Abstract
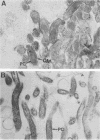
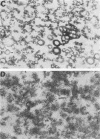
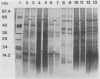
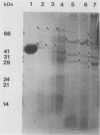
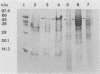
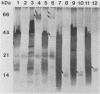
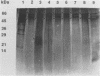
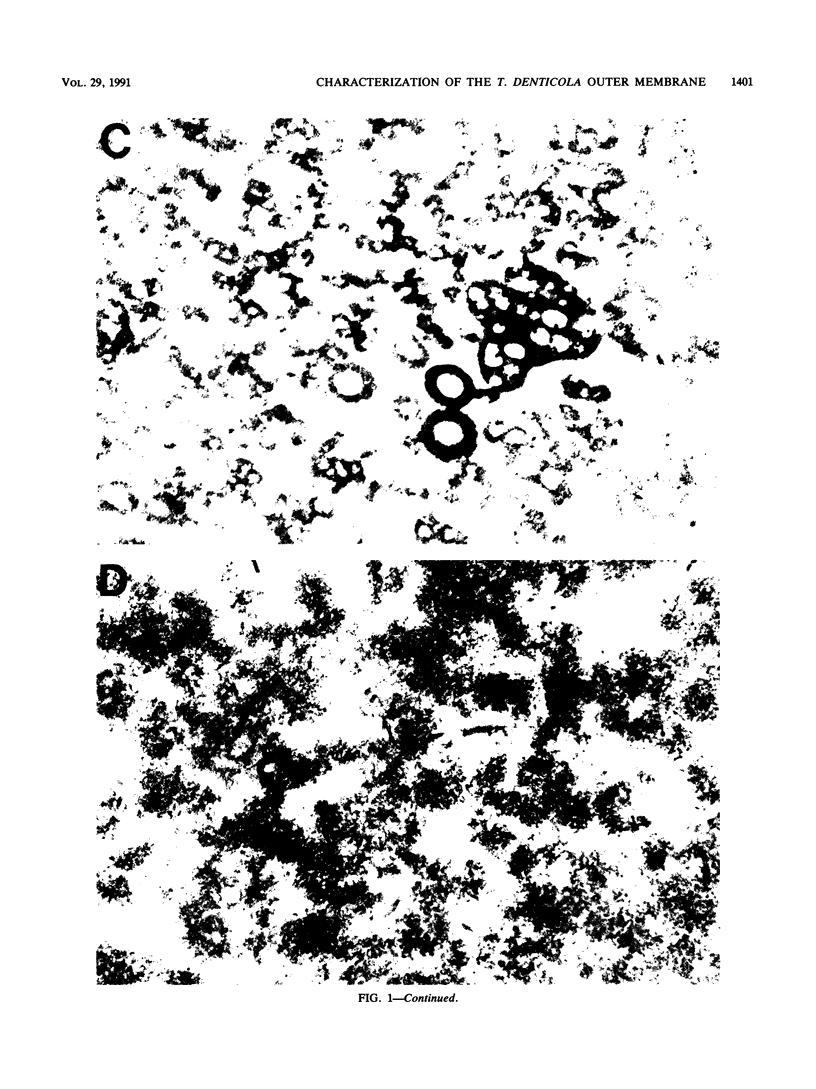
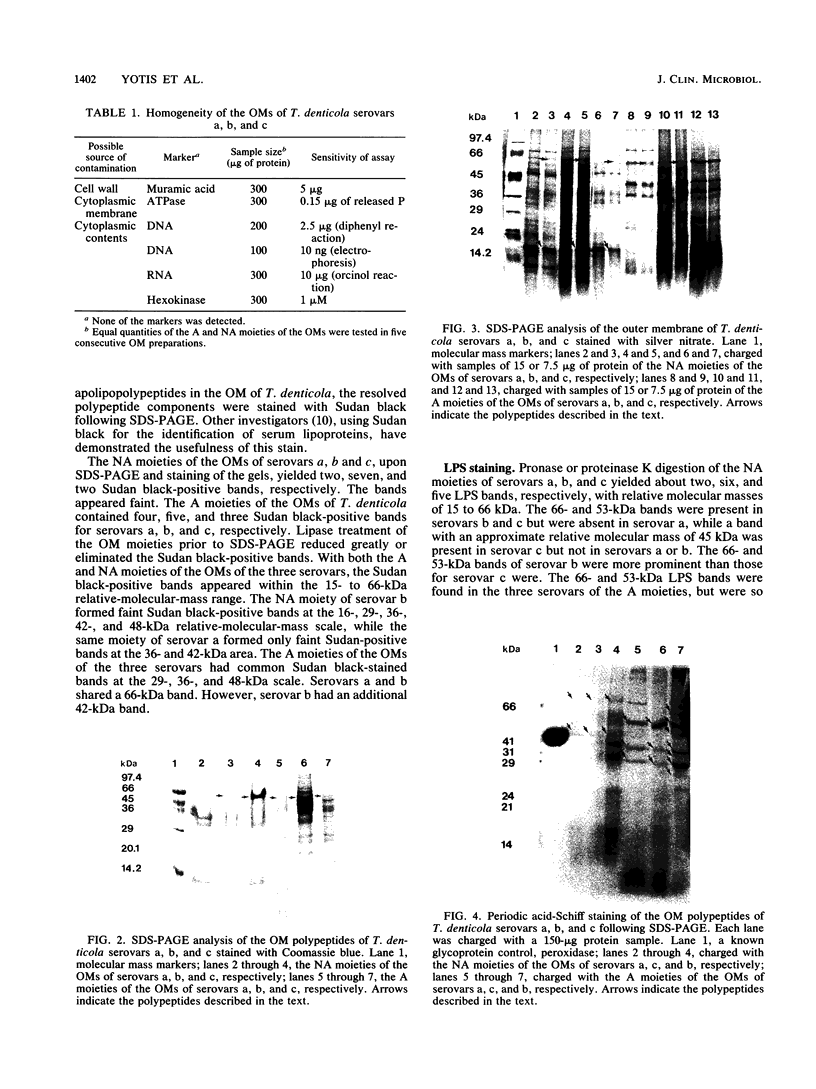
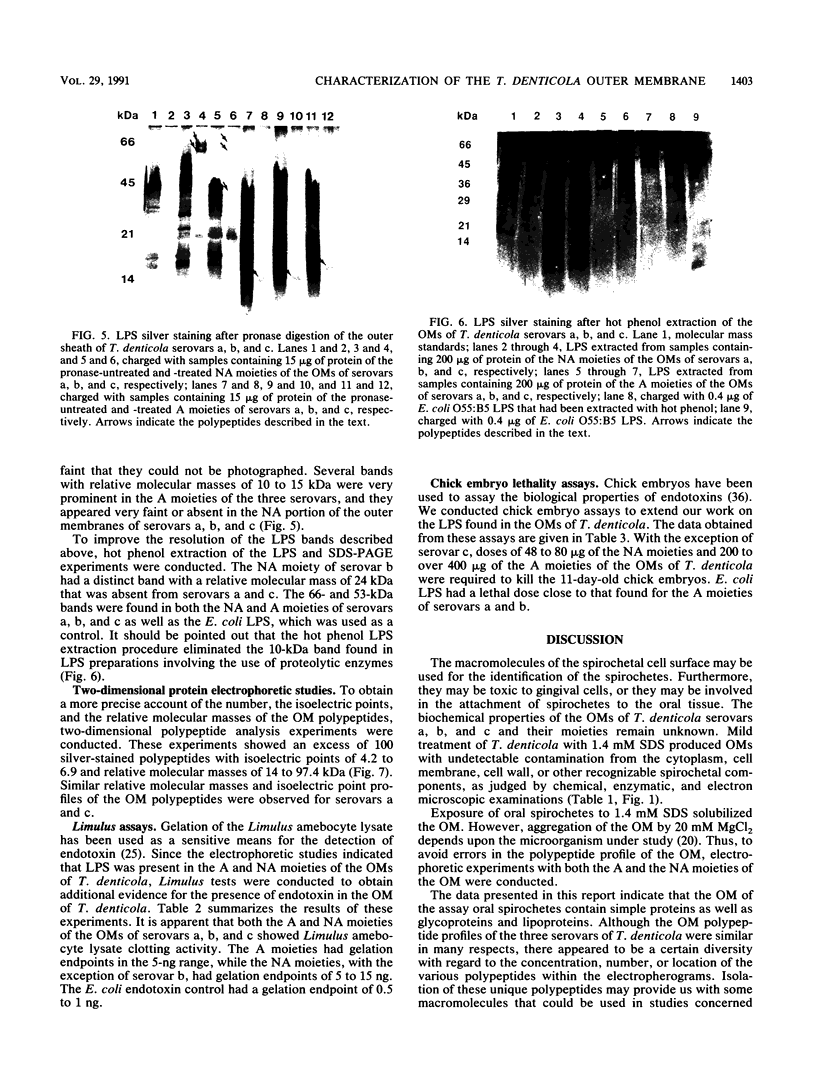
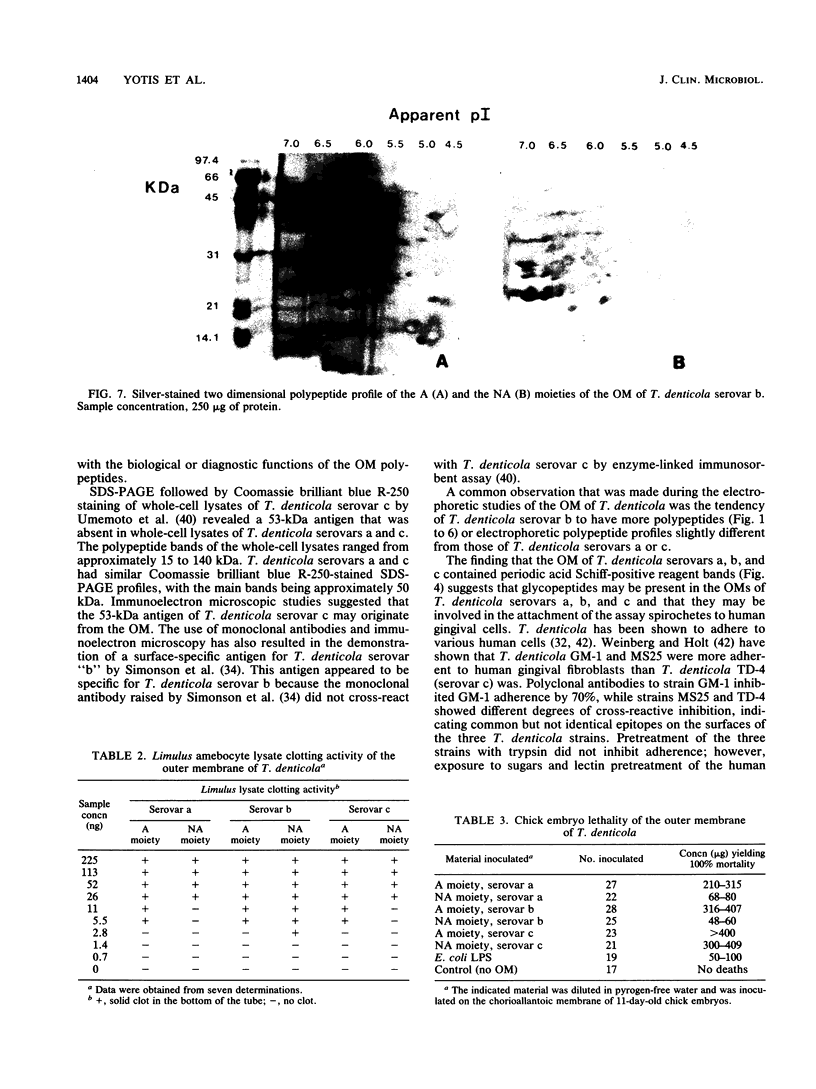
The outer membranes (OMs) from serovars a, b, and c of Treponema denticola, originally isolated from periodontal patients, were prepared. Dialysis of the OMs against 20 mM MgCl2 yielded the aggregable (A) and the nonaggregable (NA) moieties of the OMs. The absence of muramic acid, adenosine triphosphatase, hexokinase, and nucleic acid as well as electron microscopy indicated that the OM preparations were homogeneous. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis of the A and NA moieties of the OMs showed approximately 25 Coomassie brilliant blue R-250 stain-positive bands or 47 silver-stained polypeptides. The relative molecular masses ranged between 14 and 97 kDa. The electrophoretic polypeptide profiles of the A and NA moieties shared many similarities among serovars a, b, and c. However, they exhibited variation in the overall pattern, intensity, or location of the polypeptide stained zones. This was especially true for serovar b. Two-dimensional electrophoretic studies showed an excess of 100 silver-stained spots with isoelectric points of 4.6 to 7.0 and relative molecular masses in the 14- to 97-kDa range. The OMs contained simple proteins, glycoproteins, and lipoproteins. The NA moieties of the OMs contained 4 to 6, 10 to 12, and 4 to 6 glycopeptides as well as two, seven, and two lipoprotein bands for serovars a, b, and c, respectively. The A moieties of the OMs showed 7 to 9, 11 to 13 and 5 to 6 glycopeptides as well as four, five, and three lipoprotein bands for serovars a, b, and c, respectively. Lipopolysaccharide was detected in the OMs of the three serovars following removal of proteins with proteinase K, pronase and silver staining of sodium dodecyl sulfate-polyacrylamide gels, or removal of lipopolysaccharide from the OMs by hot phenol extraction. The 66- and 53-kDa bands were present in serovars b and c, while a band with a relative molecular mass of 45 kDa was present only in serovar c. Endotoxin-like activity was also shown in the OMs of the three serovars by the Limulus amebocyte clotting assay and the chick embryo lethality test. This is the first report on selected biochemical properties of the OM macromolecules of three known serovars of T. denticola.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore R. P., Canale-Parola E. Arginine catabolism by Treponema denticola. J Bacteriol. 1976 Nov;128(2):616–622. doi: 10.1128/jb.128.2.616-622.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehringer H., Taichman N. S., Shenker B. J. Suppression of fibroblast proliferation by oral spirochetes. Infect Immun. 1984 Jul;45(1):155–159. doi: 10.1128/iai.45.1.155-159.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canale-Parola E. Physiology and evolution of spirochetes. Bacteriol Rev. 1977 Mar;41(1):181–204. doi: 10.1128/br.41.1.181-204.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng S. L., Siboo R., Quee T. C., Johnson J. L., Mayberry W. R., Chan E. C. Comparative study of six random oral spirochete isolates. Serological heterogeneity of Treponema denticola. J Periodontal Res. 1985 Nov;20(6):602–612. doi: 10.1111/j.1600-0765.1985.tb00844.x. [DOI] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Fitzgerald T. J. Pathogenesis and immunology of Treponema pallidum. Annu Rev Microbiol. 1981;35:29–54. doi: 10.1146/annurev.mi.35.100181.000333. [DOI] [PubMed] [Google Scholar]
- Godolphin W. J., Stinson R. A. Isoelectrofocusing of human plasma lipoproteins in polyacrylamide gels: diagnosis of type III hyperlipoproteinemia ("broad beta" disease). Clin Chim Acta. 1974 Oct 15;56(1):97–103. doi: 10.1016/0009-8981(74)90197-1. [DOI] [PubMed] [Google Scholar]
- Hadzija O. A simple method for the quantitative determination of muramic acid. Anal Biochem. 1974 Aug;60(2):512–517. doi: 10.1016/0003-2697(74)90261-9. [DOI] [PubMed] [Google Scholar]
- Hill H. D., Straka J. G. Protein determination using bicinchoninic acid in the presence of sulfhydryl reagents. Anal Biochem. 1988 Apr;170(1):203–208. doi: 10.1016/0003-2697(88)90109-1. [DOI] [PubMed] [Google Scholar]
- Hitchcock P. J., Brown T. M. Morphological heterogeneity among Salmonella lipopolysaccharide chemotypes in silver-stained polyacrylamide gels. J Bacteriol. 1983 Apr;154(1):269–277. doi: 10.1128/jb.154.1.269-277.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochstrasser D. F., Harrington M. G., Hochstrasser A. C., Miller M. J., Merril C. R. Methods for increasing the resolution of two-dimensional protein electrophoresis. Anal Biochem. 1988 Sep;173(2):424–435. doi: 10.1016/0003-2697(88)90209-6. [DOI] [PubMed] [Google Scholar]
- Hochstrasser D. F., Patchornik A., Merril C. R. Development of polyacrylamide gels that improve the separation of proteins and their detection by silver staining. Anal Biochem. 1988 Sep;173(2):412–423. doi: 10.1016/0003-2697(88)90208-4. [DOI] [PubMed] [Google Scholar]
- Jackson S. W., Zey P. N. Ultrastructure of lipopolysaccharide isolated from Treponema pallidum. J Bacteriol. 1973 May;114(2):838–844. doi: 10.1128/jb.114.2.838-844.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson P. H., Grossman L. I. Electrophoresis of DNA in agarose gels. Optimizing separations of conformational isomers of double- and single-stranded DNAs. Biochemistry. 1977 Sep 20;16(19):4217–4225. doi: 10.1021/bi00638a014. [DOI] [PubMed] [Google Scholar]
- Johnson R. C., Wachter M. S., Ritzi D. M. Treponeme outer cell envelope: solubilization and reaggregation. Infect Immun. 1973 Feb;7(2):249–258. doi: 10.1128/iai.7.2.249-258.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kido N., Ohta M., Kato N. Detection of lipopolysaccharides by ethidium bromide staining after sodium dodecyl sulfate-polyacrylamide gel electrophoresis. J Bacteriol. 1990 Feb;172(2):1145–1147. doi: 10.1128/jb.172.2.1145-1147.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubak B. M., Yotis W. W. Staphylococcus aureus adenosine triphosphatase: inhibitor sensitivity and release from membrane. J Bacteriol. 1981 Apr;146(1):385–390. doi: 10.1128/jb.146.1.385-390.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEVIN J., BANG F. B. THE ROLE OF ENDOTOXIN IN THE EXTRACELLULAR COAGULATION OF LIMULUS BLOOD. Bull Johns Hopkins Hosp. 1964 Sep;115:265–274. [PubMed] [Google Scholar]
- LUFT J. H. Improvements in epoxy resin embedding methods. J Biophys Biochem Cytol. 1961 Feb;9:409–414. doi: 10.1083/jcb.9.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Listgarten M. A. The role of dental plaque in gingivitis and periodontitis. J Clin Periodontol. 1988 Sep;15(8):485–487. doi: 10.1111/j.1600-051x.1988.tb01019.x. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Oakley B. R., Kirsch D. R., Morris N. R. A simplified ultrasensitive silver stain for detecting proteins in polyacrylamide gels. Anal Biochem. 1980 Jul 1;105(2):361–363. doi: 10.1016/0003-2697(80)90470-4. [DOI] [PubMed] [Google Scholar]
- Ohta K., Makinen K. K., Loesche W. J. Purification and characterization of an enzyme produced by Treponema denticola capable of hydrolyzing synthetic trypsin substrates. Infect Immun. 1986 Jul;53(1):213–220. doi: 10.1128/iai.53.1.213-220.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen I. Attachment of Treponema denticola to cultured human epithelial cells. Scand J Dent Res. 1984 Feb;92(1):55–63. doi: 10.1111/j.1600-0722.1984.tb00860.x. [DOI] [PubMed] [Google Scholar]
- SMITH R. T., THOMAS L. The lethal effect of endotoxins on the chick embryo. J Exp Med. 1956 Aug 1;104(2):217–231. doi: 10.1084/jem.104.2.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonson L. G., Goodman C. H., Bial J. J., Morton H. E. Quantitative relationship of Treponema denticola to severity of periodontal disease. Infect Immun. 1988 Apr;56(4):726–728. doi: 10.1128/iai.56.4.726-728.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonson L. G., Rouse R. F., Bockowski S. W. Monoclonal antibodies that recognize a specific surface antigen of Treponema denticola. Infect Immun. 1988 Jan;56(1):60–63. doi: 10.1128/iai.56.1.60-63.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith P. K., Krohn R. I., Hermanson G. T., Mallia A. K., Gartner F. H., Provenzano M. D., Fujimoto E. K., Goeke N. M., Olson B. J., Klenk D. C. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985 Oct;150(1):76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Switzer R. C., 3rd, Merril C. R., Shifrin S. A highly sensitive silver stain for detecting proteins and peptides in polyacrylamide gels. Anal Biochem. 1979 Sep 15;98(1):231–237. doi: 10.1016/0003-2697(79)90732-2. [DOI] [PubMed] [Google Scholar]
- Tsai C. M. The analysis of lipopolysaccharide (endotoxin) in meningococcal polysaccharide vaccines by silver staining following SDS-polyacrylamide gel electrophoresis. J Biol Stand. 1986 Jan;14(1):25–33. doi: 10.1016/s0092-1157(86)80006-3. [DOI] [PubMed] [Google Scholar]
- Umemoto T., Namikawa I., Suido H., Asai S. A major antigen on the outer envelope of a human oral spirochete, Treponema denticola. Infect Immun. 1989 Aug;57(8):2470–2474. doi: 10.1128/iai.57.8.2470-2474.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Pringle J. R., Osborn M. Measurement of molecular weights by electrophoresis on SDS-acrylamide gel. Methods Enzymol. 1972;26:3–27. doi: 10.1016/s0076-6879(72)26003-7. [DOI] [PubMed] [Google Scholar]
- Weinberg A., Holt S. C. Interaction of Treponema denticola TD-4, GM-1, and MS25 with human gingival fibroblasts. Infect Immun. 1990 Jun;58(6):1720–1729. doi: 10.1128/iai.58.6.1720-1729.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]