Abstract
Liver regeneration after partial hepatectomy is a very complex and well-orchestrated phenomenon. It is carried out by the participation of all mature liver cell types. The process is associated with signaling cascades involving growth factors, cytokines, matrix remodeling, and several feedbacks of stimulation and inhibition of growth related signals. Liver manages to restore any lost mass and adjust its size to that of the organism, while at the same time providing full support for body homeostasis during the entire regenerative process. In situations when hepatocytes or biliary cells are blocked from regeneration, these cell types can function as facultative stem cells for each other.
Liver is an interesting organ with high regenerative capacity and complex functions (Michalopoulos and DeFrances, 1997; Taub, 2004; Michalopoulos and Khan, 2005c; Fausto et al., 2006). Liver receives all exiting circulation from the small and most of the large intestine, as well as spleen and pancreas, through the portal vein. Its “strategic” location in relation to the food supply via the portal vein, and the unique gene-and protein-expression patterns of hepatocytes (the main functional cells of the liver) allow it to function as a biochemical defense against toxic chemicals entering through the food and as a re-processor of absorbed food ingredients. Nutrients entering the liver are transformed into secreted proteins (albumin, most coagulation factors, several plasma carrier proteins etc. in the peripheral blood), lipids sent as lipoproteins into the other tissues, carbohydrates stored in the liver as glycogen (the main glucose reserve used for stabilization of glucose levels in the blood). Synthesis of bile is essential for absorption of fat and lipophilic nutrients. As a major regulator of plasma glucose and ammonia levels, liver is essential for optimal function of the brain. Loss of liver function leads to chronic “hepatic encephalopathy” and eventually coma. The wide array of functions performed by liver towards the rest of the body has been safeguarded by evolutionary events which imparted to liver a phenomenal capacity to regenerate. This process allows liver to recover lost mass without jeopardizing viability of the entire organism. The phenomenon of liver regeneration following loss of liver mass is seen in all vertebrate organisms, from humans to fish. It is also triggered when livers from small animals (e.g., dogs) are transplanted to large recipients of the same species. It has been recorded and mythologized in ancient times from the myth of Prometheus and libraries of clay tables picturing scarred livers of sacrificial animals, used to foretell the future in ancient Babylon and Rome (Michalopoulos and DeFrances, 1997).
Loss of liver mass can be induced by administering hepatotoxic chemicals (e.g., carbon tetrachloride). This is followed by an inflammatory response which removes tissue debris, followed by the regenerative response. Most commonly, however, regeneration of the liver is studied by performing a surgical procedure which removes 2/3 of the liver mass in rodents (rats and mice), a technique known as 2/3 partial hepatectomy (PHx) (Higgins, 1931). Due to the multi-lobe structure of the rodent liver, three of the five liver lobes (representing 2/3 of the liver mass) can be removed by an easy surgical procedure, without causing any tissue damage to the residual two lobes. The latter grow in size to restore an aggregate equivalent to the mass of the original five lobes. The process, in rats and mice, is complete within 5–7 days after surgery. The reproducibility of PHx in terms of mass removed and precision of timing of the sequence of ensuing events has made PHx the preferred approach for experimental study of liver regeneration. In a clinical setting, this procedure is also done in humans, in order to resect solitary liver metastases or repair trauma, etc.
PHx triggers a sequence of events that proceed in an orderly fashion and can be observed from the first 5 min to 5–7 days. Hepatocytes are the first cells to enter into DNA synthesis. A 2/3 PHx leaves a residual 1/3 of hepatocytes. They undergo one round of DNA synthesis (leading to 60% of hepatocytes) which peaks at 24h for the rat and at approximately 36 h for the mouse (Unless otherwise specified, times after PHx referred in this review will follow the time table of regeneration in the rat, which is more reproducible). A second smaller percent of cells enter into a second round of DNA synthesis and establish the original number of hepatocytes. A small wave of apoptosis of hepatocytes seen at the end of DNA synthesis suggests that this is a mechanism to correct an over-shooting of the regenerative response (Sakamoto et al., 1999). The proliferation of hepatocytes advances from periportal to pericentral areas of the lobule, as a wave of mitoses (Rabes, 1977). Hepatocytes surrounding the central veins (positive for glutamine synthetase (Gebhardt et al., 2007) are the last ones to undergo cell replication. Proliferation of biliary epithelial cells occurs a little later than hepatocytes. Proliferation of endothelial cells starts at 2–3 days and ends around 4–5 days after PHx. The kinetics of proliferation of stellate cells has not been fully explored. Stellate cells are cells of myofibroblastic origin, surrounding hepatocytes, located under the sinusoidal cells, producing extracellular matrix and several cytokines including HGF, and having a gene expression pattern substantially similar to the astrocytes of the brain (Neubauer et al., 1996; Cassiman et al., 2001). It should be emphasized that replacement of the lost hepatic mass is mediated through proliferation of mature adult hepatocytes and the other hepatic cell types. It is not mediated by proliferation of a selective subpopulation of stem cells (as in skin and small intestine). Normal liver weight is reestablished within 5–7 days (8–15 days in humans). At the end of regeneration, the size of the liver lobules is remarkably larger and the thickness of the hepatocyte plates is almost twice the size of the normal one cell thickness (Michalopoulos and DeFrances, 1997). Previous studies suggest that there is slow lobular reorganization taking place for several weeks, and eventually liver histology becomes indistinguishable from the original (Wagenaar et al., 1993).
Broadly defined, partial hepatectomy is a type of liver injury, though no immediate histological damage results from it. Thus, it is not surprising that the signaling pathways triggered during liver regeneration strongly resemble those of wound healing, seen in other tissues. The difference with the classic wound healing process is that the changes observed in liver occur over the entire organ (largest single organ in the body!) and that some of the signals may be derived in part from the peripheral circulation.
Hemodynamic Changes Following 2/3 Partial Hepatectomy
In a typical wound healing scenario, the injury to the tissue results in disruption of capillary vascular networks and extravasation of blood, accompanied by local release of coagulation factors, platelets, growth factors, etc. (Schafer and Werner, 2007). This is clearly not the case following 2/3 PHx. Three liver lobes are surgically removed without damage to the residual two lobes. Even though there is no damage to the residual tissue, there are big changes in hepatic blood flow patterns. There is considerable literature suggesting that the early hemodynamic changes after PHx are important, and, even though there is no extravasation of whole blood, the hemodynamic alterations after PHx induce a global spectrum of events across the entire liver that resembles a wound healing response. The arterial component of the blood supply per unit of liver tissue does not change after 2/3 PHx; the portal contribution per unit tissue, however, triples. Portal vein continues to carry the entire outflow from intestine, spleen and pancreas. The entire flow now needs to traverse through a capillary bed whose cross-section is mathematically down to 1/3 of the original. The hepatic capillaries have fenestrated endothelial cells which bring direct access of plasma through the endothelial cells to the hepatocytes. A recent study demonstrated that if these changes are prevented by keeping pressure of the portal vein constant, there is deficient activation of HGF and increased hepatocyte apoptosis, even though the kinetics of PCNA nuclear labeling do not seem to be affected (Marubashi et al., 2004). Another point that needs to be better understood is the potential impact of the change of oxygen partial pressure in the hepatic blood after PHx. Portal vein blood has a much lower oxygen concentration compared to the arterial blood. The relative increase in portal blood per unit liver tissue after PHx should result in decreased oxygen pressure in the circulating blood, perhaps triggering a hypoxic response. Recent studies however have shown that hypoxia in the liver may be regulated through pathways different than the classic ones, since HIF1α is not identified in hepatocyte nuclei, but it is instead seen in peroxisomes and mitochondria (Khan et al., 2006). The tripling of the portal vein contribution should also cause a mathematical tripling in the availability per hepatocyte of growth factors and cytokines derived from intestine and pancreas. Such factors include insulin and epidermal growth factor (EGF), endotoxin, as well as nutrients derived from the food supply (amino acids, lipids, and carbohydrates). Overall, of all aspects of liver regeneration, the importance of the hemodynamic events and the change of relative proportion of portal to arterial blood are the least studied and least understood. There is an almost universal agreement however that the aggregate changes described above trigger the better understood and better studied changes of signaling pathways in liver tissue, described below.
Early Events Occurring in Liver After PHx
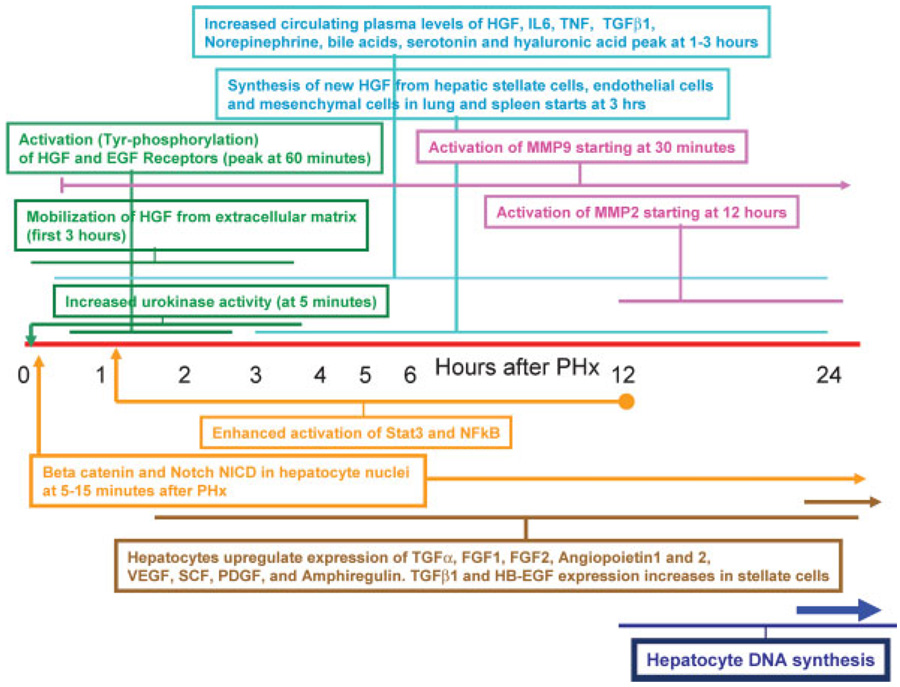
PHx induces rapid induction of more than 100 genes not expressed in normal liver (Taub, 1996, 2004). These genes relate directly or indirectly to preparative events for the entry of hepatocytes into the cell cycle. The functions served are several and many of these genes (e.g., IGFBP1) appear to play an essential role. Mice deficient in IGFBP1, for example, have deficient regenerative response (Leu et al., 2003). The precise role of the many genes expressed early in liver regeneration is not always clear and the early changes in gene expression should be viewed as serving both the entry of hepatocytes into the cell cycle as well the orchestration of specific adjustments that hepatocytes have to make, so that they can deliver all essential hepatic functions while going through cell proliferation. Given the fact that more than 95% of hepatocytes go through cell proliferation during 48 h, it is truly amazing that the support provided by liver to the whole body is not perceptibly diminished during regeneration. One of the earliest observed biochemical changes is increase in activity of urokinase plasminogen activator (uPA) (Fig. 1). This occurs over the entire tissue of the remnant lobes. As seen in early stages of wound healing (Kortlever and Bernards, 2006), there is increase in uPA activity throughout the entire liver starting as early as 5 min after PHx (Mars et al., 1995). The relationship between increase in uPA and the hemodynamic changes discussed above is not clear, but there is literature documenting increase of uPA in several cell types including endothelial cells following mechanical stress associated with increased turbulent flow (Sokabe et al., 2004). Thus, alterations of vascular flow patterns alone can trigger some of the early events. Increase in uPA activity is accompanied by activation of plasminogen to plasmin (within 10 min) and appearance of fibrinogen degradation products (Kim et al., 1997). Urokinase is known to activate matrix remodeling, seen in most tissues during wound healing and also in liver regeneration. Many proteins of the extracellular matrix are subject to turnover (Kim et al., 1997). The first evidence of activation of metalloproteinase 9 (MMP9) is seen at 30 min and further into the first 24–48 h after PHx (Kim et al., 2000). Studies from wound healing and tumor biology have shown that matrix remodeling causes signaling though integrins and is associated with release of locally bound growth factors and peptides that have signaling capabilities (Swindle et al., 2001). While there is not much proteinaceous matrix in the liver visible under the microscope, there is a great abundance of heavily glycosylated proteins in the pericellular space surrounding hepatocytes. Glycosaminoglycans are very abundant in liver and heparin, a shorter derivative, owes its name to liver (“hepar”). Overall regulation of extracellular matrix during liver regeneration is a very complex process, involving metalloproteinases and tissue inhibitors of metalloproteinases (Mohammed and Khokha, 2005a). Hepatic extracellular matrix binds many growth factors. Prominent among matrix binding growth factors in the liver is hepatocyte growth factor (HGF) (Masumoto and Yamamoto, 1991, 1993). Inactive, single-chain HGF bound to hepatic biomatrix is locally released during matrix remodeling and activated to its active heterodimeric form by uPA (Mars et al., 1993, 1995) (HGF is highly homologous to plasminogen, the recognized classic target of uPA; HGF and plasminogen have the same consensus sequence (RVV) at their activation site). Activated HGF is available locally, but it also overflows in the circulation (Lindroos et al., 1991). Pre-existing stores of inactive and active HGF rapidly diminish with the first 3 h after PHX, as HGF rises in the plasma by 10- to 20-fold (Pediaditakis et al., 2001). Metalloproteinases and TIMP levels are important in regulation of release of HGF and its availability for activation during regeneration (Mohammed et al., 2005b). Studies with hepatocytes in culture suggest that TNF may play a role in this process by inducing expression of MMP9 by hepatocytes (Haruyama et al., 2000). Overall, matrix remodeling is a very important component of liver regeneration and mice with genetic elimination of MMP9 have defective regeneration (Olle et al., 2006). Release and activation of HGF by these events results in activation of cMet within 30–60 min after PHx (Stolz et al., 1999). Other substances released locally and in the peripheral circulation shortly (within 1 h) after PHx are hyaluronic acid (a major component of hepatic biomatrix) and TGFβ1 (Michalopoulos and DeFrances, 1997). TGFβ1 is a known hepatocyte mito-inhibitor (Houck et al., 1988). When the receptor TGFβR1 is rendered inactive in normal, non-regenerating liver by injecting dominant negative DNA constructs, there is a noticeable increase in DNA synthesis of hepatocytes (Ichikawa et al., 2001). This suggests that TGFβ1 (bound to decorin) exercises a competing tonic effect against opposing effects of matrix bound growth factors, keeping hepatocytes of the normal liver in a state of quiescence. Matrix remodeling after PHx dramatically alters this balance between mitogens and mitoinhibitors, by causing release (local and in the circulation) and activation of HGF while releasing TGFβ1 massively in the circulation, where TGFβ1 is bound and inactivated by alpha-2-macroglobulin (LaMarre et al., 1991). The EGFR is also activated with the same kinetics as cMet (Stolz et al., 1999). EGF is constantly available to the liver through the portal circulation, produced by exocrine glands of the duodenum (Brunner’s glands) (Olsen et al., 1985). The similar time kinetics of activation of the HGF and EGF receptors suggest that EGF, now targeting fewer hepatocytes, has an enhanced effect; or that the EGFR becomes more sensitive to EGF. Independent work suggests that the latter may be true. Norepinephrine, which also increases in the peripheral circulation, is known to enhance the effect of HGF and EGF receptors in hepatocyte cultures through the alpha-1 adrenergic receptor (Cruise et al., 1985). Blockade of the alpha-1 receptor suppresses liver regeneration (Cruise et al., 1987). Other studies have also shown that there is a cross-talk between Met and EGFR and it is possible that activation of Met enhances activation of EGFR (Jo et al., 2000). Also increasing in circulation are the concentrations of TNF (Yamada et al., 1998), bile acids (Huang et al., 2006), IL6 (Cressman et al., 1996), and serotonin (Lesurtel et al., 2006). The changes in concentrations of all of these signaling molecules in the plasma probably account for older observations in which the signal(s) for liver regeneration is transmitted by blood in pairs of parabiotic rats (Moolten and Bucher, 1967) and in fragments of transplanted hepatic tissue (Leong et al., 1964) or isolated hepatocytes in the adipose tissue (Jirtle and Michalopoulos, 1982) when the orthotopic liver is subjected to partial hepatectomy.
Fig. 1.
Chronology of key events occurring at the early stages of liver regeneration after partial hepatectomy. Events within similarly colored boxes belong in the same category (e.g., green: growth factor related events; blue: plasma related changes, etc.). The associated horizontal lines for each box delineate the beginning and the duration of each signal.
As these events unfold outside of hepatocytes, many events are occurring inside the cell. beta catenin (Monga et al., 2001) and the Notch-1 intracellular domain (NICD) (Kohler et al., 2004) appear in hepatocyte nuclei within 15–30 min after PHx. Elimination of expression of these proteins by RNA interference (Kohler et al., 2004) decreases the regenerative response. Within 1 h after PHx, there is evidence for enhanced activation of Stat3 (Cressman et al., 1995) and NFkB (FitzGerald et al., 1995; Yamada et al., 1998). Elimination of either of these two signaling molecules does not abrogate the regenerative response, probably due to the existence of redundant pathways which complement for the loss of the proteins (e.g., Stat1 assuming the role of Stat3) (DeAngelis et al., 2001; Li et al., 2002). Despite these findings however, Stat3 and NFkB are important signaling molecules associated with cell cycle events in many cells types and their early activation in hepatocytes undoubtedly is an important contribution to the signaling pathways leading to proliferation. At 6 h after PHx there is clear evidence for activation of cyclin D1. Amino acids and TOR play a regulatory role in this process (Nelsen et al., 2003). In the rat, the first round of DNA synthesis in hepatocytes begins at 12 h after PHx, with a peak of DNA synthesis seen at 24 h. In the mouse, these events are frame-shifted later by 6–12 h (Michalopoulos and DeFrances, 1997). Throughout the first 2–3 days of regeneration, there is decrease in the ratio of C/EBPα to C/EBPβ, a process thought as underlying some of the shifts in metabolism that occur in liver during this time, such as enhanced lipid synthesis (Friedman et al., 2004). Analysis of the gene expression networks operating during liver regeneration has revealed alterations dependent on both growth factors and cytokines (Taub et al., 1999; White et al., 2005).
The events occurring in the early period of 0–5 h after PHx have often been called “priming” (Fausto, 2000). The term is a useful one, in that it denotes not only events associated for preparation for entry into the cell cycle, but also events and strategies of hepatocytes aimed at modifying patterns of gene expression so that they continue to deliver their homeostatic functions. “Priming,” however, has also been used to denote a time in which events occurring are induced only by cytokines, with events induced by growth factors occurring after the cytokine-mediated events. The findings described above, however, clearly denote that there is no demarcation point that can be ascribed to separate events induced only by cytokines or only by growth factors. The time kinetics in changes and effects of both cytokines and growth factors (see below) are intertwined and synchronous, for example, activation of growth factor receptors (Met and EGFR) occurs fully within 30 min after PHx, similar to activation of Stat3 and NFkB. A partial list of the concurrent events occurring in the first 60 min after PHx is shown in Table 1.
TABLE 1.
Chronology of concurrent early (first 1 h) signaling events after PHx
| Multiple signaling pathways involving both growth factors, cytokines, paracrine signals, and neuroendocrine factors occur simultaneously within the first 60 min after PHx. These include: |
| • Increase in urokinase activity (first 5 min) |
| • Translocation of N(otch) ICD to the nucleus (15 min) |
| • Translocation of beta-catenin to the nucleus (5–10 min to 6 h) |
| • Decrease in HGF biomatrix stores (30 min to 3 h) |
| • Activation of the HGF receptor (within 30–60 min) |
| • Activation of the EGF receptor (within 30–60 min) |
| • Increase of HGF, Norepinephrine, IL6, TNFa, TGFb1 and hyaluronic acid in the plasma (1–2 h) |
| • Activation of AP1, NFkB, and STAT3 (30–60 min) |
| • Extensive gene expression reprogramming of hepatocytes within 30 min after PHx |
Mitogenic Signals Associated With Initiation of Liver Regeneration
A key endpoint of liver regeneration is the restoration of the total number and mass of hepatocytes, the main functional cells of the liver responsible for delivering most of the hepatic functions important for body homeostasis. Hepatocytes are the first cells of the liver to enter into the cell cycle and undergo proliferation, and they produce mitogenic signals for other hepatic cell types (Fig. 2). Quiescent hepatocytes in normal liver express a variety of growth factor receptors. These include receptors for PDGF, VEGF, fibroblast growth factor receptors, c-Kit, Studies with hepatocytes in primary culture however have shown that despite the expression of many mitogenic receptors, the only mitogens for hepatocytes in chemically defined serum-free media are HGF and ligands of the EGFR (EGF, TGFα, Amphiregulin, HB-EGF, etc). These ligands are direct mitogens, in that they induce a strong mitogenic response in hepatocytes in primary culture and clonal expansion of their population (Block et al., 1996). FGF1 and FGF2 are also weak mitogens (Houck et al., 1990). HGF, EGF, and TGFα also induce hepatocyte proliferation and liver enlargement when injected alone into intact normal mice and rats (Bucher et al., 1977; Patijn et al., 1998). In addition to these proteins, however, there are other substances which, although not directly mitogenic to hepatocytes, enhance the effect of the direct mitogens. These include TNF (Webber et al., 1998), Norepinephrine (Cruise et al., 1985), and estrogens (Ni and Yager, 1994). Several studies have emphasized the role of these substances and the kinetics of their expression during liver regeneration. Recent studies have also focused on pro-regenerative effects of components of complement, bile acids, and serotonin, substances not known or tested to have direct or indirect mitogenic effects. Some of the above signaling molecules were implicated based on decreased regeneration when their signaling is eliminated (e.g., HGF, TNF, IL6, bile acids, norepinephrine, serotonin). Others have been implicated because they are mitogenic for hepatocytes in cell culture or in vivo and their signaling receptors appear activated during liver regeneration (e.g., HGF and EGF). We will briefly describe below the main line of evidence associating each of the above signaling molecules in liver regeneration. In general, however, there are two important considerations:
With the possible exception of the HGF/Met signaling pathway (see below), all other signaling pathways may temporarily dampen but do not stop liver regeneration. Other than xenobiotics such as AAF, there is no known signal whose absence or presence permanently arrests liver regeneration. Even with high doses of radiation to the liver, regeneration occurs (primarily by increase in the size of hepatocytes (Michalopoulos and DeFrances, 1997).
The fact that complete elimination of a signaling pathway does not entirely abrogate liver regeneration should not imply that the specific signaling pathway is not important. The precise orchestration of events occurring early after partial hepatectomy probably requires simultaneous presence of all the hitherto discovered extracellular signals. (Precise timing of events is important not only in experimental conditions but also in clinical settings, in which rapid regeneration of the liver makes a “life or death” difference to the organism).
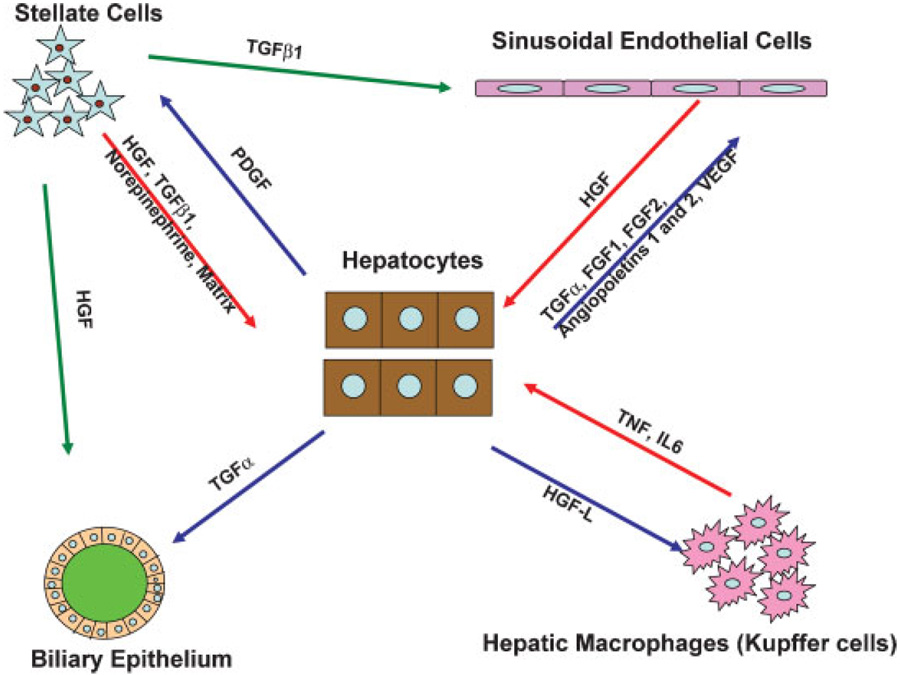
Fig. 2.
Signaling interactions between different hepatic cell types during liver regeneration.
With these two principles in mind, the main signaling pathways known today and implicated for initiation and maintenance of liver regeneration are as follows:
Hepatocyte growth factor (HGF)
It is present in liver matrix in relatively large quantities; it is also found in the matrix of other organs, such as lungs, spleen, placenta, brain, etc. (Matsumoto and Nakamura, 1996; Stella and Comoglio, 1999). Systemically injected HGF is sequestered by the liver more than any other organ (Appasamy et al., 1993). Its receptor, cMet, is expressed in most epithelial cells, endothelial cells and neurons, and mediates all the effects of HGF (Naldini et al., 1991). In addition to its mitogenic and motogenic effects, Met also binds the apoptotic receptor Fas and preventing its trimerization, thus having an anti-apoptotic effect (Wang et al., 2002). Genetic elimination of HGF or its receptor (cMet) is associated with embryonic lethality involving abnormalities in many organs, most notably in placenta (Schmidt et al., 1995; Dietrich et al., 1999; Uehara et al., 2000). Livers of the embryos are smaller than the wild type controls. HGF was isolated from the plasma of partially hepatectomized rats, from studies in search for circulating mitogens increasing in the blood after PHx (Michalopoulos et al., 1984). HGF has been implicated as involved in liver regeneration for the following reasons:
HGF levels in plasma increase 10- to 20-fold after PHx (Lindroos et al., 1991).
Active HGF is consumed from intrahepatic stores in the first 3 h after PHx, followed by new HGF synthesis from 3 to 48 h (Pediaditakis et al., 2001).
HGF causes a strong mitogenic response and clonal expansion of hepatocytes in culture (Block et al., 1996).
HGF injection in portal vein of normal rats and mice causes proliferation of hepatocytes and enlargement of the liver (Liu et al., 1994a; Patijn et al., 1998).
Liver HGF receptor (cMet) becomes activated by tyrosine phosphorylation at 30–60 min after PHx (Stolz et al., 1999).
Activation of Met in hepatocyte cultures drives activation of beta catenin by its phosphorylation on tyrosine residues and promotes its translocation to hepatocyte nucleus (Monga et al., 2002).
Targeted genetic elimination of cMet from the liver is associated with very diminished or absent regenerative response (Borowiak et al., 2004; Huh et al., 2004).
RNA interference after PHx in vivo against cMet is associated with complete blockade of the cell cycle. This effect lasts as long as the RNA interference against Met is active (Paranjpe et al., 2007). The degree of suppression of regeneration in (f) and (g) above suggests that there are unique signaling pathways associated with Met that are not compensated by EGFR or other mechanisms. It should be noted that the same dependence on c-Met has also been found in wound healing (Chmielowiec et al., 2007).
Urokinase is involved in activation of HGF in regenerating liver and mice genetically deficient in urokinase have defective liver regeneration (Roselli et al., 1998). It should be noted that HGF can also be activated by a protease with considerable homology to factor X, known as HGF activator and subject to complex regulation by anti-proteases (Shimomura et al., 1999). This protein is soluble in the plasma and there has been no role identified for it during liver regeneration.
HGF in liver is produced predominantly by the stellate cells (Schirmacher et al., 1993), but also by hepatic endothelial cells (LeCouter et al., 2003). The latter respond to production of VEGF by proliferating hepatocytes, which in turn stimulates production of HGF by endothelial cells, via the non-mitogenic VEGF Receptor I. HGF mRNA increases at 3 h after PHx not only in liver (Zarnegar et al., 1991) but also in lung and spleen (Liu et al., 1994b; Yanagita et al., 1992). The signals triggering this extrahepatic participation are not clear. Norepinephrine and IL6, both rising in the plasma after PHx, are known to stimulate production of HGF in responding cells (Broten et al., 1999; Liu et al., 1994b) are possible candidates for mediating this effect. Studies with hepatocytes in culture have suggested that part of the effects of HGF is caused by stimulation of production of TGFα (Tomiya et al., 2000). However, liver regeneration proceeds normally in TGFα deficient mice (see below) whereas the effects of inactivation of the HGF receptor (g and h above) cannot be compensated by other mitogenic receptors.
The view of HGF as an initiator of liver regeneration is bolstered by the fact that it is a direct mitogen for hepatocytes, it activates its receptor very early, and it can induce most of the changes occurring during liver regeneration (including massive hepatic enlargement) by administration in intact mice and rats. On the other hand, several events occur before HGF becomes demonstrably available (e.g., increase in urokinase, migration of NICD), etc. Currently there is no evidence that one single initiating change after PHx exists which alone leads to liver regeneration. Of all the signals participating in the very early events after PHx, however, given its properties, actions, and impact of elimination of its receptor, the signaling by HGF appears as the most irreplaceable contributor.
Ligands of EGFR
Both ligands of EGFR and the receptor itself are part of a complex signaling system of ligands and ERB receptor family members which establish mitogenic signaling through complex interactions involving receptor ligation, endocytosis, potential recycling to the plasma membrane, etc. This was discussed in the context of liver regeneration in a recent review (Michalopoulos and Khan, 2005c). Though there is high level of redundancy between receptors and ligands, the redundancy of this system is not complete. TGFα deficient mice apparently have normal liver regeneration (Russell et al., 1996), whereas mice deficient in Amphiregulin or HB EGF are reported to have deficient regeneration.
EGF is continually available to the liver through the portal vein, produced from Brunner’s glands of the duodenum and, in male mice, from circulating high levels of EGF produced by the salivary glands (Skov Olsen et al., 1988; Jones et al., 1995). Catecholamines, including epinephrine and norepinephrine, are known to stimulate production of EGF from Brunner’s glands of the duodenum (Olsen et al., 1985) and they rise in plasma after PHx. However, there has not been any direct measurement of portal vein concentration of EGF after PHx. All tested EGFR ligands are direct and strong mitogens for hepatocytes in culture. EGF given in intact animals causes hepatocyte proliferation (Bucher et al., 1977). EGFR is phosphorylated within 30–60 min after PHx. The capacity of liver to regenerate following suppression or targeted elimination of EGFR has not been tested as yet.
TGFα is produced by hepatocytes during regeneration, starting at about 2–3 h after PHx and continuing at high levels for more than 48 h (Mead and Fausto, 1989). TGFα is produced as an inactive precursor penetrating through the plasma membrane. The extracellular domain is cleaved by proteases such as TACE (Lee et al., 2003), to generate the active form. Since hepatocytes express EGFR, the possibility that TGFα production generates an autocrine loop has been considered. Mice with targeted transgenic expression of TGFα in hepatocytes have dramatic liver enlargement and develop tumors (Webber et al., 1994). On the other hand, genetic elimination of TGFα does not affect liver regeneration and despite the large increase in TGFα mRNA, the actual measured increase in protein is rather small (Russell et al., 1993, 1996). TGFα is also a mitogen for endothelial cells and bile duct epithelial cells. It is possible that TGFα production by hepatocytes triggers paracrine effects stimulated by hepatocytes and aimed to engage adjacent hepatic cells into proliferation. This is comparable to observed increases in TGFα in other normal cells in proliferation or tissue repair (e.g., keratinocytes during wound healing, mammary epithelial cells following stimulation by estrogens, and most carcinomas are known to produce high levels of TGFα (Aaronson et al., 1990; Derynck, 1992; Purup et al., 2000).
Heparin Binding EGF (HB EGF) is produced by endothelial cells and Kupffer cells (Kiso et al., 1995). HB EGF production increases within 1.5 h after PHx. HB EGF transgenic mice with liver-targeted production have enhanced regeneration (Kiso et al., 2003) whereas HB EGF knockout mice have deficient regenerative response (Mitchell et al., 2005).
Amphiregulin is another member of the family of EGFR ligands. Mice deficient in Amphiregulin have deficient liver regeneration (Berasain et al., 2005).
Tumor necrosis factor (TNF)
This is a protein known to have a variety of effects on many cells and tissues. Contrary to what its name implies, TNF can often have promitogenic effects on cells, depending on conditions which regulate activation of NFkB (Kirillova et al., 1999). If conditions favor activation of NFkB, then TNF may enhance other concurrently delivered growth signals. Alternatively, if activation of NFkB cannot be mediated by TNF, then TNF may elicit an apoptotic response (Iimuro et al., 1998). The status of free radicals (Pierce et al., 2000), energy levels and other intracellular factors determine the emergence of complex pathway involving activation of NFkB by removal of the inhibitory IkB through phosphorylation mediated by the kinase IKK (Karin et al., 2004; Luo et al., 2005; Park et al., 2005). One of the factors determining the activation of NFkB and the outcome of interaction of TNF with cells is altered integrin signaling. With all the matrix remodeling occurring during regeneration, such alterations in integrin signaling are bound to occur and they may be associated with directing the signaling of TNF towards a promitogenic effect (Chen et al., 2007). Antibodies against TNF administered at the time of hepatectomy decrease the regenerative response (Akerman et al., 1992). Mice with genetic deletions of the TNF receptor 1 (TNFR1) have slow and deficient response following PHx (Yamada et al., 1997, 1998; Yamada and Fausto, 1998). Activation of Stat3 and NFkB in these mice is diminished. Liver regeneration eventually becomes completed albeit much later. Even though deletion of NFkB components does not seem to affect liver regeneration (DeAngelis et al., 2001), given the promitogenic effects of activated NFkB in many cells and tissues, it is likely that TNF exercises its effects on liver regeneration in major part by this pathway. TNF is involved in induction of TACE, a plasma membrane associated protease which controls activation of TGFα. Enhanced activation of TGFα causes transactivation of EGFR (Argast et al., 2004). TNF is also a regulator of iNOS (Nussler et al., 1995), and mice with deficiency in iNOS have defective liver regeneration (Rai et al., 1998).
TNF is not a direct mitogen for hepatocytes. It does not induce DNA synthesis in primary cultures of hepatocytes in serum free media nor does it induce hepatocyte DNA synthesis when injected in whole animals. It does, however, enhance the mitogenic effects of direct mitogens such as HGF, both in vivo and in cell culture (Webber et al., 1998) and is mitogenic for hepatocytes with transgenic expression of TGFα (Pierce et al., 2000). TNF increases in plasma after PHx. Its cellular source is considered to be the hepatic macrophages (Kupffer cells) but production by other cell types has not been excluded. A stimulus that may induce TNF after PHx is endotoxin, produced by bacteria from the gut. Given the absence of direct mitogenic effects on hepatocytes, TNF should not be viewed as the initiator of liver regeneration, but rather as one of the many concurrent and contributory extracellular signals that all together orchestrate the early events of the response.
Interleukin 6 (IL6)
There is abundant literature documenting the crucial role of IL6 in initiation of the acute phase response in hepatocytes. This is a rapid increase in production by hepatocytes of many proteins which assist in controlling acute or chronic inflammation (Fey et al., 1991; Geisterfer et al., 1993). IL6 is produced by hepatic macrophages. Previous studies however have shown that it is produced by hepatoma cell lines, suggesting that it may also be produced by hepatocytes themselves (Northemann et al., 1990). IL6 binds to a soluble receptor, and the complex binds to the receptor gp130, which IL6 shares with other cytokines, including Oncostatin M, CNTF, LIF, etc. (Benigni et al., 1996). There was a previous report claiming that mice deficient in IL6 have deficient liver regeneration. This was associated with deficient activation of Stat3 (Cressman et al., 1996). Other studies however have shown that liver regeneration in these mice is essentially normal even though there is decreased activation of Stat3 (Sakamoto et al., 1999). Mice over-expressing both IL6 and its soluble receptor have areas of periportal hepatocyte hyperplasia (Maione et al., 1998). Mice with genetic deletions of gp130 are more sensitive to toxic effects but have essentially normal liver regeneration (Streetz et al., 2003). IL6 is not a direct mitogen for hepatocytes and does not enhance the mitogenic effect of other growth factors. It is, however, a direct mitogen for biliary cells (Liu et al., 1998) and it has important effects on integrity of the intrahepatic biliary tree by regulating production of small proline-rich proteins by cholangiocytes (Nozaki et al., 2005; Demetris et al., 2006). IL6 does increase in plasma following PHx. IL6 is probably a factor contributing to optimizing processes of the early stage of liver regeneration, but it should not be viewed as the initiator of the process.
Norepinephrine
This is a neurotransmitter in the central and peripheral autonomic nervous system. Epinephrine and norepinephrine are released in peripheral circulation from nerve endings, as well as from the adrenal medulla. Interest in the role of norepinephrine in liver regeneration arose when it was shown that norepinephrine substantially enhances the mitogenic effects of EGF and HGF in hepatocyte cultures and it decreases the mito-inhibitory effects of TGFb1 (Cruise et al., 1985; Houck et al., 1988). In cultures of hepatocytes with balanced concentrations of EGF and TGFb1 such that the final effect is neutral (EGF mitogenic effect is balanced by the mito-inhibitory effect of TGFβ1), addition of norepinephrine triggers high-level hepatocyte DNA synthesis (Houck and Michalopoulos, 1989). Norepinephrine induces synthesis of HGF in myofibroblasts (Broten et al., 1999). It is produced by and required for DNA synthesis of stellate cells in vivo and in culture (Oben et al., 2003). Another effect of norepinephrine and epinephrine of potential importance to liver regeneration is enhancement of production of EGF by Brunner’s glands of the duodenum (Olsen et al., 1985). This has not been directly linked to liver regeneration but norepinephrine rises rapidly in plasma after PHx and it may have an effect on EGF production. Blockade of the alpha-1 adrenergic receptor by prazosin inhibits DNA synthesis after PHx for 72 h (Cruise et al., 1987). It is not clear whether this effect reflects blockade of norepinephrine secreted peripherally or locally released by the stellate cells.
Bile acids and xenobiotics
It has been a long standing observation from liver pathology that hepatic cholestasis (chemically induced or due to mechanical biliary obstruction) is associated with proliferation of hepatocytes. A recent study provided evidence that bile acids increase in circulating blood after PHx and that depletion of bile acids leads to decreased regeneration (Huang et al., 2006). The elevation of bile acids in plasma occurs several hours after PHx, thus it is unlikely that they contribute to the immediate early changes after PHx described above. Nonetheless, the finding is very interesting. In the same study, mice with genetic deficiency of FXR, a transcription factor mediating nuclear events induced by bile acids, also have defective regeneration. There are several examples of xenobiotics ligating specific transcription factors or nuclear hormone receptors in hepatocytes and inducing liver enlargement. These include triiodothyronine (T3) (Short et al., 1980; Ledda-Columbano et al., 2000), agonists of PPARα (Reddy and Chu, 1996), estrogens (Yager et al., 1994), barbiturates (acting on CAR and PXR) (Columbano et al., 2005), and others. Hepatic enlargement is mediated in part by hepatocyte proliferation and in part by hepatocyte enlargement. The signaling pathways by which these chemicals exert these effects, are not clear. These pathways have not been shown so far to be associated with signaling patterns seen during liver regeneration (Columbano and Shinozuka, 1996; Columbano et al., 1997; Menegazzi et al., 1997; Ledda-Columbano et al., 2002). FXR is the first nuclear hormone receptor to be associated with proliferative events leading to regeneration of the liver and it sets a paradigm for discovery of other such nuclear hormone receptors as potentially having similar effects.
Serotonin
Mice with decreased platelet numbers have attenuated liver regeneration. Platelets contain many bioactive substances, including HGF, TGFβ1 and serotonin. A recent study (Lesurtel et al., 2006) demonstrated that supplementation of thrombocytopenic mice with serotonin reversed many of the effects of platelet depletion. Mice with low levels of serotonin (deficient in tryptophan hydroxylase 1) have low levels of platelet serotonin and they also have deficient regeneration. The mechanisms by which serotonin exerts these effects are not clear. Serotonin is not a direct or indirect mitogen for hepatocytes in culture, thus its effects on this process are likely to be indirect. It may affect the concentration and/or release of other platelet components (HGF, TGFβ1) known to have an effect on regeneration. It effects on hepatocytes in culture need to be investigated, as with norepinephrine, in order to sort out the mechanism of its action.
Components of complement
Mice deficient in components of complement C3 and C5 have defective regenerative responses to both PHx and to recovery from centrilobular necrosis following injury with CCl4. Administration of the missing components restores the efficiency of the regenerative response (Strey et al., 2003; DeAngelis et al., 2006; Tsonis et al., 2006). The mechanism for this is not clear and the phenomenon warrants detail mechanistic investigation.
Leptin, steatosis, and liver regeneration
There is emerging literature to suggest that excessive accumulation of fat in hepatocytes interferes with liver regeneration (Torbenson et al., 2002; Diehl, 2005). Leptin deficient db/db mice have excessive hepatic steatosis and impaired liver regeneration (Yamauchi et al., 2003). The mechanisms are not clear. In a similar model (ob/ob mice), neither administration of leptin nor correction of steatosis correct the regeneration response (Leclercq et al., 2006). Further complicating the issue, regenerating liver hepatocytes normally accumulate fat micro-droplets. This transient fatty liver occurs in the rat from 24 to 72 h after PHx and disappears by itself (Michalopoulos and DeFrances, 1997). The process is thought to represent a metabolic adaptation of hepatocytes so that the emerging new cells have readily available energy as well as materials they can use to build cellular membranes etc. Recent studies have shown that this phenomenon is an essential component of the regenerative process and that interference with the accumulation of fat actually blocks liver regeneration (Shteyer et al., 2004). This physiologic accumulation of fat micro-droplets is dependent on caveolin, and caveolin-knockout mice have defective liver regeneration (Fernandez et al., 2006).
Notch and jagged
There are several members to this family of proteins and they compose a complex network mediating ligand–receptor interactions between cells, in tissues undergoing differentiation and proliferation related changes (Mumm and Kopan, 2000; Baron et al., 2002; Baron, 2003). Notch proteins are considered to be the receptors, but both Notch and Jagged protein family members are anchored on the plasma membrane with a transmembrane domain. Binding of Jagged to Notch leads to a complex cascade of proteolytic events whereby the intracellular domain of Notch (NICD) is cleaved and migrates to the nucleus, where it functions as a transcription factor (co-activator) and mediates expression of several genes related to cell cycle, including Myc and Cyclin D1 (Ronchini and Capobianco, 2001). Mutations in Jagged-1 are the cause of Alagille syndrome in humans, characterized, among other symptoms, with paucity of intrahepatic bile ducts. The genes HES-1 and HES-5 are directly regulated by Notch. As mentioned above, NICD migrates to hepatocyte nuclei at 15 min after PHx (Kohler et al., 2004). HES-1 and HES-5 are also upregulated within 30 min after PHx. There is increased expression of Notch-1 and Jagged-1 from 3 h to 4 days after PHx. Treatment with RNAi against either Notch-1 or Jagged-1 partially suppressed regeneration and recombinant Jagged-1 induced DNA synthesis in hepatocyte cultures. On the other hand, elimination of Notch using a Cre/Lox system soon after birth from the entire body resulted in an adaptive hepatic nodular hyperplasia (Croquelois et al., 2005). These mice, however, also had a defective liver regeneration. The Notch/Jagged system needs to be further studied in relation to regeneration and it is likely to mediate many direct cell–cell interactions involving hepatocytes and other cell types. The story is likely to be complex since hepatocytes and biliary cells express both Notch-1 and Jagged-1.
Insulin
Liver is the first recipient of all the insulin produced by the endocrine pancreas, since it is delivered through the portal vein. Diabetes (either through insulin deficiency or through insulin resistance) causes a mild to severe steatohepatitis that may even lead to cirrhosis. Diversion of the portal vein flow to vena cava (portacaval shunt) forces insulin to bypass the liver. Liver atrophies to about 1/3 of its size (Bucher, 1976; Bucher and Weir, 1976; Thompson et al., 1983; Evarts et al., 1986). Administration of insulin directly to the liver in animals with experimental portacaval shunts reverses hepatic atrophy, and is associated with rapid hepatocyte proliferation. Hepatocytes in culture have diminished response to mitogens in the absence of insulin (Cruise et al., 1985). Insulin is clearly a very important regulator of hepatocyte functions at all times and it is very likely involved in the metabolic adaptations that hepatocytes have to undergo to provide homeostatic functions during liver regeneration. Insulin, however, is not a direct mitogen for hepatocytes.
Signaling Interactions Between Different Hepatic Cell Types During Liver Regeneration
There is myriad of complex interactions between different cell types in normal quiescent liver. A whole new pattern of interactions is activated at different stages of liver regeneration, based on production of growth factors and cytokines which exercise paracrine effects. A summary of these interactions is shown in Figure 2. The overall changes seen are mimicking themes seen in wound healing and tumor biology. In those conditions, the main epithelial cells of the tissue (or tumor) generate paracrine signals inducing proliferation of stromal cells, in order to provide connective tissue and blood vessels for tumor growth of wound healing. Hepatocytes are the first to undergo proliferation, based on external stimuli from a variety of sources, as discussed above. HGF is rapidly becoming available to hepatocytes very rapidly through matrix remodeling and local release and activation induced by uPA (see above). Stellate cells and endothelial cells are sources of new HGF, synthesized after 3 h following PHx. A very important aspect of signaling that aims to restore true hepatic tissue is mediated by the host of growth factors produced by hepatocytes during regeneration. Hepatocytes produce growth factors mitogenic for stellate cells (PDGF) (Pinzani, 2002) as well for endothelial cells. The latter include VEGF, FGF1, FGF2, SCF, Angiopoietins 1 and 2, and TGFα (Ross et al., 2001; Sato et al., 2001; Yu et al., 2003; Shimizu et al., 2005; Papastefanou et al., 2007). Proliferation of endothelial cells aimed to restore the network of sinusoids occurs during a broad period of time, from days 3–6 after PHx. The prevalent view is that endothelial cells attracted by VEGF penetrate clusters of newly proliferated yet not fully vascularized hepatocytes and re-establish a sinusoidal network. Increases in VEGF receptors 1 and 2, angiopoietin receptors Tie-1, Tie-2, and platelet-derived growth factor receptor beta (PDGF-Rβ) have been observed in the proliferating endothelial cells. Increase in Tie-1 was specifically seen in endothelial cells surrounding clusters of newly proliferated hepatocytes (Ross et al., 2001). It may mediate a paracrine response to Angiopoietin 1 produced by the hepatocytes, in order to facilitate neovascularization. It is of interest that administration of VEGF in whole animals induces proliferation of both endothelial cells and hepatocytes, whereas VEGF is not mitogenic in pure hepatocyte cultures. This is due to the fact that endothelial cells themselves produce HGF, stimulated by VEGF (produced by hepatocytes) acting through VEGFR1 (LeCouter et al., 2003). Thus, hepatocytes and endothelial cells establish a network of mutually assisted proliferation. Kupffer cells have not been clearly proven to proliferate during regeneration and their numbers may be affected by migration from precursor cells in the bone marrow. Kupffer cells, however, do produce TNF and IL6, which appear to have contributory roles on Stat3 and NFkB activation during the early stages of liver regeneration. Depletion of Kupffer cells by gadolinium chloride causes delay and attenuation of regeneration (Webber et al., 1998).
Despite the large number of signaling networks already discovered, the number of cytokines involved in this process is likely to continue to grow. Nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF), neurotrophin 3 (NT-3), neurotrophin 4/5 (NT-4/5), the low-affinity NGF receptor p75, and the high-affinity tyrosine kinase receptors (Trk) B and C have been found in hepatic stellate cells, well known for their gene expression pattern similarity to brain astrocytes (Cassiman et al., 2001). Regeneration of stellate cells is dependent on expression of neurotrophin receptors and hepatic regeneration is not proceeding normally in mice with genetic deficiency in the neurotrophin receptor p75NTR (Passino et al., 2007). These stellate cells are also deficient in production of HGF, which may correlate with decreased hepatocyte proliferation seen under some conditions (not including PHx) in these mice. Stellate cells also can produce norepinephrine (Oben et al., 2003). NGF is regulating apoptosis in stellate cells (Asai et al., 2006). Overall, the pathways and cellular kinetics controlling proliferation of stellate cells during liver regeneration have not been fully studied and need to be better understood.
TGFβ1: An Essential Regenerative Cytokine or a Regeneration Terminator?
Few cytokines have elicited as much interest and contradiction in liver growth biology as TGFβ1. It is produced predominantly by stellate cells (Ikeda et al., 1998), but most cells in all tissues have been shown to produce TGFβ1 at some point in their life cycle. It is not clear whether hepatocytes actually produce TGFβ1, though it is produced by most carcinomas derived from hepatocytes or hepatoblasts (Luo et al., 2006). TGFβ1 is mito-inhibitory for most epithelial cells and it is produced by mesenchymal cells in most tissues. It stimulates synthesis of multiple extracellular matrix proteins from mesenchymal cells (Roberts et al., 1992). It inhibits proliferation of hepatocytes in culture (Houck and Michalopoulos, 1989), suppresses production of HGF (Gohda et al., 1992) and suppresses expression of urokinase and activation of HGF (Mars et al., 1996). When administered in high doses, TGFβ1 delays or partially suppresses the peak of DNA synthesis at 24h after PHx (Russell et al., 1988). HGF and EGF, whose receptors are activated fully prior to the upregulation of TGFβ1, are known to induce synthesis of TGFβ1 in hepatic organoid cultures and may be the signals behind the TGFβ1 rise during regeneration (Michalopoulos et al., 2001). Given its mito-inhibitory properties on hepatocytes, it is enigmatic that TGFβ1 is produced at high levels during liver regeneration. New TGFβ1 synthesis starts at 2–3 h after PHx and it remains elevated until 72 h (Jakowlew et al., 1991). Similar to other cytokines involved in this process, TGFβ1 levels also rise in the plasma very shortly after PHx and remain elevated for several hours (Michalopoulos and DeFrances, 2005b). Hepatocytes from regenerating liver are resistant to the mito-inhibitory effects of TGFβ1, and the expression of TGFβ1 receptors I, II decreases in the first 48 h after PHx (Houck and Michalopoulos, 1989; Chari et al., 1995). Resistance to TGFβ1 might be conferred by the decrease in expression of its receptors, or by the high levels of norepinephrine (see above) or by the fact that regenerating hepatocytes produce TGFα. Whatever the mechanism, regenerating hepatocytes manage to escape the mito-inhibitory effect, leaving the question as to the function served by TGFβ1. To better understand (or, more effectively speculate) on the role of TGFβ1, we need to examine changes related to it both at the beginning and at the end of the regenerative process. Immunohistochemistry for TGFβ1 shows that the protein is gradually being removed as a wave from the periportal to the pericentral regions (Jirtle et al., 1991). This has not as yet been correlated with similar changes in TGFβ1 binding proteins, such as decorin. Of interest, behind the edge of TGFβ1 removal, there is a wave of proliferating hepatocytes in mitosis. This suggests that removal of TGFβ1 protein from the liver parenchyma (corresponding with its rise in the plasma) is a necessary step to allow hepatocytes to proliferate. The experiments with dominant negative constructs against receptors for either TGFβ1 or activin (see III above) also suggest that TGFβ1 plays an active role in normal quiescent liver in keeping hepatocytes in G0 phase, by exercising a “tonic” effect and enabling them to carry their differentiated functions (Kogure et al., 2000; Ichikawa et al., 2001). Removal and inactivation of TGFβ1 (by binding to alpha-2-macroglobulin in the plasma (LaMarre et al., 1991), and mobilization and activation of HGF (Pediaditakis et al., 2001) create an imbalance of agonists and antagonists that undoubtedly contributes to the mitogenic signaling cascade for hepatocytes.
Given its mito-inhibitory properties and its upregulated expression as regeneration advances, TGFβ1 has been thought as being the natural stimulus that terminates liver regeneration. While this hypothesis is attractive, there has not been much experimental support for it. Mice with transgenic over-expression of TGFβ1 in hepatocytes do regenerate almost normally, despite the presence of very high TGFβ1 levels in liver and plasma (Sanderson et al., 1995). Knockout mouse strains with elimination of TGFβ1 receptors have normal ending of liver regeneration, unless there is concurrent elimination of activin by administration of follistatin (Oe et al., 2004). Activin is also a mito-inhibitor for hepatocytes (Ho et al., 2004). It is possible that termination of regeneration requires the combined effect of both activin and TGFβ1.
Regardless of its potential as a regeneration terminator, TGFβ1 is thought to play an important role to play in the assembly of hepatic tissue towards the end of regeneration. TGFβ1 stimulates production of many extracellular matrix proteins in many tissues including liver. TGFβ1 also stimulates tubulogenesis and formation of neovascular structures in endothelial cells in collagen gels (Pepper et al., 1993; Holifield et al., 2004). New extracellular matrix is being synthesized at the end of regeneration, and the process of forming the new sinusoidal capillary network also occurs after 3 days post-PHx, both at a time when TGFβ1 expression is at its highest levels. TGFβ1 also rises at the end of wound healing in association with similar events (Murphy et al., 2006). It is also produced by tumors at high levels, and it is thought to be associated with production of tumor matrix by the stromal cells.
Termination of Liver Regeneration
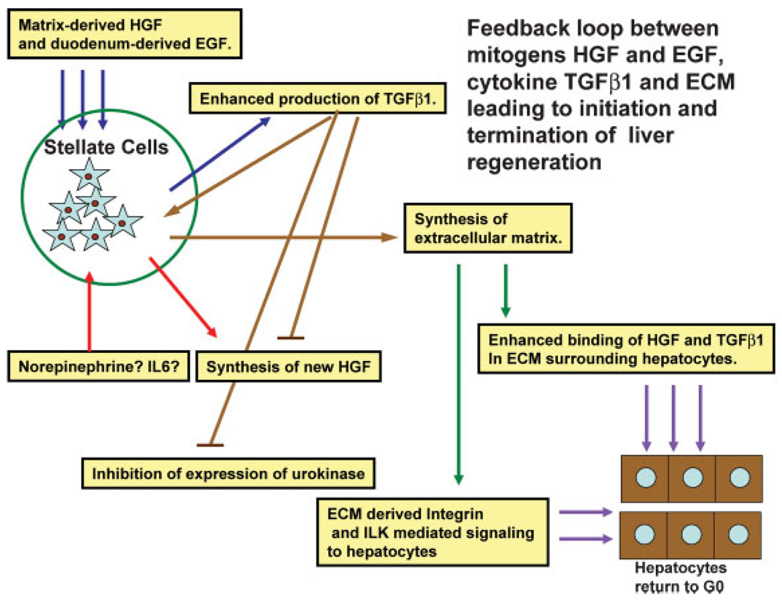
Though much less studied and even less understood, pathways leading to termination of liver regeneration should be equally as important as those initiating the process. There is evidence that the number of hepatocytes produced during regeneration may exceed the original. A small wave of apoptosis in hepatocytes occurs at the end of regeneration (Sakamoto et al., 1999). Liver weight of the expanded lobes becomes almost identical to the original total of the five lobes (in rats and mice). Livers from large animals (dogs) transplanted to smaller animals decrease in size, while the opposite is also true (as shown with livers from small to large dogs and from baboons to humans (Starzl et al., 1993). These findings have raised the issue of an existence of a “hepatostat” control system, which ensures that liver weight is as it should be (and not more) for the performance of its homeostatic functions. With considerable evidence discounting the role of any single cytokine (e.g., TGFβ1) being the terminator of regeneration, attention has been paid to other hepatocyte mito-inhibitors. Complex preparations of extracellular matrix inhibit cell proliferation and enhance differentiation of hepatocytes in culture. This has been shown with matrix extracts from the mouse EHS sarcoma (“Matrigel” and with hydrated type I collagen gels (Rana et al., 1994; Michalopoulos et al., 1999). Extracellular matrix signaling involves Integrin Linked Kinase (Gkretsi et al., 2007a). It is tempting to speculate that the reassembly of the extracellular matrix and the sinusoidal capillary network provides matrix-driven signaling that terminates the regenerative process (Fig. 3). This may be direct signaling through integrins or signaling induced by TGFβ1 (bound to the newly synthesized decorin and again exerting a “tonic” mito-inhibitory effect). Synthesis of new decorin, perlecan, and syndecan and collagen types I and III dramatically increase during regeneration (Gallai et al., 1996; Rudolph et al., 1999). Newly synthesized matrix would also be capable of binding HGF (a protein with high affinity to glycosaminoglycans and heparin) and preventing it from being activated by urokinase, which is disappearing anyway at the end of regeneration and its expression is inhibited by TGFβ1 (Mars et al., 1996). This set of events would bring hepatocytes back into a state of quiescence, surrounded by HGF (bound to glycosaminoglycans) and TGFβ1 (bound to decorin). In this scenario, early mitogenic stimuli such HGF and EGF drive both hepatocyte proliferation as well enhanced expression of TGFβ1. Proliferating hepatocytes become resistant to TGFβ1; the latter however stimulates production of extracellular matrix and formation of hepatic sinusoids. It also inhibits expression of urokinase and HGF (see above). New extracellular matrix synthesis by stellate cells stimulated by TGFβ1 restores binding of both HGF and TGFβ1 and reestablishes quiescence of hepatocytes in G0. From this perspective, TGFβ1 is not a direct terminator of regeneration, but it orchestrates multiple events as part of a large feedback loop. This loop starts with the early mitogenic signals after PHx, involves triggering of TGFβ1 synthesis by the mitogens (HGF, EGF) and eventually results in termination of the regenerative process by synthesis of new extracellular matrix. As for the components of the “hepatostat,” it is also likely that increased flow of blood through the portal vein (in the small liver to large animal) or vice versa would tend to initiate (or eliminate) some of the stimuli of the regenerative process, albeit at a smaller scale. As an example, small grafts for the size of the recipient are associated with increased plasma levels of both HGF and TGFβ1 in the human, as with response to PHx in the rat (Ninomiya et al., 2003).
Fig. 3.
Schematic of a feedback loop between growth factors, TGFβ1, and extracellular matrix, controlling early and late stages of regeneration. Mitogens (HGF and EGF) upregulate expression of TGFβ1 by stellate cells. The latter stimulates synthesis of new extracellular matrix, while eventually blocking synthesis of new HGF and expression of urokinase. The newly synthesized extracellular matrix supports binding of single chain HGF and TGFβ1 around hepatocytes and restoration of quiescence (G0 phase). Arrows of the same color denote similar origin of the input and output of the same signaling process.
The liver weight changes associated with physiologic events (e.g., hepatic enlargement during pregnancy) are likely to be mediated not through the classic pathways associated with regeneration, but through hepatic enlargement mediated by nuclear hormone receptors. Estrogens (acting through the ER receptors) have such an effect. Of interest, estradiol also enhances effects of mitogens and suppresses effects of TGFβ1 in hepatocytes cultures, similar to norepinephrine (see above). Many xenobiotics cause liver enlargement. The pathways leading to this are not fully understood and it seems that transcription factors such as PPAR (for peroxisome proliferators) and CAR may play a role (Ueda et al., 2002). Some of the liver enlargement is caused by direct effects on the size of hepatocytes. Phenobarbital, associated with a dramatic enlargement of liver in humans and rodents, causes primarily hepatocyte hypertrophy. This is probably due to induction and enhanced nuclear translocation of HNF4 by phenobarbital (Bell and Michalopoulos, 2006). This effect is independent of CAR or PXR. Removal of the xenobiotic causing liver enlargement is always associated with a return to the original liver size, mediated by a wave of hepatocyte apoptosis until liver weight returns to normal (Reddy et al., 1978). Extracellular matrix signaling may be involved. Acute removal of ILK and matrix signaling from hepatocytes causes massive hepatic apoptosis (Gkretsi et al., 2007b). This process of liver weight adjustment by apoptosis is very little understood.
If Hepatocytes Cannot Proliferate, What Are the Regenerative Alternatives?
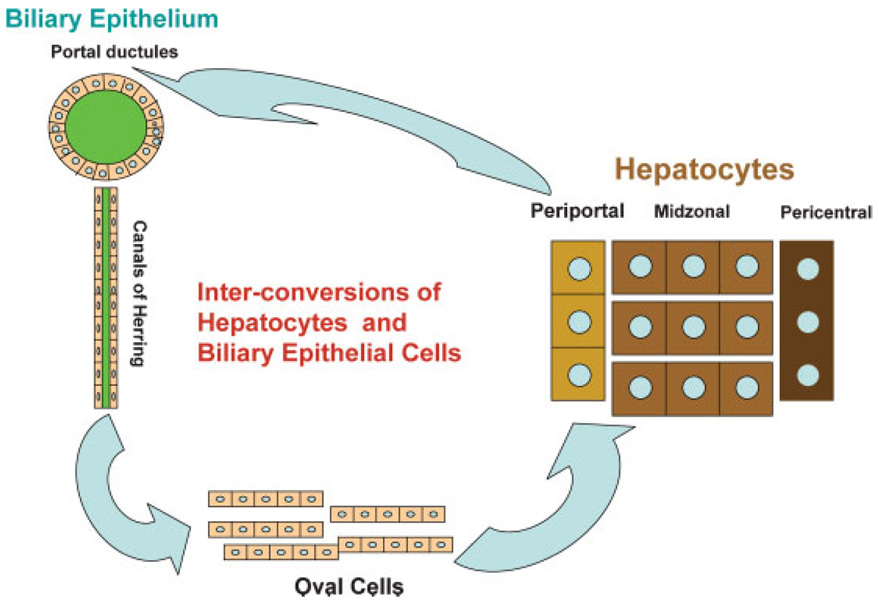
Hepatocyte proliferation may be blocked if the tissue injury is too severe, as in humans with fulminant hepatitis. Experimentally, hepatocyte proliferation is blocked by the use of the chemical AAF (N-acetylaminofluorene). On long-term administration, AAF is a carcinogenic substance. Given for a short period of time, it blocks hepatocyte proliferation, probably by forming AAF-DNA adducts which trigger proliferative arrest via p53 and p21 (Ohlson et al., 1998). When PHx is performed in animals given AAF, hepatocytes cannot proliferate. Starting at day 2–3 after PHx, biliary epithelial cells of the portal ductules and canals of Herring (small tubules lined by epithelium with biliary morphology, which connect the network of hepatocyte bile canaliculi to the portal biliary ductules) begin expressing hepatocyte-associated transcription factors (Nagy et al., 1994). Shortly thereafter, there is an increasing number of cells with mixed biliary and hepatocytic gene expression patterns, as well as some markers of their own (Sell, 1994). These cells have been called “oval” cells, from the shape of their nucleus. Oval cells proliferate intensely in the periportal areas of the hepatic lobule and they are heavily infiltrated by stellate cells; the latter intertwine with the oval cells and produce HGF, FGF1, FGF2, and VEGF (Evarts et al., 1993; Fujio et al., 1994). Other factors, such as somatostatin, stromal cell derived factor 1 (SDF1) and connective tissue growth factor also play a role (Pi et al., 2005; Jung et al., 2006; Zheng et al., 2006). Oval cells express both albumin and alpha fetoprotein. Four to five days after their expansion as a population, they become basophilic hepatocytes and eventually mature hepatocytes, and they restore liver size and histology (Evarts et al., 1989, 1996). The origin of the oval cells has been much debated. A strong argument for their origin from biliary cells is their early gene expression patterns which strongly resemble biliary cells, and the fact that biliary cells begin expressing hepatocyte-associated transcription factors before oval cells appear. Additionally, when DAPM (a toxin which selectively destroys biliary cells), is given before the initiation of the AAF/PHx protocol, it destroys the biliary epithelium and prevents the appearance of the oval cells (Petersen et al., 1997). There is no histologic observation demonstrating an oval cell population in any non-biliary compartment in a normal liver. Cells equivalent to oval cells, called “ductular hepatocytes,” are also seen in humans during fulminant hepatitis following extensive liver injury (by chemicals, viruses, etc.) and they are assumed to pay a role similar to oval cells in restoring hepatocyte populations. It should be noted that pancreatic ductules have also been viewed as the source of progenitor cells for both acinar cells and islet cells of the pancreas (Rao and Reddy, 1995).
Hepatocytes and Biliary Epithelial Cells as Facultative Stem Cells for Each Other
As discussed above, biliary epithelial cells can become oval cells and they in turn become hepatocytes, restoring liver regeneration when hepatocytes cannot proliferate. This imparts properties of facultative stem cells (Alison et al., 2001) to biliary cells for hepatocytes. The term “facultative” implies that biliary cells under normal circumstances perform their normal functions (transport of bile). Under selective circumstances, however, they can become stem cells for hepatocytes. Clinical histologic observations have suggested that periportal hepatocytes may also be facultative stem cells for biliary cells, transforming into biliary cells when the latter cannot proliferate to repair biliary epithelium during chronic injury (e.g., primary biliary cirrhosis, primary sclerosing cholangitis) (Crosby et al., 1998). This phenomenon has now been demonstrated experimentally in rats with chimeric livers (Laconi et al., 1998). Periportal hepatocytes can transform into biliary epithelial cells when the latter are destroyed by DAPM and bile ducts are simultaneously obstructed. Biliary obstruction is known to lead to bile ductule proliferation and, under the conditions described above, more than 50% of the newly emerging ductules carry markers unique to one of the two populations of the hepatocytes of the chimeric liver (Michalopoulos et al., 2005a). These findings clearly demonstrate that hepatocytes are also facultative stem cells for the biliary epithelium. As shown in Figure 4, the two types of epithelial cells of the liver (hepatocytes and biliary cells) constitute a bipolar system of facultative stem cells for each other, fully capable of repairing liver histology even when the classic regeneration fails.
Fig. 4.
Cells from the biliary compartment (portal ductules and canals of Herring) transform into oval cells and these become hepatocytes when proliferation of hepatocytes is inhibited during regeneration. Periportal hepatocytes can also convert to biliary cells when there is injury to biliary cells but their capacity for self-repair is inhibited. Hepatocytes and biliary cells are facultative stem cells for each other.
Stems Cells in the Liver: Do They Exist? Are They Needed? Where do They Come From?
The findings for hepatocytes and biliary cells discussed above raise questions about the very existence of full-time, committed, stem cells in the liver. Such cells exist in the epidermis (basal layer), intestine (cells of the crypts), and bone marrow (hematopoietic stem cells, isolated and demonstrable by cell sorting). In these other tissues, the stem cells of the tissue exist on a full-time basis, they can be seen by casual examination of the histology of the tissue and they are committed to “stemness” as their single function. Liver histology does not contain any cells that appear to be full-time, committed, stem cells. The term “progenitor cells” has often being used for oval cells, and it is a useful term as long as it is remembered that these cells normally do not exist. It is quite possible that there are subpopulations of both hepatocytes (periportal cells?) and biliary cells (canals of Herring?) which may have a higher propensity to function as facultative stem cells compared to other cells of their kind. All these cells, however, function as hepatocytes and biliary epithelial cells under normal circumstances.
There also has been considerable discussion that bone marrow derived hematopoietic stem cells can trans-differentiate into hepatocytes or oval cells and rescue the liver when all other means of regeneration fail (Petersen et al., 1999). A dramatic demonstration of this phenomenon was the rescue of mice with tyrosinemia (due to congenital deficiency of the enzyme FAH, involved in the degradation of tyrosine, see below). Injection of bone marrow from normal mice gave rise to a large number of normal appearing hepatocytes and rescued the liver (Lagasse et al., 2000). Subsequent studies, however, showed that hematopoietic stem cells have the capacity to fuse with other cell targets (Terada et al., 2002). Karyotypic analysis of the hepatocytes derived from bone marrow in the FAH model showed that the apparently normal hepatocytes were the result of fusion between the bone marrow (FAH+/+) cells and the FAH−/− hepatocytes of the host mouse (Wang et al., 2003). This was found to also be true in other tissues (heart, muscle) in which hematopoietic stem cells apparently give rise to other non-hematologic cell types. It is now generally agreed upon that if there are circumstances in which hematopoietic stem cells give rise to hepatocytes in the liver by trans-differentiation, the phenomenon must be extremely rare (Fausto, 2004). On the other hand, there are several examples of bone marrow cell cultures in which, under the influence of HGF, hematopoietic cells apparently transform into cells very similarto hepatocytes, producing albumin (Miyazaki et al., 2004). This implies that, under conditions hitherto unknown and unavailable, this trans-differentiation of hemopoietic cells to hepatocytes could at some point become a controlled and useful process. Similar trans-differentiation into hepatocyte-like cells in culture has also been shown for amniotic cells (Miki and Strom, 2006). Despite the ups and downs in the story of bone marrow cells trans-differentiating into hepatocytes in vivo, the phenomenon is actively being investigated (Oh et al., 2007) and its full potential remains a target for further study.
Do Hepatocytes Have Unusual and Unique Growth Properties In Vivo?
Hepatocytes in humans and rodents are polyploid cells with highly complex gene expression patterns. Their behavior during liver regeneration shows that they can undergo one or two rounds of cell proliferation. The literature reports one record number of 12 sequential hepatectomies being performed without failure of the regenerative potential of the liver (Stocker et al., 1973). A more extreme paradigm, however, was demonstrated in the mouse tyrosinemia model (FAH−/− mice). Due to absence of the enzyme FAH, a toxic metabolite is generated which causes hepatocyte death and liver failure. These mice can survive if given the drug NTBC, which blocks another enzyme of tyrosine catabolism upstream of FAH and prevents the formation of the toxic metabolite. When NTBC is withdrawn, mice rapidly enter into liver failure. At that point, injection of hepatocytes from normal mice rescues the liver (and the animal), because the injected normal hepatocytes enter into rapid proliferation, re-establish liver mass, and restore normal liver histology. Normal hepatocytes isolated from the first generation of rescued mice can be used in the same manner to rescue a second generation of mice. This was repeated seriatim and up to 10 consecutive generations of mice were rescued from hepatocytes isolated from one original mouse. It was calculated that under the mathematics of the system one hepatocyte from a normal mouse could give rise to 50 mouse livers! (Overturf et al., 1997). With exception of neoplastic cells, and perhaps hematopoietic stem cells, there is no other example of a cell that can expand as much, in vivo or in cell culture. Cell fusion between FAH+/+ and FAH −/− hepatocytes could be the only alternative explanation, but it has been apparently ruled out. The phenomenon needs to be better understood, but it does place the hepatocytes in a unique category of proliferative capability compared to other cells in the body. Hepatocytes in culture are capable of reactivating telomerase functions and this may underlie their unique (almost endless) capacity to proliferate in vivo, despite their very complex phenotype (Nozawa et al., 1999).
Liver Regeneration in the Embryo?
Signaling patterns involving cardiac mesenchyme and ventral endoderm are very complex and they result in formation of the liver primordium. There are several excellent studies in this subject, and they have implicated FGF growth factor family members and BMP family members, as inducing with hepatic anlagen commitment, specification, and differentiation (Lee et al., 2005; Battle et al., 2006; Zaret, 2006). Several transcription factors are induced in a sequential manner to control phenomena orchestrating this process. These studies are not part of this review. A recent study however showed that partial destruction of hepatic precursor cells immediately after their emergence results in an embryonic liver which initially is much smaller than. It does expand rapidly however, and at birth, the size of the liver of treated mice is indistinguishable to that of the control (Stanger et al., 2007). This suggests that, even though the signaling pathways controlling hepatic embryogenesis appear to be different from those of regeneration, the embryonic liver has the capacity to undergo a regenerative expansion even during embryonic development. In the same study, this was found not to be true for pancreas.
Conclusion
Despite multiple studies of liver regeneration, many aspects of this phenomenon remain to be further understood. The changes associated with PHx that constitute the “first trigger” (prior to urokinase elevation, Notch and beta catenin nuclear migration, etc.) are still not clear, but it is inevitable that the large hemodynamic alterations seen after in the remnant liver after PHx must play a role. Equally unclear but slightly better understood are the signaling pathways leading to the end of regeneration at the right time. In skin, intestine and blood, tissue restoration occurs by proliferation of stem cells, and it is a “local” affair. Wound healing and associated phenomena, with which liver regeneration has many similarities, is also a “local” affair. With liver, this is not the case. Liver function affects the entire body and consequences of liver failure are anything but local. The fully differentiated hepatocytes continue to carry the burden of maintaining homeostasis for the entire body and at the same time, restoring liver mass. Many recent studies are focusing on the signaling pathways that allow hepatocytes to maintain most of their homeostatic functions and proliferate at the same time. This aspect of liver regeneration, as well as the events of the very beginning and the (well regulated) end of regeneration can be better studied, now that a solid framework of growth factors, cytokines and cellular events regulating regeneration has been laid out.
Acknowledgments
This study was supported by grants from NIH CA035373, CA103958 (GKM) and the Rangos Fund for the enhancement of research in Pathology.
Literature Cited
- Aaronson SA, Rubin JS, Finch PW, Wong J, Marchese C, Falco J, Taylor WG, Kraus MH. Growth factor-regulated pathways in epithelial cell proliferation. Am Rev Respir Dis. 1990;142:S7–S10. doi: 10.1164/ajrccm/142.6_Pt_2.S7. [DOI] [PubMed] [Google Scholar]
- Akerman P, Cote P, Yang SQ, McClain C, Nelson S, Bagby GJ, Diehl AM. Antibodies to tumor necrosis factor-alpha inhibit liver regeneration after partial hepatectomy. Am J Physiol. 1992;263:G579–G585. doi: 10.1152/ajpgi.1992.263.4.G579. [DOI] [PubMed] [Google Scholar]
- Alison MR, Poulsom R, Forbes SJ. Update on hepatic stem cells. Liver. 2001;21:367–373. doi: 10.1034/j.1600-0676.2001.210601.x. [DOI] [PubMed] [Google Scholar]
- Appasamy R, Tanabe M, Murase N, Zarnegar R, Venkataramanan R, Van Thiel DH, Michalopoulos GK. Hepatocyte growth factor, blood clearance, organ uptake, and biliary excretion in normal and partially hepatectomized rats. Lab Invest. 1993;68:270–276. [PubMed] [Google Scholar]
- Argast GM, Campbell JS, Brooling JT, Fausto N. Epidermal growth factor receptor transactivation mediates tumor necrosis factor-induced hepatocyte replication. J Biol Chem. 2004;279:34530–34536. doi: 10.1074/jbc.M405703200. [DOI] [PubMed] [Google Scholar]
- Asai K, Tamakawa S, Yamamoto M, Yoshie M, Tokusashi Y, Yaginuma Y, Kasai S, Ogawa K. Activated hepatic stellate cells overexpress p75NTR after partial hepatectomy and undergo apoptosis on nerve growth factor stimulation. Liver Int. 2006;26:595–603. doi: 10.1111/j.1478-3231.2006.01267.x. [DOI] [PubMed] [Google Scholar]
- Baron M. An overview of the Notch signalling pathway. Semin Cell Dev Biol. 2003;14:113–119. doi: 10.1016/s1084-9521(02)00179-9. [DOI] [PubMed] [Google Scholar]
- Baron M, Aslam H, Flasza M, Fostier M, Higgs JE, Mazaleyrat SL, Wilkin MB. Multiple levels of Notch signal regulation (review) Mol Membr Biol. 2002;19:27–38. doi: 10.1080/09687680110112929. [DOI] [PubMed] [Google Scholar]
- Battle MA, Konopka G, Parviz F, Gaggl AL, Yang C, Sladek FM, Duncan SA. Hepatocyte nuclear factor 4alpha orchestrates expression of cell adhesion proteins during the epithelial transformation of the developing liver. Proc Natl Acad Sci U S A. 2006;103:8419–8424. doi: 10.1073/pnas.0600246103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell AW, Michalopoulos GK. Phenobarbital regulates nuclear expression of HNF-4alpha in mouse and rat hepatocytes independent of CAR and PXR. Hepatology. 2006;44:186–194. doi: 10.1002/hep.21234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benigni F, Fantuzzi G, Sacco S, Sironi M, Pozzi P, Dinarello CA, Sipe JD, Poli V, Cappelletti M, Paonessa G, Pennica D, Panayotatos N, Ghezzi P. Six different cytokines that share GP130 as a receptor subunit, induce serum amyloid A and potentiate the induction of interleukin-6 and the activation of the hypothalamus-pituitary-adrenal axis by interleukin-1. Blood. 1996;87:1851–1854. [PubMed] [Google Scholar]
- Berasain C, Garcia-Trevijano ER, Castillo J, Erroba E, Lee DC, Prieto J, Avila MA. Amphiregulin: An early trigger of liver regeneration in mice. Gastroenterology. 2005;128:424–432. doi: 10.1053/j.gastro.2004.11.006. [See comment] [DOI] [PubMed] [Google Scholar]
- Block GD, Locker J, Bowen WC, Petersen BE, Katyal S, Strom SC, Riley T, Howard TA, Michalopoulos GK. Population expansion, clonal growth, and specific differentiation patterns in primary cultures of hepatocytes induced by HGF/SF, EGF and TGF alpha in a chemically defined (HGM) medium. J Cell Biol. 1996;132:1133–1149. doi: 10.1083/jcb.132.6.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borowiak M, Garratt AN, Wustefeld T, Strehle M, Trautwein C, Birchmeier C. Met provides essential signals for liver regeneration. Proc Natl Acad Sci U S A. 2004;101:10608–10613. doi: 10.1073/pnas.0403412101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broten J, Michalopoulos G, Petersen B, Cruise J. Adrenergic stimulation of hepatocyte growth factor expression. Biochem Biophys Res Commun. 1999;262:76–79. doi: 10.1006/bbrc.1999.1183. [DOI] [PubMed] [Google Scholar]
- Bucher NL. Insulin, glucagon, and the liver. Adv Enzyme Regul. 1976;15:221–230. doi: 10.1016/0065-2571(77)90018-8. [DOI] [PubMed] [Google Scholar]
- Bucher NL, Patel U, Cohen S. Hormonal factors concerned with liver regeneration. Ciba Found Symp. 1977;55:95–107. doi: 10.1002/9780470720363.ch5. [DOI] [PubMed] [Google Scholar]
- Bucher NL, Weir GC. Insulin, glucagon, liver regeneration, and DNA synthesis. Metabolism. 1976;25:1423–1425. doi: 10.1016/s0026-0495(76)80156-4. [DOI] [PubMed] [Google Scholar]
- Cassiman D, Denef C, Desmet VJ, Roskams T. Human and rat hepatic stellate cells express neurotrophins and neurotrophin receptors. Hepatology. 2001;33:148–158. doi: 10.1053/jhep.2001.20793. [DOI] [PubMed] [Google Scholar]
- Chari RS, Price DT, Sue SR, Meyers WC, Jirtle RL. Down-regulation of transforming growth factor beta receptor type I, II, and III during liver regeneration. Am J Surg. 1995;169:126–131. doi: 10.1016/s0002-9610(99)80120-2. discussion 126–131. [DOI] [PubMed] [Google Scholar]
- Chen CC, Young JL, Monzon RI, Chen N, Todorovic V, Lau LF. Cytotoxicity of TNFalpha is regulated by integrin-mediated matrix signaling. EMBO J. 2007;26:1257–1267. doi: 10.1038/sj.emboj.7601596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chmielowiec J, Borowiak M, Morkel M, Stradal T, Munz B, Werner S, Wehland J, Birchmeier C, Birchmeier W. c-Met is essential for wound healing in the skin. J Cell Biol. 2007;177:151–162. doi: 10.1083/jcb.200701086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Columbano A, Ledda-Columbano GM, Pibiri M, Cossu C, Menegazzi M, Moore DD, Huang W, Tian J, Locker J. Gadd45beta is induced through a CAR-dependent, TNF-independent pathway in murine liver hyperplasia. Hepatology. 2005;42:1118–1126. doi: 10.1002/hep.20883. [DOI] [PubMed] [Google Scholar]
- Columbano A, Ledda-Columbano GM, Pibiri M, Piga R, Shinozuka H, De Luca V, Cerignoli F, Tripodi M. Increased expression of c-fos, c-jun and LRF-1 is not required for in vivo priming of hepatocytes by the mitogen TCPOBOP. Oncogene. 1997;14:857–863. doi: 10.1038/sj.onc.1200891. [DOI] [PubMed] [Google Scholar]
- Columbano A, Shinozuka H. Liver regeneration versus direct hyperplasia. Faseb J. 1996;10:1118–1128. doi: 10.1096/fasebj.10.10.8751714. [DOI] [PubMed] [Google Scholar]
- Cressman DE, Diamond RH, Taub R. Rapid activation of the Stat3 transcription complex in liver regeneration. Hepatology. 1995;21:1443–1449. [PubMed] [Google Scholar]
- Cressman DE, Greenbaum LE, DeAngelis RA, Ciliberto G, Furth EE, Poli V, Taub R. Liver failure and defective hepatocyte regeneration in interleukin-6-deficient mice. Science. 1996;274:1379–1383. doi: 10.1126/science.274.5291.1379. [DOI] [PubMed] [Google Scholar]
- Croquelois A, Blindenbacher A, Terracciano L, Wang X, Langer I, Radtke F, Heim MH. Inducible inactivation of Notch1 causes nodular regenerative hyperplasia in mice. Hepatology. 2005;41:487–496. doi: 10.1002/hep.20571. [See comment] [DOI] [PubMed] [Google Scholar]
- Crosby HA, Hubscher S, Fabris L, Joplin R, Sell S, Kelly D, Strain AJ. Immunolocalization of putative human liver progenitor cells in livers from patients with end-stage primary biliary cirrhosis and sclerosing cholangitis using the monoclonal antibody OV-6. Am J Pathol. 1998;152:771–779. [PMC free article] [PubMed] [Google Scholar]
- Cruise JL, Houck KA, Michalopoulos GK. Induction of DNA synthesis in cultured rat hepatocytes through stimulation of alpha 1 adrenoreceptor by norepinephrine. Science. 1985;227:749–751. doi: 10.1126/science.2982212. [DOI] [PubMed] [Google Scholar]
- Cruise JL, Knechtle SJ, Bollinger RR, Kuhn C, Michalopoulos G. Alpha 1-adrenergic effects and liver regeneration. Hepatology. 1987;7:1189–1194. doi: 10.1002/hep.1840070604. [DOI] [PubMed] [Google Scholar]
- DeAngelis RA, Kovalovich K, Cressman DE, Taub R. Normal liver regeneration in p50/nuclear factor kappaB1 knockout mice. Hepatology. 2001;33:915–924. doi: 10.1053/jhep.2001.23192. [DOI] [PubMed] [Google Scholar]
- DeAngelis RA, Markiewski MM, Lambris JD. Liver regeneration: A link to inflammation through complement. Adv Exp Med Biol. 2006;586:17–34. doi: 10.1007/0-387-34134-X_2. [DOI] [PubMed] [Google Scholar]
- Demetris AJ, Lunz JG, 3rd, Specht S, Nozaki I. Biliary wound healing, ductular reactions, and IL-6/gp130 signaling in the development of liver disease. World J Gastroenterol. 2006;12:3512–3522. doi: 10.3748/wjg.v12.i22.3512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derynck R. The physiology of transforming growth factor-alpha. Adv Cancer Res. 1992;58:27–52. doi: 10.1016/s0065-230x(08)60289-4. [DOI] [PubMed] [Google Scholar]
- Diehl AM. Lessons from animal models of NASH. Hepatol Res. 2005;33:138–144. doi: 10.1016/j.hepres.2005.09.022. [DOI] [PubMed] [Google Scholar]
- Dietrich S, Abou-Rebyeh F, Brohmann H, Bladt F, Sonnenberg-Riethmacher E, Yamaai T, Lumsden A, Brand-Saberi B, Birchmeier C. The role of SF/HGF and c-Met in the development of skeletal muscle. Development. 1999;126:1621–1629. doi: 10.1242/dev.126.8.1621. [DOI] [PubMed] [Google Scholar]
- Evarts RP, Hu Z, Fujio K, Marsden ER, Thorgeirsson SS. Activation of hepatic stem cell compartment in the rat: Role of transforming growth factor alpha, hepatocyte growth factor, and acidic fibroblast growth factor in early proliferation. Cell Growth Differ. 1993;4:555–561. [PubMed] [Google Scholar]
- Evarts RP, Hu Z, Omori N, Omori M, Marsden ER, Thorgeirsson SS. Precursor-product relationship between oval cells and hepatocytes: Comparison between tritiated thymidine and bromodeoxyuridine as tracers. Carcinogenesis. 1996;17:2143–2151. doi: 10.1093/carcin/17.10.2143. [DOI] [PubMed] [Google Scholar]
- Evarts RP, Nagy P, Nakatsukasa H, Marsden E, Thorgeirsson SS. In vivo differentiation of rat liver oval cells into hepatocytes. Cancer Res. 1989;49:1541–1547. [PubMed] [Google Scholar]
- Evarts RP, Raab M, Marsden E, Thorgeirsson SS. Histochemical changes in livers from portacaval-shunted rats. J Natl Cancer Inst. 1986;76:731–738. doi: 10.1093/jnci/76.4.731. [DOI] [PubMed] [Google Scholar]
- Fausto N. Liver regeneration. J Hepatol. 2000;32:19–31. doi: 10.1016/s0168-8278(00)80412-2. [DOI] [PubMed] [Google Scholar]
- Fausto N. Liver regeneration and repair: Hepatocytes, progenitor cells, and stem cells. Hepatology. 2004;39:1477–1487. doi: 10.1002/hep.20214. [DOI] [PubMed] [Google Scholar]
- Fausto N, Campbell JS, Riehle KJ. Liver regeneration. Hepatology. 2006;43:S45–S53. doi: 10.1002/hep.20969. [DOI] [PubMed] [Google Scholar]
- Fernandez MA, Albor C, Ingelmo-Torres M, Nixon SJ, Ferguson C, Kurzchalia T, Tebar F, Enrich C, Parton RG, Pol A. Caveolin-1 is essential for liver regeneration. Science. 2006;313:1628–1632. doi: 10.1126/science.1130773. [DOI] [PubMed] [Google Scholar]
- Fey GH, Hattori M, Hocke G, Brechner T, Baffet G, Baumann M, Baumann H, Northemann W. Gene regulation by interleukin 6. Biochimie. 1991;73:47–50. doi: 10.1016/0300-9084(91)90073-a. [DOI] [PubMed] [Google Scholar]
- FitzGerald MJ, Webber EM, Donovan JR, Fausto N. Rapid DNA binding by nuclear factor kappa B in hepatocytes at the start of liver regeneration. Cell Growth Differ. 1995;6:417–427. [PubMed] [Google Scholar]
- Friedman JR, Larris B, Le PP, Peiris TH, Arsenlis A, Schug J, Tobias JW, Kaestner KH, Greenbaum LE. Orthogonal analysis of C/EBPbeta targets in vivo during liver proliferation. Proc Natl Acad Sci U S A. 2004;101:12986–12991. doi: 10.1073/pnas.0402875101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujio K, Evarts RP, Hu Z, Marsden ER, Thorgeirsson SS. Expression of stem cell factor and its receptor, c-kit, during liver regeneration from putative stem cells in adult rat. Lab Invest. 1994;70:511–516. [PubMed] [Google Scholar]
- Gallai M, Sebestyen A, Nagy P, Kovalszky I, Onody T, Thorgeirsson SS. Proteoglycan gene expression in rat liver after partial hepatectomy. Biochem Biophys Res Commun. 1996;228:690–694. doi: 10.1006/bbrc.1996.1718. [DOI] [PubMed] [Google Scholar]
- Gebhardt R, Baldysiak-Figiel A, Krugel V, Ueberham E, Gaunitz F. Hepatocellular expression of glutamine synthetase: An indicator of morphogen actions as master regulators of zonation in adult liver. Prog Histochem Cytochem. 2007;41:201–266. doi: 10.1016/j.proghi.2006.12.001. [DOI] [PubMed] [Google Scholar]
- Geisterfer M, Richards C, Baumann M, Fey G, Gywnne D, Gauldie J. Regulation of IL-6 and the hepatic IL-6 receptor in acute inflammation in vivo. Cytokine. 1993;5:1–7. doi: 10.1016/1043-4666(93)90017-y. [DOI] [PubMed] [Google Scholar]
- Gkretsi V, Bowen WC, Yang Y, Wu C, Michalopoulos GK. Integrin-linked kinase is involved in matrix-induced hepatocyte differentiation. Biochem Biophys Res Commun. 2007a;353:638–643. doi: 10.1016/j.bbrc.2006.12.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gkretsi V, Mars WM, Bowen WC, Barua L, Yang Y, Guo L, St-Arnaud R, Dedhar S, Wu C, Michalopoulos GK. Loss of integrin linked kinase from mouse hepatocytes in vitro and in vivo results in apoptosis and hepatitis. Hepatology. 2007b;45:1025–1034. doi: 10.1002/hep.21540. [DOI] [PubMed] [Google Scholar]
- Gohda E, Matsunaga T, Kataoka H, Yamamoto I. TGF-beta is a potent inhibitor of hepatocyte growth factor secretion by human fibroblasts. Cell Biol Int Rep. 1992;16:917–926. doi: 10.1016/s0309-1651(06)80171-2. [DOI] [PubMed] [Google Scholar]
- Haruyama T, Ajioka I, Akaike T, Watanabe Y. Regulation and significance of hepatocyte-derived matrix metalloproteinases in liver remodeling. Biochem Biophys Res Commun. 2000;272:681–686. doi: 10.1006/bbrc.2000.2837. [DOI] [PubMed] [Google Scholar]
- Higgins GM, Anderson RM. Experimental pathology of the liver, 1:Restoration of the liver of the white rat following partial surgical removal. Arch Pathol. 1931;12:186–202. [Google Scholar]
- Ho J, de Guise C, Kim C, Lemay S, Wang XF, Lebrun JJ. Activin induces hepatocyte cell growth arrest through induction of the cyclin-dependent kinase inhibitor p15INK4B and Sp1. Cell Signal. 2004;16:693–701. doi: 10.1016/j.cellsig.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Holifield JS, Arlen AM, Runyan RB, Tomanek RJ. TGF-beta1, -beta2 and -beta3 cooperate to facilitate tubulogenesis in the explanted quail heart. J Vasc Res. 2004;41:491–498. doi: 10.1159/000081805. [DOI] [PubMed] [Google Scholar]
- Houck KA, Cruise JL, Michalopoulos G. Norepinephrine modulates the growth-inhibitory effect of transforming growth factor-beta in primary rat hepatocyte cultures. J Cell Physiol. 1988;135:551–555. doi: 10.1002/jcp.1041350327. [DOI] [PubMed] [Google Scholar]
- Houck KA, Michalopoulos GK. Altered responses of regenerating hepatocytes to norepinephrine and transforming growth factor type beta. J Cell Physiol. 1989;141:503–509. doi: 10.1002/jcp.1041410308. [DOI] [PubMed] [Google Scholar]
- Houck KA, Zarnegar R, Muga SJ, Michalopoulos GK. Acidic fibroblast growth factor (HBGF-1) stimulates DNA synthesis in primary rat hepatocyte cultures. J Cell Physiol. 1990;143:129–132. doi: 10.1002/jcp.1041430117. [DOI] [PubMed] [Google Scholar]
- Huang W, K M, J Z, M Q, J C, J L, B D, X H, DD M. Nuclear receptor-dependent bile acid signaling is required for normal liver regeneration. Science. 2006;312:233–236. doi: 10.1126/science.1121435. [DOI] [PubMed] [Google Scholar]
- Huh CG, Factor VM, Sanchez A, Uchida K, Conner EA, Thorgeirsson SS. Hepatocyte growth factor/c-met signaling pathway is required for efficient liver regeneration and repair. Proc Natl Acad Sci U S A. 2004;101:4477–4482. doi: 10.1073/pnas.0306068101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichikawa T, Zhang YQ, Kogure K, Hasegawa Y, Takagi H, Mori M, Kojima I. Transforming growth factor beta and activin tonically inhibit DNA synthesis in the rat liver. Hepatology. 2001;34:918–925. doi: 10.1053/jhep.2001.29132. [DOI] [PubMed] [Google Scholar]
- Iimuro Y, Nishiura T, Hellerbrand C, Behrns KE, Schoonhoven R, Grisham JW, Brenner DA. NFkappaB prevents apoptosis and liver dysfunction during liver regeneration [published erratum appears in J Clin Invest 1998 Apr 1;101(7):1541] J Clin Invest. 1998;101:802–811. doi: 10.1172/JCI483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda H, Nagoshi S, Ohno A, Yanase M, Maekawa H, Fujiwara K. Activated rat stellate cells express c-met and respond to hepatocyte growth factor to enhance transforming growth factor beta1 expression and DNA synthesis. Biochem Biophys Res Commun. 1998;250:769–775. doi: 10.1006/bbrc.1998.9387. [DOI] [PubMed] [Google Scholar]
- Jakowlew SB, Mead JE, Danielpour D, Wu J, Roberts AB, Fausto N. Transforming growth factor-beta (TGF-beta) isoforms in rat liver regeneration: Messenger RNA expression and activation of latent TGF-beta. Cell Regul. 1991;2:535–548. doi: 10.1091/mbc.2.7.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jirtle RL, Carr BI, Scott CD. Modulation of insulin-like growth factor-II/mannose 6-phosphate receptors and transforming growth factor-beta 1 during liver regeneration [published erratum appears in J Biol Chem 1991 Dec 25;266(36):24860] J Biol Chem. 1991;266:22444–22450. [PubMed] [Google Scholar]
- Jirtle RL, Michalopoulos G. Effects of partial hepatectomy on transplanted hepatocytes. Cancer Res. 1982;42:3000–3004. [PubMed] [Google Scholar]
- Jo M, Stolz DB, Esplen JE, Dorko K, Michalopoulos GK, Strom SC. Cross-talk between epidermal growth factor receptor and c-Met signal pathways in transformed cells. J Biol Chem. 2000;275:8806–8811. doi: 10.1074/jbc.275.12.8806. [DOI] [PubMed] [Google Scholar]
- Jones DE, Jr, Tran-Patterson R, Cui DM, Davin D, Estell KP, Miller DM. Epidermal growth factor secreted from the salivary gland is necessary for liver regeneration. Am J Physiol. 1995;268:G872–G878. doi: 10.1152/ajpgi.1995.268.5.G872. [DOI] [PubMed] [Google Scholar]
- Jung Y, Oh SH, Zheng D, Shupe TD, Witek RP, Petersen BE. A potential role of somatostatin and its receptor SSTR4 in the migration of hepatic oval cells. Lab Invest. 2006;86:477–489. doi: 10.1038/labinvest.3700410. [DOI] [PubMed] [Google Scholar]
- Karin M, Yamamoto Y, Wang QM. The IKK NF-kappa B system: A treasure trove for drug development. Nat Rev Drug Discov. 2004;3:17–26. doi: 10.1038/nrd1279. [DOI] [PubMed] [Google Scholar]
- Khan Z, Michalopoulos GK, Stolz DB. Peroxisomal localization of hypoxia-inducible factors and hypoxia-inducible factor regulatory hydroxylases in primary rat hepatocytes exposed to hypoxia-reoxygenation. Am J Pathol. 2006;169:1251–1269. doi: 10.2353/ajpath.2006.060360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim TH, Mars WM, Stolz DB, Michalopoulos GK. Expression and activation of pro-MMP-2 and pro-MMP-9 during rat liver regeneration. Hepatology. 2000;31:75–82. doi: 10.1002/hep.510310114. [DOI] [PubMed] [Google Scholar]
- Kim TH, Mars WM, Stolz DB, Petersen BE, Michalopoulos GK. Extracellular matrix remodeling at the early stages of liver regeneration in the rat. Hepatology. 1997;26:896–904. doi: 10.1002/hep.510260415. [DOI] [PubMed] [Google Scholar]
- Kirillova I, Chaisson M, Fausto N. Tumor necrosis factor induces DNA replication in hepatic cells through nuclear factor kappaB activation. Cell Growth Differ. 1999;10:819–828. [PubMed] [Google Scholar]
- Kiso S, Kawata S, Tamura S, Higashiyama S, Ito N, Tsushima H, Taniguchi N, Matsuzawa Y. Role of heparin-binding epidermal growth factor-like growth factor as a hepatotrophic factor in rat liver regeneration after partial hepatectomy. Hepatology. 1995;22:1584–1590. [PubMed] [Google Scholar]
- Kiso S, Kawata S, Tamura S, Inui Y, Yoshida Y, Sawai Y, Umeki S, Ito N, Yamada A, Miyagawa J, Higashiyama S, Iwawaki T, Saito M, Taniguchi N, Matsuzawa Y, Kohno K. Liver regeneration in heparin-binding EGF-like growth factor transgenic mice after partial hepatectomy. Gastroenterology. 2003;124:701–707. doi: 10.1053/gast.2003.50097. [DOI] [PubMed] [Google Scholar]
- Kogure K, Zhang YQ, Maeshima A, Suzuki K, Kuwano H, Kojima I. The role of activin and transforming growth factor-beta in the regulation of organ mass in the rat liver. Hepatology. 2000;31:916–921. doi: 10.1053/he.2000.6100. [DOI] [PubMed] [Google Scholar]
- Kohler C, Bell AW, Bowen WC, Monga SP, Fleig W, Michalopoulos GK. Expression of Notch-1 and its ligand Jagged-1 in rat liver during liver regeneration. Hepatology. 2004;39:1056–1065. doi: 10.1002/hep.20156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kortlever RM, Bernards R. Senescence, wound healing and cancer: The PAI-1 connection. Cell cycle. 2006;5:2697–2703. doi: 10.4161/cc.5.23.3510. [DOI] [PubMed] [Google Scholar]
- Laconi E, Oren R, Mukhopadhyay DK, Hurston E, Laconi S, Pani P, Dabeva MD, Shafritz DA. Long-term, near-total liver replacement by transplantation of isolated hepatocytes in rats treated with retrorsine. Am J Pathol. 1998;153:319–329. doi: 10.1016/S0002-9440(10)65574-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagasse E, Connors H, Al-Dhalimy M, Reitsma M, Dohse M, Osborne L, Wang X, Finegold M, Weissman IL, Grompe M. Purified hematopoietic stem cells can differentiate into hepatocytes in vivo. Nat Med. 2000;6:1229–1234. doi: 10.1038/81326. [See comment] [DOI] [PubMed] [Google Scholar]
- LaMarre J, Hayes MA, Wollenberg GK, Hussaini I, Hall SW, Gonias SL. An alpha 2-macroglobulin receptor-dependent mechanism for the plasma clearance of transforming growth factor-beta 1 in mice. J Clin Invest. 1991;87:39–44. doi: 10.1172/JCI114998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leclercq IA, Vansteenberghe M, Lebrun VB, VanHul NK, Abarca-Quinones J, Sempoux CL, Picard C, Starkel P, Horsmans YL. Defective hepatic regeneration after partial hepatectomy in leptin-deficient mice is not rescued by exogenous leptin. Lab Invest. 2006;86:1161–1171. doi: 10.1038/labinvest.3700474. [DOI] [PubMed] [Google Scholar]
- LeCouter J, Moritz DR, Li B, Phillips GL, Liang XH, Gerber HP, Hillan KJ, Ferrara N. Angiogenesis-independent endothelial protection of liver: Role of VEGFR-1. Science. 2003;299:890–893. doi: 10.1126/science.1079562. [DOI] [PubMed] [Google Scholar]
- Ledda-Columbano GM, Perra A, Loi R, Shinozuka H, Columbano A. Cell proliferation induced by triiodothyronine in rat liver is associated with nodule regression and reduction of hepatocellular carcinomas. Cancer Res. 2000;60:603–609. [PubMed] [Google Scholar]
- Ledda-Columbano GM, Pibiri M, Concas D, Cossu C, Tripodi M, Columbano A. Loss of cyclin D1 does not inhibit the proliferative response of mouse liver to mitogenic stimuli. Hepatology. 2002;36:1098–1105. doi: 10.1053/jhep.2002.36159. [DOI] [PubMed] [Google Scholar]
- Lee CS, Friedman JR, Fulmer JT, Kaestner KH. The initiation of liver development is dependent on Foxa transcription factors. Nature. 2005;435:944–947. doi: 10.1038/nature03649. [DOI] [PubMed] [Google Scholar]
- Lee DC, Sunnarborg SW, Hinkle CL, Myers TJ, Stevenson MY, Russell WE, Castner BJ, Gerhart MJ, Paxton RJ, Black RA, Chang A, Jackson LF. TACE/ADAM17 processing of EGFR ligands indicates a role as a physiological convertase. Ann NY Acad Sci. 2003;995:22–38. doi: 10.1111/j.1749-6632.2003.tb03207.x. [DOI] [PubMed] [Google Scholar]
- Leong GF, Grisham JW, Hole BV, Albright ML. Effect of Partial Hepatectomy on DNA Synthesis and Mitosis in Heterotopic Partial Autografts of Rat Liver. Cancer Res. 1964;24:1496–1501. [PubMed] [Google Scholar]
- Lesurtel M, Graf R, Aleil B, Walther B, Tian Y, Jochum W, Gaget C, Bader M, Clavien P. Platelet-derived serotonin mediates liver regeneration. Science. 2006;312:104–107. doi: 10.1126/science.1123842. [DOI] [PubMed] [Google Scholar]
- Leu JI, Crissey MA, Craig LE, Taub R. Impaired hepatocyte DNA synthetic response posthepatectomy in insulin-like growth factor binding protein 1-deficient mice with defects in C/EBP beta and mitogen-activated protein kinase/extracellular signal-regulated kinase regulation. Mol Cell Biol. 2003;23:1251–1259. doi: 10.1128/MCB.23.4.1251-1259.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Liang X, Kellendonk C, Poli V, Taub R. STAT3 contributes to the mitogenic response of hepatocytes during liver regeneration. J Biol Chem. 2002;277:28411–28417. doi: 10.1074/jbc.M202807200. [DOI] [PubMed] [Google Scholar]
- Lindroos PM, Zarnegar R, Michalopoulos GK. Hepatocyte growth factor (hepatopoietin A) rapidly increases in plasma before DNA synthesis and liver regeneration stimulated by partial hepatectomy and carbon tetrachloride administration. Hepatology. 1991;13:743–750. [PubMed] [Google Scholar]
- Liu ML, Mars WM, Zarnegar R, Michalopoulos GK. Collagenase pretreatment and the mitogenic effects of hepatocyte growth factor and transforming growth factor-alpha in adult rat liver. Hepatology. 1994a;19:1521–1527. [PubMed] [Google Scholar]
- Liu Y, Michalopoulos GK, Zarnegar R. Structural and functional characterization of the mouse hepatocyte growth factor gene promoter. J Biol Chem. 1994b;269:4152–4160. [PubMed] [Google Scholar]
- Liu Z, Sakamoto T, Ezure T, Yokomuro S, Murase N, Michalopoulos G, Demetris AJ. Interleukin-6, hepatocyte growth factor, and their receptors in biliary epithelial cells during a type I ductular reaction in mice: Interactions between the periductal inflammatory and stromal cells and the biliary epithelium. Hepatology. 1998;28:1260–1268. doi: 10.1002/hep.510280514. [DOI] [PubMed] [Google Scholar]
- Luo JH, Ren B, Keryanov S, Tseng GC, Rao UN, Monga SP, Strom S, Demetris AJ, Nalesnik M, Yu YP, Ranganathan S, Michalopoulos GK. Transcriptomic and genomic analysis of human hepatocellular carcinomas and hepatoblastomas. Hepatology. 2006;44:1012–1024. doi: 10.1002/hep.21328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo JL, Kamata H, Karin M. IKK/NF-kappaB signaling: Balancing life and death--a new approach to cancer therapy. J Clin Invest. 2005;115:2625–2632. doi: 10.1172/JCI26322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maione D, Di Carlo E, Li W, Musiani P, Modesti A, Peters M, Rose-John S, Della Rocca C, Tripodi M, Lazzaro D, Taub R, Savino R, Ciliberto G. Coexpression of IL-6 and soluble IL-6R causes nodular regenerative hyperplasia and adenomas of the liver. EMBO J. 1998;17:5588–5597. doi: 10.1093/emboj/17.19.5588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mars WM, Kim TH, Stolz DB, Liu ML, Michalopoulos GK. Presence of urokinase in serum-free primary rat hepatocyte cultures and its role in activating hepatocyte growth factor. Cancer Res. 1996;56:2837–2843. [PubMed] [Google Scholar]
- Mars WM, Liu ML, Kitson RP, Goldfarb RH, Gabauer MK, Michalopoulos GK. Immediate early detection of urokinase receptor after partial hepatectomy and its implications for initiation of liver regeneration. Hepatology. 1995;21:1695–1701. [PubMed] [Google Scholar]
- Mars WM, Zarnegar R, Michalopoulos GK. Activation of hepatocyte growth factor by the plasminogen activators uPA and tPA. Am J Pathol. 1993;143:949–958. [PMC free article] [PubMed] [Google Scholar]
- Marubashi S, Sakon M, Nagano H, Gotoh K, Hashimoto K, Kubota M, Kobayashi S, Yamamoto S, Miyamoto A, Dono K, Nakamori S, Umeshita K, Monden M. Effect of portal hemodynamics on liver regeneration studied in a novel portohepatic shunt rat model. Surgery. 2004;136:1028–1037. doi: 10.1016/j.surg.2004.03.012. [DOI] [PubMed] [Google Scholar]
- Masumoto A, Yamamoto N. Sequestration of a hepatocyte growth factor in extracellular matrix in normal adult rat liver. Biochem Biophys Res Commun. 1991;174:90–95. doi: 10.1016/0006-291x(91)90489-t. [DOI] [PubMed] [Google Scholar]
- Masumoto A, Yamamoto N. Stimulation of DNA synthesis in hepatocytes by hepatocyte growth factor bound to extracellular matrix. Biochem Biophys Res Commun. 1993;191:1218–1223. doi: 10.1006/bbrc.1993.1347. [DOI] [PubMed] [Google Scholar]
- Matsumoto K, Nakamura T. Emerging multipotent aspects of hepatocyte growth factor. J Biochem. 1996;119:591–600. doi: 10.1093/oxfordjournals.jbchem.a021283. [DOI] [PubMed] [Google Scholar]
- Mead JE, Fausto N. Transforming growth factor alpha may be a physiological regulator of liver regeneration by means of an autocrine mechanism. Proc Natl Acad Sci U S A. 1989;86:1558–1562. doi: 10.1073/pnas.86.5.1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menegazzi M, Carcereri-De Prati A, Suzuki H, Shinozuka H, Pibiri M, Piga R, Columbano A, Ledda-Columbano GM. Liver cell proliferation induced by nafenopin and cyproterone acetate is not associated with increases in activation of transcription factors NF-kappaB and AP-1 or with expression of tumor necrosis factor alpha. Hepatology. 1997;25:585–592. doi: 10.1002/hep.510250316. [DOI] [PubMed] [Google Scholar]
- Michalopoulos G, Houck KA, Dolan ML, Leutteke NC. Control of hepatocyte replication by two serum factors. Cancer Res. 1984;44:4414–4419. [PubMed] [Google Scholar]
- Michalopoulos GK, Barua L, Bowen WC. Transdifferentiation of rat hepatocytes into biliary cells after bile duct ligation and toxic biliary injury. Hepatology. 2005a;41:535–544. doi: 10.1002/hep.20600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalopoulos GK, Bowen WC, Mule K, Stolz DB. Histological organization in hepatocyte organoid cultures. Am J Pathol. 2001;159:1877–1887. doi: 10.1016/S0002-9440(10)63034-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalopoulos GK, Bowen WC, Zajac VF, Beer-Stolz D, Watkins S, Kostrubsky V, Strom SC. Morphogenetic events in mixed cultures of rat hepatocytes and nonparenchymal cells maintained in biological matrices in the presence of hepatocyte growth factor and epidermal growth factor. Hepatology. 1999;29:90–100. doi: 10.1002/hep.510290149. [see comments] [DOI] [PubMed] [Google Scholar]
- Michalopoulos GK, DeFrances M. Liver regeneration. Adv Biochem Eng Biotechnol. 2005b;93:101–134. doi: 10.1007/b99968. [DOI] [PubMed] [Google Scholar]
- Michalopoulos GK, DeFrances MC. Liver regeneration. Science. 1997;276:60–66. doi: 10.1126/science.276.5309.60. [DOI] [PubMed] [Google Scholar]
- Michalopoulos GK, Khan Z. Liver regeneration, growth factors, and amphiregulin. Gastroenterology. 2005c;128:503–506. doi: 10.1053/j.gastro.2004.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miki T, Strom SC. Amnion-derived pluripotent/multipotent stem cells. Stem Cell Rev. 2006;2:133–142. doi: 10.1007/s12015-006-0020-0. [DOI] [PubMed] [Google Scholar]
- Mitchell C, Nivison M, Jackson LF, Fox R, Lee DC, Campbell JS, Fausto N. Heparin-binding epidermal growth factor-like growth factor links hepatocyte priming with cell cycle progression during liver regeneration. J Biol Chem. 2005;280:2562–2568. doi: 10.1074/jbc.M412372200. [DOI] [PubMed] [Google Scholar]
- Miyazaki M, Masaka T, Akiyama I, Nakashima E, Sakaguchi M, Huh NH. Propagation of adult rat bone marrow-derived hepatocyte-like cells by serial passages in vitro. Cell Transplant. 2004;13:385–391. doi: 10.3727/000000004783983800. [DOI] [PubMed] [Google Scholar]
- Mohammed FF, Khokha R. Thinking outside the cell: Proteases regulate hepatocyte division. Trends Cell Biol. 2005a;15:555–563. doi: 10.1016/j.tcb.2005.08.009. [DOI] [PubMed] [Google Scholar]
- Mohammed FF, Pennington CJ, Kassiri Z, Rubin JS, Soloway PD, Ruther U, Edwards DR, Khokha R. Metalloproteinase inhibitor TIMP-1 affects hepatocyte cell cycle via HGF activation in murine liver regeneration. Hepatology. 2005b;41:857–867. doi: 10.1002/hep.20618. [DOI] [PubMed] [Google Scholar]
- Monga SP, Mars WM, Pediaditakis P, Bell A, Mule K, Bowen WC, Wang X, Zarnegar R, Michalopoulos GK. Hepatocyte growth factor induces Wnt-independent nuclear translocation of beta-catenin after Met-beta-catenin dissociation in hepatocytes. Cancer Res. 2002;62:2064–2071. [PubMed] [Google Scholar]
- Monga SP, Pediaditakis P, Mule K, Stolz DB, Michalopoulos GK. Changes in WNT/beta-catenin pathway during regulated growth in rat liver regeneration. Hepatology. 2001;33:1098–1109. doi: 10.1053/jhep.2001.23786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moolten FL, Bucher NL. Regeneration of rat liver: Transfer of humoral agent by cross circulation. Science. 1967;158:272–274. doi: 10.1126/science.158.3798.272. [DOI] [PubMed] [Google Scholar]
- Mumm JS, Kopan R. Notch signaling: From the outside in. Dev Biol. 2000;228:151–165. doi: 10.1006/dbio.2000.9960. [DOI] [PubMed] [Google Scholar]
- Murphy MO, Ghosh J, Fulford P, Khwaja N, Halka AT, Carter A, Turner NJ, Walker MG. Expression of growth factors and growth factor receptor in non-healing and healing ischaemic ulceration. Eur J Vasc Endovasc Surg. 2006;31:516–522. doi: 10.1016/j.ejvs.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Nagy P, Bisgaard HC, Thorgeirsson SS. Expression of hepatic transcription factors during liver development and oval cell differentiation. J Cell Biol. 1994;126:223–233. doi: 10.1083/jcb.126.1.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naldini L, Vigna E, Narsimhan RP, Gaudino G, Zarnegar R, Michalopoulos GK, Comoglio PM. Hepatocyte growth factor (HGF) stimulates the tyrosine kinase activity of the receptor encoded by the proto-oncogene c-MET. Oncogene. 1991;6:501–504. [PubMed] [Google Scholar]
- Nelsen CJ, Rickheim DG, Tucker MM, Hansen LK, Albrecht JH. Evidence that cyclin D1 mediates both growth and proliferation downstream of TOR in hepatocytes. J Biol Chem. 2003;278:3656–3663. doi: 10.1074/jbc.M209374200. [DOI] [PubMed] [Google Scholar]
- Neubauer K, Knittel T, Aurisch S, Fellmer P, Ramadori G. Glial fibrillary acidic protein—A cell type specific marker for Ito cells in vivo and in vitro. J Hepatol. 1996;24:719–730. doi: 10.1016/s0168-8278(96)80269-8. [DOI] [PubMed] [Google Scholar]
- Ni N, Yager JD. Comitogenic effects of estrogens on DNA synthesis induced by various growth factors in cultured female rat hepatocytes. Hepatology. 1994;19:183–192. [PubMed] [Google Scholar]
- Ninomiya M, Harada N, Shiotani S, Hiroshige S, Minagawa R, Soejima Y, Suehiro T, Nishizaki T, Shimada M, Sugimachi K. Hepatocyte growth factor and transforming growth factor beta1 contribute to regeneration of small-for-size liver graft immediately after transplantation. Transpl Int. 2003;16:814–819. doi: 10.1007/s00147-003-0628-9. [DOI] [PubMed] [Google Scholar]
- Northemann W, Hattori M, Baffet G, Braciak TA, Fletcher RG, Abraham LJ, Gauldie J, Baumann M, Fey GH. Production of interleukin 6 by hepatoma cells. Mol Biol Med. 1990;7:273–285. [PubMed] [Google Scholar]
- Nozaki I, Lunz JG, III, Specht S, Stolz DB, Taguchi K, Subbotin VM, Murase N, Demetris AJ. Small proline-rich proteins 2 are noncoordinately upregulated by IL-6/STAT3 signaling after bile duct ligation. Lab Invest. 2005;85:109–123. doi: 10.1038/labinvest.3700213. [DOI] [PubMed] [Google Scholar]
- Nozawa K, Kurumiya Y, Yamamoto A, Isobe Y, Suzuki M, Yoshida S. Up-regulation of telomerase in primary cultured rat hepatocytes. J Biochem. 1999;126:361–367. doi: 10.1093/oxfordjournals.jbchem.a022458. [DOI] [PubMed] [Google Scholar]
- Nussler AK, Di Silvio M, Liu ZZ, Geller DA, Freeswick P, Dorko K, Bartoli F, Billiar TR. Further characterization and comparison of inducible nitric oxide synthase in mouse, rat, and human hepatocytes. Hepatology. 1995;21:1552–1560. [PubMed] [Google Scholar]
- Oben JA, Yang S, Lin H, Ono M, Diehl AM. Norepinephrine and neuropeptide Y promote proliferation and collagen gene expression of hepatic myofibroblastic stellate cells. Biochem Biophys Res Commun. 2003;302:685–690. doi: 10.1016/s0006-291x(03)00232-8. [DOI] [PubMed] [Google Scholar]
- Oe S, Lemmer ER, Conner EA, Factor VM, Leveen P, Larsson J, Karlsson S, Thorgeirsson SS. Intact signaling by transforming growth factor beta is not required for termination of liver regeneration in mice. Hepatology. 2004;40:1098–1105. doi: 10.1002/hep.20426. [DOI] [PubMed] [Google Scholar]
- Oh SH, Witek RP, Bae SH, Zheng D, Jung Y, Piscaglia AC, Petersen BE. Bone marrow-derived hepatic oval cells differentiate into hepatocytesin 2-acetylaminofluorene/partial hepatectomy-induced liver regeneration. Gastroenterology. 2007;132:1077–1087. doi: 10.1053/j.gastro.2007.01.001. [DOI] [PubMed] [Google Scholar]
- Ohlson LC, Koroxenidou L, Hallstrom IP. Inhibition of in vivo rat liver regeneration by 2-acetylaminofluorene affects the regulation of cell cycle-related proteins. Hepatology. 1998;27:691–696. doi: 10.1002/hep.510270309. [DOI] [PubMed] [Google Scholar]
- Olle EW, Ren X, McClintock SD, Warner RL, Deogracias MP, Johnson KJ, Colletti LM. Matrix metalloproteinase-9 is an important factor in hepatic regeneration after partial hepatectomy in mice. Hepatology. 2006;44:540–549. doi: 10.1002/hep.21314. [DOI] [PubMed] [Google Scholar]
- Olsen PS, Poulsen SS, Kirkegaard P. Adrenergic effects on secretion of epidermal growth factor from Brunner’s glands. Gut. 1985;26:920–927. doi: 10.1136/gut.26.9.920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overturf K, al-Dhalimy M, Ou CN, Finegold M, Grompe M. Serial transplantation reveals the stem-cell-like regenerative potential of adult mouse hepatocytes. Am J Pathol. 1997;151:1273–1280. [PMC free article] [PubMed] [Google Scholar]
- Papastefanou VP, Bozas E, Mykoniatis MG, Grypioti A, Garyfallidis S, Bartsocas CS, Nicolopoulou-Stamati P. VEGF isoforms and receptors expression throughout acute acetaminophen-induced liver injury and regeneration. Arch Toxicol. 2007 doi: 10.1007/s00204-007-0201-x. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- Paranjpe S, Bowen WC, Bell AW, Nejak-Bowen K, Luo JH, Michalopoulos GK. Cell cycle effects resulting from inhibition of hepatocyte growth factor and its receptor c-Met in regenerating rat livers by RNA interference. Hepatology. 2007;45:1471–1477. doi: 10.1002/hep.21570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JM, Greten FR, Wong A, Westrick RJ, Arthur JS, Otsu K, Hoffmann A, Montminy M, Karin M. Signaling pathways and genes that inhibit pathogen-induced macrophage apoptosis--CREB and NF-kappaB as key regulators. Immunity. 2005;23:319–329. doi: 10.1016/j.immuni.2005.08.010. [DOI] [PubMed] [Google Scholar]
- Passino MA, Adams RA, Sikorski SL, Akassoglou K. Regulation of hepatic stellate cell differentiation by the neurotrophin receptor p75NTR. Science. 2007;315:1853–1856. doi: 10.1126/science.1137603. [DOI] [PubMed] [Google Scholar]
- Patijn GA, Lieber A, Schowalter DB, Schwall R, Kay MA. Hepatocyte growth factor induces hepatocyte proliferation in vivo and allows for efficient retroviral-mediated gene transfer in mice. Hepatology. 1998;28:707–716. doi: 10.1002/hep.510280317. [DOI] [PubMed] [Google Scholar]
- Pediaditakis P, Lopez-Talavera JC, Petersen B, Monga SP, Michalopoulos GK. The processing and utilization of hepatocyte growth factor/scatter factor following partial hepatectomy in the rat. Hepatology. 2001;34:688–693. doi: 10.1053/jhep.2001.27811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepper MS, Vassalli JD, Orci L, Montesano R. Biphasic effect of transforming growth factor-beta 1 on in vitro angiogenesis. Exp Cell Res. 1993;204:356–363. doi: 10.1006/excr.1993.1043. [DOI] [PubMed] [Google Scholar]
- Petersen BE, Bowen WC, Patrene KD, Mars WM, Sullivan AK, Murase N, Boggs SS, Greenberger JS, Goff JP. Bone marrow as a potential source of hepatic oval cells. Science. 1999;284:1168–1170. doi: 10.1126/science.284.5417.1168. [DOI] [PubMed] [Google Scholar]
- Petersen BE, Zajac VF, Michalopoulos GK. Bile ductular damage induced by methylene dianiline inhibits oval cell activation. Am J Pathol. 1997;151:905–909. [PMC free article] [PubMed] [Google Scholar]
- Pi L, Oh SH, Shupe T, Petersen BE. Role of connective tissue growth factor in oval cell response during liver regeneration after 2-AAF/PHx in rats. Gastroenterology. 2005;128:2077–2088. doi: 10.1053/j.gastro.2005.03.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce RH, Campbell JS, Stephenson AB, Franklin CC, Chaisson M, Poot M, Kavanagh TJ, Rabinovitch PS, Fausto N. Disruption of redox homeostasis in tumor necrosis factor-induced apoptosis in a murine hepatocyte cell line. Am J Pathol. 2000;157:221–236. doi: 10.1016/S0002-9440(10)64533-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinzani M. PDGF and signal transduction in hepatic stellate cells. Front Biosci. 2002;7:d1720–d1726. doi: 10.2741/A875. [DOI] [PubMed] [Google Scholar]
- Purup S, Vestergaard M, Sejrsen K. Involvement of growth factors in the regulation of pubertal mammary growth in cattle. Adv Exp Med Biol. 2000;480:27–43. doi: 10.1007/0-306-46832-8_4. [DOI] [PubMed] [Google Scholar]
- Rabes HM. Kinetics of hepatocellular proliferation as a function of the microvascular structure and functional state of the liver. Ciba Found Symp. 1977:31–53. doi: 10.1002/9780470720363.ch3. [DOI] [PubMed] [Google Scholar]
- Rai RM, Lee FY, Rosen A, Yang SQ, Lin HZ, Koteish A, Liew FY, Zaragoza C, Lowenstein C, Diehl AM. Impaired liver regeneration in inducible nitric oxide synthasedeficient mice. Proc Natl Acad Sci U S A. 1998;95:13829–13834. doi: 10.1073/pnas.95.23.13829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rana B, Mischoulon D, Xie Y, Bucher NL, Farmer SR. Cell-extracellular matrix interactions can regulate the switch between growth and differentiation in rat hepatocytes: Reciprocal expression of C/EBP alpha and immediate-early growth response transcription factors. Mol Cell Biol. 1994;14:5858–5869. doi: 10.1128/mcb.14.9.5858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao MS, Reddy JK. Hepatic trans differentiation in the pancreas. Semin Cell Biol. 1995;6:151–156. doi: 10.1006/scel.1995.0021. [DOI] [PubMed] [Google Scholar]
- Reddy JK, Azarnoff DL, Sirtori CR. Hepatic peroxisome proliferation: Induction by BR-931, a hypolipidemic analog of WY-14,643. Arch Int Pharmacodyn Ther. 1978;234:4–14. [PubMed] [Google Scholar]
- Reddy JK, Chu R. Peroxisome proliferator-induced pleiotropic responses: Pursuit of a phenomenon. Ann N Y Acad Sci. 1996;804:176–201. doi: 10.1111/j.1749-6632.1996.tb18616.x. [DOI] [PubMed] [Google Scholar]
- Roberts AB, McCune BK, Sporn MB. TGF-beta: Regulation of extracellular matrix. Kidney Int. 1992;41:557–559. doi: 10.1038/ki.1992.81. [DOI] [PubMed] [Google Scholar]
- Ronchini C, Capobianco AJ. Induction of cyclin Dl transcription and CDK2 activity by Notch(ic): Implication for cell cycle disruption in transformation by Notch(ic) Mol Cell Biol. 2001;21:5925–5934. doi: 10.1128/MCB.21.17.5925-5934.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roselli HT, Su M, Washington K, Kerins DM, Vaughan DE, Russell WE. Liver regeneration is transiently impaired in urokinase-deficient mice. Am J Physiol. 1998;275:G1472–G1479. doi: 10.1152/ajpgi.1998.275.6.G1472. [DOI] [PubMed] [Google Scholar]
- Ross MA, Sander CM, Kleeb TB, Watkins SC, Stolz DB. Spatiotemporal expression of angiogenesis growth factor receptors during the revascularization of regenerating rat liver. Hepatology. 2001;34:1135–1148. doi: 10.1053/jhep.2001.29624. [DOI] [PubMed] [Google Scholar]
- Rudolph KL, Trautwein C, Kubicka S, Rakemann T, Bahr MJ, Sedlaczek N, Schuppan D, Manns MP. Differential regulation of extracellular matrix synthesis during liver regeneration after partial hepatectomy in rats. Hepatology. 1999;30:1159–1166. doi: 10.1002/hep.510300502. [DOI] [PubMed] [Google Scholar]
- Russell WE, Coffey RJ, Jr, Ouellette AJ, Moses HL. Type beta transforming growth factor reversibly inhibits the early proliferative response to partial hepatectomy in the rat. Proc Natl Acad Sci U S A. 1988;85:5126–5130. doi: 10.1073/pnas.85.14.5126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell WE, Dempsey PJ, Sitaric S, Peck AJ, Coffey RJ., Jr Transforming growth factor-alpha (TGF alpha) concentrations increase in regenerating rat liven Evidence for a delayed accumulation of mature TGF alpha. Endocrinology. 1993;133:1731–1738. doi: 10.1210/endo.133.4.8404616. [DOI] [PubMed] [Google Scholar]
- Russell WE, Kaufmann WK, Sitaric S, Luetteke NC, Lee DC. Liver regeneration and hepatocarcinogenesis in transforming growth factor-alpha-targeted mice. Mol Carcinog. 1996;15:183–189. doi: 10.1002/(SICI)1098-2744(199603)15:3<183::AID-MC4>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Sakamoto T, Liu Z, Murase N, Ezure T, Yokomuro S, Poli V, Demetris AJ. Mitosis and apoptosis in the liver of interleukin-6-deficient mice after partial hepatectomy. Hepatology. 1999;29:403–411. doi: 10.1002/hep.510290244. [DOI] [PubMed] [Google Scholar]
- Sanderson N, Factor V, Nagy P, Kopp J, Kondaiah P, Wakefield L, Roberts AB, Sporn MB, Thorgeirsson SS. Hepatic expression of mature transforming growth factor beta 1 in transgenic mice results in multiple tissue lesions. Proc Natl Acad Sci U S A. 1995;92:2572–2576. doi: 10.1073/pnas.92.7.2572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato T, El-Assal ON, Ono T, Yamanoi A, Dhar DK, Nagasue N. Sinusoidal endothelial cell proliferation and expression of angiopoietin/Tie family in regenerating rat liver. J Hepatol. 2001;34:690–698. doi: 10.1016/s0168-8278(00)00109-4. [DOI] [PubMed] [Google Scholar]
- Schafer M, Werner S. Transcriptional Control of Wound Repair. Annu Rev Cell Dev Biol. 2007 doi: 10.1146/annurev.cellbio.23.090506.123609. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- Schirmacher P, Geerts A, Jung W, Pietrangelo A, Rogler CE, Dienes HP. The role of Ito cells in the biosynthesis of HGF-SF in the liver. Exs. 1993;65:285–299. [PubMed] [Google Scholar]
- Schmidt C, Bladt F, Goedecke S, Brinkmann V, Zschiesche W, Sharpe M, Gherardi E, Birchmeier C. Scatter factor/hepatocyte growth factor is essential for liver development. Nature. 1995;373:699–702. doi: 10.1038/373699a0. [DOI] [PubMed] [Google Scholar]
- Sell S. Liver stem cells. Mod Pathol. 1994;7:105–112. [PubMed] [Google Scholar]
- Shimizu H, Mitsuhashi N, Ohtsuka M, Ito H, Kimura F, Ambiru S, Togawa A, Yoshidome H, Kato A, Miyazaki M. Vascular endothelial growth factor and angiopoietins regulate sinusoidal regeneration and remodeling after partial hepatectomy in rats. World J Gastroenterol. 2005;11:7254–7260. doi: 10.3748/wjg.v11.i46.7254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimomura T, Denda K, Kawaguchi T, Matsumoto K, Miyazawa K, Kitamura N. Multiple sites of proteolytic cleavage to release soluble forms of hepatocyte growth factor activator inhibitor type 1 from a transmembrane form. J Biochem. 1999;126:821–828. doi: 10.1093/oxfordjournals.jbchem.a022522. [DOI] [PubMed] [Google Scholar]
- Short J, Wedmore R, Kibert L, Zemel R. Triidothyronine: On its role as a specific hepatomitogen. Cytobios. 1980;28:165–177. [PubMed] [Google Scholar]
- Shteyer E, Liao Y, Muglia LJ, Hruz PW, Rudnick DA. Disruption of hepatic adipogenesis is associated with impaired liver regeneration in mice. Hepatology. 2004;40:1322–1332. doi: 10.1002/hep.20462. [DOI] [PubMed] [Google Scholar]
- Skov Olsen P, Boesby S, Kirkegaard P, Therkelsen K, Almdal T, Poulsen SS, Nexo E. Influence of epidermal growth factor on liver regeneration after partial hepatectomy in rats. Hepatology. 1988;8:992–996. doi: 10.1002/hep.1840080503. [DOI] [PubMed] [Google Scholar]
- Sokabe T, Yamamoto K, Ohura N, Nakatsuka H, Qin K, Obi S, Kamiya A, Ando J. Differential regulation of urokinase-type plasminogen activator expression by fluid shear stress in human coronary artery endothelial cells. Am J Physiol. 2004;287:H2027–H2034. doi: 10.1152/ajpheart.00260.2004. [DOI] [PubMed] [Google Scholar]
- Stanger BZ, Tanaka AJ, Melton DA. Organ size is limited by the number of embryonic progenitor cells in the pancreas but not the liver. Nature. 2007;445:886–891. doi: 10.1038/nature05537. [DOI] [PubMed] [Google Scholar]
- Starzl TE, Fung J, Tzakis A, Todo S, Demetris AJ, Marino IR, Doyle H, Zeevi A, Warty V, Michaels M. Baboon-to-human liver transplantation. Lancet. 1993;341:65–71. doi: 10.1016/0140-6736(93)92553-6. [See comment] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stella MC, Comoglio PM. HGF: A multifunctional growth factor controlling cell scattering. Int J Biochem Cell Biol. 1999;31:1357–1362. doi: 10.1016/s1357-2725(99)00089-8. [DOI] [PubMed] [Google Scholar]
- Stocker E, Wullstein HK, Brau G. Capacity of regeneration in liver epithelia of juvenile, repeated partially hepatectomized rats. Autoradiographic studies after continous infusion of 3H-thymidine (author’s transl) Virchows Archiv. 1973;14:93–103. [PubMed] [Google Scholar]
- Stolz DB, Mars WM, Petersen BE, Kim TH, Michalopoulos GK. Growth factor signal transduction immediately after two-thirds partial hepatectomy in the rat. Cancer Res. 1999;59:3954–3960. [PubMed] [Google Scholar]
- Streetz KL, Tacke F, Leifeld L, Wustefeld T, Graw A, Klein C, Kamino K, Spengler U, Kreipe H, Kubicka S, Muller W, Manns MP, Trautwein C. Interleukin 6/gpl30-dependent pathways are protective during chronic liver diseases. Hepatology. 2003;38:218–229. doi: 10.1053/jhep.2003.50268. [DOI] [PubMed] [Google Scholar]
- Strey CW, Markiewski M, Mastellos D, Tudoran R, Spruce LA, Greenbaum LE, Lambris JD. The proinflammatory mediators C3a and C5a are essential for liver regeneration. J Exp Med. 2003;198:913–923. doi: 10.1084/jem.20030374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swindle CS, Tran KT, Johnson TD, Banerjee P, Mayes AM, Griffith L, Wells A. Epidermal growth factor (EGF)-like repeats of human tenascin-C as ligands for EGF receptor. J Cell Biol. 2001;154:459–468. doi: 10.1083/jcb.200103103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taub R. Liver regeneration 4: Transcriptional control of liver regeneration. Faseb J. 1996;10:413–427. [PubMed] [Google Scholar]
- Taub R. Liver regeneration: From myth to mechanism. Nat Rev Mol Cell Biol. 2004;5:836–847. doi: 10.1038/nrm1489. [DOI] [PubMed] [Google Scholar]
- Taub R, Greenbaum LE, Peng Y. Transcriptional regulatory signals define cytokine-dependentand -independent pathways in liver regeneration. Semin Liver Dis. 1999;19:117–127. doi: 10.1055/s-2007-1007104. [DOI] [PubMed] [Google Scholar]
- Terada N, Hamazaki T, Oka M, Hoki M, Mastalerz DM, Nakano Y, Meyer EM, Morel L, Petersen BE, Scott EW. Bone marrow cells adopt the phenotype of other cells by spontaneous cell fusion. Nature. 2002;416:542–545. doi: 10.1038/nature730. [DOI] [PubMed] [Google Scholar]
- Thompson JS, Porter KA, Hayashida N, McNamara DJ, Parker TS, Russell WJ, Francavilla A, Starzl TE. Morphologic and biochemical changes in dogs after portacaval shunt plus bile fistula or ileal bypass: Failure of bile fistula or ileal bypass to prevent hepatocyte atrophy. Hepatology. 1983;3:581–587. doi: 10.1002/hep.1840030418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomiya T, Ogata I, Yamaoka M, Yanase M, Inoue Y, Fujiwara K. The mitogenic activity of hepatocyte growth factor on rat hepatocytes is dependent upon endogenous transforming growth factor-alpha. Am J Pathol. 2000;157:1693–1701. doi: 10.1016/s0002-9440(10)64806-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torbenson M, Yang SQ, Liu HZ, Huang J, Gage W, Diehl AM. STAT-3 overexpression and p2l up-regulation accompany impaired regeneration of fatty livers. Am J Pathol. 2002;161:155–161. doi: 10.1016/S0002-9440(10)64167-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsonis PA, Lambris JD, Del Rio-Tsonis K. To regeneration … with complement. Adv Exp Med Biol. 2006;586:63–70. doi: 10.1007/0-387-34134-X_5. [DOI] [PubMed] [Google Scholar]
- Ueda A, Hamadeh HK, Webb HK, Yamamoto Y, Sueyoshi T, Afshari CA, Lehmann JM, Negishi M. Diverse roles of the nuclear orphan receptor CAR in regulating hepatic genes in response to phenobarbital. Mol Pharmacol. 2002;61:1–6. doi: 10.1124/mol.61.1.1. [DOI] [PubMed] [Google Scholar]
- Uehara Y, Mori C, Noda T, Shiota K, Kitamura N. Rescue of embryonic lethality in hepatocyte growth factor/scatter factor knockout mice. Genesis. 2000;27:99–103. [PubMed] [Google Scholar]
- Wagenaar GT, Chamuleau RA, Pool CW, de Haan JG, Maas MA, Korfage HA, Lamers WH. Distribution and activity of glutamine synthase and carbamoylphosphate synthase upon enlargement of the liver lobule by repeated partial hepatectomies. J Hepatol. 1993;17:397–407. doi: 10.1016/s0168-8278(05)80224-7. [DOI] [PubMed] [Google Scholar]
- Wang X, DeFrances MC, Dai Y, Pediaditakis P, Johnson C, Bell A, Michalopoulos GK, Zarnegar R. A mechanism of cell survival: Sequestration of Fas by the HGF receptor Met. Mol Cell. 2002;9:411–421. doi: 10.1016/s1097-2765(02)00439-2. [DOI] [PubMed] [Google Scholar]
- Wang X, Willenbring H, Akkari Y, Torimaru Y, Foster M, Al-Dhalimy M, Lagasse E, Finegold M, Olson S, Grompe M. Cell fusion is the principal source of bone-marrow-derived hepatocytes. Nature. 2003;422:897–901. doi: 10.1038/nature01531. [See comment] [DOI] [PubMed] [Google Scholar]
- Webber EM, Bruix J, Pierce RH, Fausto N. Tumor necrosis factor primes hepatocytes for DNA replication in the rat. Hepatology. 1998;28:1226–1234. doi: 10.1002/hep.510280509. [DOI] [PubMed] [Google Scholar]
- Webber EM, Wu JC, Wang L, Merlino G, Fausto N. Overexpression of transforming growth factor-alpha causes liver enlargement and increased hepatocyte proliferation in transgenic mice. Am J Pathol. 1994;145:398–408. [PMC free article] [PubMed] [Google Scholar]
- White P, Brestelli JE, Kaestner KH, Greenbaum LE. Identification of transcriptional networks during liver regeneration. J Biol Chem. 2005;280:3715–3722. doi: 10.1074/jbc.M410844200. [DOI] [PubMed] [Google Scholar]
- Yager JD, Zurlo J, Sewall CH, Lucier GW, He H. Growth stimulation followed by growth inhibition in livers of female rats treated with ethinyl estradiol. Carcinogenesis. 1994;15:2117–2123. doi: 10.1093/carcin/15.10.2117. [DOI] [PubMed] [Google Scholar]
- Yamada Y, Fausto N. Deficient liver regeneration after carbon tetrachloride injury in mice lacking type 1 but not type 2 tumor necrosis factor receptor. Am J Pathol. 1998;152:1577–1589. [PMC free article] [PubMed] [Google Scholar]
- Yamada Y, Kirillova I, Peschon JJ, Fausto N. Initiation of liver growth by tumor necrosis factor: Deficient liver regeneration in mice lacking type 1 tumor necrosis factor receptor. Proc Natl Acad Sci U S A. 1997;94:1441–1446. doi: 10.1073/pnas.94.4.1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada Y, Webber EM, Kirillova I, Peschon JJ, Fausto N. Analysis of liver regeneration in mice lacking type 1 or type 2 tumor necrosis factor receptor Requirement for type 1 but not type 2 receptor. Hepatology. 1998;28:959–970. doi: 10.1002/hep.510280410. [comment] [DOI] [PubMed] [Google Scholar]
- Yamauchi H, Uetsuka K, Okada T, Nakayama H, Doi K. Impaired liver regeneration after partial hepatectomy in db/db mice. Exp Toxicol Pathol. 2003;54:281–286. doi: 10.1078/0940-2993-00265. [DOI] [PubMed] [Google Scholar]
- Yanagita K, Nagaike M, Ishibashi H, Niho Y, Matsumoto K, Nakamura T. Lung may have an endocrine function producing hepatocyte growth factor in response to injury of distal organs. Biochem Biophys Res Commun. 1992;182:802–809. doi: 10.1016/0006-291x(92)91803-x. [DOI] [PubMed] [Google Scholar]
- Yu C, Wang F, Jin C, Huang X, Miller DL, Basilico C, McKeehan WL. Role of fibroblast growth factor type 1 and 2 in carbon tetrachloride-induced hepatic injury and fibrogenesis. Am J Pathol. 2003;163:1653–1662. doi: 10.1016/S0002-9440(10)63522-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaret KS. Stability and evolution of transcriptional regulatory networks. Mol Syst Biol. 2006;2:0021. doi: 10.1038/msb4100066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarnegar R, DeFrances MC, Kost DP, Lindroos P, Michalopoulos GK. Expression of hepatocyte growth factor mRNA in regenerating rat liver after partial hepatectomy. Biochem Biophys Res Commun. 1991;177:559–565. doi: 10.1016/0006-291x(91)92020-k. [DOI] [PubMed] [Google Scholar]
- Zheng D, Oh SH, Jung Y, Petersen BE. Oval cell response in 2-acetylaminofluorene/partial hepatectomy rat is attenuated by short interfering RNA targeted to stromal cell-derived factor-1. Am J Pathol. 2006;169:2066–2074. doi: 10.2353/ajpath.2006.060211. [DOI] [PMC free article] [PubMed] [Google Scholar]