3 knockin mice. A) WT and NL3 displayed similar latencies to locate the hidden platform for both acquisition and reversal of the Morris spatial navigation task. B) NL3 mice swam a shorter distance to find the hidden platform during acquisition but there was no genotype difference in distance swam during reversal. C) Both WT and NL3 spent more time in the previously trained quadrant (black bars) than the other three quadrants (white bars) during the probe trial following the initial acquisition phase. Both WT and NL3 spent more time in the previously trained quadrant than the left and right quadrants, but not the opposite quadrant, during the probe trial following the reversal phase. D) Both WT and NL3 crossed the platform in the previously trained quadrant (black bars) significantly more than the platform locations in other three quadrants (white bars) during the probe trial following the acquisition phase. WT crossed the platform in the previously trained quadrant (black bars) significantly more than the platform locations in other three quadrants (white bars) during the probe trial following the acquisition phase, but NL3 crossed the platform in the trained quadrant more than the the corresponding location in the quadrant to the right, but not the left and opposite locations, during the probe trial following the reversal phase. N = 31 WT, N = 25 NL3. E) Contextual and cued fear conditioning. WT and NL3 did not differ in freezing, a species-specific fear responses measured as the amount of time spent immobile, before training and following training. WT and NL3 did not differ in freezing to the identical context 24 h after training. WT and NL3 did not differ in freezing to a novel context 48 h after training, either in the absence (pre-CS) or presence of the conditioned auditory cue. N = 38 WT, N = 24 NL3.
Reversal
Data from the reversal phase of the Morris water maze are shown in Figure 3 panels A-D (right side of graphs). During reversal training both WT and NL3 mice improved across day of training in latency to reach the hidden platform (F4,220 = 40.065, p<0.0001) but there was no main effect of genotype (F1,55 = 1.05, NS) and no genotype × day of training (F4,220 = 0.956, NS). Total distance traveled showed a significant main effect of day of training (F4,220 = 36.863, p<0.0001) but not of genotype (F1,55 = 2.59, NS) or genotype × day of training (F4,220 = 0.51, NS). Swim speed was significant for day of training (F4,220 = 7.68, p<0.0001) but not genotype (F1,55 = 2.55, NS) or genotype × day of training (F4,220 = 0.48, NS). Thigmotaxis (Figure 4SB) was significant for day of training (F4,220 = 3.00, p<0.05) but not for genotype (F1,55 = 2.86, NS) nor genotype × day of training (F4,220 = 0.53, NS). Probe trial testing revealed that all genotypes spent significantly more time (all comparison p values < 0.05) in the reversal training quadrant as compared to the left and right adjacent quadrants. However, time spent in the opposite quadrant, where the hidden platform was originally located, was similar to time spent in the new quadrant where the hidden platform was located during the reversal trial in some cases (F3,165= 14.25, p <0.0001, post hoc comparison for left and right quadrant p values < 0.05, opposite quadrant NS). The WT group made significantly more platform crosses in the trained quadrant than in the other three quadrants during the reversal probe trial (F3,93 = 12.58, p<0.0001, post hoc all comparison p values < 0.05). While the NL3 group showed a significant effect of platform crosses (F3,72 = 3.48, p<0.05), the NL3 group crossed the reversal training platform significantly more than the right platform location but not any of the other platform locations.
Contextual and Cued Fear Conditioning
As shown in Figure 3E, no genotype differences were detected on contextual or cued fear conditioning in the standard delay learning and memory emotional task in male NL3 and WT mice. In the training phase, significantly more freezing was apparent during the 2 min after the cue-shock pairings than in the 2 min before the cue-shock pairings (F1,59=92.39, p<0.0001). There was no main effect of genotype on freezing during training (F1,59=3.10, NS), and no genotype × cue-shock pairing interaction (F1,59=2.72, NS). On the next day, when trained mice were placed back in the identical context, but with no footshock administered, there was no main effect of genotype on the levels of freezing (F1,60=0.31, NS). On the subsequent day, when trained mice were placed in a novel environmental context and given the auditory tone that had been paired with footshock during training, both genotypes displayed some freezing to the novel context and similarly high levels of freezing to the auditory cue (F1,60=156.58, p<0.0001), with no significant differences between genotypes (F1,60=0.10, NS), and no interaction of cue × genotype (F1,60=0.41, NS).
Pup Ultrasonic Vocalizations to Separation from the Mother and Siblings
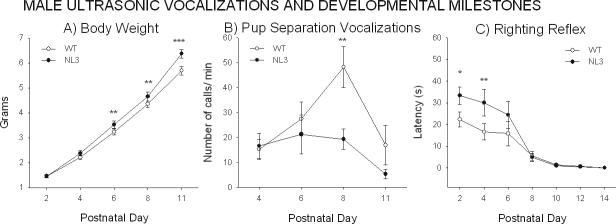
Baseline measurements of ultrasonic vocalizations revealed a different profile of emission between male NL3 and WT at one time point during the first two postnatal weeks (Figure 4B). On pnd 8, WT pups emitted significantly more calls (genotype: F1,27= 3.47, p=0.07; day: F3,81= 6.79, p<0.001; genotype × day interaction, F3,81=2.85, p=0.04, pnd 8: p<0.001), indicating a temporally shifted developmental profile of these distress calls. Duration, peak frequency and amplitude of the calls did not differ between groups (Duration, F1,27=1.61, NS; Peak frequency, F1,27=1.75, NS; Peak amplitude, F1,27=0.80, NS; Figure 5S panels A-C). No differences were detected between the body temperatures of WT and NL3 mice (F1,27= 0.01, NS) as measured after each separation test. Female pups are showed no significant genotype differences on any of the vocalization parameters (Figure 5S panels D-G).
Figure 4.
Ontogenetic profile at postnatal (PND) ages 2 to 14 days of age. A) Body weight (PNDs 2−11), B) Ultrasonic vocalizations (PNDs 4−11) and C) Righting reflex (PNDs 2−14) in WT and NL3 male pups. NL3 pups had significantly higher body weights from PND 6 through PND 11. NL3 emitted fewer ultrasonic vocalizations at PND 8 than WT. N = 16 WT, N = 13 NL3. Differences in righting reflex were noted in NL3 pups displayed slower righting reflex latencies as compared with WT pups at PNDs 2, 4, and 6, which normalized by PND 8. N = 16 WT, N = 16 NL3.
Homing test (postnatal 9)
In the homing test (Figure 6S), which measures the tendency of pups placed in a novel arena to move toward a familiar social odor, i.e. a location containing nesting material from the home cage, no significant effect of genotype was found in males on latency to reach the area containing the nest litter (F1,30=0.26, NS) or time spent over the area containing the nest litter (F1,30=0.05, NS). No significant effect of genotype was detected on general locomotor activity, as measured by number of line-crossings in the arena (F1,30=2.85, NS; data not shown). Females (Figure 6S panels C-D) displayed a significant genotype effect on latency to reach the area containing the nest litter (ANOVA F2,33=3.81, p=0.03; Kruskal-Wallis p=0.02). Post hoc comparisons revealed that female NL3 pups showed shorter latencies to reach the nest area in comparison with WT pups (p<0.01). Female NL3 pups spent significantly more time in the nest area (F2,33=4.24, p=0.02, p=0.01 for NL3 vs WT). ANOVA did not detect a significant genotype effect on general locomotor activity, indicating that the three genotypes had similar activity levels in the homing arena (F2,33=2.60, NS, data not shown).
Open Field Exploration
Figures 5A and 8S panel A-C, show open field exploratory locomotion in adult male WT and NL3. Both groups were more active at the beginning of the test as compared to the end. Figure 5A illustrates a genotype difference in vertical activity (F1,58 = 8.40, p<0.01) and of 5 min bin (F5,290 = 5.43, p<0.0001) but no interaction of genotype and 5 min bin (F5,290 = 0.98, NS), indicating that the NL3 made fewer upright rears. There was a significant effect of time on horizontal activity (F5,295 = 56.76, p<0.0001; Figure 8S panel A) and interaction of genotype by 5 min bin (F5,295 = 5.43, p<0.0001) but no effect of genotype (F1,59 = 0.099, NS). Newman-Keuls post hoc analysis did not detect any significant differences between the genotypes on any specific day (p >0.05). There was a significant effect of 5 min time bins on total distance (F5,295 = 75.87, p<0.0001) but no effect of genotype (F1,59 = 0.001, NS) or interaction of genotype by 5 min bin (F5,295 = 2.02, NS; Figure 8S panel B). There was no effect of genotype on % center time (F1,95 = 0.175, NS; Figure 8S, panel C) or interaction of genotype × 5 min bin (F5,295 = 0.798, NS), but there was a significant effect of 5 min bin (F5,295 = 6.05, p<0.0001). The majority of the open field results were similar in the females, although genotype differences were detected in males but not in females on vertical activity (Figure 8S panels D-G).
Figure 5.
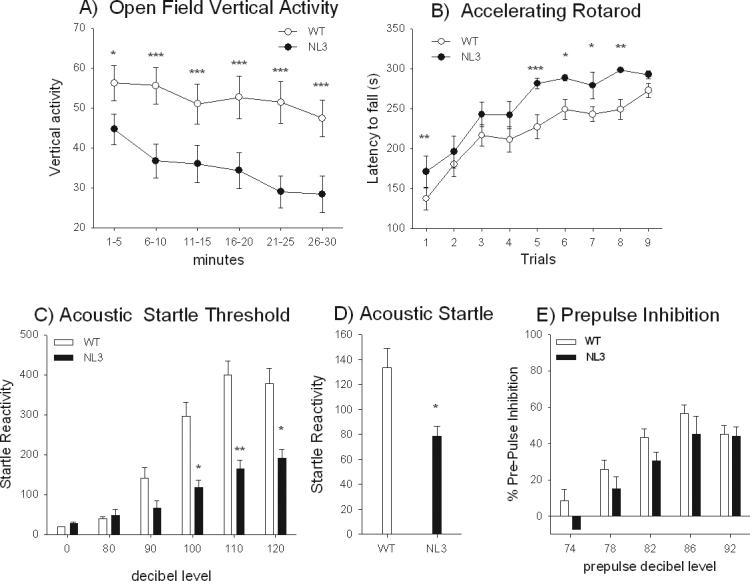
Motor performance, sensorimotor gating, and startle activity. A) Open field. NL3 overall displayed significantly less vertical activity than WT throughout the 30 min automated open field session. N = 36 WT, N=25 NL3. Rotarod. B) NL3 showed longer latencies to fall from the accelerating rotarod than WT across all trials. N = 19 WT, N = 15 NL3. C) NL3 mice had significantly less startle response as compared to WT in the acoustic startle threshold test at the 100, 110 and 120 dB stimuli, but no differences at lower startle levels. N = 19 WT, N = 15 NL3. D) Within the prepulse inhibition session, another cohort of NL3 mice similarly showed less startle to 110 dB than WT. E) No genotype difference was detected in prepulse inhibition of acoustic startle. N = 30 WT, N = 19 NL3.
Accelerating Rotarod
Adult male NL3 mice displayed better performance on the rotarod than WT, as seen by longer latencies to fall (Figure 5B). There was a significant effect of genotype (F1,32= 8.31, p<0.01), day of training (F2,64=52.70, p<0.0001), trial (F2,64=23.11, p<0.0001) and interaction of day and trial (F4,128=4.88, p<0.01) but no interaction of genotype × day of training (F2,64=0.50, NS), trial (F2,64=0.34, NS), or day × trial (F4,128=1.09, NS). No genotype differences were found in the females (Figure 9S).
Acoustic startle, Acoustic Startle Threshold, and Prepulse Inhibition (PPI) of Acoustic Startle
Adult male NL3 showed less startle response to sudden loud acoustic stimuli than WT (Figure 5C). There was a significant effect of genotype (F1,32 = 21.55, p<0.0001), trial type (F5,160 = 66.58, p<0.0001), and interaction of genotype and trial type (F5,160 = 13.66, p<0.0001). Newman-Keuls post hoc examination revealed significant differences between the genotypes on the 100, 110, and 120 dB trials (p<0.05). Both genotypes showed minimal startle at 80 dB, suggesting similar acoustic thresholds. A separate cohort of NL3 mice showed a lower response to a 110 dB acoustic startle stimulus (F1,47 = 6.79, p<0.05; Figure 5D) but normal prepulse inhibition (F1,47 = 2.58, NS; Figure 5E). PPI increased with prepulse intensity up to 86 dB regardless of genotype (F4,188 = 46.81, p<0.0001). There was no interaction between genotype and prepulse intensity (F4,188 = 0.86, NS). Similar results for females on acoustic startle and PPI are shown in Figure 10S panels A-C.
General Health Measures
NL3 and WT were similar on almost all measures of general health, reflexes and sensory function (Table 1). General health included body weight, temperature, fur condition, body and limb tone. Empty cage observations included transfer freezing, wild running, stereotypies, exploration and self-grooming. Motor reflexes included passivity, wire hang, grip strength and trunk curl. The only genotype difference in males was detected in the wire hang where the WT hung on longer than the NL3 mice. The only genotype differences in females were on body weight (Table S1) and forepaw width in the footprint test (Table S2). General reactivity was assessed with petting escape, struggling and/or vocalizations, and dowel biting. There were no significant differences between the genotypes. Observations of home cage behaviors in the vivarium indicated that NL3 mutants were normal on home cage activity, nest building, huddling and grooming.
Table 1.
General health and neurological screening of male R451C neuroligin 3 knockin (NL3) and their wildtype littermate controls (WT) at age 5−12 weeks. Data are expressed as percentages, 3 point rating scales, or mean +/− standard error of the mean. No genotype differences were detected.
| Genotypes | WT | NL3 | P-value |
|---|---|---|---|
| N=38 | N=25 | ||
| General Health | |||
| Body Weight (g) | 26.0 +/− .67 | 24.5 +/− .77 | NS |
| Body Temperature (°C) | 38.1 +/− .10 | 38.2 +/− .11 | NS |
| Fur Condition (3 pt scale) | 2 | 2 | NS |
| Bald patches (%) | 0 | 8 | NS |
| Missing whiskers (%) | 0 | 0 | NS |
| Piloerection (%) | 0 | 0 | NS |
| Body tone (3 pt scale) | 2 | 2 | NS |
| Limb tone (3 pt scale) | 2 | 2 | NS |
| Empty Cage Behavior | |||
| Transfer freezing (%) | 8 | 8 | NS |
| Wild running (%) | 0 | 4 | NS |
| Stereotypies (%) | 0 | 0 | |
| Exploration (3 pt scale) | 1.92 | 1.92 | NS |
| Grooming (3 pt scale) | 2.18 | 2.4 | NS |
| Motoric abilities | |||
| Positional passivity (%) | 42 | 28 | NS |
| Wire Hang (sec) | 59.13 +/− 0.65 | 52.99 +/− 3.170 | <.05 |
| Grip Strength (force) | 113 +/− 3.3 | 110 +−/ 3.8 | NS |
| Trunk curl (%) | 97 | 100 | NS |
| Reflexes | |||
| Forepaw reach (%) | 100 | 100 | NS |
| Righting reflex (%) | 100 | 100 | NS |
| Corneal (%) | 95 | 96 | NS |
| Pinna (%) | 100 | 100 | NS |
| Vibrissae (%) | 100 | 100 | NS |
| Reactivity | |||
| Petting escape (%) | 45 | 32 | NS |
| Struggle/vocalization (%) | 53 | 36 | NS |
| Dowel biting (3 pt scale) | 0.61 | 0.52 | NS |
At young ages, body weight differed significantly between male WT and NL3 and between female genotypes (Figure 4A, 7SA). ANOVA results showed that somatic growth rates differed between groups (genotype effect, F1,27= 3.67, p=0.07; days, F4,108= 1098.27, p<0.0001; genotype × days interaction, F4,108= 5.16, p<0.0008). Post hoc comparisons revealed that NL3 pups had a significantly greater weight increase starting from pnd 6 through pnd 11 (p<0.001 for NL3 versus WT, Figure 4A). In contrast with male data, NL3 female pups showed a significantly lower body weight on pnd 6, 8, and 11 (genotype effect, F2,31= 2.15, NS; days, F4,124= 1167.36, p<0.0001; genotype × age interaction, F8,124=2.13, p=0.03; p<0.001 for NL3 versus WT; Figure 7SA). The same profile was seen for Het pups starting from pnd 8 to 11 (p<0.05 for Het versus WT).
No significant differences in body length (F1,27= 0.06, NS), length of the tail (F1,27= 0.54, NS), eyelid opening (F1,27= 3.13, NS), incisors eruption (F1,27= 2.97, NS) and fur development (F1,27= 0.82, NS) between WT and NL3 were observed. Almost all reflex measurements were similar between the pup groups, including negative geotaxis (F1,27= 0.29, NS), cliff aversion (F1,27= 0.72, NS), forelimb stick grasp reflex (F1,27= 1.03, NS), forelimb placing reflex (F1,27= 0.01, NS), level and vertical screen test (F1,27= 1.32, NS; F1,27= 0.26, NS), and bar holding (F1,27= 0.18, NS) (data not shown).
Transient delays in righting reflex were noted in NL3 compared with WT pups, leading to a different pattern of acquisition of the righting reflex (genotype × day interaction, F6,180=2,18, p=0.04) (Figure 4C). Newman-Keuls post hoc analysis revealed that righting reflex was significantly slower in the NL3 than in the WT on days 2 and 4 (p<0.05 and 0.01 respectively), although both genotypes displayed the full righting reflex by day 8, indicating that the subsequent ontogenetic progression was similar in the two genotypes. Female NL3 showed similarly slower righting reflex latencies at earlier postnatal days, but no genotype differences in subsequent ontogenetic progression (Figure 7S, Panel B).
Anxiety-Related Traits
As described in the Supplementary Results and shown in Figure 11S, genotypes were generally similar on both the elevated plus-maze and light ↔ dark transitions anxiety-related tests.
Tactile Sensory Abilities, Grooming, and Gait
Sensory abilities in adult males showed no significant effects of genotype (Table 2). NL3 and WT were similar on latency to respond to thermal stimuli during testing in the hot plate (F1,59=0.06, NS) and tail flick (F1,59=0.77, NS) tests, on olfactory ability to locate buried food (F1,71=2.23, NS), and on time spent grooming in an empty cage (F1,37=1.54, NS). There was no significant effect of genotype on gait, as measured by forepaw width (F1,32=3.58, NS ), hindpaw width (F1,32=1.13, NS) or stride length (F1,32=1.30, NS) in the footprint test.
Table 2.
Measures of sensory function in male neuroligin 3 knockin (NL3) and wildtype (WT) littermates. Data are expressed as mean +/− standard error of the mean. No genotype differences were detected.
| Genotypes | WT | NL3 | P-Value |
|---|---|---|---|
| Pain Sensitivity | N=38 | N=25 | |
| Hot Plate (latency - sec) | 8.90 +/− 0.44 | 9.08 +/− 0.62 | NS |
| Tail Flick (latency - sec) | 2.02+/− 0.09 | 2.16 +/− 0.13 | NS |
| Olfactory Sensitivity | N=17 | N=13 | |
| Buried Food (latency to eat -sec) | 35.44 +/− 14.24 | 83.77 +/− 30.31 | NS |
| Self-Grooming | N=24 | N=15 | |
| Time spent grooming (sec) | 72.38 +/− 6.1 | 89.64 +/− 14.8 | NS |
| Footprint test | N=19 | N=15 | |
| Forepaw Width (cm) | 1.80 +/− .08 | 1.99 +/− .05 | NS |
| Hindpaw Width (cm) | 2.90 +/− .08 | 2.78 +/− .07 | NS |
| Stride Length (cm) | 5.00 +/− .15 | 4.76 +/− .15 | NS |
Discussion
Comprehensive behavioral phenotyping of multiple cohorts of neuroligin-3 R451C knockin mice and their littermate controls revealed that the NL3 mutation did not significantly affect reciprocal social interactions in juveniles or social approach in adults. No perseveration was detected in any genotype on ability to reverse a spatial position habit in the water maze. The great majority of measures of developmental milestones, pup separation vocalizations, general health, home cage behaviors, reflexes, sensory abilities including olfaction and nociception, and motor functions including locomotion, balance, and gait, were normal in NL3 male and female mice. Taken together, these corroborating results do not support the hypothesis that an R451C point mutation in NL3 produces behaviors in mice that are analogous to the first and third diagnostic symptoms of autism, abnormal reciprocal social interactions and repetitive restricted interests. Furthermore, generally similar scores in NL3 and WT on the initial acquisition of the location of a hidden platform using distal environmental cues in the Morris water maze spatial task, along with similar scores on emotional learning and memory in contextual and cued fear conditioning, do not support the hypothesis that the R451C NL3 mutation directly affects learning and memory.
Our findings of generally normal developmental milestones, physical attributes, simple procedural abilities, and complex behavioral traits in NL3 may be viewed in the broader context of the multiple neurexin and neuroligin proteins and the specific binding of their splice variants during synaptic formation (Chih et al., 2005; Chubykin et al., 2005; Varoqueaux et al., 2006; Budreck and Scheiffele, 2007; Chubykin et al., 2007; Conroy et al., 2007; Craig and Kang, 2007; Levinson and El-Husseini, 2007; Sheng and Hoogenraad, 2007). Reduction in the cell surface expression of the neuroligin-3 protein may not be sufficient to affect a large number of synapses relevant to social and cognitive abilities, if other neuroligins remain available. However, this does not appear to be the case for neuroligin-4, as an elegant study of behavioral phenotypes in neuroligin-4 knockout mice reported reduced social interactions and ultrasonic vocalizations (Jamain et al., 2008).
The present findings are at variance with some of the results and interpretations of behavioral phenotypes in another line of NL3 R451C mice (Tabuchi et al., 2007). Both lines were normal on measures of general health, motor abilities, and anxiety-related behaviors. However, social deficits and improved learning were reported in the other line (Tabuchi et al., 2007). Careful examination of the social behavior results shown in the previous report (Figure 5, Tabuchi et al., 2007), reveals that the NL3 R451C mutant did not differ from their WT controls on most measures of social behaviors. The first social task reported, shown in Tabuchi et al. Figure 5A and B, employed separate sequential test sessions. The subject mouse was first given five minutes to explore a novel object, and then given five minutes to explore a novel mouse. Unlike the many excellent and well-validated assays of social interaction used routinely by behavioral neuroscientists and behavioral neuroendocrinologists, this design of separate sequential opportunities is not a validated measure of interest in a social partner. Too many other factors could influence time spent with the novel mouse, such as generally increased exploration or heightened interest in the wire mesh container during the second five minutes.
Tabuchi et al. Figure 5C employed the three-chambered social approach task which we originally developed and validated (Moy et al., 2004; Nadler et al., 2004; Yang et al., 2007; McFarlane et al. 2008). The habituation and test sessions were performed using methods consistent with the ones in this manuscript. However, the statistical analyses were improperly conducted, using only pairwise t-tests. In fact, the data in Figure 5C of Tabuchi et al., 2007 appear to show that both genotypes spent more time with the novel mouse than with the novel object. It seems likely that both strains exhibited significant sociability, if properly compared with ANOVA statistics. This evidence for significant sociability in the NL3 group argues against a social deficit in the NL3 line tested in Tabuchi et al.
Further, in the conventional test for initial reciprocal social interactions between unfamiliar pairs of mice, there was no difference between WT and NL3 in the previous report (Figure 5 panel D, Tabuchi et al., 2007), consistent with the lack of genotype differences between WT and NL3 on reciprocal social interactions during the juvenile test session in the present findings. The test of direct social interaction in Tabuchi et al. employed only a 2 minute session, which is considerably shorter than social interaction tests used in the great majority of behavioral neuroscience assays. Further, the single parameter shown in Figure 5D, time spent in interaction, is a very gross composite measure. Most behavioral neuroscientists use a large number of specific parameters to characterize direct social interaction, as shown in our Figures 2 and 1S. The data shown in Tabuchi et al. Figure 5D, left bars, clearly demonstrate that both genotypes showed the same amount of social interaction. This lack of genotype difference in their best measure of social behaviors supports an interpretation that their R451C NL3 knockins display normal social behaviors.
The data shown in Tabuchi et al. Figure 5D, right bars, represents a social memory task. Again, the statistical analysis was incorrectly conducted, using a pairwise t-test. As a result, a misleading interpretation was given. Given the standard error bars of Figure 5D, it appears unlikely that there was a genotype difference in social memory if the proper ANOVA statistical analysis had been conducted.
Faster learning curves on both the initial acquisition and reversal learning in the Morris water maze were reported for one cohort of NL3 in Tabuchi et al. (2007) but not seen in the two large cohorts tested in the present studies, although distance traveled during acquisition was somewhat shorter in NL3 during the initial acquisition in both the present study and in Tabuchi et al. (2007). Different techniques for generating the knockin mutation, and different genetic backgrounds for the two NL3 lines, C57BL/6J in the present line from the Heintz laboratory versus a mixed C57BL/6J and 129/ImJ in the line from the Sudhof laboratory, along with methodological differences between the two laboratories in conducting the cognitive assays, may have contributed to the divergent results on spatial learning.
In the present studies, NL3 differed from their WT littermates on several minor but potentially interesting developmental measures. NL3 mice displayed slower righting reflexes during the first few days after birth, emitted fewer ultrasonic vocalizations at postnatal day 8, varied from WT in body weights, engaged in less vertical activity in the open field, had longer latencies to fall from the rotarod, and spent less time near the walls in the Morris water maze. Females displayed more homing behaviors and a wider stance in the footprint test. Both males and females responded less strongly to acoustic startle stimuli. The lower startle response is not necessarily indicative of impaired hearing, as acoustic thresholds were similar across genotypes, and electrophysiological recordings during the startle response indicate no direct correlation between hearing and amplitude of startle response (Willott et al., 1984). It remains possible that the few significant differences detected were simply the outcome of a large number of statistical comparisons. However, it seems likely that the several minor genotype differences, taken together, represent small developmental abnormalities, and/or decreased arousal levels in response to sensory and motor stimuli, caused by the NL3 mutation.
Supplementary Material
Acknowledgments
The authors thank Drs. Joanna Hill, Kathleen Bailey and Mu Yang for training on several of the behavioral tasks and statistical analyses, Dr. Laura Kus for helpful discussions and expert assistance, and Muayad Almahariq and Jason James and for their excellent technical support in the generation and genotyping of the mutant mice. Supported by the National Institute of Mental Health Intramural Research Program, Simons Foundation (NH and SG) and ISS-NIH 0F14 (MLS). NH is a Howard Hughes Medical Institute Investigator.
References
- Alarcon M, Abrahams BS, Stone JL, Duvall JA, Perederiy JV, Bomar JM, Sebat J, Wigler M, Martin CL, Ledbetter DH, Nelson SF, Cantor RM, Geschwind DH. Linkage, association, and gene-expression analyses identify CNTNAP2 as an autism-susceptibility gene. Am J Hum Genet. 2008;82:150–159. doi: 10.1016/j.ajhg.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arac D, Boucard AA, Ozkan E, Strop P, Newell E, Sudhof TC, Brunger AT. Structures of neuroligin-1 and the neuroligin-1/neurexin-1 beta complex reveal specific protein-protein and protein-Ca2+ interactions. Neuron. 2007;56:992–1003. doi: 10.1016/j.neuron.2007.12.002. [DOI] [PubMed] [Google Scholar]
- Arking DE, Cutler DJ, Brune CW, Teslovich TM, West K, Ikeda M, Rea A, Guy M, Lin S, Cook EH, Chakravarti A. A common genetic variant in the neurexin superfamily member CNTNAP2 increases familial risk of autism. Am J Hum Genet. 2008;82:160–164. doi: 10.1016/j.ajhg.2007.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Autism Genome Project Consortium Mapping autism risk loci using genetic linkage and chromosomal rearrangements. Nat Genet. 2007;39:319–328. doi: 10.1038/ng1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey KR, Pavlova MN, Rohde AD, Hohmann JG, Crawley JN. Galanin receptor subtype 2 (GalR2) null mutant mice display an anxiogenic-like phenotype specific to the elevated plus-maze. Pharmacol Biochem Behav. 2007;86:8–20. doi: 10.1016/j.pbb.2006.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blasi F, Bacchelli E, Pesaresi G, Carone S, Bailey AJ, Maestrini E. Absence of coding mutations in the X-linked genes neuroligin 3 and neuroligin 4 in individuals with autism from the IMGSAC collection. American Journal of Medical Genetics Part B Neuropsychiatric Genetics. 2006;141:220–221. doi: 10.1002/ajmg.b.30287. [DOI] [PubMed] [Google Scholar]
- Budreck EC, Scheiffele P. Neuroligin-3 is a neuronal adhesion protein at GABAergic and glutamatergic synapses. Eur J Neurosci. 2007;26:1738–1748. doi: 10.1111/j.1460-9568.2007.05842.x. [DOI] [PubMed] [Google Scholar]
- Chih B, Engelman H, Scheiffele P. Control of excitatory and inhibitory synapse formation by neuroligins. Science. 2005;307:1324–1328. doi: 10.1126/science.1107470. [DOI] [PubMed] [Google Scholar]
- Chubykin AA, Atasoy D, Etherton MR, Brose N, Kavalali ET, Gibson JR, Sudhof TC. Activity-dependent validation of excitatory versus inhibitory synapses by neuroligin-1 versus neuroligin-2. Neuron. 2007;54:919–931. doi: 10.1016/j.neuron.2007.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chubykin AA, Liu X, Comoletti D, Tsigelny I, Taylor P, Sudhof TC. Dissection of synapse induction by neuroligins: effect of a neuroligin mutation associated with autism. J Biol Chem. 2005;280:22365–22374. doi: 10.1074/jbc.M410723200. [DOI] [PubMed] [Google Scholar]
- Comoletti D, De Jaco A, Jennings LL, Flynn RE, Gaietta G, Tsigelny I, Ellisman MH, Taylor P. The Arg451Cys-neuroligin-3 mutation associated with autism reveals a defect in protein processing. J Neurosci. 2004;24:4889–4893. doi: 10.1523/JNEUROSCI.0468-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conroy WG, Nai Q, Ross B, Naughton G, Berg DK. Postsynaptic neuroligin enhances presynaptic inputs at neuronal nicotinic synapses. Dev Biol. 2007;307:79–91. doi: 10.1016/j.ydbio.2007.04.017. [DOI] [PubMed] [Google Scholar]
- Craig AM, Kang Y. Neurexin-neuroligin signaling in synapse development. Curr Opin Neurobiol. 2007;17:43–52. doi: 10.1016/j.conb.2007.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawley J, Goodwin FK. Preliminary report of a simple animal behavior model for the anxiolytic effects of benzodiazepines. Pharmacol Biochem Behav. 1980;13:167–170. doi: 10.1016/0091-3057(80)90067-2. [DOI] [PubMed] [Google Scholar]
- Crawley JN. Behavioral Phenotyping of Transgenic and Knockout Mice. John Wiley & Sons, Inc.; Hoboken: 2007. What's Wrong With My Mouse? [Google Scholar]
- Crawley JN, Chen T, Puri A, Washburn R, Sullivan TL, Hill JM, Young NB, Nadler JJ, Moy SS, Young LJ, Caldwell HK, Young WS. Social approach behaviors in oxytocin knockout mice: comparison of two independent lines tested in different laboratory environments. Neuropeptides. 2007;41:145–163. doi: 10.1016/j.npep.2007.02.002. [DOI] [PubMed] [Google Scholar]
- De Jaco A, Comoletti D, Kovarik Z, Gaietta G, Radic Z, Lockridge O, Ellisman MH, Taylor P. A mutation linked with autism reveals a common mechanism of endoplasmic reticulum retention for the alpha,beta-hydrolase fold protein family. J Biol Chem. 2006;281:9667–9676. doi: 10.1074/jbc.M510262200. [DOI] [PubMed] [Google Scholar]
- Durand CM, Betancur C, Boeckers TM, Bockmann J, Chaste P, Fauchereau F, Nygren G, Rastam M, Gillberg IC, Anckarsater H, Sponheim E, Goubran-Botros H, Delorme R, Chabane N, Mouren-Simeoni MC, de Mas P, Bieth E, Roge B, Heron D, Burglen L, Gillberg C, Leboyer M, Bourgeron T. Mutations in the gene encoding the synaptic scaffolding protein SHANK3 are associated with autism spectrum disorders. Nat Genet. 2007;39:25–27. doi: 10.1038/ng1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garber K. Neuroscience. Autism's cause may reside in abnormalities at the synapse. Science. 2007;317:190–191. doi: 10.1126/science.317.5835.190. [DOI] [PubMed] [Google Scholar]
- Gauthier J, Bonnel A, St-Onge J, Karemera L, Laurent S, Mottron L, Fombonne E, Joober R, Rouleau GA. NLGN3/NLGN4 gene mutations are not responsible for autism in the Quebec population. Am J Med Genet B Neuropsychiatr Genet. 2005;132:74–75. doi: 10.1002/ajmg.b.30066. [DOI] [PubMed] [Google Scholar]
- Jamain S, Quach H, Betancur C, Rastam M, Colineaux C, Gillberg IC, Soderstrom H, Giros B, Leboyer M, Gillberg C, Bourgeron T. Mutations of the X-linked genes encoding neuroligins NLGN3 and NLGN4 are associated with autism. Nat Genet. 2003;34:27–29. doi: 10.1038/ng1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamain S, Radyushkin K, Hammerschmidt K, Granon S, Boretius S, Varoqueaux F, Ramanantsoa N, Gallego J, Ronnenberg A, Winter D, Frahm J, Fischer J, Bourgeron T, Ehrenreich H, Brose N. Reduced social interaction and ultrasonic communication in a mouse model of monogenic heritable autism. Proc Natl Acad Sci U S A. 2008;105:1710–1715. doi: 10.1073/pnas.0711555105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffries AR, Curran S, Elmslie F, Sharma A, Wenger S, Hummel M, Powell J. Molecular and phenotypic characterization of ring chromosome 22. Am J Med Genet A. 2005;137:139–147. doi: 10.1002/ajmg.a.30780. [DOI] [PubMed] [Google Scholar]
- Kim HG, Kishikawa S, Higgins AW, Seong IS, Donovan DJ, Shen Y, Lally E, Weiss LA, Najm J, Kutsche K, Descartes M, Holt L, Braddock S, Troxell R, Kaplan L, Volkmar F, Klin A, Tsatsanis K, Harris DJ, Noens I, Pauls DL, Daly MJ, MacDonald ME, Morton CC, Quade BJ, Gusella JF. Disruption of neurexin 1 associated with autism spectrum disorder. Am J Hum Genet. 2008;82:199–207. doi: 10.1016/j.ajhg.2007.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laumonnier F, Bonnet-Brilhault F, Gomot M, Blanc R, David A, Moizard MP, Raynaud M, Ronce N, Lemonnier E, Calvas P, Laudier B, Chelly J, Fryns JP, Ropers HH, Hamel BC, Andres C, Barthelemy C, Moraine C, Briault S. X-linked mental retardation and autism are associated with a mutation in the NLGN4 gene, a member of the neuroligin family. Am J Hum Genet. 2004;74:552–557. doi: 10.1086/382137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson-Yuen A, Saldivar JS, Sommer S, Picker J. Familial deletion within NLGN4 associated with autism and Tourette syndrome. Eur J Hum Genet. 2008 doi: 10.1038/sj.ejhg.5202006. [DOI] [PubMed] [Google Scholar]
- Levinson JN, El-Husseini A. A crystal-clear interaction: relating neuroligin/neurexin complex structure to function at the synapse. Neuron. 2007;56:937–939. doi: 10.1016/j.neuron.2007.12.003. [DOI] [PubMed] [Google Scholar]
- McFarlane HG, Kusek GK, Yang M, Phoenix JL, Bolivar VJ, Crawley JN. Autism-like behavioral phenotypes in BTBR T+tf/J mice. Genes Brain Behav. 2008;7:152–163. doi: 10.1111/j.1601-183X.2007.00330.x. [DOI] [PubMed] [Google Scholar]
- Moessner R, Marshall CR, Sutcliffe JS, Skaug J, Pinto D, Vincent J, Zwaigenbaum L, Fernandez B, Roberts W, Szatmari P, Scherer SW. Contribution of SHANK3 mutations to autism spectrum disorder. Am J Hum Genet. 2007;81:1289–1297. doi: 10.1086/522590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moy SS, Nadler JJ, Young NB, Perez A, Holloway LP, Barbaro RP, Barbaro JR, Wilson LM, Threadgill DW, Lauder JM, Magnuson TR, Crawley JN. Mouse behavioral tasks relevant to autism: phenotypes of 10 inbred strains. Behav Brain Res. 2007;176:4–20. doi: 10.1016/j.bbr.2006.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scattoni ML, McFarlane HG, Zhodzishsky V, Caldwell HK, Young WS, Ricceri L, Crawley JN. Reduced ultrasonic vocalizations in vasopressin 1b knockout mice. Behav Brain Res. 2008;187:371–378. doi: 10.1016/j.bbr.2007.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng M, Hoogenraad CC. The postsynaptic architecture of excitatory synapses: a more quantitative view. Annu Rev Biochem. 2007;76:823–847. doi: 10.1146/annurev.biochem.76.060805.160029. [DOI] [PubMed] [Google Scholar]
- Tabuchi K, Blundell J, Etherton MR, Hammer RE, Liu X, Powell CM, Sudhof TC. A neuroligin-3 mutation implicated in autism increases inhibitory synaptic transmission in mice. Science. 2007;318:71–76. doi: 10.1126/science.1146221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talebizadeh Z, Bittel DC, Veatch OJ, Butler MG, Takahashi TN, Miles JH. Do known mutations in neuroligin genes (NLGN3 and NLGN4) cause autism? J Autism Dev Disord. 2004;34:735–736. doi: 10.1007/s10803-004-5295-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talebizadeh Z, Lam DY, Theodoro MF, Bittel DC, Lushington GH, Butler MG. Novel splice isoforms for NLGN3 and NLGN4 with possible implications in autism. J Med Genet. 2006;43:e21. doi: 10.1136/jmg.2005.036897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varoqueaux F, Aramuni G, Rawson RL, Mohrmann R, Missler M, Gottmann K, Zhang W, Sudhof TC, Brose N. Neuroligins determine synapse maturation and function. Neuron. 2006;51:741–754. doi: 10.1016/j.neuron.2006.09.003. [DOI] [PubMed] [Google Scholar]
- Vincent JB, Kolozsvari D, Roberts WS, Bolton PF, Gurling HM, Scherer SW. Mutation screening of X-chromosomal neuroligin genes: no mutations in 196 autism probands. Am J Med Genet B Neuropsychiatr Genet. 2004;129:82–84. doi: 10.1002/ajmg.b.30069. [DOI] [PubMed] [Google Scholar]
- Wermter AK, Kamp-Becker I, Strauch K, Schulte-Korne G, Remschmidt H. No evidence for involvement of genetic variants in the X-linked neuroligin genes NLGN3 and NLGN4X in probands with autism spectrum disorder on high functioning level. Am J Med Genet B Neuropsychiatr Genet. 2008 doi: 10.1002/ajmg.b.30618. [DOI] [PubMed] [Google Scholar]
- Willott JF, Kulig J, Satterfield T. The acoustic startle response in DBA/2 and C57BL/6 mice: relationship to auditory neuronal response properties and hearing impairment. Hear Res. 1984;16:161–167. doi: 10.1016/0378-5955(84)90005-4. [DOI] [PubMed] [Google Scholar]
- Yan J, Oliveira G, Coutinho A, Yang C, Feng J, Katz C, Sram J, Bockholt A, Jones IR, Craddock N, Cook EH, Jr., Vicente A, Sommer SS. Analysis of the neuroligin 3 and 4 genes in autism and other neuropsychiatric patients. Mol Psychiatry. 2005;10:329–332. doi: 10.1038/sj.mp.4001629. [DOI] [PubMed] [Google Scholar]
- Yang M, Zhodzishsky V, Crawley JN. Social deficits in BTBR T+tf/J mice are unchanged by cross-fostering with C57BL/6J mothers. Int J Dev Neurosci. 2007;25:515–521. doi: 10.1016/j.ijdevneu.2007.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ylisaukko-oja T, Rehnstrom K, Auranen M, Vanhala R, Alen R, Kempas E, Ellonen P, Turunen JA, Makkonen I, Riikonen R, Nieminen-von Wendt T, von Wendt L, Peltonen L, Jarvela I. Analysis of four neuroligin genes as candidates for autism. Eur J Hum Genet. 2005;13:1285–1292. doi: 10.1038/sj.ejhg.5201474. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.