Abstract
Myeloid dermal dendritic cells (DCs) accumulate in chronically inflamed tissues such as psoriasis. The importance of these cells for psoriasis pathogenesis is suggested by comparative T cell and DC cell counts, where DCs outnumber T cells. We have previously identified CD11c+BDCA-1+ cells as the main resident dermal DC population found in normal skin. We now show that psoriatic lesional skin has two populations of dermal DCs: 1) CD11c+BDCA-1+ cells which are phenotypically similar to those contained in normal skin, and 2) CD11c+BDCA-1− cells which are phenotypically immature and produce inflammatory cytokines. While BDCA-1+ DCs are not increased in number in psoriatic lesional skin compared to normal skin, BDCA-1− DCs are increased 30-fold. For functional studies, we FACS-sorted psoriatic dermal single cell suspensions to isolate these two cutaneous DC populations, and cultured them as stimulators in an allo-MLR. Both BDCA-1+ and BDCA-1− myeloid dermal DC populations induced T cell proliferation, and polarized T cells to become Th1 and Th17 cells. In addition, psoriatic dermal DCs induced a population of activated T cells that simultaneously produced IL-17 and IFN-γ, which was not induced by normal skin dermal DCs. As psoriasis is believed to be a mixed Th17/Th1 disease, it is possible that induction of these IL-17+IFNγ+ cells is pathogenic. These cytokines, the T cells that produce them, and the inducing inflammatory DCs may all be important new therapeutic targets in psoriasis.
Keywords: Psoriasis, BDCA-1, Tip-DC, dermis, inflammation, skin, myeloid dendritic cell
Introduction
Psoriasis is a common chronic inflammatory skin disease, which results in great morbidity for severely affected patients. In recent years much progress has been made understanding the pathogenesis and treatment of this disease. We now appreciate that psoriasis results from complex interactions between T cells, dendritic cells (DCs), and keratinocytes (Lowes et al., 2007). Until recently, psoriasis has been considered a classical Type 1 autoimmune disease, with a strong IFNγ Th1 signal. However, a new subset of T cells, Th17 cells, have now been described in murine models of autoimmune inflammation (Weaver et al., 2007), and we have reported the presence of these cells in psoriasis (Lowes et al., 2008). Th17 cells produce IL-17, IL-22, and have other important downstream pro-inflammatory effects in skin (Liang et al., 2006; Zheng et al., 2007). DCs may be very central pathogenic players in psoriasis, both by activating T cells and by producing amplifying cytokines and chemokines during inflammation. In the skin the main DC populations include epidermal DCs (Langerhans cells) and dermal DCs (myeloid DCs and plasmacytoid DCs). We were interested in further characterizing dermal myeloid DCs in psoriasis, as these cells may be an important therapeutic target.
Recently, we described that the best marker for identifying dermal myeloid DCs in normal skin is CD11c (Zaba et al., 2007b). Also, we have previously reported that there is a large increase in CD11c+ cells in psoriasis (Abrams et al., 1999; Gottlieb et al., 2005; Lowes et al., 2005). These CD11c+ cells include a subset of inflammatory DCs called TNF- and inducible nitric oxide synthase (iNOS) -producing DCs, or Tip-DCs (Lowes et al., 2005). Tip-DCs were first described in the spleen during a murine model of Listeria monocytogenes infection (Serbina et al., 2003; Tam and Wick, 2004). Pathogenicity of these Tip-DCs in psoriasis is suggested by the rapid downmodulation of Tip-DC products TNF, iNOS, IL-20, and IL-23 during effective treatment with TNF blocking drugs (Zaba et al., 2007a).
In normal skin, CD11c+ cells are nearly all blood dendritic cell antigen (BDCA)-1+ (Zaba et al., 2007b). BDCA-1 is also known as CD1c, and this molecule is part of the CD1 family of invariant MHC molecules that are important in presentation of lipid antigens to T cells (Barral and Brenner, 2007). In this manuscript, we show that unlike normal skin, most of these CD11c+ cells in psoriatic plaques are actually BDCA-1−. They are relatively immature DCs, with little DC-LAMP (dendritic cell -lysosomal-membrane associated protein) and DEC-205/CD205 co-expression in psoriatic lesions. Hence, in situ there were two main types of dermal DCs in psoriasis lesions: CD11c+BDCA-1+ resident DCs, and CD11c+BDCA-1− inflammatory DCs. Dermal single cell suspensions for phenotype analysis and functional studies showed that both populations were allo-stimulatory and were able to polarize allogeneic T cells into IL-17 producing Th17 cells.
Results
Psoriatic myeloid dermal DCs are CD11c+BDCA-1−BDCA-3−
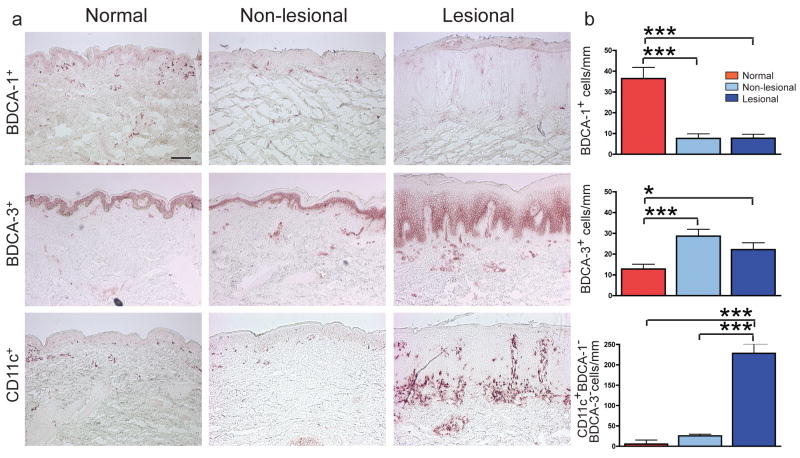
To quantify cells in each dermal DC compartment, we performed immunohistochemistry on normal skin and psoriasis paired lesional/non-lesional samples (n=20) (Figure 1). Both non-lesional and lesional psoriasis samples had 5-fold fewer BDCA-1+ DCs (Figure 1a,b) (p<0.001). However, BDCA-1 cell counts did not change significantly in a group of psoriatic patients treated with etanercept (Supplementary figure 1a) (Zaba et al., 2007a). There were 2-fold more BDCA-3+ DCs compared to normal skin (p<0.001 and p<0.05, respectively) (Figure 1a,b). The BDCA-3+ antibody gave some non-specific keratinocyte staining, as seen by others (Narbutt et al., 2006), but it is currently the only available BDCA-3 antibody. In the dermis, there was a leukocyte pattern of distribution and a dendritic cell morphology with this antibody, and only dermal cells were counted. CD11c+BDCA-1−BDCA-3− cell numbers were calculated by subtracting BDCA-1 and BDCA-3 cell counts from CD11c cell counts. While lesional and non-lesional psoriasis sections contained similar numbers of BDCA-1+ and BDCA-3+ cells, CD11c+BDCA-1−BDCA-3− cells were increased 10-fold in psoriasis plaques compared to non-lesional skin (p<0.001), and 30-fold compared to normal skin (Figure 1b) (p<0.001). In addition, we performed FACS on whole blood from normal (n=6) and psoriasis (n=6) subjects, and found that BDCA-1+ and BDCA-3+ myeloid DC subsets (MacDonald et al., 2002) were decreased in peripheral blood of psoriasis patients compared to normal volunteers (Supplementary Figure 1c, d). Negative control staining (without primary antibody) is shown in Supplementary Figure 1e.
Figure 1. CD11c+ dermal DCs are the major DC population accumulating in psoriasis lesional skin.
(a) Representative immunohistochemistry of BDCA-1+ cells, BDCA-3+ cells, and CD11c+ cells in normal, non-lesional and lesional psoriatic skin. (b) Quantification of myeloid DCs per mm skin stained by immunohistochemistry of normal skin (red boxes; n=20), non-lesional skin (light blue boxes; n=20), and matched psoriatic lesional skin (dark blue boxes; n=20). CD11c+BDCA-1−BDCA-3− cell numbers were calculated by subtracting BDCA-1 and BDCA-3 cell counts from CD11c cell counts. Error bars indicate SEM. (*) p<0.05, (***) p<0.001. Size bar = 100μm.
We next evaluated these populations in situ by 2 color immunofluorescence. In previous studies on normal human skin, we have characterized two populations of myeloid CD11c+ dermal DCs: BDCA-1+ dermal DCs comprise approximately 90% of all CD11c+ dermal cells, and the remaining 10% of CD11c+ cells are BDCA-1− (Zaba et al., 2007b). We found that in psoriasis, there was a reversal of this ratio of BDCA-1+ cells, as the minority of the CD11c+ cells co-expressed BDCA-1 (Figure 2a). BDCA-1+ cells aggregated together in dermal clumps (Figure 2a,b), compared to CD11c+ cells, which were located mostly in the upper reticular dermis and dermal papillae. BDCA-3 identifies an additional population of myeloid DCs in the circulation (MacDonald et al., 2002) and in psoriatic dermis (Figure 2b). This marker was expressed on CD11c+ cells scattered throughout the dermis and also on blood vessels (Figure 2c). Similarly, in normal skin dermis the few BDCA-3+ cells that were present were CD11c+, and the BDCA-1 and BDCA-3 identified discrete populations (n=4) (Supplementary figure 1b). As CD11c+BDCA-1+ cells are the major resident dermal DC population in normal skin, the remainder of our study compares resident CD11c+BDCA-1+ DCs in normal skin with CD11c+BDCA-1+ and CD11c+BDCA-1− DCs in the psoriatic inflammatory infiltrate.
Figure 2. Most CD11c+ myeloid DCs are BDCA-1− in psoriasis lesional skin.
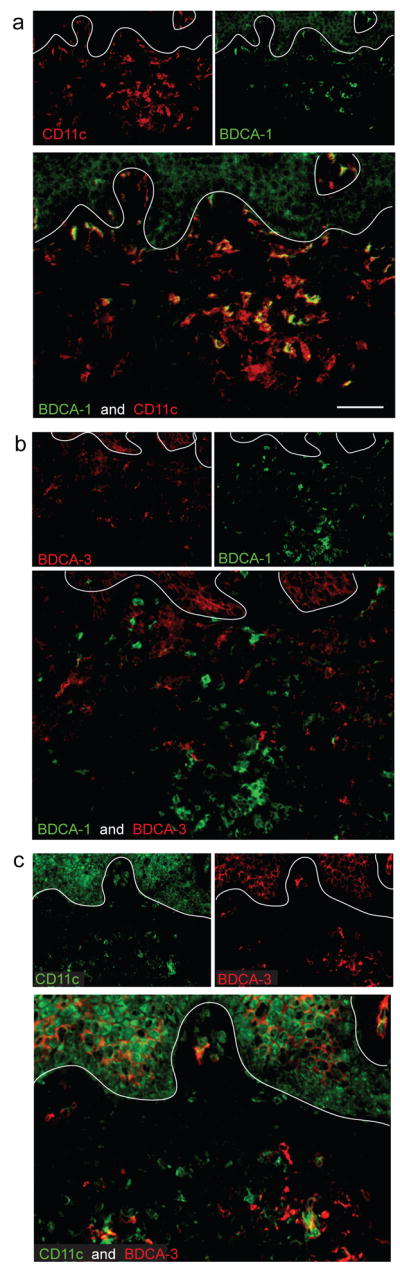
(a) The majority of CD11c+ cells in psoriatic dermis were BDCA-1−, while a small subset of CD11c+ cells co-expressed BDCA-1+. (b) BDCA-1 and BDCA-3 identified separate myeloid DC populations in the dermis. (c) Most BDCA-3+ cells co-expressed CD11c, and some BDCA-3 staining was observed on blood vessels. In all IF figures, single stained controls are above the merged image, white line denotes dermo-epidermal junction, dermal collagen fibers gave green autofluorescence, and antibodies conjugated with a fluorochrome often gave background epidermal fluorescence. Size bar = 100μm.
CD11c+ BDCA-1− myeloid dermal DCs include TNF-and-iNOS producing dendritic cell (Tip-DC) population
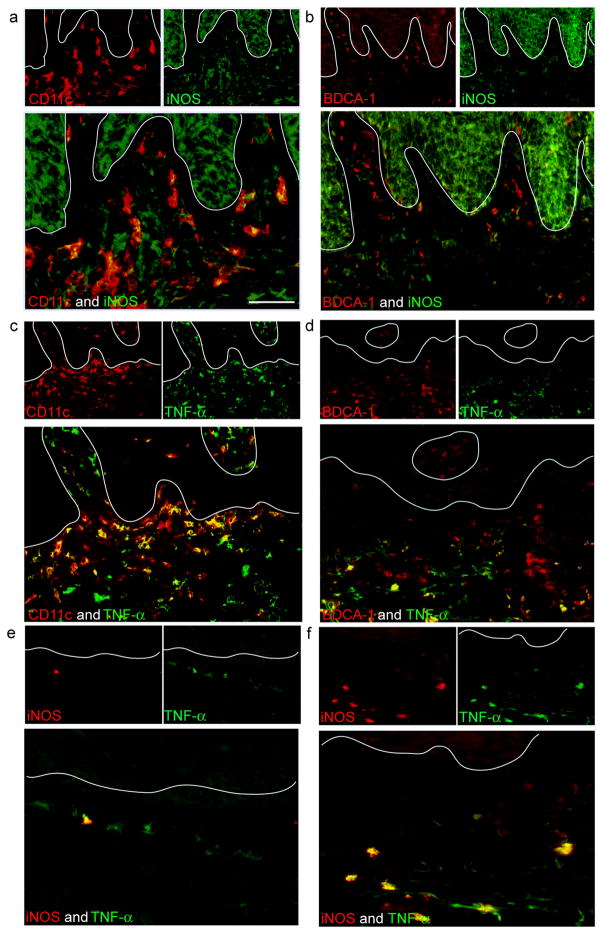
In psoriasis lesional tissue, the majority of CD11c+ cells were iNOS producing (Figure 3a), and the cellular iNOS expression appeared punctate and intracytoplasmic. Resident CD11c+ BDCA-1+ dermal DCs had <10% iNOS co-expression (Figure 3b). Similarly, TNF was expressed by >90% of CD11c+ cells (Figure 3c) and <20% of CD11c+ BDCA-1+ dermal DCs (Figure 3d). In addition, iNOS and TNF identified occasional non-lesional Tip-DCs (Figure 3e) and many lesional Tip-DCs (Figure 3f). In conclusion, Tip-DCs were identified as a subpopulation contained predominantly within the CD11c+ BDCA-1− myeloid DC population.
Figure 3. CD11c+BDCA-1− DCs contain the Tip-DC population.
(a) Most CD11c+ dermal DCs in psoriatic lesional skin co-expressed iNOS compared to (b) BDCA-1+ cells. (c) Most CD11c+ dermal DCs in lesional psoriatic skin co-expressed TNF compared to (d) BDCA-1+ cells. Approximately 25% of BDCA-1+ cells co-expressed TNF. (e,f) iNOS and TNF were co-expressed in the same cell, identifying Tip-DCs in situ. Few Tip-DCs were observed in (e) psoriatic non-lesional skin compared to (f) lesional skin. Size bar = 100μm.
CD11c+ BDCA-1− inflammatory DCs show some expression of macrophage markers compared to BDCA-1+ cells
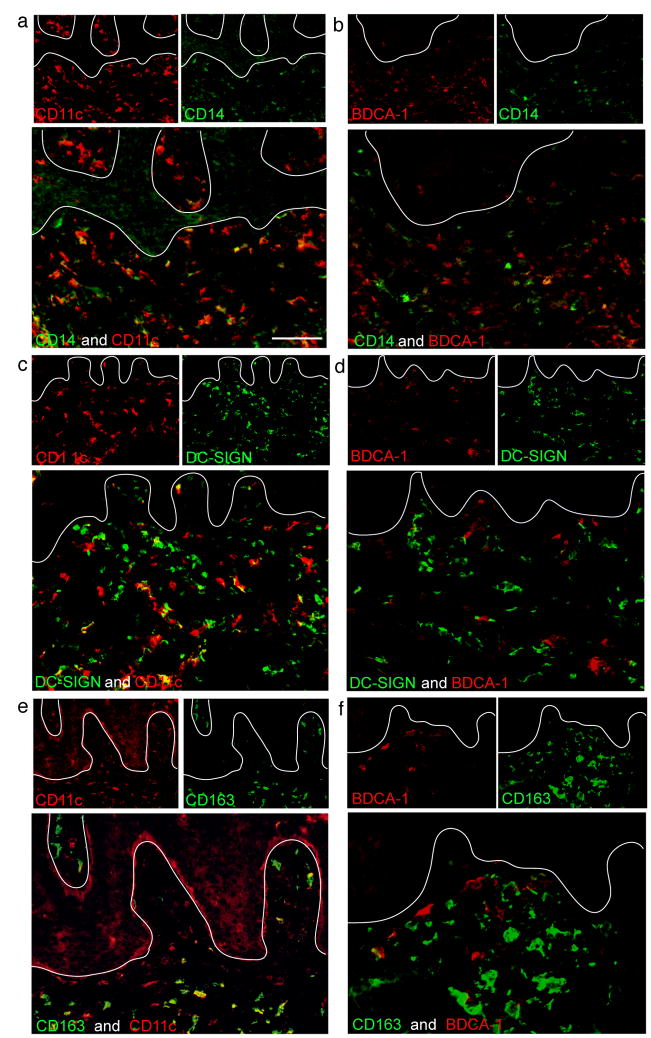
In order to further characterize the CD11c+ dermal DCs, we evaluated co-expression with a series of myeloid cell markers (Figures 4, 5). Over half of CD11c+ DCs expressed low level CD14 (Figure 4a). Most CD11c+ cells in the papillary dermis were CD14−, while most in the reticular dermis were CD14lo. In contrast, only occasional BDCA-1+ cells expressed CD14 (Figure 4b). DC-SIGN/CD209, a marker of both immature DCs and macrophages in normal human skin (Zaba et al., 2007b), partially co-localized with CD11c (Figure 4c) but did not co-localize with BDCA-1 (Figure 4d). Similarly, CD163, a marker of normal dermal macrophages (Zaba et al., 2007b), blood monocytes, and blood DCs (Maniecki et al., 2006), was expressed on some CD11c+ cells (Figure 4e), but not on CD11c+BDCA-1+ DCs (Figure 4f). In normal skin, CD11c is not expressed on CD163+ macrophages (Zaba et al., 2007b), despite the meyloid origin of macrophages.
Figure 4. CD11c+BDCA-1− inflammatory dermal DCs express CD14 and DC-SIGN.
(a) A subset of CD11c+ cells in the reticular dermis expressed low level CD14. (b) Few BDCA-1+ dermal DCs co-expressed CD14, and (c) 80% of CD11c+ cells were DC-SIGN+. This was not an exclusive myeloid DC marker as some DC-SIGN+ cells were not CD11c+. (d) BDCA-1+ cells did not co-express DC-SIGN. (e) CD163+ macrophages showed some CD11c co-expression. (f) BDCA-1+ cells did not express CD163. Size bar = 100μm.
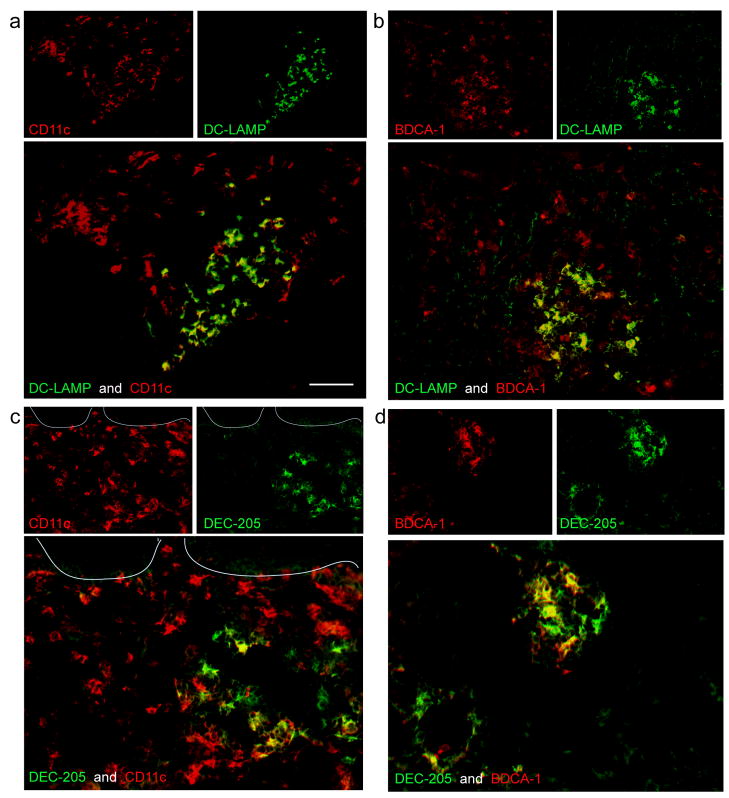
Figure 5. CD11c+BDCA-1+ cells are phenotypically mature dermal DCs in psoriasis.
DC-LAMP and DEC-205 identified mature DCs, often located in dermal aggregates. (a) DC-LAMP+ cells were CD11c+, and (b) BDCA-1+. (c) DEC-205+ cells were (c) CD11c+, and (d) BDCA-1+. There were many CD11c+ cells that were not positive for these two markers in the psoriatic dermis. Size bar = 100μm.
Single antigens specific for mature DCs include DC-LAMP/CD208 and endocytic receptor DEC-205/CD205 (Figure 5). Nearly all DC-LAMP+ and DEC-205+ cells were in dermal aggregates, and co-expressed CD11c and BDCA-1. While most BDCA-1+ cells co-expressed these two mature DC markers, there were many CD11c+ cells that did not, identifying BDCA-1− myeloid dermal DCs as less mature than BDCA-1+ cells.
Myeloid DCs obtained from psoriatic dermis are immunostimulatory and activate Th17 and Th1 cells
Single cell suspensions of normal skin (n=3) and psoriasis lesions (n=3) were obtained by enzymatic splitting of the epidermis, and then culturing the dermis for 2–3 days to allow the leukocytes to migrate out of the dermal scaffold. For functional experiments, we then FACS-sorted this bulk dermal single cell suspension, obtaining 10,000–50,000 cells in each population.
(a) DC phenotype
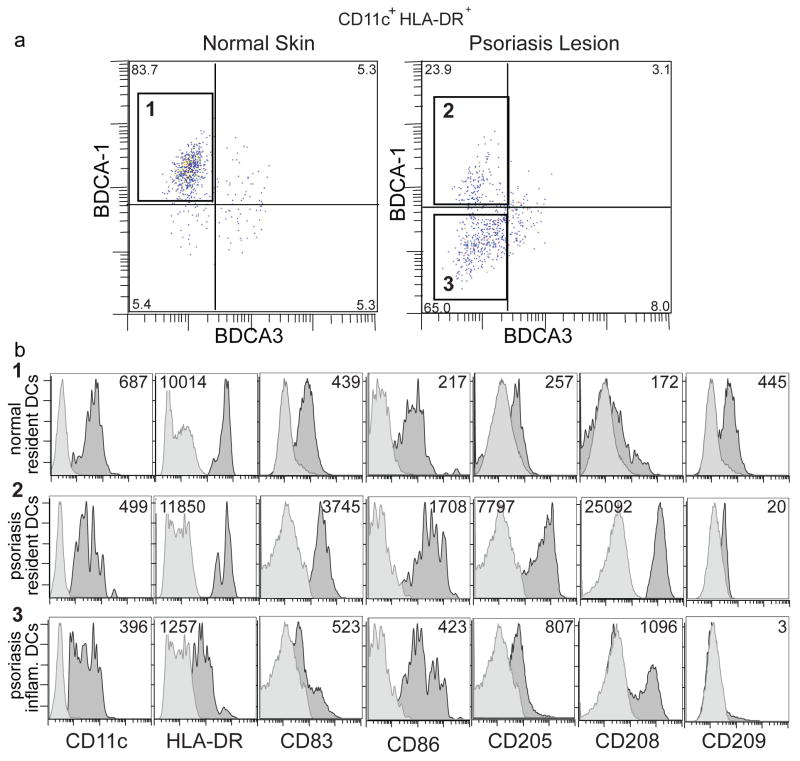
For surface phenotyping of normal and psoriatic dermal myeloid DCs by FACS, large cells were gated on CD11c+HLA-DR+, a classic definition of a myeloid DC, and further gated according to BDCA-1 expression (Figure 6a). Phenotypic characterization of BDCA-1+ cells from normal skin (Figure 6a, box 1) and psoriasis lesions (Figure 6a, box 2), as well as psoriatic BDCA-1− cells (Figure 6a, box 3) is shown in Figure 6b and Supplemetary Figure 2.
Figure 6. Psoriatic inflammatory dermal DCs (CD11c+ BDCA-1−) are less mature than resident BDCA-1+ dermal DCs.
Flow cytometric analysis of single cell suspensions of dermal émigrés from normal dermis or psoriatic dermis (n=3 for each). (a) Large cells gated on CD11c+ HLA-DRhi. In normal dermis, most myeloid DCs were BDCA-1+(box 1). In psoriatic dermis, myeloid DCs were either BDCA-1+ (box 2), or BDCA-1− (box 3). Percent of myeloid cells indicated for each quadrant. (b) Histograms in each row were gated on boxes (1–3) as identified above in Figure 6a. Dark grey histogram represents antigen expression, light grey is isotype. MFI is indicated in the upper right or upper left corner of each histogram. Resident BDCA-1+ myeloid DCs from normal and psoriatic dermis were phenotypically similar, while the additional population of BDCA-1− DCs in psoriasis showed lower HLA-DR, and were smaller cells. Psoriatic BDCA-1+ DCs demonstrated the highest expression of DC-LAMP/CD208, and DEC-205/CD205.
CD11c+BDCA-1+ resident dermal DCs from both normal (Figure 6b, row 1) and psoriasis skin (Figure 6b, row 2) had similar levels of the lineage marker CD11c, and HLA-DR MHC-II protein. In contrast, CD11c+BDCA-1− psoriatic dermal DCs (Figure 6b, row 3) had 10-fold decreased HLA-DR expression. Expression of co-stimulatory molecules CD83 and CD86 was highest on psoriatic CD11c+BDCA-1+ cells. Expression of the mature DC markers DEC-205/CD205 and CD208/DC-LAMP was highest on the psoriatic CD11c+BDCA-1+ cells, with a gradient of expression on psoriatic CD11c+BDCA-1− cells, and normal skin resident CD11c+BDCA-1+ cells. CD209/DC-SIGN showed the highest expression on normal skin CD11c+BDCA-1+ cells, and was not present on BDCA-1− psoriatic DCs (unlike in situ).
CD40 was low on all three populations (Supplementary figure 2). CD11c+BDCA-1− cells from psoriatic lesions showed 3–6 fold increased CD14 LPS receptor expression, and were 20% smaller than both CD11c+ BDCA-1+ populations. Blood monocytes expressed similar levels of CD11c and HLA-DR as CD11c+ BDCA-1− psoriatic dermal DCs (data not shown), but were 99% CD14hi (Figure S2b, row 3, black line no fill) unlike CD11c+ BDCA-1− psoriatic dermal DCs which were CD14mid. CD16 Fc γRIIIa expression was similar in all three populations. Thus, psoriatic CD11c+BDCA-1+ dermal DCs are phenotypically mature and closer to normal resident CD11c+BDCA-1+ dermal DCs, whereas psoriatic CD11c+BDCA-1− cells share phenotypic features of both DCs and monocytes.
(b) Allo-MLR
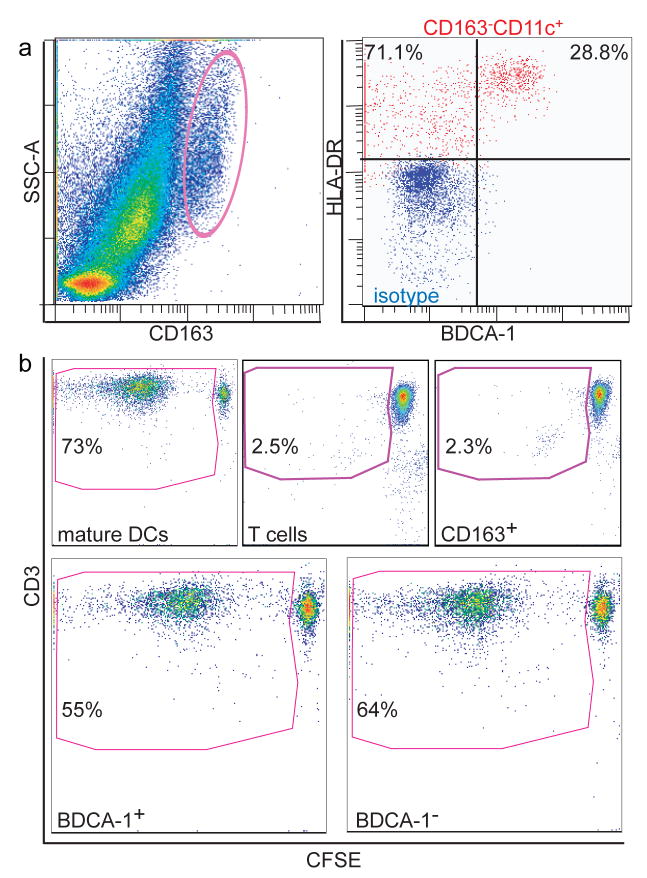
To test the immunostimulatory capacity of psoriatic dermal DC populations, we FACS-sorted DCs (CD11c+HLA-DRhiBDCA-1+ or CD11c+HLA-DRhiBDCA-1−) and macrophages (CD163hi) for co-culture as stimulators in an allogeneic mixed leukocyte reactions (allo-MLR) (Figure 7). Mature monocyte derived DCs (MoDCs) and T cells alone served as positive and negative controls, respectively. Figure 7 shows a representative experiment (n=3). 73.2% of living T cells stimulated with MoDCs at a stimulator/responder ratio of 1:50 on day 8 post sort had undergone extensive proliferation (Figure 7b). MLRs without a stimulator population (T cells) alone had 2.5% background proliferation. CD163hi cells were not more immunostimulatory than T cells alone (2.3%), whereas both CD11c+BDCA-1+ resident DCs and CD11c+BDCA-1− inflammatory DCs were similarly immunostimulatory (55.3% and 64.5%, respectively). Bulk psoriatic dermal single cell suspensions stimulate 60% (data not shown). These results suggest that CD163hi cells in psoriasis are non-immunostimulatory macrophages expressing low level CD11c (Figure 4e), and that although CD11c+BDCA-1− are phenotypically less mature than the CD11c+BDCA-1+ cells, they are comparably immunostimulatory.
Figure 7. Both psoriatic CD11c+BDCA-1+ resident DCs and CD11c+BDCA-1− inflammatory DCs were immunostimulatory in an allo-MLR.
Single-cell suspensions of psoriatic dermal émigrés were sorted into (a, left) CD163hi or (a, right), CD3−CD45+HLA-DRhiCD11chiBDCA-1+ or CD3−CD45+HLA-DRhiCD11chiBDCA-1− compared to isotype (red). (b) Gate contains CD3+ proliferating T cells with left-shifted CFSE as cells proliferated. Positive control (monocyte-derived mature DCs) on day 8-post sort at 1:50 stimulator to responder ratio. Background proliferation of T cells alone (2.5%), CD163hi cells did not stimulate above background. BDCA-1+ and BDCA-1− cells stimulated T cell proliferation similarly (both >55%). Representative graphs from 3 experiments.
(c) Induction of intracellular cytokines in T cells
We recently demonstrated the presence of Th17 cells from the dermis of psoriatic plaques (Lowes et al., 2008). We now show that CD11c+ BDCA-1+ and CD11c+ BDCA-1− psoriatic dermal DCs induce IL-17 production in allogeneic CD4+ T cells. Psoriatic dermal émigrés (n=3) were sorted as previously described into CD11c+ BDCA-1− DCs, CD11c+ BDCA-1+ DCs, or CD163+ macrophages and mixed with normal donor T cells at a 1:10 stimulator: responder ratio. Normal skin dermal émigrés (n=2) were sorted into BDCA-1+ and CD163+ populations. Cells were cultured for 9 days before analysis of T cell intracellular cytokines and IL-17 and IFNγ cytokine production in the supernatant. Figure 8 shows a representative experiment.
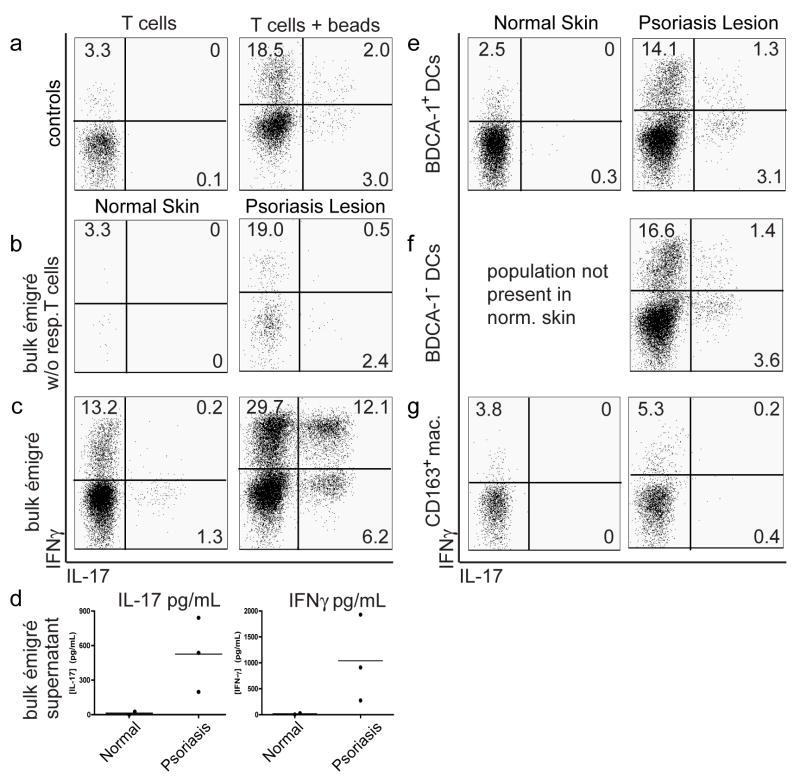
Figure 8. Psoriatic dermal DCs induce IL-17/IFNγ producing T cells.
Allogeneic T cell responders were mixed with FACS-sorted stimulator populations described in Figure 6, or with bulk émigrés from either normal or psoriatic dermal skin, at 1:10 ratio for 9 days. Intracellular cytokine expression of T cells (live CD3+CD4+CD8− cells) measured by flow cytometry (a,b,c,e,f,g) or protein supernatant (d). (b,c,e,f,g) Comparison of T cell polarization of normal dermal DCs (left panels) with psoriatic dermal DCs (right panels). (a) Controls: T cells alone (left panel) demonstrated baseline intracellular cytokine expression and T cells + beads (right panel) measured intracellular cytokine expressionfollowing CD3/CD28 bead stimulation. (b) Bulk émigrés w/o responder T cells measured baseline T cell cytokine production in single cell suspensions. Other stimulator populations mixed with responder T cells were (c) unsorted bulk émigrés, (e) sorted HLA-DR+CD11c+BDCA-1+ dermal DCs, (f) sorted HLA-DR+CD11c+BDCA-1− dermal DCs, or (g) sorted CD163+ macrophages. Psoriatic bulk dermal DCs induced a population of IL-17/IFNγ producing T cells, as did BDCA-1+ and BDCA-1− DCs (although to a lesser degree, possibly due to sorting). Quadrant percentages are given, representative graphs from 3 experiments. (d) Supernatant of the same cultures was collected for analysis of cytokine protein expression Bulk psoriatic dermal émigrés mixed with allogeneic T cells induced IL-17 and IFN-γ production, while normal dermal DCs did not.
There were few T cells positive for IL-17 or IFNγ when the T cells were cultured alone (Figure 8a, left), with marked increases when cells were stimulated with CD3/28 beads (Figure 8a, right). Culture of the dermal single cell suspensions alone, without addition of the allogeneic T cells, gave similar results to T cells alone, indicating the capability of the syngeneic T cells in the suspension (Figure 8b). There was marked increase in the intracellular cytokine staining when the dermal DCs were cultured with allogeneic T cells (Figure 8c), much more so for the psoriasis lesions. We particularly noted the increase of the IL-17+IFNγ+ cells induced by the psoriatic DCs, from 0.2% to 12.1%. The psoriatic dermal single cell suspensions also induced more IL-17 and IFNγ protein than normal skin (Figure 8d). This was similar to controls of T cells alone, and T cells stimulated with CD3/CD28 (data not shown).
The psoriatic BDCA-1+ and BDCA-1− populations were able to induce similar percentages of these IL-17+IFNγ+ cells, although less than the bulk dermal single cell suspensions (Figure 8e, 8f, right). This reduction is likely due to the process of FACS-sorting, as sorting reduces the ability of the DCs to polarize T cells by an average of 6-fold (analysis of the ability of bulk single cell suspensions before and after sorting, data not shown). Also, it was technically difficult to titrate an exact DC:T cell ratio in bulk émigrés making it difficult to compare results with pure sorted cells. The BDCA-1+ DCs from normal skin did not induce IL-17+ IFNγ+ cells (Figure 8e, left), nor did the normal or psoriatic skin macrophages (Figure 8g).
In summary, normal skin dermal émigrés polarized few Th17 cells, none of which were IL-17 and IFNγ producing, while psoriatic bulk émigrés and dermal DCs (BDCA-1+ and BDCA-1−) polarized Th17 cells producing both IL-17 and IFNγ. CD163+ macrophages from either normal or psoriatic skin were not able to polarize T cells to produce IL-17.
Discussion
In psoriasis as well as normal skin, myeloid DCs identified by BDCA-1 were present, and their numbers remained the same during a course of etanercept therapy. These resident BDCA-1+ DCs were often in clumps in the dermis, and were positive for mature markers such as DC-LAMP and DEC-205. In psoriasis, there was a marked increase in CD11c+ cells currently best identified as BDCA-1−, and we have termed these BDCA-1− dermal cells “inflammatory” myeloid DCs. This group of inflammatory cells may be heterogeneous, including the Tip-DCs, IL-20-producing DCs (Wang et al., 2006), and IL-23-producing DCs (Lee et al., 2004; Zaba et al., 2007a). The success of anti-TNF therapies to reduce all these cytokines and mediators and reverse the psoriatic phenotype supports the potential pathogenic role of these DCs (Zaba et al., 2007a). A similar finding was recently described in normal and diseased kidney, with a stable number of BDCA-1+ cells but increased DC-SIGN+ cells during inflammation (Woltman et al., 2007).
We have interpreted our in situ data to indicate that there is a distinction between BDCA-1+ and BDCA-1− myeloid DCs. However, there was little difference in the function of these two FACS-sorted populations ex vivo, as both BDCA-1+ and BDCA-1− DCs were immunostimulatory in the allo-MLR and induced Th1, Th17 and a mixed Th1/Th17 cell type. We interpret these findings as confirmation of their DC function, and an indication that the CD11c+BDCA-1− cells are not monocytes or macrophages (which cannot stimulate in these tests). Their ability to perform in these assays indicates their antigen-presenting potential, rather than being a true characterization of their role in situ during inflammation. This lack of difference in their function is perhaps surprising: we had expected the BDCA-1− population to be less stimulatory. The similar function of these two populations may be due to maturation of the BDCA-1− cells during migration out of the dermis, demonstrated by acquisition of DC-LAMP by a proportion of BDCA-1− cells in the single cell suspensions, with concomitant up-regulation of DC-defining functions. Thus, although there are several limitations to this system, it is currently the only method available to study the potential functions of these cells. Another possibility for the similar capability of the BDCA-1+ versus the BDCA-1− populations is that the CD11c+BDCA-1− FACS-sorted cells contain a small number of immunostimulatory BDCA-3+ cells. However, there are too few BDCA-3+ cells to separate for functional studies, and in future sorting strategies, we will exclude these cells.
There are several possible mechanisms to explain an increase in inflammatory myeloid DCs in psoriasis lesional skin, including arising from in situ DCs in non-lesional skin, from circulating DC-precursors or monocytes. These cells may arise from BDCA-1+ resident myeloid DCs that down-regulate their BDCA-1 surface expression, or even Langerhans cells. This is supported by the xenotransplant psoriasis model, where human transplanted non-lesional skin from psoriasis patients is grafted onto immunocompetent mice and the grafts become psoriatic without further intervention (Nestle et al., 2005). In this model, resident cells in the non-lesional skin are sufficient and capable of inducing the psoriatic phenotype, without any contribution from circulating cells. However, lesional skin immunocytes may behave differently in this xenotransplant system and may take on unconventional roles.
Secondly, these inflammatory myeloid DCs may arise from peripheral blood DC precursors, including hematopoietic stem-cell precursors (HSC), multipotent progenitor (MPP), common myeloid precursor (CMP), circulating pre-DC (CD11c+ HLA-DRhi, CD16+), or monocytes (Massberg et al., 2007; Piccioli et al., 2007; Randolph et al., 2002; Serbina et al., 2003; Tacke and Randolph, 2006). It is possible that any of these precursor cells are “pre-inflammatory DCs”, migrating into the skin in response to a chemokine gradient or other stimulus, supported by our observation of a reduction of some circulating DC subsets in psoriasis blood compared to normal. The concept that the inflammatory DCs arise from circulating precursors rather than in situ DCs is also consistent with other murine models demonstrating that during steady state, Langerhans cells and dermal DCs are able to locally proliferate, but during active inflammation there is migration of peripheral blood DC precursors into the skin (Bogunovic et al., 2006; Ledgerwood et al., 2007; Liu et al., 2007; Massberg et al., 2007).
CD11c+BDCA-1− dermal DCs retain some phenotypic features of their likely peripheral blood precursors, including smaller size, low-level expression of CD14, and CD163 – supporting the concept that these cells migrate into the skin from the blood. CD163hi cells, however, when sorted from psoriasis dermis are not immunostimulatory, thus they can be distinguished functionally from the immunostimulatory CD11c+BDCA-1− DC population. Other phenotypic markers that have been attributed to both inflammatory DCs and inflammatory macrophages include CD68 (Wang et al., 2006), CD32 (Dhodapkar et al., 2007), and DC-SIGN/CD209 (Granelli-Piperno et al., 2005). The co-localization of some of these antigens may also be due to plasticity between these immature DC and macrophage populations within the tissue during inflammation.
In addition to characterization of psoriatic myeloid dermal DCs, this paper presents evidence for Th17 polarization by psoriatic DCs. Both psoriatic CD11c+BDCA-1+ resident DCs and CD11c+BDCA-1− DCs had the capacity to polarize Th17 cells, although most polarization was induced by bulk psoriatic dermal cells not damaged by sorting. Moreover, bulk psoriatic émigrés induced Th17 cells producing both IL-17 and IFNγ compared to normal skin. Since psoriasis is now thought of as a mixed Th17/Th1 disease with strong IL-17 and IFNγ signatures (Blauvelt, 2007; Ghoreschi et al., 2007; Lowes et al., 2007), it is possible that these IL-17/IFNγ positive T cells induced by psoriatic DCs are pathogenic. However, Th17 cell induction could also be due to an allo-response, so future studies are planned to evaluate autologous effects of these DCs, and also to study other skin diseases to assess psoriasis-specific effects.
In summary, we have demonstrated a marked increase in CD11c+BDCA-1− myeloid DCs in psoriasis, and this group of inflammatory DCs contains Tip-DCs. These cells are immunostimulatory and capable of Th17 polarization, but their most essential contribution may be to secrete inflammatory products such as iNOS, TNF, IL-20, and IL-23. We hypothesize that the resident BDCA-1+ are likely the myeloid DCs capable of classic antigen-presentation to cutaneous T cells, although their dermal location and organization into clumps associated with T cells suggests this function may occur within the skin environment (“ectopic lymphoid tissue”) rather than in an extracutaneous lymphoid organ such as a lymph node (Lew et al., 2004). In contrast, while BDCA-1− DCs are certainly capable of robust antigen presentation in an allo-MLR and have the ability to polarize T cells (DC-defining characteristics), their main role may actually be as an inflammatory mediator production house, amplifying and maintaining psoriatic inflammation. Further studies need to be performed to prove this, dependant on new tools to study DCs in situ. We also need new markers to identify these inflammatory DCs in a positive manner, rather than as a negative population. We need to understand how and where these non-resident CD11c+BDCA-1− DCs are generated, in order to be able to specifically shut down their production of pro-inflammatory mediators, and bring about rapid resolution of clinical disease.
Materials & Methods
Skin Samples
Skin punch biopsies (6 mm diameter) were obtained from normal volunteers and psoriasis patients under a Rockefeller University IRB-approved protocol. Informed consent was obtained and the study was performed in adherence with the Declaration of Helsinki Principles. Lesional and non-lesional samples used for immunohistochemistry and immunofluorescence were from patients enrolled in an etanercept clinical trial as previously described (Zaba et al., 2007a). Biopsies were frozen in OTC (Sakura) and stored at −80°C until required. Dermal single cells suspensions from normal human skin were obtained from waste abdominoplasty skin as previously described (Zaba et al., 2007b). Dermal single cell suspensions from psoriasis shave biopsies were obtained following overnight incubation in dispase (Invitrogen Life Technologies) 1mg/mL at 4°C, to separate the epidermis. The dermis was cultured for 36–48h at 37°C in RPMI 1640 (Gibco-BRL Life Technologies) supplemented with 10% pooled human serum (Mediatech Inc.), 0.1% gentamicin reagent solution (Gibco-BRL Life Technologies), and 1% 1M Hepes buffer (Sigma) (Lowes et al., 2005).
Antibodies
All antibodies used for immunohistochemistry, immunofluorescence, and FACS are listed in Supplementary Table 1 a and b.
Immunohistochemistry
Skin sections were stained as previously described (Zaba et al., 2007b). Positive cells per mm were counted manually using computer-assisted image analysis (NIH IMAGE 6.1).
Immunofluorescence
Skin sections were stained as previously described (Zaba et al., 2007b). Images were acquired using appropriate filters of a Zeiss Axioplan 2I microscope with Plan Apochromat 20 × 0.7 numerical aperture lens and a Hagamatsu orca ER-cooled charge-coupled device camera, controlled by METAVUE software (Universal Imaging). Images in each figure are presented both as single color stains (green and red) located above the merged image, so that one can appreciate the localization of two markers on similar or different cells. Cells that co-express the two markers in a similar location are yellow in color. A white line denotes the dermo-epidermal junction. Dermal collagen fibers gave green autofluorescence, and antibodies conjugated with a fluorochrome often gave background epidermal fluorescence.
FACS
Cells from dermal cell suspensions or from whole blood were stained with the antibodies listed in Supplementary Table 1b. Briefly, cells were stained for 20 min. at 4°C, whole blood samples were lysed with FACSlyse (BD Biosciences) for 20 min., all samples were washed with FACSwash (PBS 0.1% sodium azide and 2% FBS) (BD Biosciences) and re-suspended in 1.3% formaldehyde (Fisher Scientific) in FACSwash. For intracellular cytokine staining assays, cells were incubated in aqua marina live/dead dye (BD Biosciences) for 30 min. on ice, washed, fixed with 4% paraformaldehyde (BD Biosciences) for 20 min. on ice, blocked in 1:100 mouse serum (BD Biosciences), permeabilized in FACSPerm (BD Biosciensces), incubated simultaneously with intracellular cytokine and extracellular antibodies, washed, and collected. All samples were acquired using an LSR-II (BD Biosciences) and analyzed with FlowJo (Treestar). Appropriate isotype controls were used.
FACS sort and Mixed Leukocyte Reaction (MLR)
This was performed as previously described (Zaba et al., 2007b), n=3. Dermal cells from single cell suspensions of psoriatic lesional skin were stained with CD3, CD45, CD11c, HLA-DR, BDCA-1, and CD163 antibodies (Supplementary Table 1b), and sorted on a FACSAria (BD Biosciences) using a low-pressure setting. Three populations were obtained: CD163+macrophages, CD3−CD45+CD11c+HLA-DR+BDCA-1+DCs (“resident myeloid dermal DCs”), and CD3−CD45+CD11c+HLA-DR+BDCA-1− DCs (“inflammatory myeloid dermal DCs”). Responding T cells were obtained from a normal volunteer by density centrifugation over Ficoll-Paque Plus (Amersham Biosciences), followed by T cell purification using a T cell negative selection kit (Dynal). Sorted stimulator populations were co-cultured with 10μm carboxy fluoroscein succinimidyl ester (CFSE) labeled T cells at a 1:50 ratio and T cell proliferation was analyzed on day 8 post-sort. Proliferation assays were harvested, stained with 250 ng/ml propidium iodide to label dead cells, and CD3-APC for 15 min. at room temperature. Propidium iodide negative cells were gated and then plotted as CFSE versus CD3+ cells, where proliferating cells diluted their content of CFSE and move to the left of the non-proliferating cells. The CFSE low cells were quantified as a percentage of live cells in the culture. CFSE-labeled T cells alone were used as a negative control, and co-culture with monocyte-derived mature DCs were used as a positive control. The process for making mature DCs has been previously described (Lee et al., 2002).
T cell polarization assays
Stimulator DC populations were prepared as above for the MLR (n=3 for psoriasis dermis, n=2 for normal dermis). Unlabeled bead-separated (Dynal) T cells were used as responding cells. Stimulator and responder cells were cultured at a ratio of 1:10. for 9 days. Cells were then re-suspended in RPMI media and activated for 4 hours using 25 ng/ml phorbol myristate acetate (PMA) and 2 μg/ml ionomycin, in the presence of 10 μg/ml brefeldin A (all Sigma Aldrich) at 37°C. Unactivated controls were treated with brefeldin A only. Ethylenediaminetetraacetic acid (2 mM, Fisher Scientific) was added for 10 min. at 37°C to stop activation. T cell intracellular cytokine expression was analyzed using the following antibodies: CD3, CD4, CD8, IFNγ, IL-17, and aqua live-dead stain. T cells alone were used as a negative control, and T cells stimulated with CD3/28 beads (Dynal:1/2 bead per T cell) were used as a positive control. Before activation, T cell polarization assay plates were centrifuged and the supernatant collected for measurement of cytokine protein concentration using the human cytokine 25-plex kit (Invitrogen) on a luminex CS1000 autoplex analyzer (Luminex corporation). The assay was performed in duplicate according to the manufacturer’s instruction, and results were averaged.
Statistical analysis
Two-tail paired Students t-test was used to compare normal versus psoriasis non-lesional and lesional cell counts for BDCA-1+, BDCA-3+, and CD11c+BDCA-1-BDCA-3− DC populations. The two-tail p-values are designated as p<0.05 (*), p<0.01 (**), and p<0.001 (***).
Supplementary Material
Acknowledgments
Research was supported by NIH grants R01 AI-49572, AI-49832, and UL1 RR024143, and AI40045 (RMS). LZ is supported by NIH MSTP grant GM07739, KP is supported by the Dana Foundation (Human Immunology Consortium Grant), and MAL is supported by 1 K23 AR052404-01A1. We thank Patricia Gilleaudeau and Mary Whalen-Sullivan for excellent care of our patients, and plastic surgeons Drs. AN LaBruna and DM Senderoff for their generous donation of abdominoplasty surgical waste. We also appreciate the assistance and advice of the Flow Cytometry Core Facility (Dr S. Mazel) and Bio-imaging Resource Center (Dr A. North) at Rockefeller University.
Abbreviations
- BDCA
blood dendritic cell antigen
- DC-LAMP
dendritic cell-lysosomal-associated membrane glycoprotein
- allo-MLR
allogeneic mixed leukocyte reaction
- DC-SIGN
dendritic cell-specific ICAM-3-grabbing non-integrin
- DC-LAMP
dendritic cell - lysosomal-membrane associated protein
- MFI
median fluorescence intensity
- DC
dendritic cell
- PMA
phorbol myristate acetate
- Tip-DC
TNF-and-iNOS producing dendritic cell
- iNOS
inducible nitric oxide synthase
Footnotes
Conflict of Interest
The authors do not have any financial interest related to this work.
References
- Abrams JR, Lebwohl MG, Guzzo CA, Jegasothy BV, Goldfarb MT, Goffe BS, et al. CTLA4Ig-mediated blockade of T-cell costimulation in patients with psoriasis vulgaris. J Clin Invest. 1999;103:1243–1252. doi: 10.1172/JCI5857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barral DC, Brenner MB. CD1 antigen presentation: how it works. Nat Rev Immunol. 2007;7:929–941. doi: 10.1038/nri2191. [DOI] [PubMed] [Google Scholar]
- Blauvelt A. New concepts in the pathogenesis and treatment of psoriasis: key roles for IL-23, IL-17A and TGF-beta1. Expert Rev Dermatol. 2007;2:69–78. [Google Scholar]
- Bogunovic M, Ginhoux F, Wagers A, Loubeau M, Isola LM, Lubrano L, et al. Identification of a radio-resistant and cycling dermal dendritic cell population in mice and men. J Exp Med. 2006;203:2627–2638. doi: 10.1084/jem.20060667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhodapkar KM, Banerjee D, Connolly J, Kukreja A, Matayeva E, Veri MC, et al. Selective blockade of the inhibitory Fcgamma receptor (FcgammaRIIB) in human dendritic cells and monocytes induces a type I interferon response program. J Exp Med. 2007;204:1359–1369. doi: 10.1084/jem.20062545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghoreschi K, Weigert C, Rocken M. Immunopathogenesis and role of T cells in psoriasis. Clin Dermatol. 2007;25:574–580. doi: 10.1016/j.clindermatol.2007.08.012. [DOI] [PubMed] [Google Scholar]
- Gottlieb AB, Chamian F, Masud S, Cardinale I, Abello MV, Lowes MA, et al. TNF inhibition rapidly down-regulates multiple proinflammatory pathways in psoriasis plaques. J Immunol. 2005;175:2721–2729. doi: 10.4049/jimmunol.175.4.2721. [DOI] [PubMed] [Google Scholar]
- Granelli-Piperno A, Pritsker A, Pack M, Shimeliovich I, Arrighi JF, Park CG, et al. Dendritic cell-specific intercellular adhesion molecule 3-grabbing nonintegrin/CD209 is abundant on macrophages in the normal human lymph node and is not required for dendritic cell stimulation of the mixed leukocyte reaction. J Immunol. 2005;175:4265–4273. doi: 10.4049/jimmunol.175.7.4265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledgerwood LG, Lal G, Zhang N, Garin A, Esses SJ, Ginhoux F, et al. The sphingosine 1-phosphate receptor 1 causes tissue retention by inhibiting the entry of peripheral tissue T lymphocytes into afferent lymphatics. Nat Immunol. 2007 doi: 10.1038/ni1534. [DOI] [PubMed] [Google Scholar]
- Lee AW, Truong T, Bickham K, Fonteneau JF, Larsson M, Da Silva I, et al. A clinical grade cocktail of cytokines and PGE2 results in uniform maturation of human monocyte-derived dendritic cells: implications for immunotherapy. Vaccine. 2002;20(Suppl 4):A8–A22. doi: 10.1016/s0264-410x(02)00382-1. [DOI] [PubMed] [Google Scholar]
- Lee E, Trepicchio WL, Oestreicher JL, Pittman D, Wang F, Chamian F, et al. Increased Expression of Interleukin 23 p19 and p40 in Lesional Skin of Patients with Psoriasis Vulgaris. J Exp Med. 2004;199:125–130. doi: 10.1084/jem.20030451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lew W, Bowcock AM, Krueger JG. Psoriasis vulgaris: cutaneous lymphoid tissue supports T-cell activation and ‘Type 1’ inflammatory gene expression. Trends Immunol. 2004;25:295–305. doi: 10.1016/j.it.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Liang SC, Tan XY, Luxenberg DP, Karim R, Dunussi-Joannopoulos K, Collins M, et al. Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. J Exp Med. 2006;203:2271–2279. doi: 10.1084/jem.20061308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu K, Waskow C, Liu X, Yao K, Hoh J, Nussenzweig M. Origin of dendritic cells in peripheral lymphoid organs of mice. Nat Immunol. 2007;8:578–583. doi: 10.1038/ni1462. [DOI] [PubMed] [Google Scholar]
- Lowes MA, Bowcock AM, Krueger JG. Pathogenesis and therapy of psoriasis. Nature. 2007;445:866–873. doi: 10.1038/nature05663. [DOI] [PubMed] [Google Scholar]
- Lowes MA, Chamian F, Abello MV, Fuentes-Duculan J, Lin SL, Nussbaum R, et al. Increase in TNF-alpha and inducible nitric oxide synthase-expressing dendritic cells in psoriasis and reduction with efalizumab (anti-CD11a) Proc Natl Acad Sci U S A. 2005;102:19057–19062. doi: 10.1073/pnas.0509736102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowes MA, Kikuchi T, Fuentes-Duculan J, Cardinale I, Zaba LC, Haider AS, et al. Psoriasis vulgaris lesions contain discrete Th1 and Th17 T cell populations. JID. 2008 doi: 10.1038/sj.jid.5701213. (In Press) [DOI] [PubMed] [Google Scholar]
- MacDonald KP, Munster DJ, Clark GJ, Dzionek A, Schmitz J, Hart DN. Characterization of human blood dendritic cell subsets. Blood. 2002;100:4512–4520. doi: 10.1182/blood-2001-11-0097. [DOI] [PubMed] [Google Scholar]
- Maniecki MB, Moller HJ, Moestrup SK, Moller BK. CD163 positive subsets of blood dendritic cells: the scavenging macrophage receptors CD163 and CD91 are coexpressed on human dendritic cells and monocytes. Immunobiology. 2006;211:407–417. doi: 10.1016/j.imbio.2006.05.019. [DOI] [PubMed] [Google Scholar]
- Massberg S, Schaerli P, Knezevic-Maramica I, Kollnberger M, Tubo N, Moseman EA, et al. Immunosurveillance by Hematopoietic Progenitor Cells Trafficking through Blood, Lymph, and Peripheral Tissues. Cell. 2007;131:994–1008. doi: 10.1016/j.cell.2007.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narbutt J, Lesiak A, Sysa-Jedrzejowska A, Smolewski P, Robak T, Zalewska A. The number and distribution of blood dendritic cells in the epidermis and dermis of healthy human subjects. Folia Histochem Cytobiol. 2006;44:61–63. [PubMed] [Google Scholar]
- Nestle FO, Conrad C, Tun-Kyi A, Homey B, Gombert M, Boyman O, et al. Plasmacytoid predendritic cells initiate psoriasis through interferon-alpha production. J Exp Med. 2005;202:135–143. doi: 10.1084/jem.20050500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccioli D, Tavarini S, Borgogni E, Steri V, Nuti S, Sammicheli C, et al. Functional specialization of human circulating CD16 and CD1c myeloid dendritic-cell subsets. Blood. 2007;109:5371–5379. doi: 10.1182/blood-2006-08-038422. [DOI] [PubMed] [Google Scholar]
- Randolph GJ, Sanchez-Schmitz G, Liebman RM, Schakel K. The CD16(+) (FcgammaRIII(+)) subset of human monocytes preferentially becomes migratory dendritic cells in a model tissue setting. J Exp Med. 2002;196:517–527. doi: 10.1084/jem.20011608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serbina NV, Salazar-Mather TP, Biron CA, Kuziel WA, Pamer EG. TNF/iNOS-producing dendritic cells mediate innate immune defense against bacterial infection. Immunity. 2003;19:59–70. doi: 10.1016/s1074-7613(03)00171-7. [DOI] [PubMed] [Google Scholar]
- Tacke F, Randolph GJ. Migratory fate and differentiation of blood monocyte subsets. Immunobiology. 2006;211:609–618. doi: 10.1016/j.imbio.2006.05.025. [DOI] [PubMed] [Google Scholar]
- Tam MA, Wick MJ. Dendritic cells and immunity to Listeria: TipDCs are a new recruit. Trends Immunol. 2004;25:335–339. doi: 10.1016/j.it.2004.05.004. [DOI] [PubMed] [Google Scholar]
- Wang F, Lee E, Lowes MA, Haider AS, Fuentes-Duculan J, Abello MV, et al. Prominent production of IL-20 by CD68+/CD11c+ myeloid-derived cells in psoriasis: Gene regulation and cellular effects. J Invest Dermatol. 2006;126:1590–1599. doi: 10.1038/sj.jid.5700310. [DOI] [PubMed] [Google Scholar]
- Weaver CT, Hatton RD, Mangan PR, Harrington LE. IL-17 family cytokines and the expanding diversity of effector T cell lineages. Annu Rev Immunol. 2007;25:821–852. doi: 10.1146/annurev.immunol.25.022106.141557. [DOI] [PubMed] [Google Scholar]
- Woltman AM, de Fijter JW, Zuidwijk K, Vlug AG, Bajema IM, van der Kooij SW, et al. Quantification of dendritic cell subsets in human renal tissue under normal and pathological conditions. Kidney Int. 2007;71:1001–1008. doi: 10.1038/sj.ki.5002187. [DOI] [PubMed] [Google Scholar]
- Zaba LC, Cardinale I, Gilleaudeau P, Sullivan-Whalen M, Suarez Farinas M, Fuentes-Duculan J, et al. Amelioration of epidermal hyperplasia by TNF inhibition is associated with reduced Th17 responses. J Exp Med. 2007a doi: 10.1084/jem.20071094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaba LC, Fuentes-Duculan J, Steinman RM, Krueger JG, Lowes MA. Normal human dermis contains distinct populations of CD11cBDCA-1 dendritic cells and CD163FXIIIA macrophages. J Clin Invest. 2007b;117:2517–2525. doi: 10.1172/JCI32282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y, Danilenko DM, Valdez P, Kasman I, Eastham-Anderson J, Wu J, et al. Interleukin-22, a T(H)17 cytokine, mediates IL-23-induced dermal inflammation and acanthosis. Nature. 2007;445:648–651. doi: 10.1038/nature05505. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.