Abstract
Background and Purpose
The purpose of this study was to examine regional cerebral blood flow (rCBF) in normal cognitive-performing subjects with hypertension (HTN) using continuous arterial spin-labeled MRI. The most common explanation for the effect of blood pressure on cognition is that HTN increases the risk of cerebrovascular disease, and it may increase the risk for Alzheimer disease possibly through small vessel disease, ischemia, oxidative stress, and inflammation. However, few studies to date have examined the rCBF of cognitively normal subjects with HTN in population-based cohorts, and none have used continuous arterial spin-labeled MRI. This is a noninvasive technique that does not require either injections or ionizing radiation and can measure absolute rCBF rates over the entire brain.
Methods
rCBF was measured at 1.5 T using continuous arterial spin-labeled MRI in 41 cognitively normal subjects who were participating in the Cardiovascular Health Study Cognition Study. A deformable atrophy-corrected registration method was used to warp the rCBF maps to the standard colin27 brain space. Image and cluster-based statistical analyses were performed between subject groups.
Results
Cognitively normal subjects with HTN (n=19) had decreased rCBF in the putamen, globus pallidus, bilaterally, and in the left hippocampus compared with normotensives (n=22). In addition, decreased rCBF was observed in the right and left anterior cingulate gyrus with extension to the subcallosal region, left posterior cingulate gyrus and medial precuneus, left lateral inferior and superior frontal, and inferior parietal, left orbitofrontal, and left superior temporal cortices
Conclusions
rCBF is affected in normal subjects with HTN, not only in the subcortical regions, but also in limbic and paralimbic structures. We hypothesize that the HTN creates a vulnerability state for the development of neurodegenerative disorders, especially Alzheimer disease.
Keywords: CASL, cerebral blood flow, cognition, hypertension, MRI
Hypertension (HTN) is frequent in the elderly: 30% to 40% prevalence among individuals age 65 or greater.1 It has a significant impact on cardiovascular function and on cerebral structural integrity and associated cognitive deterioration.2-4 The most common explanation for the deleterious effect of HTN on cognition is that HTN increases the risk of cerebrovascular disease.5 Long-term HTN can cause vascular hypertrophy and microvascular remodeling by promoting arteriosclerosis in large vessels and lipohyalinosis in penetrating arterioles with subsequent regional cerebral blood flow (rCBF) dysfunction.6 This can lead to lacunar infarcts and white matter disease7 and eventually to neuronal loss.
Cross-sectional studies have found both positive8 and negative9 correlations between HTN and cognitive impairment. However, longitudinal studies have shown a positive association between HTN and cognitive decline; that is, individuals with HTN were more likely to have cognitive impairment or lower mental status examination scores. These effects are independent of clinical strokes,2-4 and this association is stronger in individuals who do not use antihypertensive drug therapy.2
HTN is a risk factor for cognitive decline and for dementia, especially Alzheimer disease (AD),10-12 likely secondary to a vulnerability state caused by cerebrovascular disease. However, the factors that predispose individuals with HTN to developed AD are unknown. As a first step in understanding this process, it is crucial to study the consequences of HTN on brain structure and function in cognitively normal subjects.
Positron emission tomography studies conducted in middle-aged, cognitively normal subjects with HTN showed a pattern of reduced rCBF and compensation.13,14 Hypertensive individuals had less activation when engaged in memory tasks than normotensives in the middle posterior watershed area, parietal lobes, and thalamus. Hypertensive subjects with normal performance in memory tasks had increased rCBF in the amygdala/hippocampus area. Other positron emission tomography scan studies showed decreased metabolism in the striatum and thalamus.15,16 A recent longitudinal study (7 years) conducted in 14 highly selected hypertense (mean age, 60 years) and cognitively normal subjects using biannual O15 positron emission tomography scans showed decreasing rCBF over time in the middle and inferior prefrontal cortex, anterior cingulate gyrus, and occipital–temporal cortices.17 Functional MRI studies find a correlation between activation in the anterior cingulate gyrus, insula, thalamus, and periaqueductal gray matter and blood pressure when cognitively normal individuals with HTN performed the Stroop test.18
Volumetric MRI studies showed that elevated systolic pressures in untreated hypertensive and cognitively normal individuals (mean age, 61.3 years) correlate with decreased gray matter volumes in superior frontal, anterior cingulate, and middle temporal gyri.19 In addition, the gray matter volume was associated with memory and executive function test performance, and untreated midlife HTN was associated with decreased hippocampal volume 30 years later.20 These studies suggest that HTN can alter brain structure and rCBF beyond the expected cerebral regions localized at the end of the tree of the perforant arteries. These limbic and paralimbic areas are critical for higher cognitive functions and are targeted by AD pathology.21
The purpose of this study was to investigate rCBF in cognitively normal subjects, identified through a population-based study, using continuous arterial spin-labeled MRI (CASL-MRI). CASL-MRI is a noninvasive technique that does not require either injections or ionizing radiation and can measure absolute rCBF rates over the entire brain. CASL-MRI achieves sufficient perfusion signal-to-noise ratio and image resolution to be used in the identification and analysis of cerebrovascular and neurodegenerative diseases.22-25 The features and performance of CASL-MRI make it the preferred quantitative over alternative rCBF methods, including dynamic susceptibility contrast MRI, H2 15O-positron emission tomography, single photon emission CT, and Xe.26,27 Based on the existing data, we hypothesize that the rCBF of hypertensive subjects will be altered in multiple brain regions, reflecting a vulnerability state caused by HTN.
Materials and Methods
Cardiovascular Health Study Cognition Study
The Pittsburgh Cardiovascular Health Study Cognition Study (CHS-CS) started in 1992 to 1994, and in 2002 to 2003 we began a longitudinal study to determine the incidence of dementia and mild cognitive impairment in a group of normal and mild cognitive impairment subjects identified in 1998 to 1999 in the CHS-CS in Pittsburgh.28 Of the 924 participants examined since 1992 to 1994, a total of 532 normal and mild cognitive impairment subjects were available for study. These subjects had annual cognitive tests from 1992 to 1999 (ie, Digit Symbol Substitution Tests,29 Benton Visual Retention Test,29 Modified Mini-Mental State Examination)30 and complete neurological and neuropsychological examinations in 1998 to 1999 and 2002 to 2003.28 An MRI of the brain was obtained in 1992 to 1994, repeat MRI in 1998 to 1999, and 150 participants had a third MRI in 2002 to 2003. CASL-MRI was obtained only in the participants assessed in 2002 to 2003, and these formed the basis for the present study. The characteristics of the total CHS cohort and the Pittsburgh CHS-CS have been described previously31 as well as the details concerning the longitudinal follow-up of the CHS participants.31
Subjects
The CHS-CS acquires an MRI of the brain from all subjects who convert from normal to mild cognitive impairment, normal to dementia, or mild cognitive impairment to dementia. In addition, we acquire an MRI of the brain from 25 to 30 normal subjects every year. Of the 51 normal individuals who had an MRI of the brain in 2002 to 2003, we selected 41 (80%) for this study. These participants had no radiological evidence of structural central nervous system lesions (eg, central nervous system neoplasms, MRI-identified infarcts, prior brain surgery) or a history of clinical strokes or head trauma encephalopathy. Additional exclusion criteria to maximize case reliability and validity were: consumption of caffeine within 8 hours before examination, inability to segment images using semiautomated tools, placement of the labeling plane was not orthogonal to both carotid arteries and/or the difference between left and right carotid arterial mean velocities exceeded 20% of the mean, excessive patient motion as evidenced in structural images, or excessive image artifact (eg, hair oil or dental implant).
Hypertension
HTN was considered as present when a participant had been told by their doctor that they had HTN and were placed on antihypertensive treatment. Individuals with systolic blood pressure >140 or diastolic >90 mm Hg in 2 separate measurements were also considered as having HTN. All subjects with HTN included in the study were taking antihypertensive medication. Sixteen (84%) participants had the diagnosis of HTN before 1992 to 1994 and 3 (16%) between 1995 to 1996 and 1998 to 1999.
Continuous Arterial Spin-Labeled MRI
All MRI data were acquired using a 1.5-T GE Signa system (LX version; Milwaukee, Wisc) after each subject provided informed consent either directly or by their caregiver and passed the Society of Magnetic Resonance Imaging standardized MRI screenings. Blood flow velocities, perfusion rates, and T1 relaxation times were measured in each subject using phase contrast cine, multislice CASL perfusion MRI, and saturation recovery MRI, respectively. CASL used alternating single and double adiabatic inversions (3.7-second pulse train at 92% duty cycle) and ramp-sampled echo planar imaging (EPI) to acquire 19 contiguous axial slices (64×64 matrix, 20-cm field of view, 5-mm slice thickness, 0 spacing, 21-ms echo time [ie, minimum full], 76-kHz effective receiver bandwidth, 1-second acquisition time, 700-ms transit delay, 90° flip angle). Images were acquired sequentially from superior to inferior brain to avoid radiofrequency perturbation of the endogenous tracer as it moved superiorly into the brain and to minimize intensity discontinuities associated with interleaved acquisitions. The adiabatic inversions pulse sequence was repeated 50 times for signal averaging of the pairs of label and control acquisitions. The inversion efficiencies in the internal carotid arteries were calculated for each subject based on B1 maps and phase contrast cine velocimetry at the label plane. Coronal T1-weighted spoiled gradient-recalled echo (SPGR) images (256×192 matrix, 124 coronal slices, 1.5-mm slice thickness, 0 spacing, 21-ms echo time, 25-ms repetition time, 40° flip angle) covering the whole brain were acquired for gray and white matter segmentations and for CASL image coregistration.
EPI images were constructed offline from the raw k-space data using a reconstruction program written in C. EPI and SPGR images were converted into the Analyze format for processing with Statistical Parametric Mapping (Wellcome Department of Imaging Neuroscience). EPI gray and white matter masks were created from segmentation of the SPGR images and coregistration of the SPGR and EPI images.
Statistical Parametric Mapping image realignment corrected physiological motion between CASL acquisitions. Nonoverlapping volumes that normally appear on the superior slice (first slice) or the inferior slice (the 19th slice) tended to be discarded in the realignment process. We usually discarded the superior slice due to the lack of gray matter content.
Absolute rCBF maps of gray matter were calculated using the tracer kinetic convolution model of CASL assuming: (1) the gray matter rCBF was a global constant for each subject (ie, in the absence of significant cerebrovascular disease), (2) the endogenous tracer arrival time occurs when the normalized CASL difference (control – label) signal reaches its maxima (excluding intraluminal signals), and (3) the tracer decays before exchanging with tissue water. Only gray matter perfusion was used for the CBF analysis because of the low perfusion signal-to-noise ratio and the long tracer arrival time of white matter. The image subtraction and normalization associated with CASL intrinsically corrects for changes associated with signal amplification and coil loading. In addition, we measure B1 for each subject and use B1 to correct for intersubject (and B1-dependent) differences in labeling efficiency, coil positioning, and residual magnetization transfer effects that may affect quantification.
Statistics
The mean EPI image for each subject was registered to the corresponding SPGR image using Statistical Parametric Mapping, and the results of the alignment were applied to all CASL images to align them to the anatomical image. The SPGR images were then aligned to the standard colin27 brain using a fully deformable registration method. The fully deformable registration method algorithm allows a higher degree of deformation, thus enabling more accurate regional alignment to the standard brain. This method was adopted to compensate for the region-related volumetric loss and the decreased perfusion due to the brain atrophy.
Having warped the SPGR images into colin27 space, the deformations were used to transform the CBF maps to same the standard space. For technical reasons, not every voxel in every CASL map contained valid data for every subject. Therefore, we restricted our analysis to those voxels that contained valid perfusion data in the majority of the subjects (ie, greater than 8 of the 19 in the hypertensive subjects and greater than 11 of the 22 normotensives). Using this information, we created a gray matter volume mask, and this accounted for 88% of the total gray matter. The rCBF maps for each subject were smoothed using an isotropic 6-mm Gaussian filter after application of the brain mask.
We used a customized t test algorithm, written in MATLAB (MathWorks Inc), to evaluate group differences (HTN versus normotensives) on a voxel-by-voxel basis. A 2-tailed probability value of 0.01 was chosen for the voxel-level analysis to identify significant differences between groups. The t maps were further masked using the brain volume mask to avoid a smoothing artifact. Clusters of significant voxels were thresholded at a corrected cluster level of P<0.01. The cluster-level correction was performed to guard against false-positives from multiple comparisons and took into account the voxel-level height threshold and the size and shape of the cluster. The corrected cluster-level probability value (the probability that a cluster happened by chance) was obtained by calling the Statistical Parametric Mapping subroutine for each cluster. The significant clusters (P<0.01) were displayed on the colin27 brain.
A volume of interest-based CBF analysis was also performed to validate the voxel-level analysis. The candidate volumes of interest resulted from the statistically significant clusters (with the cluster-level threshold at P<0.01). The average rCBF for each volume of interest was calculated for each individual using those voxels with valid gray matter rCBF values. Individuals with fewer than 100 voxels in the volume of interest were excluded from further analyses. We compared the hypertensive and the normotensive group factors using the multivariate linear regression model controlling for age, gender, heart disease, antihypertensive drugs, and white matter lesions (as per CHS visual rating scale).
Results
There were no statistical differences between groups in terms of age, gender, education level, Modified Mini-Mental State Examination30 scores, heart disease, diabetes mellitus, and other medication intake (beta blockers, calcium channel blockers, other vasodilators) other than convertase II inhibitor and diuretic use (see Table 1). Similarly, no statistical differences were noted in terms of white matter grade (per CHS Visual Rating Scale), high-density lipoprotein cholesterol level, and plasma Aβ40 and Aβ42 amyloid levels. The diastolic and systolic blood pressures were higher in subjects with HTN compared with normotensives.
Table 1.
Demographic and Clinical Characteristics of Cognitively Normal Subjects With and Without HTN
| HTN | Normotensive | χ2/t Test | |
|---|---|---|---|
| No. of subjects | 19 | 22 | |
| Age, mean±SD | 82.6±3.6 | 82.2±3.7 | −0.30 |
| Age range | 75–89 | 77–92 | |
| Education level, >high school (%) | 12 (63) | 16 (73) | 0.43 |
| Race, whites (%) | 12 (63) | 20 (91) | 4.05‡ |
| Male/female | 7/12 | 7/15 | 0.11 |
| 3MSE, mean±SD | 94.2±3.1 | 95.8±4.2 | 0.110 |
| Heart disease* (%) | 2 (10.5) | 4 (18) | 0.47 |
| Diabetes mellitus† (%) | 1 (5) | 2 (9) | 0.22 |
| Smoking, ever+current (%) | 5 (26) | 5 (23) | 0.07 |
| Systolic blood pressure, mean±SD | 139.1±20.8 | 120.0±18.0 | −2.93§ |
| Diastolic blood pressure, mean±SD | 72.8±10.4 | 65.1±9.4 | −2.70§ |
| Convertase II inhibitors (%) | 9 (47) | 0 (0) | 13.3∥ |
| Beta blockers (%) | 5 (26) | 3 (14) | 1.04 |
| Calcium channel blocker (%) | 7 (37) | 6 (27) | 0.43 |
| Diuretics (%) | 12 (63) | 1 (4.5) | 16.5∥ |
| MRI white matter lesion grade >3 (%) | 5 (26) | 4 (18) | 0.39 |
| High-density lipoprotein cholesterol, mg/dL | 52.7±12.7 | 58.0±17.2 | 0.79 |
| Plasma amyloid levels | |||
| Aβ-40, pg/mL, mean±SD | 161.4±55.5 | 160.4±61.5 | 0.057 |
| Aβ-42, pg/mL, mean±SD | 22.0±12.2 | 25.8±14.3 | 0.73 |
History of angina, congestive heart failure, or myocardial infarction.
By American Diabetes Association.
P<0.05.
P<0.01.
P<0.001.
3MSE indicates Modified Mini-Mental State Examination.
Continuous Arterial Spin-Labeled MRI
Cluster-level statistics for all clusters and the Talairach coordinates are shown in Table 2. There were significant clusters of hypoperfusion in the hypertensive compared with normotensive subjects (see the Figure). These included the right and left anterior cingulate gyrus with extension to the subcallosal region, left posterior cingulate gyrus and medial precuneus, left lateral inferior and superior frontal, inferior parietal, left orbitofrontal, and left superior and middle temporal cortices, left hippocampus and bilateral putamen, and globus pallidus. There were no areas of hyperperfusion in the participants with HTN.
Table 2.
Summary of Cluster-Level Statistics for Hypoperfusion Clusters
| Talairach Coordinates |
|||||
|---|---|---|---|---|---|
| Region | Cluster Size | Cluster P Value | x | y | z |
| Left inferior frontal, left putamen, and globus pallidus | 8123 | <0.001 | −34 | 6 | 15 |
| Left anterior cingulate gyrus | 3287 | <0.001 | −8 | 54 | 25 |
| Left lateral frontal | 893 | <0.001 | −35 | 38 | 28 |
| Right inferior frontal | 491 | 0.001 | 29 | 22 | 13 |
| Right putamen and globus pallidus | 931 | <0.001 | 19 | 6 | 7 |
| Right anterior cingulate gyrus | 1252 | <0.001 | 4 | 34 | 35 |
| Left posterior cingulate and medial precuneus | 1587 | <0.001 | −47 | −61 | 31 |
| Left superior temporal | 371 | 0.001 | −57 | 4 | 1 |
| Left orbitofrontal | 429 | 0.001 | −38 | 38 | −6 |
| Left middle temporal | 860 | <0.001 | −59 | −17 | −3 |
| Left middle hippocampus | 226 | 0.003 | −29 | −23 | −11 |
Figure.
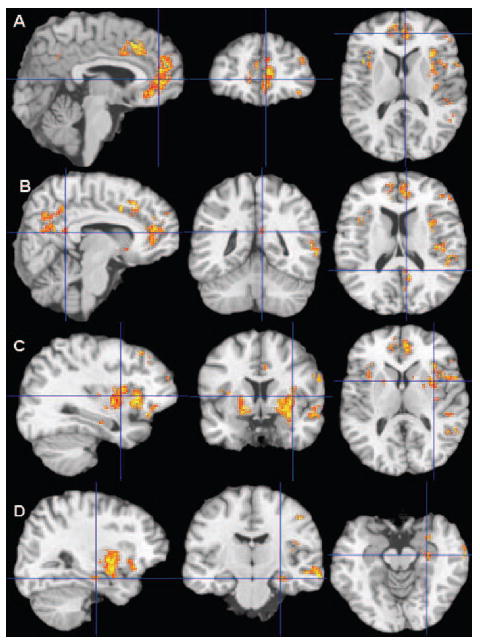
Statistically significant decreases in rCBF (only gray matter was quantified and tested for significance) in hypertensive compared with normotensive subjects by t test (cluster-level P<0.01) are in color on the surface section of the colin27 brain. A, Anterior cingulate gyrus, with extension to subcallosal area, and orbitofrontal and lateral superior frontal cortices; (B) left inferior parietal, posterior cingulate gyrus, and medial precuneus; (C) bilateral putamen and globus pallidus, left superior and lateral inferior frontal, orbitofrontal, superior temporal, and inferior parietal cortices; (D) left medial hippocampus and superior and middle temporal cortices.
Multivariate linear regression analyses were used to test for group differences after controlling for the potential confounding factors (age, gender, heart disease, race, antihypertensive use, and white matter lesions). The regression analyses also tested for the all possible 2-way interactions between the risk factors; no significant interactions were found for any of the volumes of interest. Table 3 shows the mean perfusion values and 2-tailed probability values after adjusting for the factors that can alter rCBF in normotensive and hypertensive individuals. Heart disease was found to be associated with hypoperfusion in the right inferior frontal lobe (P=0.02) and white matter lesions with the right anterior cingulate gyrus (P=0.009).
Table 3.
Adjusted Mean rCBF in 10 Volumes of Interest in Normotensive and Hypertensive Subjects
| Region | Hypertensive | Normotensive | P Value |
|---|---|---|---|
| Left anterior cingulate gyrus | 25.5±8.6 | 52.4±13.9 | <0.001 |
| Left superior frontal | 25.3±5.6 | 42.6±10.4 | 0.001 |
| Right inferior frontal | 22.2±7.3 | 42.0±9.6 | 0.01 |
| Right putamen and globus pallidus | 23.1±7.3 | 42.6±12.6 | 0.002 |
| Left inferior frontal, putamen, and globus pallidus | 25.0±7.0 | 44.0±9.6 | 0.01 |
| Right anterior cingulate gyrus | 21.0±5.6 | 42.9±11.7 | 0.01 |
| Left posterior cingulate and medial precuneus | 36.6±12.8 | 60.7±11.0 | 0.008 |
| Left superior temporal | 22.1±8.8 | 46.9±9.2 | 0.006 |
| Left orbitofrontal | 27.5±10.1 | 39.9±10.0 | 0.03 |
| Left middle temporal | 21.0±9.0 | 45.3±13.2 | 0.006 |
| Left middle hippocampus | 30.0±11.9 | 51.8±12.0 | .004 |
Adjusted for age, gender, race, heart disease, antihypertensive medication, and white lesions grade >3.
rCBF levels are expressed in millimeters per 100 g per minute (mL/100 g/min).
Discussion
This is the first study using CASL-MRI in subjects with HTN; rCBF is reduced in normal elderly individuals with HTN, not only in the subcortical regions, but also in limbic and paralimbic structures, even when the subjects are receiving antihypertensive treatment. These data are consistent with previous observations of diminished rCBF in subcortical and cortical areas in subjects with HTN15-17 with the association between HTN and hippocampal and amygdala atrophy20,32 and with the data showing that HTN can affect cerebral structures that are targeted by AD pathology,13,14,17,18 possibly increasing the vulnerability of hypertensive subjects to develop AD.
Abnormal rCBF in the cingulate gyrus, hippocampus, orbitofrontal, parietal, temporal, and hippocampal areas observed in HTN individuals were previously described in patients with AD as well. Functional neuroimaging studies in patients with AD have shown decreased rCBF or glucose metabolism in the temporal, parietal, and frontal heteromodal association areas, mesial temporal lobe, and in the posterior cingulate gyrus.33,34 Some studies found decreased CBF in the anterior cingulate gyrus with extension to the subcallosal area.35,36 CASL-MRI studies24,25 have shown atrophy and diminished CBF in the same areas detected with other functional methods. Recent positron emission tomography technologies that allow us to detect in vivo amyloid deposits in the brain have shown amyloid deposits in the frontal and posterior cingulate gyrus and in the ventral striatum of patients with mild AD.37
These findings suggest that HTN can alter rCBF beyond the expected cerebral regions localized at the end of the tree of the perforant arteries in subcortical regions (striatum). These findings are consistent with previous studies conducted in cognitively normal subjects with HTN that showed a diminished cerebrovascular dilative response to physiological stimuli13,14,18 and a pattern of reduced rCBF and compensation in middle-aged subjects with HTN. Volumetric MRI studies have shown that elevated systolic blood pressure in untreated hypertensive, cognitively normal subjects correlated with gray matter volumes in superior frontal, anterior cingulate, and middle temporal gyri.19
HTN can contribute to cognitive deficits in the absence of radiological evidence of brain infracts,2,11 and cerebrovascular disease can modulate AD clinical manifestation38 by expressing the clinical symptoms of dementia with fewer AD pathological changes.39 HTN plays a critical role in this process and our data suggest one mechanism that could account for these observations. However, our study is limited by the lack of information about the duration of antihypertensive medication, which could affect the risk of dementia and modify structural brain changes.
One dilemma in the assessment of the effects of HTN on rCBF is whether to include the actual systolic and diastolic values in the analysis (ie, to adjust for current blood pressure levels). All of these subjects were receiving antihypertensive medication, and their HTN was considered under control. No significant correlations were found between antihypertensive use and systolic (rho= −0.14, P=0.36) or diastolic (rho= −0.20, P=0.19) blood pressure. Therefore, we limited our analysis to adjustment for the use of antihypertensives. Nevertheless, had we included mean blood pressure as a continuous variable in the analysis, the associations would have remained unchanged for all regions, except for the left orbitofrontal cortex (P=0.48), with attenuation in the left superior temporal cortex (P=0.05), and hippocampus (P=0.05).
This is a cross-sectional study of the relationship between HTN and rCBF. We did not examine duration of treatment or duration of HTN in relation to changes in rCBF. Nevertheless, 84% of the hypertensive participants were diagnosed for more than 10 years, so we are likely detecting the long-term effect of HTN on the central nervous system. Furthermore, it is possible that the use of antihypertensives to treat other disease processes (eg, heart disease) in the normotensive participants masked the presence of mild HTN. These issues pose a limitation to our analysis, and additional longitudinal studies are necessary to examine the duration of treatment and HTN and its effects on rCBF.
The relationship between CASL-MRI in hypertensive patients and incident dementia needs to be examined to identify an rCBF pattern that may predict dementia in healthy individuals with HTN. Because there are no proven therapies to prevent dementia, prevention and better control of blood pressure could have a major effect not only on stroke risk and vascular dementia, but also on the development of AD.
Acknowledgments
Sources of Funding This research was supported by grants AG15928 and AG20098 from the National Institute on Aging.
Footnotes
Disclosures None.
References
- 1.Wolf-Maier K, Cooper RS, Banegas J, Giampaoli S, Hense HW, Joffres M, Kastarinen MPN, Primatesta P. Hypertension prevalence and blood pressure levels in 6 European countries, Canada, and the United States. JAMA. 2003;289:2362–2369. doi: 10.1001/jama.289.18.2363. [DOI] [PubMed] [Google Scholar]
- 2.Kilander L, Nyman H, Boberg M, Hansson L, Lithell H. Hypertension is related to cognitive impairment: a 20-year follow-up of 999 men. Hypertension. 1998;31:780–786. doi: 10.1161/01.hyp.31.3.780. [DOI] [PubMed] [Google Scholar]
- 3.Kivipelto M, Helkala E-L, Hanninen T, Laakso MP, Hallikainen M, Alhainen K, Soininen H, Tuomilehto J, Nissinen A. Midlife vascular risk factors and late-life mild cognitive impairment. Neurology. 2001;56:1683–1689. doi: 10.1212/wnl.56.12.1683. [DOI] [PubMed] [Google Scholar]
- 4.Launer LJ, Masaki K, Petrovitch H, Foley D, Havlik R. The association between mid-life blood pressure levels and late life cognitive function: the Honolulu–Asia Aging Study. JAMA. 1995;274:1846–1851. [PubMed] [Google Scholar]
- 5.Struijs J, van Genugten LL, Evers SMAA, jr, Ament AJHA, Baan CA, van den Bos GAM. Modeling the future burden of stroke in The Netherlands: impact of aging, smoking, and hypertension. Stroke. 2005;36:1648–1655. doi: 10.1161/01.STR.0000173221.37568.d2. [DOI] [PubMed] [Google Scholar]
- 6.Moossy J. Pathology of cerebral atherosclerosis. Influence of age, race, and gender. Stroke. 1993;24(suppl 12):I22–I32. [PubMed] [Google Scholar]
- 7.van Dijk EJ, Breteler MB, Schmidt R, Berger K, Nilsson L-G, Oudkerek M, Pajak A, Sans S, de Ridder MCD. The association between blood pressure, hypertension, and cerebral white matter lesions. The Cardiovascular Determinants of Dementia Study. Hypertension. 2004;44:625–630. doi: 10.1161/01.HYP.0000145857.98904.20. [DOI] [PubMed] [Google Scholar]
- 8.Stewart R, Russ C, Richards M, Brayne C, Lovestone S, Mann A. Apolipoprotein E genotype, vascular risk and early cognitive impairment in an African Caribbean population. Dement Geriatr Cogn Disord. 2001;12:251–256. doi: 10.1159/000051267. [DOI] [PubMed] [Google Scholar]
- 9.Hebert LE, Scherr PA, Bennett DA, Bienias JL, Wilson RS, Morris MC, Evans DA. Blood pressure and late-life cognitive function change: a biracial longitudinal population study. Neurology. 2004;62:2021–2024. doi: 10.1212/01.wnl.0000129258.93137.4b. [DOI] [PubMed] [Google Scholar]
- 10.Qiu C, Winblad B, Fastbom J, Fratiglioni L. Combined effects of APOE genotype, blood pressure, and antihypertensive drug use on incident AD. Neurology. 2003;61:656–660. doi: 10.1212/wnl.61.5.655. [DOI] [PubMed] [Google Scholar]
- 11.Kivipelto M, Helkala E-L, Laakso MP, Hanninen T, Hallikainen M, Alhainen K, Soininen H, Tuomilehto J, Nissinen A. Midlife vascular risk factors and Alzheimer’s disease in later life: longitudinal, population based study. BMJ. 2001;322:1447–1451. doi: 10.1136/bmj.322.7300.1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Skoog I, Lernfeldt B, Landahl S. 15-year longitudinal study of blood pressure and dementia. Lancet. 1996;347:1141–1145. doi: 10.1016/s0140-6736(96)90608-x. [DOI] [PubMed] [Google Scholar]
- 13.Jennings JR, Muldoon MF, Ryan CM, Mintun MA, Meltzer C, Townsend D, Sutton-Tyrel K, Shapiro AP, Manuck SB. Cerebral blood flow in hypertensive patients: an initial report of reduced and compensatory blood flow responses during performance of two cognitive tasks. Hypertension. 1998;31:1216–1222. doi: 10.1161/01.hyp.31.6.1216. [DOI] [PubMed] [Google Scholar]
- 14.Jennings JR, Muldoon MF, Ryan C, Price JC, Greer P, Sutton-Tyrrell K, van der Veen FM, Meltzer CC. Reduced cerebral blood flow response and compensation among patients with untreated hypertension. Neurology. 2005;64:1358–1365. doi: 10.1212/01.WNL.0000158283.28251.3C. [DOI] [PubMed] [Google Scholar]
- 15.Fujishima S, Imaizumi T, Abe I, Takeshita A, Fujishima M. Effects of intra-arterial infusion of insulin on forearm vasoreactivity in hypertensive humans. Hypertens Res. 1995;18:327–333. doi: 10.1291/hypres.18.227. [DOI] [PubMed] [Google Scholar]
- 16.Mentis MJ, Salerno J, Horwitz B, Grady CL, Schapiro MB, Murphy DC, Rapoport SI. Reduction of functional neuronal connectivity in long-term treated hypertension. Stroke. 1994;25:601–607. doi: 10.1161/01.str.25.3.601. [DOI] [PubMed] [Google Scholar]
- 17.Beason-Held LL, Moghekar A, Zonderman AB, Kraut MA, Resnick SM. Longitudinal changes in cerebral blood flow in the older hypertensive brain. Stroke. 2007;39:1766–1773. doi: 10.1161/STROKEAHA.106.477109. [DOI] [PubMed] [Google Scholar]
- 18.Gianaros PJ, Derbyshire SW, May JC, Siegle GJ, Gamalo MA, Jennings JR. Anterior cingulate activity correlates with blood pressure during stress. Psychophysiology. 2005;42:627–636. doi: 10.1111/j.1469-8986.2005.00366.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gianaros PJ, Greer PJ, Ryan CM, Jennings JR. Higher blood pressure predicts lower regional grey matter volume: consequences on short-term information processing. Neuroimage. 2006;31:754–765. doi: 10.1016/j.neuroimage.2006.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Korf ESC, White LR, Scheltens P, Launer LJ. Midlife blood pressure and the risk of hippocampal atrophy: the Honolulu Asia Aging Study. Hypertension. 2004;44:29–34. doi: 10.1161/01.HYP.0000132475.32317.bb. [DOI] [PubMed] [Google Scholar]
- 21.Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- 22.Talagala SL, Noll DC. Functional MRI using steady state arterial water labeling. Magn Reson Med. 1998;39:179–183. doi: 10.1002/mrm.1910390203. [DOI] [PubMed] [Google Scholar]
- 23.Gach HM, Dai W. Simple model of double adiabatic inversion (DAI) efficiency. Magn Redon Med. 2004;52:941–946. doi: 10.1002/mrm.20240. [DOI] [PubMed] [Google Scholar]
- 24.Alsop DC, Detre JA, Grossman M. Assessment of cerebral blood flow in Alzheimer’s disease by spin-labeled magnetic resonance imaging. Ann Neurol. 2000;47:93–100. [PubMed] [Google Scholar]
- 25.Johnson NA, Jahng G-H, Weiner MW, Miller BL, Chui HC, Jagust WJ, Gorno-Tempini ML, Schuff N. Pattern of cerebral hypoperfusion in Alzheimer disease and mild cognitive impairment measured with arterial spin-labeling MR imaging: initial experience. Radiology. 2005;234:851–859. doi: 10.1148/radiol.2343040197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brown GG, Clark CM, Liu TT. Measurement of cerebral perfusion with arterial spin labeling: part 2. Applications. J Int Neuropsychol Soc. 2007;13:1–13. doi: 10.1017/S1355617707070634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu TT, Brown GG. Measurement of cerebral perfusion with arterial spin labeling: part I. Methods. J Int Neuropsychol Soc. 2007;13:1–9. doi: 10.1017/S1355617707070646. [DOI] [PubMed] [Google Scholar]
- 28.Lopez OL, Kuller LH, Becker JT, Dulberg C, DeKosky S, Sweet R, Gach M. Incidence of dementia in mild cognitive impairment in the Cardiovascular Health Study. Arch Neurol. 2007;64:416–421. doi: 10.1001/archneur.64.3.416. [DOI] [PubMed] [Google Scholar]
- 29.Lezak M. Neuropsychological Assessment. 3. New York: Oxford University Press; 1995. [Google Scholar]
- 30.Teng EL, Chui HC. The Modified Mini-Mental State (3MS) Examination. J Clin Psychiatry. 1987;48:314–318. [PubMed] [Google Scholar]
- 31.Lopez OL, Kuller LH, Fitzpatrick A, Ives D, Becker JT, Beauchamp N. Evaluations of dementia in the Cardiovascular Health Cognition study. Neuroepidemiology. 2003;22:1–12. doi: 10.1159/000067110. [DOI] [PubMed] [Google Scholar]
- 32.den Heijer T, Launer LJ, van Dijk EJ, Vermeer SE, Hofman A, Koudstaal PJ, Breteler MMB. Association between blood pressure, white matter lesions, and atrophy of the medial temporal lobe. Neurology. 2005;64:263–267. doi: 10.1212/01.WNL.0000149641.55751.2E. [DOI] [PubMed] [Google Scholar]
- 33.Minoshima S, Giordani B, Berent S, Frey KA, Foster NL, Kuhl DE. Metabolic reduction in the posterior cingular cortex in very early Alzheimer’s disease. Ann Neurol. 1997;42:85–94. doi: 10.1002/ana.410420114. [DOI] [PubMed] [Google Scholar]
- 34.Drzezga A, Riemenschneider M, Strassner B, Grimmer T, Peller M, Knoll A, Wagenpfeil S, Minoshima S, Schwaiger M, Kurz A. Cerebral glucose metabolism in patients with AD and different APOE genotypes. Neurology. 2005;64:102–107. doi: 10.1212/01.WNL.0000148478.39691.D3. [DOI] [PubMed] [Google Scholar]
- 35.Chetelat G, Desgranges B, de la Sayette V, Viader F, Eustache F, Baron J-C. Mapping gray matter loss with voxel-based morphometry in mild cognitive impairment. NeuroReport. 2002;13:1939–1943. doi: 10.1097/00001756-200210280-00022. [DOI] [PubMed] [Google Scholar]
- 36.Karas GB, Scheltens P, Rombouts SARB, Visser PJ, van Schijndel RA, Fox NC, Barkhof F. Global and local gray matter loss in mild cognitive impairment and Alzheimer’s disease. NeuroImage. 2004;23:708–716. doi: 10.1016/j.neuroimage.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 37.Klunk WE, Engler H, Nordberg A, Wang Y, Blomqvist G, Holt DP, B M, Savitcheva I, Huang G, Estrada S. Imaging brain amyloid in Alzheimer’s disease with Pittsburgh Compond-B. Ann Neurol. 2004;55:306–319. doi: 10.1002/ana.20009. [DOI] [PubMed] [Google Scholar]
- 38.Snowdon DA, Grainer LH, Mortimer JA, Riley KP, Grainer PA, Markesbery WR. Brain infarction and the clinical expression of Alzheimer disease: the Nun study. JAMA. 1997;277:813–817. [PubMed] [Google Scholar]
- 39.Petrovitch H, White LR, Izmirilian G, Ross GW, Havlik RJ, Markesbery W, Nelson J, Davis DG, Hardman J, Foley DJ, Launer LJ. Midlife blood pressure and neuritic plaques, neurofibrillary tangles, and brain weight at death: the HAAS, Honolulu–Asia Aging Study. Neurobiol Aging. 2000;21:57–62. doi: 10.1016/s0197-4580(00)00106-8. [DOI] [PubMed] [Google Scholar]


