Abstract
Mutations in cardiac troponin T (TnT) are a cause of familial hypertrophic cardiomyopathy (FHC). Transgenic mice expressing a missense mutation (R92Q) or a splice site donor mutation (Trunc) in the cardiac TnT gene have mutation-specific phenotypes but mice of both models have smaller hearts compared to wild type and exhibit hemodynamic dysfunction. Because growth-related signaling pathways in the hearts of mice expressing TnT mutations are not known, we evaluated the impact of increased Akt or glycogen synthase kinase-3β (GSK-3β) activity in both mutant TnT mice; molecules that increase heart size via physiologic pathways and block pathologic growth, respectively. Expression of activated Akt dramatically augments heart size in both R92Q and Trunc mice; however, this increase in heart size is not beneficial, since Akt also increases fibrosis in both TnT mutants and causes some pathologic gene expression shifts in the R92Q mice. Activated GSK-3β results in further decreases in left ventricular size in both R92Q and Trunc hearts, but this decrease is associated with significant mutation-specific phenotypes. Among many pathologic consequences, activating GSK-3β in R92Q hearts decreases phosphorylation of troponin I and results in early mortality. In contrast, increased GSK-3β activity in Trunc hearts does not significantly impact cardiac phenotypes. These findings demonstrate that increased Akt and its downstream target, GSK-3β can impact both cardiac size and phenotype in a mutation-specific manner. Moreover, increased activity of these molecules implicated in beneficial cardiac phenotypes exacerbates the progression of disease in the R92Q TnT mutant.
Keywords: Familial hypertrophic cardiomyopathy, troponin T, Akt, glycogen synthase kinase-3β, GSK-3β, cardiomyopathy
1. Introduction
Familial hypertrophic cardiomyopathy (FHC) is an autosomal dominant disease resulting from mutations in genes encoding cardiac myofilament proteins [1]. Cardiac troponin T (TnT) accounts for approximately 7% to 15% of FHC cases [1, 2]. While FHC patients with mutations in cardiac TnT are typically characterized by relatively mild or no hypertrophy and a high incidence of sudden death, patients can present with diverse clinical symptoms [3]. Recapitulating human disease causing mutations, transgenic mice with 7 different TnT mutations (R92Q, R92W, Δ160E, R287C, trunc, I79N and delta 16) do not exhibit hypertrophy and in fact, have similar or smaller hearts compared to wild type [4–9].
We selected two of these models in which to explore growth related signaling pathways [4, 5]. One transgenic mouse model expresses a truncated cardiac TnT protein with a carboxy-terminal truncation (called Trunc) mimicking the product of a splice donor-site mutation [4]. The hearts from these mice are smaller than wild-type mice and exhibit severe diastolic and milder systolic dysfunction, myocellular disarray, no fibrosis, and no induction of hypertrophic markers [4]. The second transgenic mouse line expressing a missense allele (R92Q) is similar to the Trunc mice with smaller left ventricles in males but not females [5]. They do, however, differ in that the R92Q hearts have fibrosis and induction of hypertrophic markers including atrial natriuretic factor (ANF) and β-myosin heavy chain (βMyHC) [5]. The R92Q hearts are hypercontractile with severe diastolic dysfunction [5] and they exhibit enhanced Ca2+ sensitivity [10].
Among the many myocellular-signaling pathways, the Akt/GSK-3β pathway is a crucial regulator of cardiac hypertrophy (For review, see Dorn and Force [11]). Among other things, activated Akt phosphorylates and inactivates its downstream target, glycogen synthase kinase-3β (GSK-3β), thereby promoting hypertrophic growth [12]. Increased activation of each molecule in a cardiac-specific manner results in opposite effects on cardiac size with Akt inducing massive cardiac enlargement while GSK-3β attenuates pathologic cardiac growth [13, 14]. The cardiac growth associated with activated Akt has been hypothesized to be beneficial, at least in the short term, and GSK-3β has been suggested as a potential therapeutic (for a review, see Shiojima and Walsh [15]).
While it is known that mutant sarcomeric proteins lead to FHC and specific signaling pathways determine cardiac size, it is not clear what signaling pathways are altered or how changes in the activity of signaling pathways modify the progression of the FHC. The questions we set out to ask are the following: 1. Will increasing heart size in these mutant TnT mice by Akt signaling prevent some of the defects cause by the mutant TnTs? 2. Will activation of GSK-3β further decrease heart size in the TnT mutants?
In this report, we used several transgenic mouse models to show that myristolated Akt (myrAkt) stimulates heart growth in both the R92Q and Trunc mice, and constitutive active GSK-3β (caGSK-3β) reduces cardiac size. However, this increase in cardiac size in the TnT mutants expressing myrAkt, was accompanied by induction of at least one hypertrophic marker gene and fibrosis. Furthermore, caGSK-3β worsened the cardiac phenotype and caused early mortality in R92Q mice with little impact on Trunc mice. These signaling molecules show mutation-specific phenotypes and the disease progression in the TnT mutants is not improved.
2. Materials and Methods
An expanded Materials and Methods section can be found in the online Supplemental Methods.
2.1. Transgenic Mice
The transgenic mice expressing a missense allele (R92Q) or a truncated cTnT protein missing the last coding exon (Trunc) were described previously [4, 5]. Transgenic mice expressing a cardiac-specific, myristolated form of Akt (myrAkt) or a cardiac-specific, constitutively active form of GSK-3β (caGSK-3β) were detailed previously [13, 14]. It is important to note that expression levels of Akt or GSK-3β and both mutant TnT proteins were equivalent indicating that the unique phenotypes were not due to differential expression of the transgenes (data not shown). All research involving the use of mice was performed in strict accordance to approved protocols by the institutional animal use and care committee (IACUC) at the University of Colorado.
2.2. Western Blot Analysis
Left ventricular tissue was prepared and homogenized in standard lysis buffer as previously described [16]. Total protein (50μg) was separated by SDS gel electrophoresis, transferred to poyvinylidene difuoride membranes, and detected by antibodies to anti-GSK-3β (Santa Cruz Biotechnology, anti-phospho-GSK-3β (Santa Cruz Biotechnology), anti-Akt (Cell Signaling), and anti-phospho-Akt (Cell Signaling). Subsequent densitometric analysis was performed and the relative intensities were measured and calculated.
2.3. Histological Analysis
For histological analysis, hearts were rapidly excised, rinsed in cold PBS and weighed. The whole heart was fixed in 10% phosphate-buffered formalin for 24 hours at 4°C and then placed into 70% ethanol until processed. Samples were processed, embedded in paraffin, sectioned, and stained with either H&E or Masson’s trichrome stain by Premier Histology (Boulder, Colorado). Sections were then analyzed by light microscopy. Fibrosis was evaluated by blinded observers and scored as a percentage of fibrosis in each microscopic area from at least 6–8 fields per free wall of the left ventricle.
2.4. RT-PCR Analysis
Expression of hypertrophic marker genes in transgenic mice were measured by RT-PCR (see online Supplemental Methods).
2.5. Echocardiography
Contractile function was evaluated by echocardiography. All measurements and readings were performed by a single operator (see online Supplemental Methods).
2.6. Phosphorylation of myofilament proteins
Phosphorylation levels of myofilament proteins and the phosphyorylation profile of cardiac TnI were performed as previously described (see supplemental methods) [17, 18].
2.7. Data and Statistical Analysis
Comparison of survival rates was performed by Kaplan-Meier analysis. Results are presented as mean ± SE. Statistical analysis of group differences was performed by one-way ANOVA. If significance was achieved (P < 0.05), Tukey’s post hoc comparison was performed.
3. Results
3.1. Active GSK-3β attenuates Akt-mediated cardiac growth
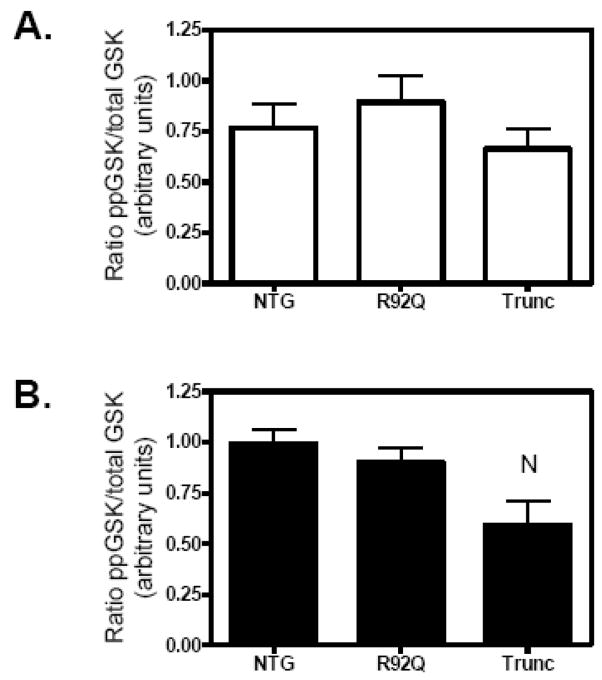
Previous investigations have demonstrated that Akt phosphorylates GSK-3β, rendering the kinase inactive and allowing for pro-hypertrophic signaling [12]. To determine whether alterations in phosphorylation of either Akt or GSK-3β were associated with the smaller hearts of the R92Q and Trunc mice, we evaluated the phosphorylation levels of both signaling molecules in the left ventricles of the R92Q and Trunc hearts. Since we have previously demonstrated that sex plays a major role in cardiac disease [19], we evaluated the cardiac phenotype of both males and females. We determined that phosphorylation of GSK-3β was significantly reduced in Trunc males and showed a trend in reduction in females; however, the reduction was not statistically significant (Fig. 1). No changes in Akt phosphorylation were detected (data not shown). This suggests that GSK-3β may be involved in the small heart phenotypes of the Trunc mice.
Fig. 1.
Decreased phosphorylation of gycogen synthase kinase-3β (GSK-3β) in Trunc males at 12 weeks of age. Western blot analysis was performed as described in the Material and Methods. Quantitation of phospho-GSK-3β to total GSK-3β levels in (A) females (open bar), and (B) males (solid bars). Values are means ± SE of 4–6 individual hearts. NP < 0.05 vs. NTG.
Given that there have been some incidences of a disconnection of a direct downsream effect on GSK by Akt, we directly tested this by crossing well-characterized genetically manipulated transgenic mouse models expressing either increased Akt or GSK-3β activity in a cardiac-specific manner. We found that the Akt/GSK-3β double transgenic mice exhibit a marked reduction in Akt-mediated left ventricular size (Fig. 1, supplemental data). Because these data indicate that Akt is operating through GSK-3β with respect to hypertrophy, we chose to investigate the impact of the Akt/GSK-3β signaling pathway on the cardiac phenotype of mice expressing different TnT mutations.
3.2. Cardiac phenotype of TnT mutant mice expressing increased Akt activity
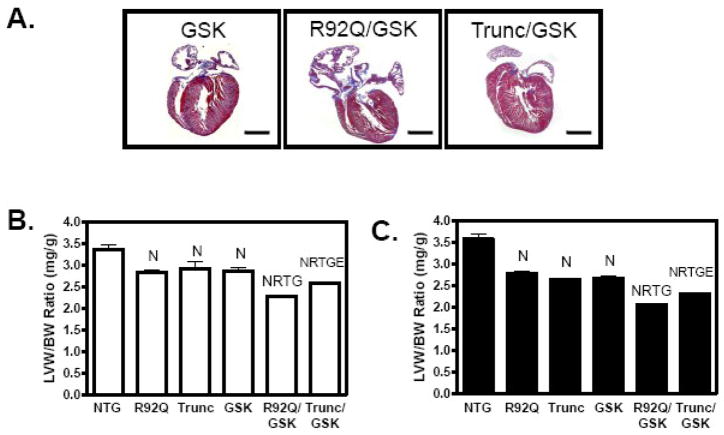
To assess whether increased Akt or GSK-3β activity would alter heart size and the progression of disease in TnT-mutant mice, transgenic mice expressing either myrAkt or caGSK-3β were crossbred into both TnT mutant transgenic mouse lines and studied at 12–14 weeks-of-age. As previously published, R92Q and Trunc mice of both sexes have smaller ventricle sizes and left ventricular weight-to-body weight (LV/BW) ratios compared to nontransgenic (NTG) mice (Figure 2 and supplemental Table 1) [4, 5]. Increased Akt activity (in the absence of TnT mutations) results in significantly larger LVs than NTG and shows no signs of pathology up to 10 months of age, as previously reported [14]. In double transgenic mice expressing either of the TnT mutations and myrAkt, LV size was enlarged in comparison to the parental TnT mutant, independent of sex (Figure 2A–C). It is important to note that even with augmented LV size in both double transgenics, LV size is significantly smaller in the doubly transgenic mice than in the Akt transgenic mice.
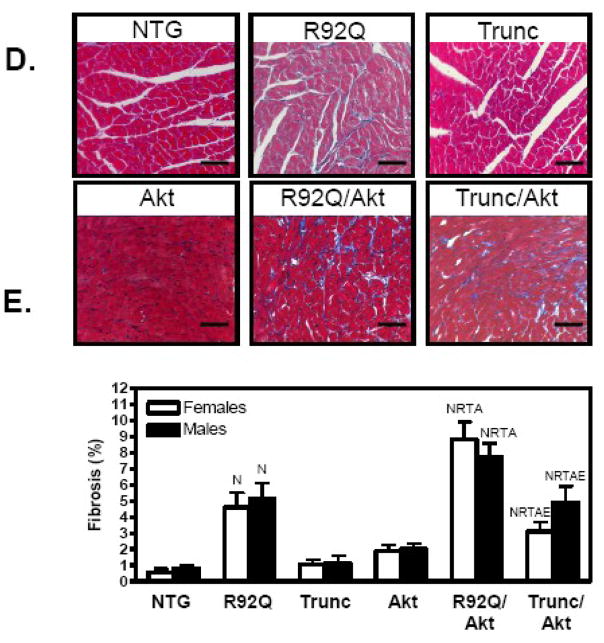
Fig. 2.
Elevated Akt activity increases LVW/BW in TnT mutant mice at 12 weeks of age. (A) Macroscopic histological sections and LVW/BW ratios revealed augmented ventricular size associated with increased activity of Akt in the hearts of TnT mutant (B) females and (C) males. The bar graph shows means ± SE of 5–14 individual hearts. (D) Microscopic histological analysis of male transgenic hearts indicates increased fibrosis in trichrome-stained sections of R92Q, R92Q/Akt, and Trunc/Akt hearts. (E) Quantification of fibrosis from trichrome-stained sections for females (open bars) and males (solid bars). Values are means ± SE of 4–6 individual hearts. (N Significantly different (P < 0.05) from NTG, R significantly different from R92Q, T significantly different from Trunc, A significantly different from Akt, E significantly different from R92Q/Akt).
Histological analysis indicates that the Akt transgenic hearts do not have significantly elevated levels of interstitial fibrosis (Figure 2D and 2E). However, there is increased interstitial fibrosis in the R92Q/Akt and Trunc/Akt mice compared to their respective parental lines (Figure 2D and 2E). It is well established that FHC is characterized by changes in gene expression of the hypertrophic-associated genes and therefore, relative mRNA levels of ANF, BNP, βMyHC, and SERCA were measured in this study. As previously determined, ANF and βMyHC mRNA levels were elevated in R92Q mice compared with age-matched NTG mice (Figure 3A and 3B). Trunc mice did not demonstrate significant changes in gene expression of the hypertrophic-associated genes (Figure 3A and 3B). In the Akt hearts, ANF and BNP mRNA levels are dramatically reduced in both sexes compared to NTG hearts (Figure 3A and 3B). In the doubly transgenic males and females of both genotypes, both ANF and BNP expression are also reduced compared to the parental lines. However, β-MyHC gene expression is dramatically elevated in both R92Q/Akt males and females compared to the R92Q transgenic mice (Figure 3A and 3B). It is important to note that there was no reduced survival in either Akt double transgenic mouse lines up to 8 months-of-age. Collectively, these data suggest that increased Akt activity significantly alters the cardiac phenoytpe of each TnT mutant and that despite larger heart sizes, this increase was not associated with an entirely beneficial profile.
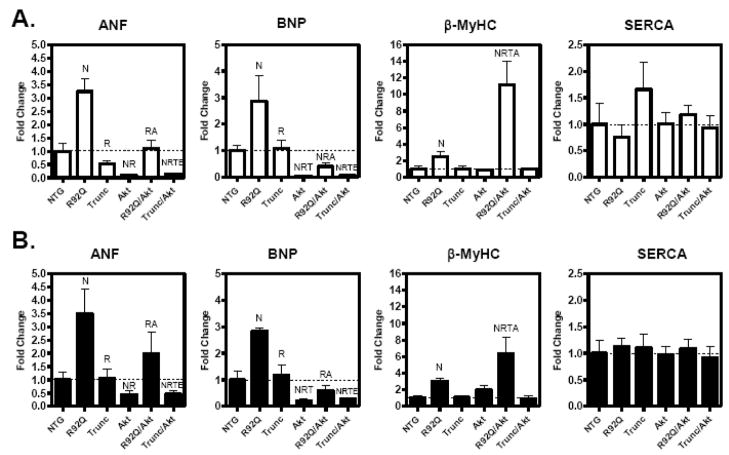
Fig. 3.
Expression of hypertrophic-associated genes is altered by increased Akt activity in 12-week-old TnT mutant mice. Quantitative analysis of hypertrophic-associated genes; atrial natriuretic peptide (ANF), β-myosin heavy chain (β-MyHC), sarcoplasmic recticulum Ca2+-ATPase (SERCA), and brain natriuretic peptide (BNP) from the left ventricle of female (A) and male (B) transgenic littermates. Expression of each respective gene was normalized to 18S and expressed as fold change versus NTG. The bar graph shows means ± SE of 5–7 individual hearts (N Significantly different (P < 0.05) from NTG; R significantly different from R92Q; T significantly different from Trunc, A significantly different from Akt, and E significantly different from R92Q/Akt).
3.3. Cardiac phenotype of TnT mutant mice expressing constitutively active GSK-3β
Examination of the offspring between the R92Q and GSK-3β mice revealed that LV mass and LV/BW ratios in the R92Q/GSK-3β males and females are significantly reduced compared to the R92Q parental lines (Figure 4 and supplemental Table 2). These doubly transgenic mice also have enlarged atria (Figure 4A). Trunc/GSK-3β males, but not females, have statistically smaller LV/BW ratios compared to the Trunc parental line. However, the impact of GSK3β is greater on the R92Q mutations since both R92Q/GSK-3β males and females have even smaller left ventricular sizes than the Trunc/GSK-3β mice.
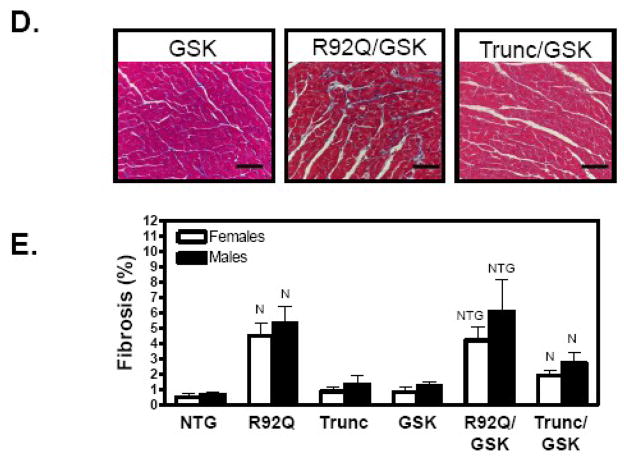
Fig. 4.
Active GSK-3β reduces LVW/BW but does not increase fibrosis in TnT mutant mice at 12–14 weeks of age. (A) Macroscopic histological sections and LVW/BW ratios revealed a reduction in ventricular size associated with expression of active GSK-3β in the hearts of TnT mutant (B) females and (C) males. The bar graph shows means ± SE of 10–21 individual hearts. (D) Microscopic histological analysis of male transgenic hearts indicates increased fibrosis in trichrome-stained sections R92Q/GSK mice. (E) Quantification of fibrosis from trichrome-stained sections for females (open bars) and males (solid bars). Values are means ± SE of 4–6 individual hearts. (N Significantly different (P < 0.05) from NTG; R significantly different from R92Q; T significantly different from Trunc, G significantly different from GSK-3β, and E significantly different from R92Q/GSK).
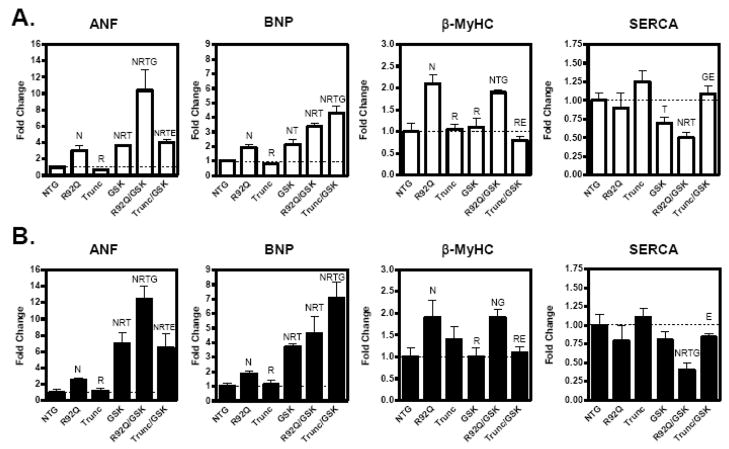
Previous investigations determined that R92Q hearts have significant levels of interstitial fibrosis, whereas, the Trunc hearts display no fibrosis [4, 5]. R92Q mice have an 8- and 10-fold increase in fibrosis compared to NTG males and females, respectively, as measured by trichrome staining (Figure 4D and 4E). Mice expressing caGSK-3β have no fibrosis (Figure 4D and 4E). Both doubly transgenic mouse lines have similar levels of fibrosis compared to their respective parental lines (Figure 4D and 4E). Evaluation of hypertrophic-associated genes indicated that R92Q/GSK-3β males and females have 5- and 3.5-fold increased ANF gene expression, respectively, while SERCA mRNA levels are reduced by approximately 50% in both sexes (Figure 5A and 5B). Interestingly, ANF and BNP expression is significantly elevated in both sexes of the Trunc/GSK-3β mice compared to the Trunc parental line (Figure 5A and 5B). Consistent with changes in the mRNA levels, protein levels of β-MyHC are increased in the R92Q and R92Q/GSK-3β hearts (Figure 5C). It is important to note that β-MyHC is elevated in the R92Q hearts and no additional expression was observed in the R92Q/GSK hearts. Overall, these data show that caGSK-3β more profoundly impacts the cardiac phenotype of the R92Q mice compared to the Trunc mice.
Fig. 5.
Expression of hypertrophic-associated genes is altered by expression of active GSK-3β in 12- to14-week-old TnT mutant mice. Quantitative analysis of hypertrophic-associated genes; atrial natriuretic peptide (ANF), β-myosin heavy chain (βMyHC), sarcoplasmic recticulum Ca2+-ATPase (SERCA), and brain natriuretic peptide (BNP) from the left ventricle of female (A) and male (B) transgenic littermates. Expression of each respective gene was normalized to 18S and expressed as fold change versus NTG. The bar graph shows means ± SE of 5–6 individual hearts. (E) Representative myosin heavy chain separation gel demonstrating increased βMyHC protein in R92Q and R92Q/GSK-3β hearts. (N Significantly different (P < 0.05) from NTG; R significantly different from R92Q; T significantly different from Trunc, G significantly different from GSK-3β, and E significantly different from R92Q/GSK).
3.4. Increased mortality, reduced fractional shortening, and decreased cTnI phosphorylation in R92Q mice expressing active GSK-3β
Both male and female R92Q/GSK-3β doubly transgenic animals have significantly increased mortality, with 50% survival at 9 and 11 weeks for males and females, respectively (Figure 6A). There is only ~20% survival at 18 weeks. Sex has no effect on survival. No differences in survival were observed in any of the littermates or offspring from Trunc/GSK-3β or TnT/Akt crosses. None of the R92Q/GSK-3β animals exhibit physical signs of congestive heart failure such as massive intraperitoneal edema, increased lung or liver weights (data not shown), respiratory distress, or loss of activity.
Fig. 6.
Active GSK-3β reduces the lifespan and contractile function in R92Q mice. (A) Kaplan-Meier survival curves for NTG, GSK, R92Q, Trunc (all four genotypes included in solid line), and R92Q/GSK males (long dash) and females (short dash) as a function of age. Doubly transgenic males and females have significantly increased mortality compared to other littermates (P < 0.001). (B) Active GSK-3β reduces percent fractional shortening in 12- to 14-week-old R92Q mice. Percent fractional shortening measurements in females and males (C). The bar graph shows means ± SE of 6–11 individual hearts (N Significantly different (P < 0.05) from NTG; R significantly different from R92Q; T significantly different from Trunc, G significantly different from GSK-3β, and E significantly different from R92Q/GSK).
To investigate whether caGSK-3β caused mutation-specific alterations in contractile function, echocardiography was performed on the TnT/GSK-3β males and females. Evaluation of cardiac dimensions demonstrated that percent fractional shortening is significantly reduced in both R92Q/GSK-3β females and males, indicating impairment of contractility (Figure 6B and 6C, respectively). It may be that the observed reduction in fractional shortening is underestimated as some mice died prior to echocardiography.
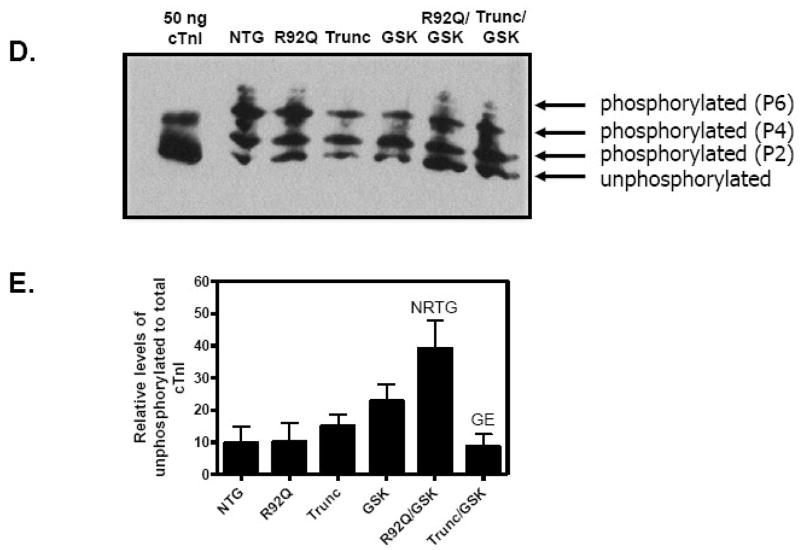
Previous studies demonstrated that the R92Q hearts have a greater increase in Ca2+ sensitivity compared to Trunc hearts [10]. Because Ca2+ sensitive contractility is modulated by phosphorylation of contractile proteins [20], we compared the phosphorylation levels of myofilament proteins in the left ventricles of male TnT/GSK-3β transgenic animals. Figure 7 shows the phosphorylation levels of myofilament proteins by Pro Q Diamond staining. These data indicate that there is a significant reduction in cTnI phosphorylation in the R92Q/GSK-3β LVs. To verify these results, we used a one-dimensional non-equilibrium isoelectric focusing method [18]. These results similarly demonstrate that the R92Q/GSK-3β mice have a greater proportion of unphosphorylated species and fewer phospho-species, indicative of decreased phosphorylation of cTnI (Figure 7D and 7E). To determine whether phosphorylation of serines 23 and 24 of cTnI was specifically reduced in the R92Q/GSK hearts, cTnI was probed with an anti-cTnI phospho-Ser 23/24 antibody and re-probed with a total cTnI antibody. Fig. 2 in the supplemental data demonstrate that phosphorylation levels are similar in all genotypes. Overall, these results suggest that expression of GSK-3β detrimentally influences functional, molecular, and biochemical changes in the R92Q hearts that lead to early mortality.
Fig. 7.
Active GSK-3β reduces troponin I (TnI) phosphorylation in R92Q mice. (A) Representative Pro Q Diamond stained myofilament proteins (MyBPC – myosin binding protein C; TnT – troponin T; tropomyosin; TnI – troponin I; LC2 – myosin light chain). (B) Coomassie blue staining of the representative gel in A. (C) Pro Q Diamond staining was normalized to CBB staining and average relative phosphorylation level of cTnI was quantitated (n = 6). (D) A representative immunoblot of the separation of cTnI phospho-species in left ventricular homogenates from 10–12 week old mice, probed with pan cTnI antibody. (E) Quantification of the levels of the unphosphorylated to phsophorylated of cTnI. (N Significantly different (P < 0.05) from NTG; R significantly different from R92Q; T significantly different from Trunc, G significantly different from GSK-3β, and E significantly different from R92Q/GSK).
4. Discussion
It is well recognized that the Akt-GSK-3β signaling pathway regulates cardiac size (reviewed in [21]). In the current study, we generated mice that co-express either increased GSK-3β or Akt activity and the R92Q or Trunc cTnT mutation. This allowed us to directly evaluate how these signaling molecules impact the cardiomyopathy in mice harboring cardiac TnT mutant proteins. We found that in both TnT mutants, Akt stimulated cardiac growth while GSK-3β reduced heart size. More importantly, we found the cardiac phenotype resulting from these signaling molecules to be dependent on the specific TnT mutation.
This is the first study to evaluate the impact of signaling molecules in conjunction with a TnT mutation. Earlier studies in these TnT mutant mice demonstrated that pharmacological administration of hypertrophic stimuli differentially affected the hypertrophic signaling and the occurrence of sudden death was sex modified [22]. They concluded that based on the primary TnT mutation, a worsened phenotype could result from superimposition of certain pathological stimuli. More specifically, they found that the Trunc mice had attenuated responses to many of the stimuli. With respect to the current studies, we initially hypothesized that GSK-3β would decrease LV size while Akt would augment left ventricular growth. However, we did not expect that increasing heart size through Akt in the TnT mice would be associated with an exacerbated cardiac phenotype.
Another interesting aspect of this study is that increased GSK-3β activity dramatically and more profoundly impacted the R92Q mice compared to the Trunc mice. In contrast, GSK-3β did not greatly alter the cardiomyopathy of the Trunc mice suggesting that heart size in TnT mutants alone is not a good prognostic indicator of cardiac disease progression. The increased susceptibility of the R92Q mice to this signaling pathway was also observed in the TnT mutants expressing myrAkt. β-MyHC gene expression was remarkably elevated in only the R92Q/Akt mice. Mouse hearts normally express exclusively α-MyHC and the switch from the fast α-to the slower β-isoform is a hallmark of pathologic cardiac hypertrophy [23]. There was no increased mortality in either Akt double transgenic mice (up to 8 months-of-age), and the functional impact of increased Akt activity in the TnT mutant hearts was not assessed.
In considering the distinct mutation-specific cardiac phenotypes, we must carefully consider the structural and functional differences resulting from the primary cardiac TnT mutation in the sarcomere. The R92Q missense mutation is a located at the tropomyosin (TM)/troponin interface [24]. It is likely that replacement of the strongly charged, polar residue (arginine) with a non-charged (neutral) amino acid (glutamine) may interfere with the association between tropomyosin and the troponin complex. The Trunc mutation is a deletion of a significant portion of the 3′ TnI-Troponin C-TM binding site [25]. At baseline, these two TnT-mutant models both demonstrate reduced ventricular mass, however, the cardiac phenotypes remain distinct with regard to systolic function, fibrosis, and gene expression [4, 5]. Subsequent studies have shown that R92Q fibers had greater sensitivity to Ca2+ at different sarcomere lengths and increased ATPase activity compared to fibers containing the Trunc mutation [10]. It was also reported that the R92Q mutation led to increased ATP utilization rates, higher than those of the NTG hearts, and impaired the ability of the heart to increase contractile performance [26]. Similar studies were not conducted on the Trunc mice. It may be that these differences play an important role in the different cardiac phenotypes observed in this study.
An interesting finding of this study is the observation that a mutation in one thin filament protein affects specific posttranslational modifications on another thin filament protein. Specifically, cardiac TnI phosphorylation is reduced in the R92Q/GSK-3β hearts and no changes in phosphorylation of cTnI were observed in the Trunc/GSK-3β mice, or other littermate genotypes.
Phosphorylation of thin filament proteins is an important short- and long-term regulatory event that directly impacts normal and diseased cardiac function [20, 27, 28]. Studies have demonstrated that at least five sites within cTnI can be phosphorylated, including Ser23, Ser24, Ser43, Ser45, and Thr144 [29–31]. Phosphorylation at these sites can influence myofilament force development, Ca2+ sensitivity, and contractility. A number of kinases, including protein kinase A and protein kinase C, phosphorylate TnI [20]. Type 2A phoshatase (PP2A) can dephosphorylate cardiac TnI and studies have demonstrated that p38 mitogen-activated protein kinase and p21-activated-kinase-1 regulate PP2A activity at the myofilament [32, 33]. Our preliminary studies suggest that GSK-3β does not associate tightly with myofilaments (data not shown) and it is unclear what the mechanism is that reduces cTnI phosphorylation in the hearts of the R92Q/GSK-3β mice. This question is relevant because there are conflicting and opposing data in the literature regarding cTnI phosphorylation levels and the impact of cTnI phosphorylation on cardiac function. Several investigations have determined that phosphorylation of cTnI contributes to cardiac dysfunction and heart failure [18, 34, 35]. On the other hand, several groups have also reported a reduction in cTnI phosphorylation in human and experimental heart failure [36–39]. We are attempting to determine what phosphorylation sites on cTnI were dephosphorylated, what the functional impact is of reduced cTnI phosphorylation, and how cTnI is dephosphorylated.
Lastly, a major unanswered question pertains to the increased mortality of the R92Q/GSK-3β mice. It is most likely that the animals die of sudden cardiac death as there were no signs of overt heart failure prior to death, nor found immediately after death (i.e. no changes in lung weight, no pleural edema, etc). It is hypothesized that sudden cardiac death results from a cardiac arrhythmia, which is most likely caused by an electrically unstable heart manifested by a confluence of structural and functional changes in the heart [40]. Future studies aimed at the elucidation of the mechanism of the decreased mortality of the R92Q/GSK-3β mice will help further our understanding of the signaling defects manifested in these animals.
Because we have observed decreased cTnI phosphorylation levels in the R92Q/GSK-3β hearts, we might expect that these hearts have increased Ca2+ responsiveness. This would result in greater myofilament activation in response to normal intracellular Ca2+ concentrations. Although increased sensitization is thought to ultimately elevate cardiac output by enhancing cardiac contractility [41], this would be detrimental for the diastolic phase of the cardiac cycle by slowing the relaxation rate. Moreover, humans with the cardiac TnT F110I mutation have an extremely poor clinical diagnosis [42]. Mice expressing this mutation have increased Ca2+ sensitivity and higher energy cost which investigators believe may be responsible for the severe phenotype [8]. It is important to remember that the R92Q mice already have severe diastolic dysfunction, enhanced contractility, increased ratio of ATPase per force, and the R92Q/GSK-3β hearts have decreased SERCA levels and no differences in phosporylation of phospholambam (data not shown). Based on the previous investigations and the data in the current study, we hypothesize that concomitant expression of active GSK-3β and the R92Q mutation further increases Ca2+ sensitivity and energy cost, and coupled with Ca2+ dysregulation, a potentially lethal arrhythmic event could occur. Investigations are underway to test this hypothesis.
This is the first study to analyze the role of increased Akt or GSK-3β activity in mice with TnT mutations. These results are interesting and provide us with a better understanding into the pathophysiological differences between the R92Q and the Trunc mutations. First, our results show that growth-related signaling pathways such as Akt and GSK-3β can influence cardiac size of mice with TnT mutations and that both of these molecules in the setting of cTnT FHC were generally associated with poor phenotypes. Second, the observed changes in cardiac disease are mutation-specific. Most striking was the combination of either increased GSK-3β or Akt activity with the R92Q mutation. Third, sex was not a major modifying factor in cardiac disease in these models. Lastly, as we continue to gain an understanding of the cardiac disease mediated by these different TnT mutations, our ability to treat patients with TnT mutations will follow.
Supplementary Material
Acknowledgments
This work was supported by NIH grant to L.A. Leinwand (HL 56510) and by a National Research Service Awards from the NIH awarded to S.W. Luckey (F32 HL 72565). We are grateful to Ping Yue for the echocardiographic measurements. We also thank Gail Ackerman and Margaret Isenhart for care of the mice. Premier Histology (Boulder, CO) processed the histology sections and staining.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Richard P, Charron P, Carrier L, Ledeuil C, Cheav T, Pichereau C, et al. Hypertrophic cardiomyopathy: distribution of disease genes, spectrum of mutations, and implications for a molecular diagnosis strategy. Circulation. 2003;107(17):2227–32. doi: 10.1161/01.CIR.0000066323.15244.54. [DOI] [PubMed] [Google Scholar]
- 2.Bonne G, Carrier L, Richard P, Hainque B, Schwartz K. Familial hypertrophic cardiomyopathy: from mutations to functional defects. Circ Res. 1998;83(6):580–93. doi: 10.1161/01.res.83.6.580. [DOI] [PubMed] [Google Scholar]
- 3.Varnava AM, Elliott PM, Baboonian C, Davison F, Davies MJ, McKenna WJ. Hypertrophic cardiomyopathy: histopathological features of sudden death in cardiac troponin T disease. Circulation. 2001;104(12):1380–4. doi: 10.1161/hc3701.095952. [DOI] [PubMed] [Google Scholar]
- 4.Tardiff JC, Factor SM, Tompkins BD, Hewett TE, Palmer BM, Moore RL, et al. A truncated cardiac troponin T molecule in transgenic mice suggests multiple cellular mechanisms for familial hypertrophic cardiomyopathy. J Clin Invest. 1998;101(12):2800–11. doi: 10.1172/JCI2389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tardiff JC, Hewett TE, Palmer BM, Olsson C, Factor SM, Moore RL, et al. Cardiac troponin T mutations result in allele-specific phenotypes in a mouse model for hypertrophic cardiomyopathy. J Clin Invest. 1999;104(4):469–81. doi: 10.1172/JCI6067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ertz-Berger BR, He H, Dowell C, Factor SM, Haim TE, Nunez S, et al. Changes in the chemical and dynamic properties of cardiac troponin T cause discrete cardiomyopathies in transgenic mice. Proc Natl Acad Sci U S A. 2005;102(50):18219–24. doi: 10.1073/pnas.0509181102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sirenko SG, Potter JD, Knollmann BC. Differential effect of troponin T mutations on the inotropic responsiveness of mouse hearts--role of myofilament Ca2+ sensitivity increase. J Physiol. 2006;575(Pt 1):201–13. doi: 10.1113/jphysiol.2006.107557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hernandez OM, Szczesna-Cordary D, Knollmann BC, Miller T, Bell M, Zhao J, et al. F110I and R278C troponin T mutations that cause familial hypertrophic cardiomyopathy affect muscle contraction in transgenic mice and reconstituted human cardiac fibers. J Biol Chem. 2005;280(44):37183–94. doi: 10.1074/jbc.M508114200. [DOI] [PubMed] [Google Scholar]
- 9.Knollmann BC, Kirchhof P, Sirenko SG, Degen H, Greene AE, Schober T, et al. Familial hypertrophic cardiomyopathy-linked mutant troponin T causes stress-induced ventricular tachycardia and Ca2+-dependent action potential remodeling. Circ Res. 2003;92(4):428–36. doi: 10.1161/01.RES.0000059562.91384.1A. [DOI] [PubMed] [Google Scholar]
- 10.Montgomery DE, Tardiff JC, Chandra M. Cardiac troponin T mutations: correlation between the type of mutation and the nature of myofilament dysfunction in transgenic mice. J Physiol. 2001;536(Pt 2):583–92. doi: 10.1111/j.1469-7793.2001.0583c.xd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dorn GW, 2nd, Molkentin JD. Manipulating cardiac contractility in heart failure: data from mice and men. Circulation. 2004;109(2):150–8. doi: 10.1161/01.CIR.0000111581.15521.F5. [DOI] [PubMed] [Google Scholar]
- 12.Morisco C, Zebrowski D, Condorelli G, Tsichlis P, Vatner SF, Sadoshima J. The Akt-glycogen synthase kinase 3beta pathway regulates transcription of atrial natriuretic factor induced by beta-adrenergic receptor stimulation in cardiac myocytes. J Biol Chem. 2000;275(19):14466–75. doi: 10.1074/jbc.275.19.14466. [DOI] [PubMed] [Google Scholar]
- 13.Antos CL, McKinsey TA, Frey N, Kutschke W, McAnally J, Shelton JM, et al. Activated glycogen synthase-3 beta suppresses cardiac hypertrophy in vivo. Proc Natl Acad Sci U S A. 2002;99(2):907–12. doi: 10.1073/pnas.231619298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matsui T, Li L, Wu JC, Cook SA, Nagoshi T, Picard MH, et al. Phenotypic Spectrum Caused by Transgenic Overexpression of Activated Akt in the Heart. J Biol Chem. 2002;277(25):22896–22901. doi: 10.1074/jbc.M200347200. [DOI] [PubMed] [Google Scholar]
- 15.Shiojima I, Walsh K. Regulation of cardiac growth and coronary angiogenesis by the Akt/PKB signaling pathway. Genes Dev. 2006;20(24):3347–65. doi: 10.1101/gad.1492806. [DOI] [PubMed] [Google Scholar]
- 16.Luckey SW, Mansoori J, Fair K, Antos CL, Olson EN, Leinwand LA. Blocking cardiac growth in hypertrophic cardiomyopathy induces cardiac dysfunction and decreased survival only in males. Am J Physiol Heart Circ Physiol. 2007;292(2):H838–845. doi: 10.1152/ajpheart.00615.2006. [DOI] [PubMed] [Google Scholar]
- 17.Kobayashi T, Yang X, Walker LA, Van Breemen RB, Solaro RJ. A non-equilibrium isoelectric focusing method to determine states of phosphorylation of cardiac troponin I: identification of Ser-23 and Ser-24 as significant sites of phosphorylation by protein kinase C. J Mol Cell Cardiol. 2005;38(1):213–8. doi: 10.1016/j.yjmcc.2004.10.014. [DOI] [PubMed] [Google Scholar]
- 18.Scruggs SB, Walker LA, Lyu T, Geenen DL, Solaro RJ, Buttrick PM, et al. Partial replacement of cardiac troponin I with a non-phosphorylatable mutant at serines 43/45 attenuates the contractile dysfunction associated with PKCepsilon phosphorylation. J Mol Cell Cardiol. 2006;40(4):465–73. doi: 10.1016/j.yjmcc.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 19.Leinwand LA. Sex is a potent modifier of the cardiovascular system. J Clin Invest. 2003;112(3):302–7. doi: 10.1172/JCI19429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sumandea MP, Burkart EM, Kobayashi T, De Tombe PP, Solaro RJ. Molecular and integrated biology of thin filament protein phosphorylation in heart muscle. Ann N Y Acad Sci. 2004;1015:39–52. doi: 10.1196/annals.1302.004. [DOI] [PubMed] [Google Scholar]
- 21.Hill JA, Olson EN. Cardiac plasticity. N Engl J Med. 2008;358(13):1370–80. doi: 10.1056/NEJMra072139. [DOI] [PubMed] [Google Scholar]
- 22.Maass AH, Ikeda K, Oberdorf-Maass S, Maier SK, Leinwand LA. Hypertrophy, fibrosis, and sudden cardiac death in response to pathological stimuli in mice with mutations in cardiac troponin T. Circulation. 2004;110(15):2102–9. doi: 10.1161/01.CIR.0000144460.84795.E3. [DOI] [PubMed] [Google Scholar]
- 23.Izumo S, Nadal-Ginard B, Mahdavi V. Protooncogene induction and reprogramming of cardiac gene expression produced by pressure overload. Proc Natl Acad Sci U S A. 1988;85(2):339–43. doi: 10.1073/pnas.85.2.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hinkle A, Goranson A, Butters CA, Tobacman LS. Roles for the troponin tail domain in thin filament assembly and regulation. A deletional study of cardiac troponin T. J Biol Chem. 1999;274(11):7157–64. doi: 10.1074/jbc.274.11.7157. [DOI] [PubMed] [Google Scholar]
- 25.Tobacman LS, Lin D, Butters C, Landis C, Back N, Pavlov D, et al. Functional consequences of troponin T mutations found in hypertrophic cardiomyopathy. J Biol Chem. 1999;274(40):28363–70. doi: 10.1074/jbc.274.40.28363. [DOI] [PubMed] [Google Scholar]
- 26.Javadpour MM, Tardiff JC, Pinz I, Ingwall JS. Decreased energetics in murine hearts bearing the R92Q mutation in cardiac troponin T. J Clin Invest. 2003;112(5):768–75. doi: 10.1172/JCI15967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Solaro RJ, Montgomery DM, Wang L, Burkart EM, Ke Y, Vahebi S, et al. Integration of pathways that signal cardiac growth with modulation of myofilament activity. J Nucl Cardiol. 2002;9(5):523–33. doi: 10.1067/mnc.2002.127626. [DOI] [PubMed] [Google Scholar]
- 28.de Tombe PP, Solaro RJ. Integration of cardiac myofilament activity and regulation with pathways signaling hypertrophy and failure. Ann Biomed Eng. 2000;28(8):991–1001. doi: 10.1114/1.1312189. [DOI] [PubMed] [Google Scholar]
- 29.Chandra M, Dong WJ, Pan BS, Cheung HC, Solaro RJ. Effects of protein kinase A phosphorylation on signaling between cardiac troponin I and the N-terminal domain of cardiac troponin C. Biochemistry. 1997;36(43):13305–11. doi: 10.1021/bi9710129. [DOI] [PubMed] [Google Scholar]
- 30.Kentish JC, McCloskey DT, Layland J, Palmer S, Leiden JM, Martin AF, et al. Phosphorylation of troponin I by protein kinase A accelerates relaxation and crossbridge cycle kinetics in mouse ventricular muscle. Circ Res. 2001;88(10):1059–65. doi: 10.1161/hh1001.091640. [DOI] [PubMed] [Google Scholar]
- 31.Noland TA, Jr, Guo X, Raynor RL, Jideama NM, Averyhart-Fullard V, Solaro RJ, et al. Cardiac troponin I mutants. Phosphorylation by protein kinases C and A and regulation of Ca(2+)-stimulated MgATPase of reconstituted actomyosin S-1. J Biol Chem. 1995;270(43):25445–54. doi: 10.1074/jbc.270.43.25445. [DOI] [PubMed] [Google Scholar]
- 32.Ke Y, Wang L, Pyle WG, de Tombe PP, Solaro RJ. Intracellular localization and functional effects of P21-activated kinase-1 (Pak1) in cardiac myocytes. Circ Res. 2004;94(2):194–200. doi: 10.1161/01.RES.0000111522.02730.56. [DOI] [PubMed] [Google Scholar]
- 33.Liu Q, Hofmann PA. Modulation of protein phosphatase 2a by adenosine A1 receptors in cardiomyocytes: role for p38 MAPK. Am J Physiol Heart Circ Physiol. 2003;285(1):H97–103. doi: 10.1152/ajpheart.00956.2002. [DOI] [PubMed] [Google Scholar]
- 34.Belin RJ, Sumandea MP, Kobayashi T, Walker LA, Rundell VL, Urboniene D, et al. Left ventricular myofilament dysfunction in rat experimental hypertrophy and congestive heart failure. Am J Physiol Heart Circ Physiol. 2006;291(5):H2344–53. doi: 10.1152/ajpheart.00541.2006. [DOI] [PubMed] [Google Scholar]
- 35.Jweied EE, McKinney RD, Walker LA, Brodsky I, Geha AS, Massad MG, et al. Depressed cardiac myofilament function in human diabetes mellitus. Am J Physiol Heart Circ Physiol. 2005;289(6):H2478–83. doi: 10.1152/ajpheart.00638.2005. [DOI] [PubMed] [Google Scholar]
- 36.van der Velden J, Papp Z, Zaremba R, Boontje NM, de Jong JW, Owen VJ, et al. Increased Ca2+-sensitivity of the contractile apparatus in end-stage human heart failure results from altered phosphorylation of contractile proteins. Cardiovasc Res. 2003;57(1):37–47. doi: 10.1016/s0008-6363(02)00606-5. [DOI] [PubMed] [Google Scholar]
- 37.Bodor GS, Oakeley AE, Allen PD, Crimmins DL, Ladenson JH, Anderson PA. Troponin I phosphorylation in the normal and failing adult human heart. Circulation. 1997;96(5):1495–500. doi: 10.1161/01.cir.96.5.1495. [DOI] [PubMed] [Google Scholar]
- 38.Zakhary DR, Moravec CS, Stewart RW, Bond M. Protein kinase A (PKA)-dependent troponin-I phosphorylation and PKA regulatory subunits are decreased in human dilated cardiomyopathy. Circulation. 1999;99(4):505–10. doi: 10.1161/01.cir.99.4.505. [DOI] [PubMed] [Google Scholar]
- 39.McConnell BK, Moravec CS, Bond M. Troponin I phosphorylation and myofilament calcium sensitivity during decompensated cardiac hypertrophy. Am J Physiol Heart Circ Physiol. 1998;274(2):H385–396. doi: 10.1152/ajpheart.1998.274.2.H385. [DOI] [PubMed] [Google Scholar]
- 40.Tomaselli GF, Zipes DP. What causes sudden death in heart failure? Circ Res. 2004;95(8):754–63. doi: 10.1161/01.RES.0000145047.14691.db. [DOI] [PubMed] [Google Scholar]
- 41.Schillinger W, Kogler H. Altered phosphorylation and Ca2+-sensitivity of myofilaments in human heart failure. Cardiovasc Res. 2003;57(1):5–7. doi: 10.1016/s0008-6363(02)00743-5. [DOI] [PubMed] [Google Scholar]
- 42.Gomes AV, Potter JD. Molecular and cellular aspects of troponin cardiomyopathies. Ann N Y Acad Sci. 2004;1015:214–24. doi: 10.1196/annals.1302.018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.