Abstract
BMP2 signaling and RUNX2 regulatory pathways converge for transcriptional control of bone formation in vivo. SMAD proteins are recruited to RUNX2 regulatory complexes via an overlapping nuclear matrix targeting signal/Smad interacting domain sequence (391–432) in Runx2. To establish the contribution of RUNX2-SMAD interaction to osteoblastogenesis, we characterized a number of point mutants. Only a triple mutation of amino acids 426–428 (HTY-AAA) results in loss of RUNX2 interactions with either BMP2- or TGF-β- responsive SMADs and fails to integrate the BMP2/TGF-β signal on target gene promoters. In a Runx2 null cell reconstitution assay, the HTY mutant did not activate the program of osteoblast differentiation (alkaline phosphatase, collagen type 1, osteopontin, bone sialoprotein and osteocalcin) in response to BMP2 signaling. Thus, subnuclear targeting function and formation of a RUNX2-SMAD osteogenic complex are functionally inseparable. Taken together, these studies provide direct evidence that RUNX2 is essential for execution and completion of BMP2 signaling for osteoblast differentiation.
Key Words: RUNX2, Transcriptional regulation, SMAD coregulatory proteins, BMP2 osteogenic signaling
Introduction
Transduction of the BMP and TGF-β signals results in the activation of target transcription factors, which is essential for differentiation and in vivo bone formation. SMAD proteins function as transducers of BMP/TGF-β signal in a variety of cell types [Xu and Massagué, 2004; Massagué et al., 2005]. There is mounting evidence that BMP signaling promotes multiple phenotypes through interaction of SMADs with other master transcription factors [Massagué et al., 2005]. The runt-related factor RUNX2 (Cbfa1/AML3) is critical for osteogenic lineage commitment and formation of the skeleton. Targeted disruption of Runx2 in mice results in the maturational arrest of osteoblasts and a complete lack of mineralized bone [Komori et al., 1997; Otto et al., 1997; Choi et al., 2001]. RUNX protein provides a platform for assembly of a multicomponent regulatory complex that controls both activation and repression of genes during cell fate determination and differentiation [Javed et al., 1999, 2001; Gutierrez et al., 2002; Young et al., 2007]. The carboxy terminus of RUNX2 mediates interactions with a number of coregulatory proteins including SMADs [Zhang et al., 2000]. RUNX2-SMAD interaction is confined to a 41-amino acid Smad interacting domain (SMID) that overlaps with the nuclear matrix targeting signal (NMTS) [Afzal et al., 2005]. SMID suffices as an autonomous module that is both necessary and sufficient for interaction with SMADs. MAPK-mediated posttranslational modification of RUNX2 is an important component of the RUNX2-SMAD regulatory complex [Afzal et al., 2005].
The precise contact points for the RUNX2/SMAD physical association are yet to be established. Here we characterize a RUNX2 mutant that controls both formation of the RUNX2-SMAD complex and functional incorporation of the BMP2 osteogenic signal.
Materials and Methods
Cell Cultures
HeLa cells were cultured and maintained as previously described [Afzal et al., 2005]. Detailed description and morphological characteristics of the established Runx2 null cell line from calvarial tissue of 17.5-day-old Runx2 null mouse embryos was reported previously [Bae et al., 2006]. Cells were maintained in α-MEM supplemented with 10% FBS and penicillin/streptomycin (100 μg/ml) at 37°C in a humidified atmosphere of 5% CO2 in air.
Plasmids and Adenoviral Constructs
The BMP2- and TGF-β-responsive flag-tagged receptor SMAD (Smad2 and 3) constructs and the 6xRunx-luciferase plasmid were reported previously [Afzal et al., 2005]. Expression constructs of hemagglutinin-tagged RUNX2, deletion Δ391, H426A, T427A, Y428A and HTY were described earlier [Afzal et al., 2005; Javed et al., 2005; Zaidi et al., 2006; Javed et al., 2008]. Wild type (WT), point and deletion mutants of Runx2 adenoviruses were described previously [Afzal et al., 2005, Javed et al., 2008]. The following MOI were used for various Runx2 adenoviruses to maintain an equal level of expression and low cytotoxicity in Runx2 null cells: WT, 50; HTY, 60; Y428A, 70; Δ391, 37.
Transient Transfection, Immunprecipitation and Luciferase Assays
All transient transfections were performed using Superfect transfection reagent. For promoter reporter assays, HeLa cells were transfected with 0.5 μg of Runx2, receptor Smad expression vectors and TBRE-luciferase reporter. Transfected cells were cultured for an additional 18 h in the presence or absence of 100 ng/ml of BMP2 or TGF-β (5 ng/ml). Cells were lysed and luciferase activity was determined. Coimmunoprecipitations were performed as previously described. Western blots were probed with RUNX2 and flag antibodies, visualized with appropriate horseradish peroxidase-conjugated secondary antibody and chemiluminescence as described earlier [Javed et al., 2008].
Reconstitution Assays
Runx2 null cell cultures at 70% confluence were infected with the Runx2 (WT, Y428A, HTY, Δ391) or β-galactosidase adenovirus in serum-free MEM medium. After 90 min, cells were washed in α-MEM without serum followed by addition of complete α-MEM. Cells were cultured in the presence or absence of BMP2 (100 ng/ml) for an additional 12 days. Two days after infection, cells were fed with osteogenic media every other day and subsequently harvested at indicated days for RNA or alkaline phosphatase (ALP) histology as described previously [Javed et al., 2008].
Results and Discussion
RUNX2-SMAD Interaction Requires Specific Residues within the SMID/NMTS
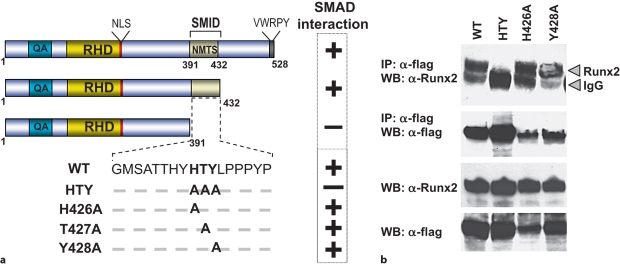
We previously documented that the SMID in RUNX2 overlaps the well-characterized NMTS, which is required for organization of RUNX2 coregulatory protein complexes in unique subnuclear foci [Javed et al., 2000; Choi et al., 2001; Afzal et al., 2005]. From X-ray crystallography studies, this domain from 391 to 432 amino acids is structurally characterized by loop-turn-loop configuration [Tang et al., 1999]. The FTY and HTY residues form the tips of the interacting surfaces of loops 1 and 2, respectively, and are conserved among other family members and species (fig. 1a; data not shown). These amino acids along with residues in both the N- and C-terminal end of the SMID of RUNX2 were mutated to find the minimal requirements for interaction with SMAD proteins. Coimmunoprecipitation studies showed no changes in SMAD1 interaction with the triple mutations of FTY residues in loop 1, while the HTY mutation in loop 2 resulted in complete loss of interaction similar to the Δ391 mutant which lacks SMID containing C terminus of Runx2 (fig. 1b) [Javed et al., 2008]. Expression levels of SMAD1 and mutant RUNX2 proteins are shown in input samples (fig. 1b). Thus, the HTY motif in loop 2 of SMID is required for formation of the RUNX2-SMAD complex.
Fig. 1.
Three residues in the SMID of RUNX2 are required for formation of the RUNX2-SMAD complex. a Schematic illustration of various conserved region and C-terminal deletion as well as SMID mutant RUNX2 protein. QA = Polyglutamic acid and alanine stretch; RHD = DNA-binding runt homology domain; NLS = nuclear localization signal. SMID resides between amino acids 391 and 432. BMP2- or TGF-β-responsive SMAD (Smad1, 2 and 3) interactions with key RUNX2 point mutants determined by coimmunoprecipitation studies are indicated. b HeLa cells were cotransfected with 5 μg of Smad1 and indicated Runx2 expression vectors and cultured in the presence of 100 ng/ml of BMP2. Only mutation of all 3 amino acids resulted in loss of RUNX2-SMAD interaction. Lower panels show expression of RUNX2 and SMAD proteins in the input sample. IP = Immunoprecipitation; WB = Western blotting.
To further establish if the HTY residues represent the minimal contact point or if there are crucial amino acids within this motif for SMAD interaction, each of 3 residues of the HTY motif were mutated individually or in pairs. These results are summarized in figure 1. Loss of the RUNX2-SMAD interaction is observed only when all 3 amino acids (HTY) are mutated together or SMID is deleted. We further found >70% loss of in situcolocalization of HTY mutant and SMAD (data not shown) [Javed et al., 2008]. Thus, the HTY mutation disrupts both biochemical and in situ association with receptor SMADs. These findings establish the HTY motif as a minimal contact point that is required for formation of the RUNX2-SMAD complex in response to BMP/TGF-β signaling.
Disruption of the RUNX2-SMAD Complex Impairs Integration of the BMP/TGF-β Signal Required for Commitment and Progression of Osteoblast Differentiation
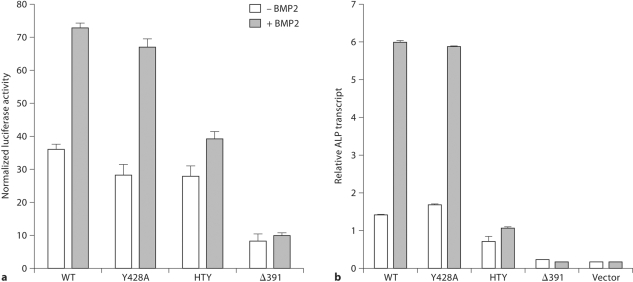
We assessed the functional consequences of the disruption of the RUNX2-SMAD interaction by several criteria. RUNX2 transcriptional activation function was examined using a promoter that contains cis-elements for both Runx and Smad (3X-TBRE-Luc). BMP2 treatment resulted in 3-fold stimulation by WT but not with the HTY mutant (fig. 2a). HTY mutation does not change DNA binding (data not shown) [Javed et al., 2008], but selectively blocks integration of the BMP/TGF-β regulatory signal for promoter activity. To address if osteoblast differentiation is critically dependent on the RUNX2-SMAD molecular complex, we used a biological assay of reconstituted Runx2 null cells. In the absence of RUNX2, the null cells cannot be induced to the osteoblast lineage by BMP2 stimulation. Reintroduction of WT or Y428A RUNX2 restored the osteoblast phenotype with a progressive increase in ALP mRNA, which was further enhanced upon BMP2 stimulation (fig. 2b). In contrast, cells reconstituted with either HTY or Δ391 RUNX2 mutant did not show any ALP expression in the absence or presence of BMP2. Expression of other osteogenic marker genes (collagen type 1, osteopontin, bone sialoprotein and osteocalcin) in response to BMP2 was similarly lost (data not shown) [Javed et al., JBC 2008]. These data demonstrate that loss of RUNX2-SMAD interaction impairs capacity of BMP2 to promote osteogenic differentiation.
Fig. 2.
Loss of osteogenic differentiation and BMP signal integration by HTY mutant RUNX2 protein. a Each TBRE element contains a Smad and an overlapping Runx DNA-binding motif. Cells were cotransfected as described and treated with 100 ng BMP2 and luciferase activity was determined. b Commitment of reconstituted Runx2 null cells to osteoblast lineages was tested by ALP expression. Adenovirus-infected cells were cultured in the presence of BMP2 and relative expression levels of ALP marker gene were monitored by real-time RT-PCR analysis.
In summary, these findings suggest that formation of the RUNX2-SMAD regulatory complex is obligatory for activation of a gene network that drives osteoblast differentiation. Furthermore, our results establish RUNX2 as a molecular endpoint that is required for execution and completion of TGF-β/BMP2 signaling in osteoblasts.
Acknowledgements
NIH sponsor contract grant numbers: AR39588, AG030228, DE12528. The contents of this paper are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
Abbreviations used in this paper
- ALP
alkaline phosphatase
- BMP
bone morphogenetic protein
- FBS
fetal bovine serum
- MEM
modified Eagle's medium
- MOI
multiplicity of infections
- NMTS
nuclear matrix targeting signal
- SMID
Smad interacting domain
- TGF-β
transforming growth factor-β
- WT
wild type
References
- Afzal F., Pratap J., Ito K., Ito Y., Stein J.L., van Wijnen A.J., Stein G.S., Lian J.B., Javed A. Smad function and intranuclear targeting share a Runx2 motif required for osteogenic lineage induction and BMP2 responsive transcription. J Cell Physiol. 2005;204:63–72. doi: 10.1002/jcp.20258. [DOI] [PubMed] [Google Scholar]
- Bae J.S., Gutierrez S., Narla R., Pratap J., Devados R., van Wijnen A.J., Stein J.L., Stein G.S., Lian J.B., Javed A. Reconstitution of Runx2/Cbfa1-null cells identifies a requirement for BMP2 signaling through a Runx2 functional domain during osteoblast differentiation. J Cell Biochem. 2007;100:434–449. doi: 10.1002/jcb.21039. [DOI] [PubMed] [Google Scholar]
- Choi J.-Y., Pratap J., Javed A., Zaidi S.K., Xing L., Balint E., Dalamangas S., Boyce B., van Wijnen A.J., Lian J.B., Stein J.L., Jones S.N., Stein G.S. Subnuclear targeting of Runx/Cbfa/AML factors is essential for tissue-specific differentiation during embryonic development. Proc Natl Acad Sci USA. 2001;98:8650–8655. doi: 10.1073/pnas.151236498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez S., Javed A., Tennant D.K., van Rees M., Montecino M., Stein G.S., Stein J.L., Lian J.B. CCAAT/enhancer-binding proteins (C/EBP) β and δ activate osteocalcin gene transcription and synergize with Runx2 at the C/EBP element to regulate bone-specific expression. J Biol Chem. 2002;277:1316–1323. doi: 10.1074/jbc.M106611200. [DOI] [PubMed] [Google Scholar]
- Javed A., Guo B., Hiebert S., Choi J.Y., Green J., Zhao S.C., Osborne M.A., Stifani S., Stein J.L., Lian J.B., van Wijnen A.J., Stein G.S. Groucho/TLE/R-esp proteins associate with the nuclear matrix and repress RUNX (CBFα/AML/PEBP2α) dependent activation of tissue-specific gene transcription. J Cell Sci. 2000;113:2221–2231. doi: 10.1242/jcs.113.12.2221. [DOI] [PubMed] [Google Scholar]
- Javed A., Barnes G.L., Pratap J., Antkowiak T., Gerstenfeld L.C., van Wijnen A.J., Stein J.L., Lian J.B., Stein G.S. Impaired intranuclear trafficking of Runx2 (AML3/CBFA1) transcription factors in breast cancer cells inhibits osteolysis in vivo. Proc Natl Acad Sci USA. 2005;102:1454–1459. doi: 10.1073/pnas.0409121102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javed A., Bae J.S., Afzal F., Gutierrez S., Pratap J., Zaidi S.K., Lou Y., van Wijnen A.J., Stein J.L., Stein G.S., Lian J.B. Structural coupling of Smad and Runx2 for execution of the BMP2 osteogenic signal. J Biol Chem. 2008;283:8412–8422. doi: 10.1074/jbc.M705578200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javed A., Gutierrez S., Montecino M., van Wijnen A.J., Stein J.L., Stein G.S., Lian J.B. Multiple Cbfa/AML sites in the rat osteocalcin promoter are required for basal and vitamin D-responsive transcription and contribute to chromatin organization. Mol Cell Biol. 1999;19:7491–7500. doi: 10.1128/mcb.19.11.7491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komori T., Yagi H., Nomura S., Yamaguchi A., Sasaki K., Deguchi K., Shimizu Y., Bronson R.T., Gao Y.-H., Inada M., Sato M., Okamoto R., Kitamura Y., Yoshiki S., Kishimoto T. Targeted disruption of Cbfa1 results in a complete lack of bone formation owing to maturational arrest of osteoblasts. Cell. 1997;89:755–764. doi: 10.1016/s0092-8674(00)80258-5. [DOI] [PubMed] [Google Scholar]
- Massague J., Seoane J., Wotton D. Smad transcription factors. Genes Dev. 2005;19:2783–2810. doi: 10.1101/gad.1350705. [DOI] [PubMed] [Google Scholar]
- Otto F., Thornell A.P., Crompton T., Denzel A., Gilmour K.C., Rosewell I.R., Stamp G.W., Beddington R.S., Mundlos S., Olsen B.R., Selby P.B., Owen M.J. Cbfa1, a candidate gene for cleidocranial dysplasia syndrome, is essential for osteoblast differentiation and bone development. Cell. 1997;89:765–771. doi: 10.1016/s0092-8674(00)80259-7. [DOI] [PubMed] [Google Scholar]
- Tang L., Guo B., Javed A., Choi J.Y., Hiebert S., Lian J.B., van Wijnen A.J., Stein J.L., Stein G.S., Zhou G.W. Crystal structure of the nuclear matrix targeting signal of the transcription factor acute myelogenous leukemia-1/polyoma enhancer-binding protein 2αB/core binding factor α2. J Biol Chem. 1999;274:33580–33586. doi: 10.1074/jbc.274.47.33580. [DOI] [PubMed] [Google Scholar]
- Xu L., Massague J. Nucleocytoplasmic shuttling of signal transducers. Nat Rev Mol Cell Biol. 2004;5:209–219. doi: 10.1038/nrm1331. [DOI] [PubMed] [Google Scholar]
- Young D.W., Hassan M.Q., Yang X.Q., Galindo M., Javed A., Zaidi S.K., Furcinitti P., Lapointe D., Montecino M., Lian J.B., Stein J.L., van Wijnen A.J., Stein G.S. Mitotic retention of gene expression patterns by the cell fate-determining transcription factor Runx2. Proc Natl Acad Sci USA. 2007;104:3189–3194. doi: 10.1073/pnas.0611419104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaidi S.K., Javed A., Pratap J., Schroeder T.M., Westendorf J., Lian J.B., van Wijnen A.J., Stein G.S., Stein J.L. Alterations in intranuclear localization of Runx2 affect biological activity. J Cell Physiol. 2006;209:935–942. doi: 10.1002/jcp.20791. [DOI] [PubMed] [Google Scholar]
- Zhang Y.W., Yasui N., Ito K., Huang G., Fujii M., Hanai J., Nogami H., Ochi T., Miyazono K., Ito Y. A RUNX2/PEBP2α A/CBFA1 mutation displaying impaired transactivation and Smad interaction in cleidocranial dysplasia. Proc Natl Acad Sci USA. 2000;97:10549–10554. doi: 10.1073/pnas.180309597. [DOI] [PMC free article] [PubMed] [Google Scholar]