Abstract
Background and purpose
There are limited data on the causes and severity of subsequent stroke in patients presenting initially with TIA or stroke attributed to intracranial arterial stenosis.
Methods
We evaluated the location, type (lacunar vs. non-lacunar), cause, and severity of stroke in patients who had an ischemic stroke endpoint in the Warfarin Aspirin Symptomatic Intracranial Disease (WASID) trial.
Results
Of the 569 patients enrolled in the WASID trial, 106 patients (18.6%) had an ischemic stroke during a mean follow-up of 1.8 years. Stroke occurred in the territory of the symptomatic artery in 77 (73%) of 106 patients. Among the 77 strokes in the territory, 70 (91%) were non-lacunar and 34 (44%) were disabling. Stroke out of the territory of the symptomatic artery occurred in 29 (27%) of 106 patients. Among these 29 strokes, 24 (83%) were non-lacunar, 14 (48%) were due to previously asymptomatic intracranial stenosis, and 9 (31%) were disabling.
Conclusions
Most subsequent strokes in patients with symptomatic intracranial artery stenosis are in the same territory and non-lacunar, and nearly half of the strokes in the territory are disabling. The most commonly identified cause of stroke out of the territory was a previously asymptomatic intracranial stenosis. Penetrating artery disease was responsible for a low number of strokes.
Indexing terms: WASID, stroke, intracranial stenosis, severity, causes and subtypes
Introduction
Previous studies of patients enrolled in carotid stenosis or atrial fibrillation trials show that 20%–48% of ischemic stroke endpoints in those trials could be attributed to cerebrovascular disorders other than carotid stenosis or atrial fibrillation 1–4. In the NASCET trial, approximately 20% of strokes in the territory of a symptomatic internal carotid artery stenosis could have been caused by cardioembolism or small vessel disease1. In the SPAF trials, 52% of strokes were probably cardioembolic, 24% were non-cardioembolic, and 24% were of uncertain cause2–4. There are a paucity of similar data on the causes of stroke in patients with intracranial arterial stenosis, a population at particularly high risk of stroke 5–7.
Accumulating data shows that different stroke subtypes are associated with different risks of recurrent stroke 8–12 and that disease specific treatments such as carotid endarterectomy for symptomatic carotid stenosis and warfarin for atrial fibrillation are very effective for lowering the risk of recurrent stroke that is directly caused by those conditions. Disease specific therapy in the form of intracranial stenting is being developed for intracranial stenosis and, if effective, is only likely to reduce the risk of recurrent stroke directly attributable to disease in the target vessel13. Thus, it is important to know what percentage of recurrent strokes in patients presenting with symptomatic intracranial stenosis may be caused by other disorders such as penetrating artery disease, extracranial large artery atherostenosis, and cardioembolism. The Warfarin Aspirin Symptomatic Intracranial Disease (WASID) trial provided a unique opportunity to determine the locations, types, potential causes, and severity of stroke in patients with symptomatic intracranial arterial stenosis enrolled in that trial.
Materials and methods
Entrance Criteria
The present study reports data gathered on 106 patients who suffered ischemic strokes of the 569 patients enrolled in the randomized, double blind, multi-center WASID trial. The design of the WASID trial and the baseline characteristics of patients enrolled in the trial have been published previously 5, 14, 15. Inclusion criteria included TIA or non-disabling stroke (modified Rankin score of 3 or less) within 90 days of enrollment attributable to angiographically confirmed 50–90% stenosis of a major intracranial artery. Exclusion criteria included extracranial carotid stenosis 50–90% tandem to a symptomatic intracranial carotid or middle cerebral artery (MCA) stenosis of 50%–99% because these patients were candidates for endarterectomy. Extracranial carotid stenosis in the presence of contralateral symptomatic intracranial carotid, middle cerebral artery (MCA) stenosis or vertebral artery or basilar artery stenosis was not an exclusion criterion. Similarly, extracranial vertebral artery stenosis tandem to an intracranial vertebral artery or basilar artery stenosis was not an exclusion criterion since revascularization of the extracranial vertebral artery has not been established as the standard of care in this situation. Other exclusion criteria included a known cardiac source of embolus (chronic or paroxysmal atrial fibrillation, mitral stenosis, mechanical valve, endocarditis, intracardiac clot or vegetation, myocardial infarction within three months of enrollment, dilated cardiomyopathy, or left atrial spontaneous echo contrast).
Diagnosis of Endpoint Ischemic Stroke
All patients with symptoms suggestive of an ischemic stroke during follow-up were evaluated by study neurologists who were blinded to treatment assignment. Ischemic stroke was diagnosed if a patient developed a new focal neurological deficit that was sudden in onset, thought to have a vascular cause, lasted at least 24 hours, and was not associated with a hemorrhage on CT or MRI of the brain. If a stroke was diagnosed, brain imaging (CT or MRI) was required by the study protocol to distinguish between ischemic and hemorrhagic stroke and to assist with localization of the stroke.
Localization of Ischemic Stroke
Ischemic strokes were classified as in or out of the territory of the symptomatic intracranial stenosis. An ischemic stroke was considered in the territory of the stenotic intracranial artery if the new neurological signs correlated with a new infarct on CT or MRI in an area of the brain supplied by the stenotic artery or if the neurological signs localized to an area of the brain supplied by the stenotic artery in the absence of new infarct on brain imaging. An ischemic stroke was considered out of the territory of the stenotic intracranial artery if any of the following applied:
If the new neurological signs correlated with a new infarct on CT or MRI in an area of the brain not supplied by the stenotic artery.
If the new neurological signs localized to an area of the brain not supplied by the stenotic artery when there was no new infarct on brain imaging.
If the new neurological signs could localize to two or more distinct vascular territories and there was no new infarct on brain imaging.
At the time of an endpoint ischemic stroke, the site investigator classified the location of the stroke. In addition, the locations of all ischemic strokes were independently determined by a central investigator (MIC) at the end of the study. In cases where there was disagreement, a second central investigator (BJS) independently determined the location and the classification made by two of the three investigators was used.
Definitions of Lacunar vs. Non-lacunar Stroke
Each ischemic stroke was classified as lacunar or non-lacunar. A lacunar stroke was defined by a typical lacunar syndrome (pure motor hemiparesis, pure sensory stroke, or sensory-motor stroke involving at least two of the following body parts: face, arm or leg; clumsy-hand dysarthria; ataxic hemiparesis; or hemiballismus) in association with a clinically appropriate subcortical infarct ≤ 1.5 cm or no infarct on head CT or brain MRI. All other strokes were defined as non-lacunar.
Potential Causes of Ischemic Stroke
Diagnostic tests to establish the cause of stroke (MRA or CTA of head and neck, carotid and transcranial doppler ultrasound, echocardiogram, laboratory tests) were encouraged but ultimately left to the discretion of the study neurologists. We requested that the results of all diagnostic tests performed for establishing the cause of ischemic stroke be submitted to the statistical coordinating center at Emory University, Atlanta, GA. Diagnostic tests done within 30 days of the ischemic stroke were evaluated for this analysis.
The definitions for each potential cause of ischemic stroke that were used for this analysis were as follows:
Large artery stenosis or occlusion was considered a potential cause of an endpoint ischemic stroke (lacunar or non-lacunar) as long as the stroke was in the territory of a stenotic (50%–99%) or occluded extracranial (carotid or vertebral artery) or intracranial artery (MCA, carotid, ACA, PCA, vertebral, basilar). If a patient had a stroke in the territory of the symptomatic stenotic intracranial artery for which the patient was enrolled in the trial, that stenosis was considered a potential cause of the patient’s stroke.
Penetrating artery disease was considered a potential cause of every endpoint lacunar stroke whether the stroke was in or out of the territory of the symptomatic stenotic intracranial artery for which the patient was enrolled in the trial.
Cardioembolism was considered a potential cause of an endpoint ischemic stroke (lacunar or non-lacunar) if any of the following cardiac abnormalities were identified as part of the diagnostic evaluation of the stroke: chronic or paroxysmal atrial fibrillation, mitral stenosis, mechanical valve, endocarditis, intracardiac clot or vegetation, myocardial infarction within three months of enrollment, dilated cardiomyopathy, or left atrial spontaneous echo contrast
The diagnosis of stroke of undetermined cause was made only in those patients with non-lacunar stroke out of the territory of the symptomatic stenotic intracranial artery for which no other potential cause of stroke could be identified.
Determination of stroke subtypes (lacunar vs. non-lacunar) and potential causes of stroke using these criteria were based on review of all the relevant diagnostic case report forms (neurological examination, brain and vascular imaging, echocardiography) by two central adjudicators (BMF and MIC) who were required to come to a consensus opinion.
Severity of Stroke
Severity of stroke was based on the site neurologist’s assessment of the NIH stroke scale, Barthel index, and Modified Rankin at the time of the endpoint stroke. Strokes were classified as disabling based on the patient having any one of the following six criteria at the time the patient was first seen for evaluation of the ischemic stroke endpoint: 1) composite NIHSS score of > or = 7, (2) NIHSS motor score of 3 or greater of an upper or lower extremity, (3) modified Rankin score of 4 or greater, (4) Barthel index score of < or = 80, (5) NIHSS best language score of 2 or greater or (6) NIHSS visual field score of 3.
Statistical Section
Percentages were compared between groups using a chi square test.
Results
Diagnostic Evaluation of Endpoint Ischemic Strokes
Of the 569 patients enrolled in WASID, 106 (18.6%) had an ischemic stroke during a mean follow-up of 1.8 years. The diagnostic evaluation available to us in these 106 patients with an endpoint ischemic stroke included a head CT or brain MRI in 101 patients (95%), at least one of the following vascular imaging studies ((cerebral angiogram, MRA, TCD, CTA or carotid ultrasound) in 61 patients (58%), and echocardiography in 10 patients (9%).
Subtypes and Causes of Stroke
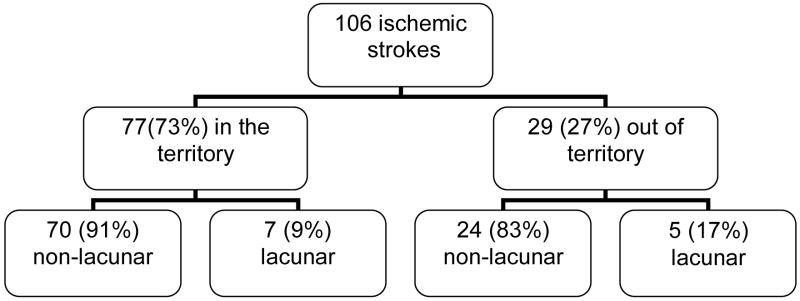
Based on the neurological examination and brain imaging findings, 77 (73%) of the 106 strokes were classified as in the territory and 29 (27%) were classified as out of the territory of the symptomatic stenotic intracranial artery (fig. 1). Table 1 summarizes the subtypes (lacunar vs. non-lacunar) of stroke in the territory of the symptomatic intracranial artery and shows all potential causes of these strokes other than the intracranial stenosis. Seventy (91%) were non-lacunar and 7 (9%) were lacunar. Of the 70 non-lacunar strokes, the symptomatic intracranial artery was the only identifiable cause of stroke in 62 patients (89%). Another potential cause was found in 8 other patients with non-lacunar stroke: 4 with extracranial carotid stenosis or occlusion, 3 with potential cardioembolism, 1 with extracranial vertebral artery stenosis.
Figure 1.
Location and subtypes of endpoint ischemic strokes in the WASID study
Table 1.
Subtypes and potential causes of stroke in the territory of the symptomatic intracranial stenosis other than intracranial stenosis
| Stroke Type | n | Other Potential Cause of Stroke | n |
|---|---|---|---|
| Lacunar | 7 | PAD | 6 |
| PAD and Extracranial Vertebral Stenosis | 1 | ||
| Non-Lacunar | 70 | No other potential cause | 62 |
| Extracranial Vertebral Stenosis | 1 | ||
| Extracranial Carotid Stenosis | 4 | ||
| Cardioembolism (1 A. Fib, 1 PFO, 1 A. Fib + PFO) | 3 |
PAD indicates penetrating artery disease; A. Fib, atrial fibrillation; PFO, patent foramen ovale
By the study definitions, the 7 lacunar strokes in the territory could have been caused by the qualifying stenotic intracranial artery (n=7), penetrating artery disease (n=7), or extracranial vertebral artery stenosis. Overall, in the territory of the symptomatic intracranial stenosis, at least 15 of 77 (19%) strokes may have been caused by another disease (penetrating artery disease, extracranial large artery disease, or cardioembolism) besides the symptomatic intracranial stenosis.
Among the 106 strokes occurring in patients with symptomatic intracranial stenosis, 29 (27%) occurred outside of the territory of the symptomatic intracranial stenosis. Table 2 summarizes the subtypes (lacunar vs. non-lacunar), potential causes, and severity of these 29 strokes. Of these 29 strokes, 24 (83%) were non-lacunar and 5 (17%) were lacunar. Among the 24 non-lacunar strokes, the potential causes of stroke identified were previously asymptomatic intracranial stenosis in 14 (58%), extracranial vertebral stenosis alone in 1, and undetermined in 9. Of the five lacunar strokes out of the territory, penetrating artery disease was a potential cause in all five (by definition), and only 1 of these patients had another potential cause (extracranial carotid occlusion). Overall, 14 of 29 (48%) of strokes out the territory may have been caused by stenosis (11) or occlusion (3) of a previously asymptomatic intracranial artery, while small vessel disease was responsible for at most 5 of 29 (17%) strokes.
Table 2.
Subtypes and potential causes of stroke out of the territory of the symptomatic intracranial stenosis
| Stroke Type | n | Potential Cause of Stroke | n |
|---|---|---|---|
| Lacunar | 5 | PAD | 4 |
| Extracranial Carotid Occlusion | 1 | ||
| Non-Lacunar | 24 | Other Intracranial Stenosis, Occlusion or Thrombus | 14* |
| Extracranial Vertebral Stenosis | 1 | ||
| Undetermined | 9 |
PAD indicates penetrating artery disease
3 had another possible cause of stroke:1 cardioembolism, 1 extracranial vertebral stenosis and 1 other (lupus anticoagulant).
Disabling Stroke
At study entry, a modified Rankin score of 0 or 1 was present in 51 of 77 patients (59%) who subsequently had a stroke in the territory during follow-up compared with 10 of 29 patients (41%) with stroke out of the territory during follow-up (p=0.035). Despite their lower modified Rankin scores at study entry, patients who had stroke in the territory during follow-up had a higher observed rate of disabling stroke in the trial (34 of 77, 44%) compared with patients who had a stroke out of the territory (9 of 39, 31%) (p=0.22). For patients with stroke in the territory, 32 of 70 (46%) non-lacunar strokes were disabling while 2 of 7 (29%) lacunar strokes were disabling. For patients with stroke out of the territory, 1 of 5 (20%) lacunar strokes were disabling and 8 of 24 (33%) non-lacunar strokes were disabling.
Discussion
Most 77 (73%) of the strokes in patients with 50%–99% intracranial stenosis in the WASID trial were in the territory of the symptomatic intracranial stenosis and 70 (91%) of these strokes were non-lacunar. These results are similar to the findings in NASCET which showed that 95% of strokes were ipsilateral in patients with 70%–99% carotid stenosis and 71% of strokes were ipsilateral in patients with 50–69% carotid stenosis 1, 16. In addition, the findings of our study are in agreement with the findings of a recent study in which 89% of recurrent strokes in patients with an index stroke classified as being secondary to large artery intracranial atherosclerosis, occurred in the same territory as the index stroke.17 In another study evaluating stroke recurrence in 120 patients with significant stenosis and occlusion of the middle cerebral artery (MCA) 8 of 11 recurrent ischemic strokes (89%) occurred in the same territory as the index stroke in patients with MCA stenosis and 3 of 3 (100%) recurrent strokes occurred in the same territory as the index stroke in patients with MCA occlusion.18. Other studies evaluating the mechanism of recurrent strokes have also shown that most recurrent ischemic strokes have the same mechanism as the index stroke.19
Among the 77 strokes that were in the territory of the symptomatic intracranial stenosis, at least 15 (19%) could have been caused by other mechanisms (e.g. penetrating artery disease, cardioembolism, extracranial arterial stenosis). This is similar to the percentage of strokes in the territory of extracranial carotid stenosis that could have been caused by non-carotid causes in NASCET1. This finding in WASID could have implications for future trials evaluating the efficacy of stenting for intracranial stenosis because stenting is unlikely to have any impact on these mechanisms of stroke.
Almost half (44%) of the strokes in the territory of the symptomatic intracranial artery were disabling whereas 31% of strokes out of the territory were disabling; (p=0.22). The high frequency of disabling strokes in the territory of the symptomatic intracranial artery is similar to the finding in NASCET that 30% of strokes ipsilateral to symptomatic 50%–69% extracranial carotid stenosis and 48% of strokes ipsilateral to symptomatic 70%–99% extracranial carotid stenosis were disabling1. The higher frequency of disabling strokes in patients with strokes in the territory occurred in spite of the lower baseline Rankin scores in this group of patients compared to patients with endpoint stroke out of the territory (p=0.035). Together these findings reiterate the high risk of severe stroke associated with symptomatic large artery atherostenosis.
Of the strokes occurring outside the territory of the symptomatic intracranial artery, almost half (48%) could have been caused by previously asymptomatic or newly developed intracranial stenosis in a different vascular territory. This suggests that patients with intracranial stenosis have a propensity for atherosclerotic stenosis at different sites within the intracranial circulation. However, it is also possible that some of these strokes attributed to previously asymptomatic or newly developed intracranial stenosis may have been caused by an embolus which partially recanalized leaving a residual stenosis. Supporting this theory are data from another WASID analysis which showed that asymptomatic intracranial stenosis that was present at study entry (coexistent with the symptomatic stenosis) was associated with a low rate of stroke (3.5 % after 1 year of follow-up)20.
Given the high burden of vascular risk factors, especially hypertension, in patients enrolled in WASID21, 221, it is surprising that penetrating artery disease was an uncommon potential cause of stroke in or out of the territory of the symptomatic intracranial stenosis. It is not surprising that cardioembolism and extracranial carotid disease were uncommon potential causes of stroke in this study given that patients with known cardioembolic sources and extracranial carotid stenosis tandem to intracranial carotid or MCA stenosis were excluded in WASID. Another possible reason for the low frequency of cardioembolic stroke in this study is that less than 10 % of patients had echocardiograms at the time of an ischemic stroke endpoint so cardioembolic mechanisms of stroke may have been missed. However, it is important to note that the most common causes of cardioembolism in the USA (atrial fibrillation, recent MI) do not require echocardiography for diagnosis.
The major limitation of this study was the incomplete diagnostic evaluation available to us in many patients with recurrent stroke. This almost certainly led to an underestimate of the number of patients that could have had causes of stroke other than intracranial stenosis. Despite the limited diagnostic evaluation in many of these patients, we were still able to identify other potential causes of stroke in 19% of patients who had recurrent stroke in the territory of the symptomatic intracranial stenosis. This strongly supports the importance of evaluating patients with symptomatic intracranial stenosis fully for other potential causes of stroke that may be amenable to disease specific treatments.
Acknowledgments
This study was funded by a research grant (1R01 NS36643, Principal Investigator: Dr Chimowitz) from the US Public Health Service, NINDS. In addition, the following General Clinical Research centers, funded by the NIH, provided local support for the evaluation of patients in the trial: Emory University (M01 RR00039), Case Western University, Metro Health Medical Center (5M01 RR00080), San Francisco General Hospital (M01 RR00083-42), Johns Hopkins University School of Medicine (M01 RR000052), Indiana University School of Medicine (5M01 RR000750-32), Cedars-Sinai Hospital (M01 RR00425), and the University of Maryland (M01 RR165001). Bristol-Myers-Squibb (after incorporating DuPont Pharma) supplied the warfarin (Coumadin) and placebo warfarin tablets, and Bayer supplied the aspirin and placebo aspirin tablets for the trial. Neither of these companies supplied direct funding for the trial. The Food and Drug Administration assigned an IND number of 57,138 for Coumadin (warfarin sodium) for this trial.
References
- 1.Barnett HJ, Gunton RW, Eliasziw M, Fleming L, Sharpe B, Gates P, Meldrum H. Causes and severity of ischemic stroke in patients with internal carotid artery stenosis. JAMA. 2000;283:1429–1436. doi: 10.1001/jama.283.11.1429. [DOI] [PubMed] [Google Scholar]
- 2.Stroke prevention in atrial fibrillation study. Final results. Circulation. 1991;84:527–539. doi: 10.1161/01.cir.84.2.527. [DOI] [PubMed] [Google Scholar]
- 3.Warfarin versus aspirin for prevention of thromboembolism in atrial fibrillation: Stroke prevention in atrial fibrillation II study. Lancet. 1994;343:687–691. [PubMed] [Google Scholar]
- 4.Adjusted-dose warfarin versus low-intensity, fixed-dose warfarin plus aspirin for high-risk patients with atrial fibrillation: Stroke prevention in atrial fibrillation iii randomised clinical trial. Lancet. 1996;348:633–638. [PubMed] [Google Scholar]
- 5.Chimowitz MI, Lynn MJ, Howlett-Smith H, Stern BJ, Hertzberg VS, Frankel MR, Levine SR, Chaturvedi S, Kasner SE, Benesch CG, Sila CA, Jovin TG, Romano JG Warfarin-Aspirin Symptomatic Intracranial Disease Trial I. Comparison of warfarin and aspirin for symptomatic intracranial arterial stenosis. New England Journal of Medicine. 2005;352:1305–1316. doi: 10.1056/NEJMoa043033. [DOI] [PubMed] [Google Scholar]
- 6.Chimowitz MI, Kokkinos J, Strong J, Brown MB, Levine SR, Silliman S, Pessin MS, Weichel E, Sila CA, Furlan AJ, et al. The Warfarin-Aspirin Symptomatic Intracranial Disease Study. Neurology. 1995;45:1488–1493. doi: 10.1212/wnl.45.8.1488. [DOI] [PubMed] [Google Scholar]
- 7.Mazighi M, Tanasescu R, Ducrocq X, Vicaut E, Bracard S, Houdart E, Woimant F. Prospective study of symptomatic atherothrombotic intracranial stenoses: The GESICA study. Neurology. 2006;66:1187–1191. doi: 10.1212/01.wnl.0000208404.94585.b2. [DOI] [PubMed] [Google Scholar]
- 8.Landi G, Cella E, Boccardi E, Musicco M. Lacunar versus non-lacunar infarcts: Pathogenetic and prognostic differences. Journal of Neurology, Neurosurgery & Psychiatry. 1992;55:441–445. doi: 10.1136/jnnp.55.6.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lovett JK, Coull AJ, Rothwell PM. Early risk of recurrence by subtype of ischemic stroke in population-based incidence studies. Neurology. 2004;62:569–573. doi: 10.1212/01.wnl.0000110311.09970.83. [DOI] [PubMed] [Google Scholar]
- 10.Norrving B. Long-term prognosis after lacunar infarction. Lancet Neurology. 2003;2:238–245. doi: 10.1016/s1474-4422(03)00352-1. [DOI] [PubMed] [Google Scholar]
- 11.Petty GW, Brown RD, Jr, Whisnant JP, Sicks JD, O’Fallon WM, Wiebers DO. Ischemic stroke subtypes: A population-based study of functional outcome, survival, and recurrence. Stroke. 2000;31:1062–1068. doi: 10.1161/01.str.31.5.1062. [DOI] [PubMed] [Google Scholar]
- 12.Kolominsky-Rabas PL, Weber M, Gefeller O, Neundoerfer B, Heuschmann PU. Epidemiology of ischemic stroke subtypes according to toast criteria: Incidence, recurrence, and long-term survival in ischemic stroke subtypes: A population-based study. Stroke. 2001;32:2735–2740. doi: 10.1161/hs1201.100209. [DOI] [PubMed] [Google Scholar]
- 13.Derdeyn CP, Chimowitz MI. Angioplasty and stenting for atherosclerotic intracranial stenosis: Rationale for a randomized clinical trial. Neuroimaging Clinics of North America. 2006;17:355–363. doi: 10.1016/j.nic.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Samuels OB, Joseph GJ, Lynn MJ, Smith HA, Chimowitz MI. A standardized method for measuring intracranial arterial stenosis. Ajnr: American Journal of Neuroradiology. 2000;21:643–646. [PMC free article] [PubMed] [Google Scholar]
- 15.Warfarin-Aspirin Symptomatic Intracranial Disease Trial I. Design, progress and challenges of a double-blind trial of warfarin versus aspirin for symptomatic intracranial arterial stenosis. Neuroepidemiology. 2003;22:106–117. doi: 10.1159/000068744. [DOI] [PubMed] [Google Scholar]
- 16.Barnett Henry JM, MD, Taylor D Wayne, MA, Eliasziw Michael, PhD, Fox Allan J, MD, Ferguson Gary G, MD, Haynes R Brian, MD, Rankin Richard N, MD, Clagett G Patrick, MD, Hachinski Vladimir C, MD, Sackett David L, MD, Thorpe Kevin E, MMath, Meldrum Heather E, BA, Spence J David, MD for The North American Symptomatic Carotid Endarterectomy Trial Collaborators. Benefit of Carotid Endarterectomy in Patients with Symptomatic Moderate or Severe Stenosis. New England Journal of Medicine. 1998;339:1415–1425. doi: 10.1056/NEJM199811123392002. [DOI] [PubMed] [Google Scholar]
- 17.Shin DH, Lee PH, Bang OY. Mechanisms of recurrence in subtypes of ischemic stroke: A hospital-based follow-up study. Archives of Neurology. 2005;62:1232–123. doi: 10.1001/archneur.62.8.1232. [DOI] [PubMed] [Google Scholar]
- 18.Kern R, Steinke W, Daffertshofer M, Prager R, Hennerici M. Stroke recurrences in patients with symptomatic vs asymptomatic middle cerebral artery disease. Neurology. 2005;65:859–864. doi: 10.1212/01.wnl.0000175983.76110.59. [DOI] [PubMed] [Google Scholar]
- 19.Yamamoto H, Bogousslavsky J. Mechanisms of second and further strokes. Journal of Neurology, Neurosurgery & Psychiatry. 1998;64:771–776. doi: 10.1136/jnnp.64.6.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nahab F, Cotsonis G, Lynn M, Feldmann E, Chaturvedi S, Hemphill JC, Zweifler R, Johnston K, Bonovich D, Kasner S, Chimowitz M. WASID Study Group. Prevalence and prognosis of coexistent asymptomatic intracranial stenosis. Stroke. 2008 Mar;39(3):1039–41. doi: 10.1161/STROKEAHA.107.499475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chaturvedi SM, Turan TNM, Lynn MJM, Kasner SEM, Romano JM, Cotsonis GM, Frankel MM, Chimowitz MIMBC For the WSG. Risk factor status and vascular events in patients with symptomatic intracranial stenosis. Neurology. 2007;69:2063–2068. doi: 10.1212/01.wnl.0000279338.18776.26. [DOI] [PubMed] [Google Scholar]
- 22.Ovbiagele B, Saver JL, Lynn MJ, Chimowitz M, Group WS. Impact of metabolic syndrome on prognosis of symptomatic intracranial atherostenosis. Neurology. 2006;66:1344–1349. doi: 10.1212/01.wnl.0000210530.46058.5c. [DOI] [PubMed] [Google Scholar]