Abstract
Atherosclerosis, the underlying cause of cardiovascular disease, is characterized by lipid accumulation, lipoprotein oxidation, and inflammation. Products of the cyclooxygenase (COX) pathway participate in acute and chronic inflammation. The inducible form of COX, COX-2, generates lipid mediators of inflammation that are pro-inflammatory and COX-2-selective inhibitors are potent anti-inflammatory agents. However, clinical data suggest an increased risk of cardiovascular side effects in patients using COX-2-selective inhibitors. In this paper, we sought to determine the affect of COX-2 deficiency on atherosclerosis-related lipoprotein metabolism in mice. We demonstrate that COX-2 deficiency resulted in i) accumulation of lipids in circulation and liver, ii) pro-inflammatory properties of HDL as measured by HDL’s increased reactive oxygen species (ROS) content, decreased paraoxonase 1 (PON1) activity, decreased serum apoA-1, reduced ability to efflux cholesterol and to prevent LDL oxidizability, and iii) increased TXB2 in circulation. Moreover, when placed on an atherogenic diet, COX-2 deficiency resulted in i) increased lipid deposition in the aorta, ii) a further dramatic imbalance in circulating eicosanoids, i.e. decreased serum PGI2 coupled with increased PGE2 and TXB2, and iii) a marked elevation of pro-inflammatory cytokines, TNF and IL-6. Our results suggest, for the first time, that COX-2 deficiency contributes to the pro-atherogenic properties of HDL in mice.
Keywords: atherosclerosis, cardiovascular diseases, cholesterol, COX-2, cytokines, high density lipoprotein, heart diseases, hyperlipidemia, inflammation, lipoproteins, and prostanoids
1. Introduction
Atherosclerosis is the primary mechanism underlying the development of coronary artery disease, and is characterized by lipoprotein accumulation and oxidation, aberrant lipoprotein metabolism, and systemic inflammation [1]. High-density lipoprotein (HDL) cholesterol levels and atherosclerosis are inversely related [2]. In recent years, ‘HDL function’ has emerged as an effective biomarker for atherosclerosis risk [3–5]. HDL exerts anti-atherogenic function by promoting reverse cholesterol transport and preventing the oxidation of low density lipoprotein (LDL) [6, 7]. We have previously shown that the anti-inflammatory functions of HDL can be impaired in humans [5], rabbits [8] and mice [9] during inflammatory processes. During inflammation HDL is characterized by i) increased reactive oxygen species (ROS) accumulation [10], ii) decreased levels and activity of anti-inflammatory, anti-oxidant factors including apolipoprotein A1 (apoA-I) and paraoxonase 1 (PON1) [11], iii) reduced potential to efflux cholesterol [12] and iv) diminished ability to prevent LDL oxidation [3].
Cyclooxygenase 2 (COX-2) is up-regulated during acute and chronic inflammation and is involved in stimulus induced prostanoid synthesis [13]. COX-2 is expressed in both human and mouse atherosclerotic lesions [14–16]. The importance of COX-2 to the development of atherosclerosis is emphasized by a number of clinical trials that have revealed an increasing incidence of cardiovascular events - heart attacks and strokes - in response to the use of COX-2-selective inhibitors [17–19]. While human data suggest a connection between COX-2 inhibition and atherosclerosis, the underlying mechanisms and mediators responsible for the deleterious cardiovascular effects of COX-2 inhibitors have not been clearly identified.
In the present study we demonstrate using COX-2 knockout mice fed chow and atherogenic diets that genetic depletion of COX-2 is associated with i) hyperlipidemia, ii) accumulation of pro-inflammatory HDL, iii) decreased levels of PGI2, and iv) increased production of pro-inflammatory mediators of inflammation including TXA2, PGE2, IL-6 and tumor necrosis factor (TNF). We further show that a short-term (10 days) treatment with rofecoxib caused accumulation of pro-inflammatory HDL in C57BL6/J mice. Our results suggest that COX-2 plays an anti-atherogenic role in mice.
2. Materials and Methods
2.1. Mice
COX-1−/−, COX-1+/+, COX-2−/− and COX-2+/+ mice on a mixed 129/C57BL6/J background were obtained from Taconic (Germantown, NY). Mice were maintained on a 6% fat chow diet. At age of 10 – 11 weeks, mice were fed chow diet or atherogenic diet containing 15.8% fat, 1.25% cholesterol, and 0.5% cholate (Harlan Teklad, Madison, WI) for 3 weeks. For experiments with rofecoxib, C57BL6/J female mice at age of 8 – 12 weeks were given commercially available rofecoxib (Vioxx®, Merck) (30mg/kg/day) by oral gavage for ten days. Control mice were given vehicle only (0.5% carboxy methylcellulose). Serum samples were isolated from overnight fasted mice, cryopreserved in 10% sucrose and kept at −80°C until use.
2.2. Lipid Deposition
Heart and proximal aorta from mice were obtained and embedded in OCT compound. Serial 10 μm-thick cryosections from the middle portion of the ventricle to the aortic arch were collected and stained with Oil Red O and hematoxylin. The lipid-containing area on each section was determined in a blinded fashion, using a microscope eyepiece grid. The average lipid area per aorta, calculated from 25 sections of each aorta, was scored.
2.3. Serum, Lipoprotein, and Liver Lipids
Serum cholesterol levels were determined by commercially available kits (Thermo, Louisville, CO). Cholesterol esters were determined by subtracting free cholesterol from total cholesterol. HDL was isolated from serum by LipiDirect HDL reagent (Polymedco, Cortland Manor, NY) according to the manufacturer’s protocol. The supernatant containing HDL was assayed for cholesterol, protein (Promega, Madison, WI) and used for cholesterol efflux within 48 hours after the isolation. VLDL/LDL cholesterol was determined by subtracting HDL cholesterol from total cholesterol. For determining the liver cholesterol content, liver homogenates were lipid extracted with chloroform/methanol (2:1). Lipid extracts were dried under nitrogen gas and further resuspended in 1% Triton X-100 in PBS, and assayed for cholesterol.
2.4. Lipoprotein Isolation
Lipoprotein samples were isolated from pooled sera by a fast protein liquid chromatography (FPLC) system consisting of dual Superose 6 columns in series (Amersham Bioscience, Piscataway, NJ). Serum (0.5 mL) was eluted with PBS at a flow rate of 0.5 mL/min and fractionated every 1 mL. Each fraction was assayed for cholesterol (Thermo) and protein (Promega) according to manufacturer’s protocols.
2.5. HDL Characterization
2.5a Reactive oxygen species (ROS)
ROS content in lipoproteins was determined with 2,7,7′dichlorofluorescein diacetate (H2DCFDA) (Invitrogen, Carlsbad, CA) as described previously [20] with minor modifications. Individual FPLC fractions (50 μL) were incubated with H2DCFDA (10 μg/mL) in methanol for 30 min at 37°C. The presence of ROS was detected by measuring fluorescence intensity at 485nm/525nm.
2.5b Paraoxonase 1 (PON1) assay
PON1 activity in individual FPLC fractions was determined as described previously [8]. Briefly, samples were incubated with paraoxon and PON1 activity was analyzed by measuring the increase in absorbance at 405 nm due to the formation of 4-nitrophenol over a period of 12 minutes (20 second intervals). A unit of PON1 activity was defined as the formation of 1 nmol of 4-ntirophenol per minute per milliliter of sample applied.
2.5c Serum ApoA-1
Serum apoA-1 was determined by direct ELISA according to the manufacturer’s protocol (Abcam, Cambridge, MA). Briefly, 96-well enzyme EIA plates (Corning Inc., Corning, NY) were coated with serum samples (20 μg/mL) diluted in PBS overnight at 4 °C. Following washes with PBS/Tween-20 (0.05 %), the plates were blocked with 5% non-fat milk, immunoblotted with primary antibody against apoA-1 at 1:5000 (Biodesign, Saco, ME) and HRP-conjugated detection antibody at 1:5000 (GE Healthcare). HRP was probed with TMB solution (KPL, Gaithersburg, MD) and OD450 was measured. Recombinant apoA-1 (Biodesign) was used as standard.
2.5d Cholesterol efflux
Cellular cholesterol efflux was performed as described previously with minor modifications [21]. Mouse macrophage RAW264.7 cells (ATCC, Manassas, VA) were cultured on 24-well tissue culture plates and grown in DMEM media (GIBCO-BRL, Grand Island, NY) with 10% FBS overnight. Cells were washed with serum free media and loaded with 3H-cholesterol (1 μCi/mL) and acetylated LDL (50 μg/mL) in media with 0.2 % fatty acid free BSA (Sigma, St. Louis, MO) overnight. Labeled cells were washed, resuspended in DMEM media with 0.2% BSA and incubated with HDL (25 μg/mL) containing supernatant (Materials and Methods, section 2.3) for 6 hours at 37°C. Radioactivity in the supernatants and total cell extracts were measured and expressed as the percentage of total radioactive counts accumulated in the supernatants during the efflux period.
2.5e Monocyte Chemotaxis Assay
Artery wall cell cocultures were used for the monocyte chemotaxis assay as described previously [7]. Cocultures of human aortic endothelial cells and human aortic smooth muscle cells were treated with native human LDL (250μg/mL) in the presence or absence of HDL for 8 hours. Cells were subsequently washed and fresh M199 media (GIBCO-BRL, Grand Island, NY) was added for an additional 8 hours. At the end of the incubation, supernatants were collected, diluted 40-fold, and tested for monocyte chemotactic activity [7]. Briefly, the supernatant was added to a standard Neuroprobe chamber (Neuroprobe, Cabin John, MD), with monocytes added to the top. The chamber was incubated for 60 min at 37°C. After incubation, the chamber was disassembled and non-migrated monocytes were wiped off. The membrane was then air dried and fixed with 1% glutaraldehyde and stained with 0.1% Crystal Violet dye. The number of migrated monocytes was determined microscopically and expressed as the mean ± SD of 9 standardized high power fields.
2.6. Eicosanoids
Serum PGE2, LTB4, TXB2, and 6-keto PGF1α were determined by competitive EIA according to manufacturer’s protocols (Assay Designs, Ann Arbor, MI, Amersham Biosciences, Piscataway, NJ).
2.7. Cytokines
All kits, instruments, and software were purchased from BD Biosciences (San Diego, CA). Serum cytokines were determined by Cytometric Bead Array (CBA) Mouse Inflammation Kit according to manufacturer’s protocols. A FACSCalibur Analytic Flow Cytometer with CellQuest software was used for data acquisition. CBA results were analyzed using Flow Cytometric Analysis Program Array Software.
2.8. Statistics
Statistical significance was determined by Student’s T-test or Mann-Whitney U test (VassarStats: faculty.vassar.edu/lowry/utest.html). Significance was defined as p<0.05.
3. Results
3.1. COX-2−/− mice are hyperlipidemic
The main goal of the present study was to determine whether COX-2 deficiency affects the development of atherosclerotic lesions. COX-2+/+ and COX-2−/− mice were placed either on a chow diet or on an atherogenic diet (n=15 for each group). Surprisingly, the atherogenic diet was lethal to COX-2−/− mice. 10 out of 15 mice on the atherogenic diet died 4 weeks into the start of the experiment. In contrast, COX-1−/−, (n=15 per group) placed on an atherogenic diet sustained the entire 15-week period of the diet protocol and did not show any differences when compared to wild-type littermates treated similarly (not shown).
Sera and lipoproteins from COX-2−/− and COX-2+/+ mice were analyzed for cholesterol and cholesterol ester content (Table 1). Cholesterol (total, HDL, VLDL/LDL) and cholesterol esters were significantly elevated (23%, 14%, 42%, and 22%. respectively) in COX-2−/− mice on chow diet when compared to wild-type mice on chow. Atherogenic diet caused significant increases in cholesterol content in sera and lipoproteins from both COX-2+/+ and COX-2−/− mice (Table 1); however, total cholesterol, HDL cholesterol (HDL-C), VLDL/LDL cholesterol (VLDL/LDL-C) and cholesterol esters, were all significantly higher in COX-2−/− mice when compared to COX-2+/+ mice (Table 1). These data suggest that COX-2−/− mice are hyperlipidemic compared to their wild-type littermates.
Table 1.
| Total Cholesterol (mg/dL) | HDL cholesterol (mg/dL) | VLDL/LDL cholesterol (mg/dL) | Cholesterol esters (mg/dL) | |
|---|---|---|---|---|
| WT(C) | 81.0 +/−13.8 | 53.1 +/− 7.0 | 27.9 +/− 7.2 | 71.3 +/− 10.9 |
| KO(C) | 100.0 +/− 14.5** | 60.3 +/− 7.5* | 39.7 +/− 8.7** | 87.3 +/− 12.0** |
| WT(A) | 178.3 +/− 25.1 | 49.4 +/− 8.3 | 130.0 +/− 26.3 | 147.0 +/− 20.4 |
| KO(A) | 226.4 +/− 38.1** | 69.4 +/− 14.2** | 157.0 +/− 29.9** | 172.4 +/− 28.7* |
N=8–12,
= p<0.01 and
= p<0.05, COX-2−/− compared to COX-2+/+ controls on the same diet. WT = COX-2+/+ (wild-type littermates). KO = COX-2−/−, (C) = chow diet, (A) = Atherogenic diet
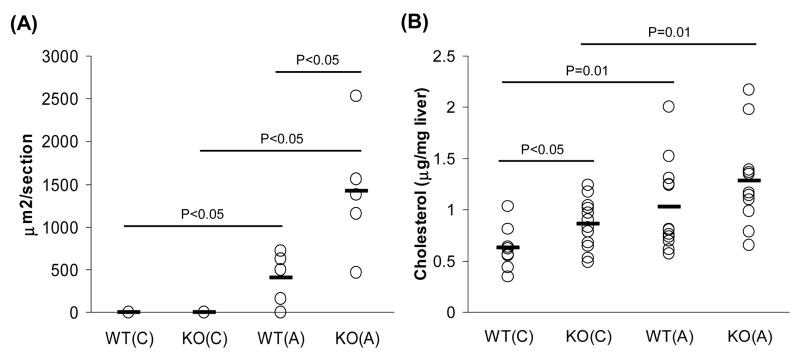
To further examine whether increased cholesterol content in circulation affected tissue lipid deposits, we analyzed aortas and liver lysates from COX-2+/+ and COX-2−/− mice for neutral lipid content and cholesterol, respectively. Aortas from COX-2−/− mice on atherogenic diet after 3 weeks had significantly larger areas of neutral lipid content as measured by Oil Red O staining (Fig. 1A). COX-2−/− mice on chow diet also had significantly increased cholesterol content in liver lysates when compared to wild-type mice (Fig. 1B). Atherogenic diet resulted, as expected, in increased liver cholesterol levels in wild-type mice with no significant differences when compared to COX-2−/− mice (Fig. 1B).
Fig. 1.
Absence of COX-2 results in increased lipid deposition in aorta and liver. (A) Lipid accumulation in the aorta (n=5 per group) and (B) liver cholesterol content (n=8–12 per group) were determined as described in Materials and Methods. Aortic lipid and liver cholesterol content are represented individually (open circles) and as averages (black bar) of each group. P-values were calculated by T-test for statistical analysis. WT = COX-2+/+ wild-type mice, KO = COX-2−/− mice, (C) = chow diet, (A) = atherogenic diet.
3.2. HDL from COX-2−/− mice is pro-inflammatory
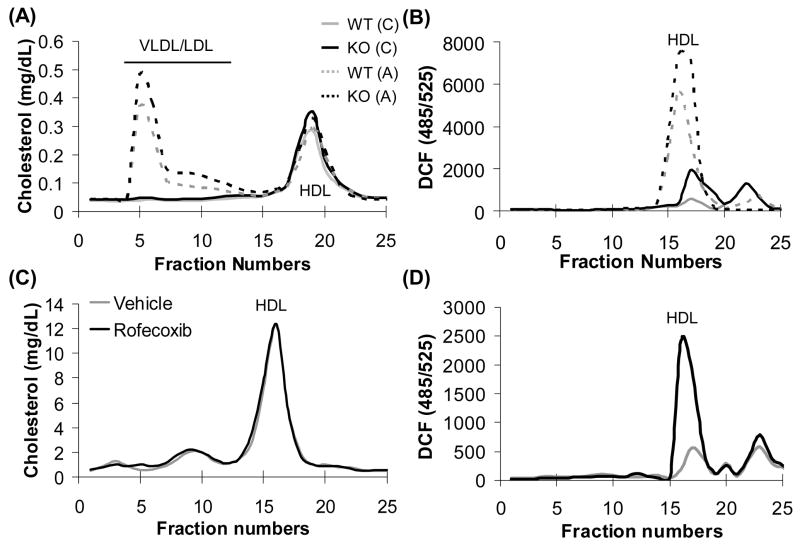
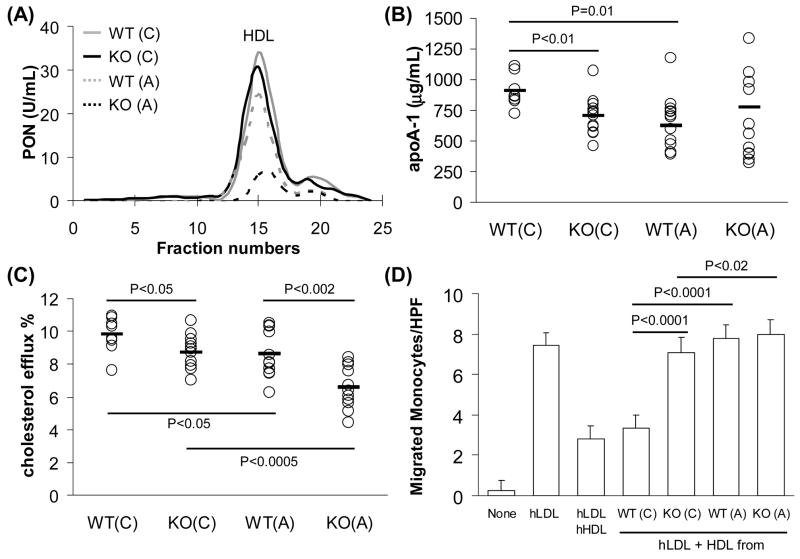
COX-2−/− mice had significantly higher HDL-C levels (Table 1). It is well established that HDL-C levels are inversely related to risk of atherosclerosis. Recent reports from others and our own laboratory suggest that ‘HDL function’ as measured by HDL’s i) ROS content, ii) PON1 activity, iii) apoA-1 level, iv) ability to induce cholesterol efflux, and v) ability to prevent LDL oxidation, is a better marker for atherosclerosis risk than HDL-C levels, reviewed in [3, 4]. Therefore, we evaluated the inflammatory properties of HDL as a measure of HDL function in all the experimental groups. Interestingly, we observed that HDL from COX-2−/− mice on a chow diet as well as C57BL6/J mice on chow treated with the COX-2 specific inhibitor rofecoxib was pro-inflammatory as indicated by significant accumulation of ROS (Fig. 2B and 2D). HDL from COX-2−/− mice also had decreased PON1 activity (Fig. 3A), decreased apoA-1 expression (Fig. 3B), reduced ability to induce cholesterol efflux (Fig. 3C), and inability to protect against LDL-induced monocyte chemotaxis (Fig. 3D). Atherogenic diet further exacerbated the pro-inflammatory properties of HDL from COX-2−/− mice when compared to wild-type mice (Figs. 2 and 3).
Fig. 2.
ROS accumulation on HDL from COX-2−/− mice and C57BL6/J wild-type mice treated with rofecoxib. Individual FPLC fractions from pooled serum samples from COX-2+/+, COX-2−/− mice (A and B) and C57BL6/J mice treated with either vehicle or rofecoxib (C and D) were assayed for cholesterol (A and C) and ROS (B and D). WT = COX-2+/+ wild-type mice, KO = COX-2−/− mice, (C) = chow diet, (A) = atherogenic diet.
Fig. 3.
HDL from COX-2−/− mice is dysfunctional and pro-inflammatory. (A) Individual FPLC fractions from pooled serum samples from COX-2+/+ and COX-2−/− mice were tested for PON activity (represented as units/mL). (B) Levels of apoA-1 in serum (20 μg/mL) were determined by ELISA. Concentrations are represented individually (open circles) and as averages (black bar) for each group (n=8–12). (C) HDL (25 μg/mL) isolated from COX-2+/+ and COX-2−/− mice was incubated for 6 hours with RAW cells preloaded with 3H-cholesterol. Percentage efflux is represented individually (open circles) and as averages (black bar) for each group (n=8–12). (D) HDL from COX-2+/+ and COX-2−/− mice was used in a monocyte chemotaxis assay as described in Materials and Methods. Data represented as average with one standard deviation of number of migrated monocytes in 9 fields for each HDL. Data are representative of three experiments. P-values were calculated by T-test for statistical analysis. WT = COX-2+/+ wild-type mice, KO = COX-2−/− mice. (C) = chow diet, (A) = atherogenic diet, hLDL = human LDL, hHDL = human HDL, none = no addition control.
3.3. Absence of COX-2 alters the inflammatory balance of circulating eicosanoids
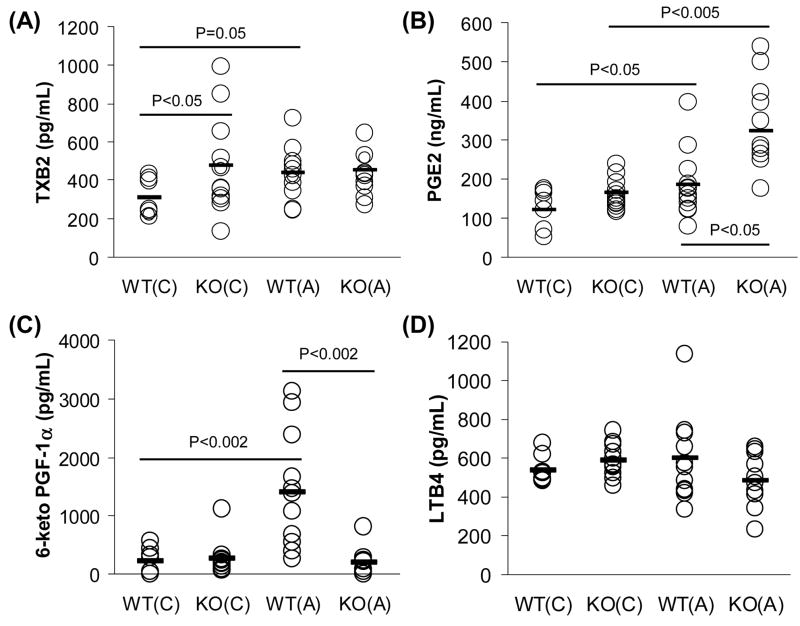
We next examined whether COX-2 deficiency affected circulating eicosanoid profiles. Serum samples from COX-2−/− and wild-type mice were assayed for TXB2 (stable metabolite of TXA2), PGE2, 6-keto PGF1α (stable metabolite of PGI2), and LTB4 (Fig. 4). Serum TXB2 is significantly increased in COX-2−/− mice on chow compared to wild-type littermates (Fig. 4A). Interestingly, the concentrations of TXB2 in COX-2−/− mice on chow (474.84 pg/mL) were comparable to those observed in wild-type mice on atherogenic diet (438.56 pg/mL) (Fig. 4A). PGE2 levels were found to be elevated in serum samples from COX-2−/− mice. Interestingly, PGE2 levels were ~1.5-fold higher in COX-2−/− mice on atherogenic diet compared to wild-type mice (Fig. 4B). In contrast, we observed a 7-fold decrease (Fig. 4C) in 6-keto PGF1α in COX-2−/− mice on atherogenic diet compared to wild-type mice. In contrast, the leukotriene pathway as measured by LTB4 levels, appeared to be unaffected in circulation of COX-2 deficient mice (Fig. 4D).
Fig. 4.
Absence of COX-2 alters the inflammatory balance of circulating eicosanoids. Concentrations of TXB2 (A), PGE2 (B), 6-keto PGF1α (C), and LTB4 (D) in serum from COX-2+/+ and COX-2−/− mice were determined by EIA kits. Eicosanoid levels are represented as individual concentrations (open circles) and averages (black bar) for each group (n=8–12). P-values were calculated by T-test for statistical analysis. WT = COX-2+/+ wild-type mice, KO = COX-2−/− mice, (C) = chow diet, (A) = atherogenic diet.
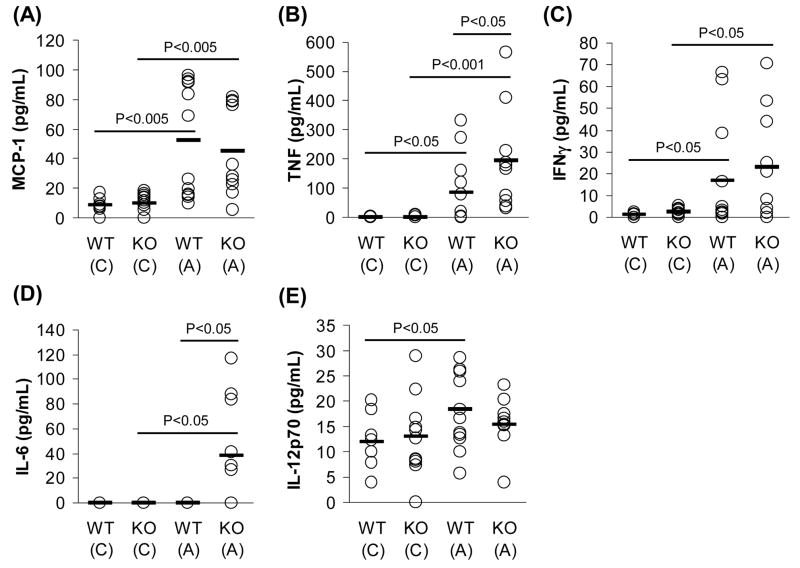
3.5. Absence of COX-2 increases pro-inflammatory cytokines in circulation
We have observed markers for a pro-atherogenic phenotype in COX-2−/− mice including hyperlipidemia (Table 1 and Fig. 1), formation of pro-inflammatory HDL (Figs. 2 and 3), and presence of a pro-inflammatory eicosanoid profile (Fig. 4). All these markers are known to be accompanied by systemic inflammation and are risk factors for atherosclerosis [1, 3–5, 22–24]. Thus, we examined whether COX-2 deficiency results in systemic inflammation when challenged with atherogenic diet. We profiled serum cytokines by a cytometric bead array and analyzed the levels of IL-6, IL-12, IL-10, TNF, MCP-1, and interferon-γ (IFN-γ) (Fig. 5). In concordance with previous literature [25–27], atherogenic diet was associated with significant elevation of MCP-1, TNF, and IFN-γ, in both groups of mice (Fig. 5A–C). Interestingly, COX-2−/− mice on atherogenic diet showed more systemic inflammation with higher levels of TNF (Fig. 5B) and IL-6 (Fig. 5D) than wild-type controls. Furthermore, IL-12 was significantly increased by atherogenic diet in wild-type controls, while COX-2 depletion completely abolished this induction (Fig. 5E). Serum IL-10 was not affected by genotype or diet (data not shown).
Fig. 5.
Absence of COX-2 increases pro-inflammatory cytokines in circulation when challenged with an atherogenic diet. Concentrations of MCP-1 (A), TNF (B), IFN-γ (C), IL-6 (D), and IL-12 (E) in serum (50 μL) from COX-2+/+ and COX-2−/− mice were determined by cytometric bead array. Cytokine levels are represented as individual concentrations (open circles) and averages (black bar) for each group (n=8–12). P-values were calculated by T-test for statistical analysis. P-value denoted by (*) was determined by Mann-Whitney U test. WT = COX-2+/+ wild-type mice, KO = COX-2−/− mice, (C) = chow, (A) = atherogenic diet.
4. Discussion
In contrast to COX-1, which has predominantly housekeeping functions, COX-2 is implicated in a number of inflammatory disorders including arthritis, cancer, and cardiovascular diseases. Since its discovery in 1991, inhibition of COX-2 activity became a major focus for the prevention and treatment of inflammatory diseases. Indeed, COX-2-selective inhibitors, celecoxib and rofecoxib, have been used to treat colon cancer [28, 29] and arthritis [30]. However, recent studies have cast doubt on the anti-inflammatory effects of COX-2-selective inhibitors because of their association with increased risk of cardiovascular events – heart attacks and strokes – with chronic use [17–19]. While the literature suggests that COX-2 inhibition may have pro-inflammatory effects in cardiovascular physiology, the underlying mechanisms are poorly understood.
In this paper, we demonstrated that COX-2−/− mice (3 – 4 months of age) harbor pro-atherogenic conditions that include i) lipid accumulation in the circulation and liver (Table 1 and Fig. 1), ii) accumulation of dysfunctional, pro-inflammatory HDL (Fig. 2 and 3), and iii) increases in TXB2 levels (Fig. 4), all of which are well-accepted pro-atherogenic factors. Moreover, upon challenge with an atherogenic diet, COX-2−/− mice developed i) increased lipid deposition in the aorta (Fig. 1), ii) a further pro-atherogenic imbalance in circulating eicosanoids, PGI2 vs. TxB2 (Fig. 4) and iii) a marked elevation of pro-inflammatory cytokines, TNF and IL-6, compared to wild-type mice (Fig. 5). Taken together, these results suggest that COX-2 deficiency may affect the cardiovascular physiology in mice.
Total cholesterol, VLDL/LDL-C and HDL-C were found to be significantly elevated on a chow diet, as well as an atherogenic diet (Table 1) in COX-2−/− mice when compared to wild-type mice. Cholesterol accumulation can promote inflammation by its ability to stimulate the production of ROS that result in the formation of pro-inflammatory oxidized phospholipids [31]. Cholesterol accumulation is also associated with increased macrophage foam cell formation [32], which is a key step in the development of atherosclerotic lesions. Indeed, a recent study by Chan et al. suggests that COX-2 inhibition alters the expression of cholesterol efflux proteins and promotes the transformation of THP-1 macrophages into foam cells [33].
The direct relationship of hyperlipidemia and the inverse relationship of HDL-C to the risk of atherosclerosis is well established [2]. However, it is also well known that a significant number of patients with normal HDL-C levels develop cardiovascular disease, including atherosclerosis [3, 4]. We previously reported that HDL from a group of patients with high HDL-C is pro-inflammatory [5]. Unlike normal/anti-inflammatory HDL, pro-inflammatory HDL is defective in reverse cholesterol transport [12] and has diminished anti-oxidant activity [3], and thus has a potential to exacerbate atherosclerosis [3, 4].
Although COX-2−/− mice had elevated HDL-C, their HDL was pro-inflammatory in every tested measure, with excessive ROS content, decreased apoA-1 levels, deficient PON1 activity, decreased cholesterol efflux potential, and inability to prevent LDL-induced monocyte chemotactic activity (Fig. 2 and 3). The inability of HDL from COX-2 deficient mice to promote cholesterol efflux correlates with the observed cholesterol accumulation in peripheral tissues, including liver and aorta, a primary site of atherogenesis in mice. The importance of COX-2 activity in maintaining normal, anti-inflammatory HDL function is further solidified by the observation that C57BL6/J mice accumulate pro-inflammatory HDL in a relatively short time (ten days) of rofecoxib treatment (Fig. 2D). Our results are in agreement with a recent study by Metzner et al., which demonstrated that treatment of apoE−/− mice on a chow diet with COX-2-selective inhibitors, rofecoxib and celecoxib, for 16 weeks, results in increased atherosclerotic lesions [34]. In the same study, Metzner et al. did not find significant differences in lesion sizes in apoE−/− mice on a western diet. Since apoE−/− mice on a western diet develop advanced lesions, the authors concluded that inhibition of COX-2 promotes initiation of atherogenesis and may not affect the late stages.
Lack of COX-2 activity also results in an imbalance of prostanoid production, a possible pro-atherogenic mechanism [35]. COX-2−/− mice on a chow diet showed significantly higher levels of TXA2 (measured by stable metabolite TXB2) compared to wild-type mice (Fig. 4), and more interestingly, the levels on a chow diet were as high as those seen in wild-type group on an atherogenic diet, suggesting that COX-2−/− mice are more prone to aggregation in the circulation. More interestingly, COX-2−/− mice on an atherogenic diet for three weeks showed a 7-fold decrease in the anti-inflammatory prostanoid prostacyclin (measured by the stable metabolite 6-keto PGF1α) coupled with concomitant increase in the pro-thrombotic and pro-nflammatory eicosanoids TXA2 and PGE2. This aberrant shift in eicosanoid profile may be due to either augmented or unopposed COX-1 activity in the absence of COX-2 [36, 37]. Because of short term feeding we are not certain whether the pro-inflammatory eicosanoid profile in circulation in the COX-2 deficient mice is sufficient to aggravate atherosclerotic lesions. However, COX-2 specific regulation of anti-atherogenic prostanoids such as prostacyclin [22, 24] and anti-inflammatory prostaglandins such as 15-deoxy-Δ12,14-PGJ2 [38] suggest that COX-2 activity is required to maintain an anti-inflammatory balance of eicosanoids. Moreover, in the studies by Metzner et al. [34] discussed above, apoE−/− mice on a chow diet that were treated with COX-2 selective inhibitors, rofecoxib and celecoxib, showed a 50% reduction in prostacyclin metabolites in urine, suggesting once again the importance of COX-2 in the maintenance of anti-atherogenic prostanoid balance.
Based on evidence of hyperlipidemia, pro-inflammatory HDL, and imbalance of prostanoids in COX-2−/− mice on chow, we suspected an exaggerated systemic inflammatory response in COX-2−/− mice upon challenge with atherogenic diet. Atherogenic diet resulted in an increase in MCP-1, TNF, IFN-γ, in both groups and IL-12 in the wild-type group, suggesting that 3-week feeding of atherogenic diet itself is sufficient to induce inflammation. Although the absence of COX-2 exacerbated inflammation as evidenced by increased TNF and IL-6 in circulation (Fig. 5), some of the changes in cytokine profiles on an atherogenic diet, could be, in part, caused by the intestinal inflammation that COX-2−/− mice develop on an atherogenic diet (reported in the accompanying manuscript).
In conclusion, we identified a novel anti-atherogenic role for COX-2 by showing that COX-2−/− mice on a chow diet and wild-type mice on a COX-2 inhibitor accumulate dysfunctional/pro-inflammatory HDL. In recent years, pro-inflammatory HDL has not only emerged as a new biomarker for atherosclerosis but has also become an excellent target for therapeutic intervention of cardiovascular diseases. Future studies based on the findings in this paper will no doubt provide new insights into the cardiovascular side effects of COX-2 selective inhibitors.
Acknowledgments
This study was supported in part by American Heart Association Fellowship 0515039Y (AN), and National Institutes of Health awards 5R33DK070328 (JL), 1RO1HL71776 (STR), and HL-30568 (MN and STR). Flow cytometry was performed in the UCLA Jonsson Comprehensive Cancer Center (JCCC) and Center for AIDS Research Flow Cytometry Core Facility that is supported by National Institutes of Health awards CA-16042 and AI-28697, and by the JCCC, the UCLA AIDS Institute, and the David Geffen School of Medicine at UCLA.
Abbreviations
- apoA-1
apolipoprotein A-1
- apoE
apolipoprotein E
- BSA
bovine serum albumin
- COX
cyclooxygenase
- ELISA
enzyme-linked immunosorbent assay
- EIA
enzyme immunoassay
- HDL
high-density lipoprotein
- IFN
interferon
- IL
interleukin
- LDL
low-density lipoprotein
- PON
paraoxonase
- ROS
reactive oxygen species
- TNF
tumor necrosis factor
- VLDL
very low-density lipoprotein
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lusis AJ. Atherosclerosis. Nature. 2000;407:233–41. doi: 10.1038/35025203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Castelli WP, Garrison RJ, Wilson PW, Abbott RD, Kalousdian S, Kannel WB. Incidence of coronary heart disease and lipoprotein cholesterol levels. The Framingham Study. Jama. 1986;256:2835–8. [PubMed] [Google Scholar]
- 3.Navab M, Ananthramaiah GM, Reddy ST, et al. The double jeopardy of HDL. Ann Med. 2005;37:173–8. doi: 10.1080/07853890510007322. [DOI] [PubMed] [Google Scholar]
- 4.Navab M, Anantharamaiah GM, Reddy ST, Van Lenten BJ, Ansell BJ, Fogelman AM. Mechanisms of disease: proatherogenic HDL--an evolving field. Nat Clin Pract Endocrinol Metab. 2006;2:504–11. doi: 10.1038/ncpendmet0245. [DOI] [PubMed] [Google Scholar]
- 5.Ansell BJ, Navab M, Hama S, et al. Inflammatory/antiinflammatory properties of high-density lipoprotein distinguish patients from control subjects better than high-density lipoprotein cholesterol levels and are favorably affected by simvastatin treatment. Circulation. 2003;108:2751–6. doi: 10.1161/01.CIR.0000103624.14436.4B. [DOI] [PubMed] [Google Scholar]
- 6.Navab M, Hama SY, Anantharamaiah GM, et al. Normal high density lipoprotein inhibits three steps in the formation of mildly oxidized low density lipoprotein: steps 2 and 3. J Lipid Res. 2000;41:1495–508. [PubMed] [Google Scholar]
- 7.Navab M, Hama SY, Cooke CJ, et al. Normal high density lipoprotein inhibits three steps in the formation of mildly oxidized low density lipoprotein: step 1. J Lipid Res. 2000;41:1481–94. [PubMed] [Google Scholar]
- 8.Van Lenten BJ, Hama SY, de Beer FC, et al. Anti-inflammatory HDL becomes pro-inflammatory during the acute phase response. Loss of protective effect of HDL against LDL oxidation in aortic wall cell cocultures. J Clin Invest. 1995;96:2758–67. doi: 10.1172/JCI118345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Van Lenten BJ, Wagner AC, Nayak DP, Hama S, Navab M, Fogelman AM. High-density lipoprotein loses its anti-inflammatory properties during acute influenza a infection. Circulation. 2001;103:2283–8. doi: 10.1161/01.cir.103.18.2283. [DOI] [PubMed] [Google Scholar]
- 10.Navab M, Berliner JA, Subbanagounder G, et al. HDL and the inflammatory response induced by LDL-derived oxidized phospholipids. Arterioscler Thromb Vasc Biol. 2001;21:481–8. doi: 10.1161/01.atv.21.4.481. [DOI] [PubMed] [Google Scholar]
- 11.Mackness MI, Durrington PN, Mackness B. The role of paraoxonase 1 activity in cardiovascular disease: potential for therapeutic intervention. Am J Cardiovasc Drugs. 2004;4:211–7. doi: 10.2165/00129784-200404040-00002. [DOI] [PubMed] [Google Scholar]
- 12.Hayek T, Oiknine J, Brook JG, Aviram M. Role of HDL apolipoprotein E in cellular cholesterol efflux: studies in apo E knockout transgenic mice. Biochem Biophys Res Commun. 1994;205:1072–8. doi: 10.1006/bbrc.1994.2775. [DOI] [PubMed] [Google Scholar]
- 13.Herschman HR. Prostaglandin synthase 2. Biochim Biophys Acta. 1996;1299:125–40. doi: 10.1016/0005-2760(95)00194-8. [DOI] [PubMed] [Google Scholar]
- 14.Baker CS, Hall RJ, Evans TJ, et al. Cyclooxygenase-2 is widely expressed in atherosclerotic lesions affecting native and transplanted human coronary arteries and colocalizes with inducible nitric oxide synthase and nitrotyrosine particularly in macrophages. Arterioscler Thromb Vasc Biol. 1999;19:646–55. doi: 10.1161/01.atv.19.3.646. [DOI] [PubMed] [Google Scholar]
- 15.Burleigh ME, Babaev VR, Oates JA, et al. Cyclooxygenase-2 promotes early atherosclerotic lesion formation in LDL receptor-deficient mice. Circulation. 2002;105:1816–23. doi: 10.1161/01.cir.0000014927.74465.7f. [DOI] [PubMed] [Google Scholar]
- 16.Schonbeck U, Sukhova GK, Graber P, Coulter S, Libby P. Augmented expression of cyclooxygenase-2 in human atherosclerotic lesions. Am J Pathol. 1999;155:1281–91. doi: 10.1016/S0002-9440(10)65230-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mukherjee D, Nissen SE, Topol EJ. Risk of cardiovascular events associated with selective COX-2 inhibitors. Jama. 2001;286:954–9. doi: 10.1001/jama.286.8.954. [DOI] [PubMed] [Google Scholar]
- 18.Solomon SD, Pfeffer MA, McMurray JJ, et al. Effect of celecoxib on cardiovascular events and blood pressure in two trials for the prevention of colorectal adenomas. Circulation. 2006;114:1028–35. doi: 10.1161/CIRCULATIONAHA.106.636746. [DOI] [PubMed] [Google Scholar]
- 19.McGettigan P, Henry D. Cardiovascular risk and inhibition of cyclooxygenase: a systematic review of the observational studies of selective and nonselective inhibitors of cyclooxygenase 2. Jama. 2006;296:1633–44. doi: 10.1001/jama.296.13.jrv60011. [DOI] [PubMed] [Google Scholar]
- 20.Navab M, Hama SY, Hough GP, Subbanagounder G, Reddy ST, Fogelman AM. A cell-free assay for detecting HDL that is dysfunctional in preventing the formation of or inactivating oxidized phospholipids. J Lipid Res. 2001;42:1308–17. [PubMed] [Google Scholar]
- 21.Navab M, Anantharamaiah GM, Reddy ST, et al. Oral D-4F causes formation of pre-beta high-density lipoprotein and improves high-density lipoprotein-mediated cholesterol efflux and reverse cholesterol transport from macrophages in apolipoprotein E-null mice. Circulation. 2004;109:3215–20. doi: 10.1161/01.CIR.0000134275.90823.87. [DOI] [PubMed] [Google Scholar]
- 22.Egan KM, Lawson JA, Fries S, et al. COX-2-derived prostacyclin confers atheroprotection on female mice. Science. 2004;306:1954–7. doi: 10.1126/science.1103333. [DOI] [PubMed] [Google Scholar]
- 23.Cheng Y, Austin SC, Rocca B, et al. Role of prostacyclin in the cardiovascular response to thromboxane A2. Science. 2002;296:539–41. doi: 10.1126/science.1068711. [DOI] [PubMed] [Google Scholar]
- 24.Wang M, Zukas AM, Hui Y, Ricciotti E, Pure E, FitzGerald GA. Deletion of microsomal prostaglandin E synthase-1 augments prostacyclin and retards atherogenesis. Proc Natl Acad Sci U S A. 2006;103:14507–12. doi: 10.1073/pnas.0606586103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lopez-Garcia E, Schulze MB, Meigs JB, et al. Consumption of trans fatty acids is related to plasma biomarkers of inflammation and endothelial dysfunction. J Nutr. 2005;135:562–6. doi: 10.1093/jn/135.3.562. [DOI] [PubMed] [Google Scholar]
- 26.Esposito K, Giugliano D. Diet and inflammation: a link to metabolic and cardiovascular diseases. Eur Heart J. 2006;27:15–20. doi: 10.1093/eurheartj/ehi605. [DOI] [PubMed] [Google Scholar]
- 27.Giugliano D, Ceriello A, Esposito K. The Effects of Diet on Inflammation: Emphasis on the Metabolic Syndrome. J Am Coll Cardiol. 2006;48:677–85. doi: 10.1016/j.jacc.2006.03.052. [DOI] [PubMed] [Google Scholar]
- 28.Oshima M, Dinchuk JE, Kargman SL, et al. Suppression of intestinal polyposis in Apc delta716 knockout mice by inhibition of cyclooxygenase 2 (COX-2) Cell. 1996;87:803–9. doi: 10.1016/s0092-8674(00)81988-1. [DOI] [PubMed] [Google Scholar]
- 29.Koehne CH, Dubois RN. COX-2 inhibition and colorectal cancer. Semin Oncol. 2004;31:12–21. doi: 10.1053/j.seminoncol.2004.03.041. [DOI] [PubMed] [Google Scholar]
- 30.Schnitzer TJ, Hochberg MC. COX-2-selective inhibitors in the treatment of arthritis. Cleve Clin J Med. 2002;69(Suppl 1):SI20–30. doi: 10.3949/ccjm.69.suppl_1.si20. [DOI] [PubMed] [Google Scholar]
- 31.Reddy ST, Anantharamaiah GM, Navab M, et al. Oral amphipathic peptides as therapeutic agents. Expert Opin Investig Drugs. 2006;15:13–21. doi: 10.1517/13543784.15.1.13. [DOI] [PubMed] [Google Scholar]
- 32.Goldstein JL, Ho YK, Basu SK, Brown MS. Binding site on macrophages that mediates uptake and degradation of acetylated low density lipoprotein, producing massive cholesterol deposition. Proc Natl Acad Sci U S A. 1979;76:333–7. doi: 10.1073/pnas.76.1.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chan ES, Zhang H, Fernandez P, et al. Effect of COX inhibition on cholesterol efflux proteins and atheromatous foam cell transformation in THP-1 human macrophages: a possible mechanism for increased cardiovascular risk. Arthritis Res Ther. 2007;9:R4. doi: 10.1186/ar2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Metzner J, Popp L, Marian C, et al. The effects of COX-2 selective and non-selective NSAIDs on the initiation and progression of atherosclerosis in ApoE(−/−) mice. J Mol Med. 2007 doi: 10.1007/s00109-007-0162-9. [DOI] [PubMed] [Google Scholar]
- 35.Fitzgerald GA. Coxibs and cardiovascular disease. N Engl J Med. 2004;351:1709–11. doi: 10.1056/NEJMp048288. [DOI] [PubMed] [Google Scholar]
- 36.Zhang J, Goorha S, Raghow R, Ballou LR. The tissue-specific, compensatory expression of cyclooxygenase-1 and -2 in transgenic mice. Prostaglandins Other Lipid Mediat. 2002;67:121–35. doi: 10.1016/s0090-6980(01)00177-0. [DOI] [PubMed] [Google Scholar]
- 37.Kirtikara K, Morham SG, Raghow R, et al. Compensatory prostaglandin E2 biosynthesis in cyclooxygenase 1 or 2 null cells. J Exp Med. 1998;187:517–23. doi: 10.1084/jem.187.4.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gilroy DW, Colville-Nash PR, Willis D, Chivers J, Paul-Clark MJ, Willoughby DA. Inducible cyclooxygenase may have anti-inflammatory properties. Nat Med. 1999;5:698–701. doi: 10.1038/9550. [DOI] [PubMed] [Google Scholar]