Abstract
Background and Purpose
Although plasma total homocysteine (tHcy) levels are associated with cardiovascular disease (CVD), it remains unclear whether homocysteine is a cause or a marker of atherosclerotic vascular disease. We determined whether reduction of tHcy levels with B-vitamin supplementation reduces subclinical atherosclerosis progression.
Methods
In this double-blind clinical trial, 506 participants 40–89 years of age with an initial tHcy >8.5 μmol/L without diabetes and CVD were randomized to high-dose B-vitamin supplementation (folic acid 5 mg + vitamin B12 0.4 mg + vitamin B6 50 mg) or matching placebo for 3.1 years. Subclinical atherosclerosis progression across 3 vascular beds was assessed using high-resolution B-mode ultrasonography to measure carotid artery intima-media thickness (primary outcome) and multidetector spiral computed tomography to measure aortic and coronary artery calcium (secondary outcome).
Results
Although the overall carotid artery intima-media thickness progression rate was lower with B-vitamin supplementation than with placebo, statistically significant between-group differences were not found (p=0.31). However, among subjects with baseline tHcy≥9.1 μmol/L, those randomized to B-vitamin supplementation had a statistically significant lower average rate of carotid artery intima-media thickness progression compared with placebo (p=0.02); among subjects with a baseline tHcy <9.1 μmol/L there was no significant treatment effect (p-value for treatment interaction=0.02). B-vitamin supplementation had no effect on progression of aortic or coronary artery calcification overall or within subgroups.
Conclusion
High-dose B-vitamin supplementation significantly reduces progression of early stage subclinical atherosclerosis (carotid artery intima-media thickness) in well-nourished healthy B-vitamin “replete” individuals at low-risk for CVD with a fasting tHcy >9.1 μmol/L.
Keywords: Atherosclerosis, Computed tomography, Folate, Homocysteine, Intima-media thickness, Randomized controlled trials, Vitamin B12
INTRODUCTION
Fasting plasma total homocysteine (tHcy) and abnormal homocysteine metabolism unmasked by methionine loading are independently associated with cardiovascular disease (CVD).1–5 Observational studies and meta-analyses indicate that tHcy is a strong, independent graded risk factor for CVD, with a 40%-60% increased risk for each 3–5 μmol/L increase in tHcy.1,2 Cardiovascular risk associated with fasting tHcy substantially increases when plasma levels exceed 8–9 μmol/L.6,7 Additionally, dietary deficiencies of folic acid, vitamin B12 and vitamin B6 are risk factors for CVD.8,9 It remains unclear whether tHcy is a cause or a marker of atherosclerotic vascular disease.
Recent trials failed to show reduction of recurrent cardiovascular events with homocysteine-lowering therapy of folic acid and vitamin B12 with or without vitamin B6.10,11 It remains unknown whether B-vitamin supplementation reduces subclinical atherosclerosis progression or CVD in individuals without pre-existing CVD. The B-Vitamin Atherosclerosis Intervention Trial (BVAIT) was designed to determine the impact of reducing fasting tHcy and post-methionine loading tHcy (PML) with B-vitamin supplementation on subclinical atherosclerosis progression in a CVD-free population.
METHODS
Study Population and Design
BVAIT was a randomized, double-blind, placebo-controlled trial conducted from November 6, 2000 to June 1, 2006. Subjects were men and postmenopausal women ≥40 years old with fasting tHcy≥8.5 μmol/L and no clinical signs/symptoms of CVD. Exclusion criteria were fasting triglycerides>5.64 mmol/L (500 mg/dL), diabetes mellitus or fasting serum glucose>6.99 mmol/L (126 mg/dl), systolic blood pressure≥160 mmHg and/or diastolic blood pressure≥100 mmHg, untreated thyroid disease, creatinine clearance<70 ml/min, life-threatening illness with prognosis<5 years or >5 alcoholic drinks daily. The University of Southern California Institutional Review Board approved the study protocol; all participants provided written informed consent.
Computer-generated random numbers were used to assign participants to daily supplementation with folic acid 5 mg + vitamin B12 0.4 mg + vitamin B6 50 mg or matching placebo in 1 of 2 strata defined by baseline carotid artery intima-media thickness (CIMT) (0.75 mm, ≥0.75 mm). Within each stratum, blocked randomization occurred with a block size of 4. Participants, clinical staff, imaging specialists and data monitors were masked to treatment assignment.
Clinic visits occurred every 3 months and vital signs, clinical events, diet and non-study medication and supplement/nutraceutical use were ascertained. Treatment adherence was assessed at each visit by pill compliance and every 6 months by measuring tHcy and B-vitamin levels. Every 6 months carotid ultrasonography, oral methionine loading and fasting blood samples were obtained. A chemistry panel and complete blood count were obtained prior to randomization and annually.
The initial 2.5-year treatment period was extended on average 1–2 years by the External Data and Safety Monitoring Board based on evolving results from secondary prevention B-vitamin trials. Interim analyses of the primary trial endpoint were not performed.
The primary trial endpoint was the rate of change in the right distal common carotid artery intima-media thickness (CIMT). Carotid ultrasonography occurred at baseline and every 6 months throughout the original 2.5-year trial period and trial extension. Secondary trial endpoints were changes in calcium in the coronary arteries (CAC) and abdominal aorta (AC); CT scans were obtained at baseline and at the end of the original 2.5-year trial.
Sample size based on CIMT progression required 176 subjects/arm to detect a moderate effect size of 0.30 at 0.05-significance (2-sided) with 0.80 power. A total of 506 subjects were recruited to accommodate anticipated dropouts and initiation of lipid-lowering medications on-trial.
Assessment of Atherosclerosis Progression
High resolution B-mode ultrasound images of the right common carotid artery were obtained with a 7.5-MHz linear array transducer attached to a Toshiba SSH 140A ultrasonography system (Toshiba Corp., Tokyo, Japan). Ultrasound imaging and measurement of far wall CIMT were completed as previously described.12–15 The coefficient of variation of repeated baseline CIMT measurements was <1%.
Multidetector spiral computed tomography (CT) (Philips Mx-8000 4-S-CT scanner, Cleveland, Ohio) was used to image the coronary arteries and abdominal aorta. Heart scanning began at the carina and proceeded through the cardiac apex; a hydroxyapatite calibration phantom pad was placed under each participant’s thorax.16,17 Simultaneous acquisition of 4 slices and fast rotation time restricted breath-hold to <15 seconds. Electrocardiographic triggering (set at 50% of the expected next RR interval) in sequential slice mode at 120 kV and 165 mAs was used to acquire contiguous, noninterlaced slices with a table increment of 20 mm for every series of 4 slices. Scanning of the abdominal aorta began at the tip of the xyphoid process and proceeded through the level of the umbilicus with a single breath-hold. Helical scanning mode at 120 kV and 180 mAs with a table speed of 3 cm/sec and pitch of 6 was used for scanning the abdominal aorta and included a calibration phantom pad under each participant’s abdomen.16,17 Scans were analyzed without knowledge of treatment assignment using validated calcium scoring software.18,19 A separate calcium score for the coronary arteries and abdominal aorta was derived.20
Laboratory Measurements
Participants fasted 8 hours before sample collections. Plasma lipids were measured using an enzymatic method under the CDC Standardization Program; LDL-C was calculated.21 Apolipoprotein A-1 and B were measured by electroimmunoassay.22,23 tHcy was determined by reverse phase high performance liquid chromatography.24 Plasma folate and vitamin B12 were determined by radioimmunoassay kit (Bio-Rad Quanta Phase I and II; Bio-Rad Laboratories, Hercules, CA). Pyridoxal-5′-phosphate, the active cofactor derived from pyridoxine (vitamin B6) was determined ezymatically using a tyrosine decarboxylase based method.25 The coefficient of variation for tHcy, folate, vitamin B12 and vitamin B6 measurements was 7.8%, 7%, 7% and 16%, respectively.
The oral methionine loading test used 100 mg L-methionine/kg body weight in 8-ounces of unsweetened orange/apple juice. Following a fasting blood draw, subjects drank the methionine within 5 minutes. Exactly 2 hours after ingestion, the second blood sample was drawn.
An independent laboratory tested each lot of B-vitamin pills for content uniformity and dissolution before release as well as stability of the pill components every 3 months for the first year and then every 6 months for years 2–3.
Statistical Analysis
Pre-randomization characteristics were compared between treatment groups with 2-sample t-tests for continuous variables and chi-square tests for categorical variables. Percentage pill compliance, tHcy and B-vitamin levels were averaged over the trial period. Average on-trial levels and changes from baseline were compared between groups with 2-sample t-tests; changes from baseline were tested within treatment groups using paired t-tests. PML results were summarized as difference in post-load minus fasting tHcy.
An intention-to-treat analysis was performed for all participants who had carotid ultrasonography at baseline and at least 1 follow-up visit. A linear mixed effects model was used to compare treatment groups on average CIMT change rates. CIMT was regressed on follow-up time (in years), with adjustment for the randomization stratification factor (baseline CIMT). The regression coefficient associated with trial follow-up time estimated the average CIMT annual rate of change. A treatment × follow-up time interaction term evaluated whether the treatment groups differed in average CIMT progression rates.
In ancillary analyses, mixed effects models were used to evaluate the association of baseline and on-trial levels of fasting tHcy, PML and B-vitamins (all modeled as continuous variables) with the rate of CIMT progression. Interaction terms with follow-up time evaluated whether these variables were significantly associated with CIMT progression.
Absolute change in CAC and AC was calculated for each subject as: final baseline calcium score. Treatment group differences were tested using the Wilcoxon rank sum test. Among subjects who had no measurable CAC on the baseline CT scan, the proportion of subjects who developed measurable calcium on the endpoint scan were compared between treatment groups using Fisher’s exact test.
Treatment group comparisons of adverse events among all randomized subjects used the Fisher’s exact test. Adverse events included deaths, cardiovascular events, cerebrovascular events, arterial revascularization procedures and cancers. The occurrence of white blood cell (WBC) count below laboratory normal limit (4,000 cell/μL) was also compared between treatment groups.
Statistical analyses used SAS 9.0 software (SAS, Inc., Cary, North Carolina); statistical testing was conducted at the 0.05 significance level.
RESULTS
Baseline Characteristics
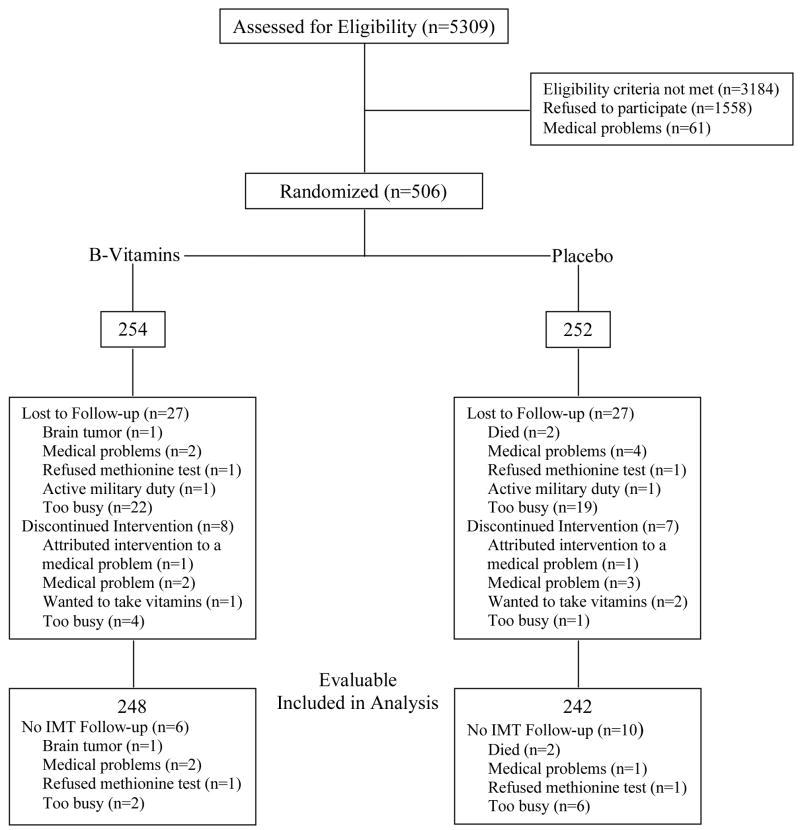
Of the 5309 subjects prescreened by telephone (Figure 1), 506 were randomized (254 B-vitamin, 252 placebo). Of the randomized subjects, 446 (223 B-vitamin, 223 placebo) completed the initially planned 2.5-year trial period; 280 (143 B-vitamin, 137 placebo) participated in the trial extension. 490 subjects (248 B-vitamin, 242 placebo) contributed to the primary end point analysis; 443 subjects (224 B-vitamin, 219 placebo) had baseline and end-of-trial calcium measures.
Trial profile.
Treatment groups did not significantly differ at baseline for demographic, clinical and atherosclerosis characteristics (Table 1). The average age was 61.4 years, 61% of subjects were male and 35% were from an ethnic minority.
Table 1.
Baseline Demographic, Clinical and Atherosclerosis Characteristics
| Variable | B-Vitamins (n=254) | Placebo (n=252) | p-value* |
|---|---|---|---|
| Age (years) | 61.7 (10.1)† | 61.1 (9.6) | 0.48 |
| Gender | 1.00 | ||
| Male | 155 (61%) | 154 (61%) | |
| Female | 99 (39%) | 98 (39%) | |
| Race | 0.76 | ||
| White | 170 (67%) | 159 (63%) | |
| Black | 36 (14%) | 38 (15%) | |
| Hispanic | 24 (9%) | 30 (12%) | |
| Asian | 21 (8%) | 24 (10%) | |
| American Indian | 2 (1%) | 1 (<1%) | |
| Education | 0.16 | ||
| High school graduate or less | 12 (5%) | 20 (8%) | |
| Some college | |||
| Trade or Business school | 74 (30%) | 73 (29%) | |
| Bachelor degree | 13 (5%) | 10 (4%) | |
| Post graduate or professional degree | 56 (22%) 94 (38%) |
73 (29%) 76 (30%) |
|
| Smoking history | 0.52 | ||
| Current | 7 (3%) | 10 (4%) | |
| Former | 81 (32%) | 89 (36%) | |
| Never smoked | 162 (65%) | 151 (60%) | |
| Blood pressure (mmHg) | |||
| Systolic | 128 (14) | 129 (15) | 0.45 |
| Diastolic | 80 (8) | 80 (8) | 0.97 |
| Body mass index (kg/m2) | 27.9 (5.0) | 28.3 (4.8) | 0.46 |
| Lipids (mg/dL) | |||
| Total cholesterol | 217 (34) | 217 (33) | 0.84 |
| LDL-cholesterol | 133 (31) | 134 (30) | 0.81 |
| HDL-cholesterol | 58 (16) | 56 (14) | 0.12 |
| Triglycerides | 128 (60) | 132 (63) | 0.44 |
| Apolipoproteins (mg/dL) | |||
| A-1 | 149 (24) | 148 (21) | 0.51 |
| B | 105 (18) | 105 (18) | 0.81 |
| Creatinine clearance (ml/min)‡ | 77.5 (22.8) | 78.2 (24.5) | 0.73 |
| CIMT (mm) | 0.75 (0.13) | 0.76 (0.16) | 0.42 |
| Coronary artery calcium§ | 0 (0, 52) | 0 (0, 90) | 0.23 |
| Aortic calcium§ | 202 (3, 1539) | 253 (0, 1561) | 0.52 |
| Lipid-lowering medications | 39 (15.4%) | 36 (14.3%) | 0.74 |
| Blood pressure medications | 69 (27.2%) | 74 (29.4%) | 0.58 |
Group comparisons utilized the independent Student t-tests for continuous variables or chi-square test for categorical variables. Calcium measures are compared by Wilcoxon rank sum test.
Mean (SD) or n (%)
Calcium measures are median (interquartile range)
CIMT Progression Rates
The 490 subjects with evaluable CIMT data had a mean (range) of 3.14 (0.48–4.56) years of follow-up in the B-vitamin group and 3.07 (0.46–5.0) years of follow-up in the placebo group (p=0.63). Participants contributed an average of 8.2 (range, 3–11) CIMT measures in the B-vitamin group and 8.2 (range, 3–11) measures in the placebo group (p=0.69). A statistically significant difference between the overall CIMT progression rate in the B-vitamin-treated group and the placebo-treated group was not found (Table 2). However, in a post-hoc analysis, among subjects with initial fasting tHcy at or above the median (≥9.1 μmol/L), the B-vitamin-treated group had statistically significant lower average rates of CIMT progression than the placebo-treated group (p=0.02); among subjects with a baseline fasting tHcy<9.1 μmol/L there was no significant treatment effect (p-value for treatment interaction=0.02). Subjects with baseline tHcy≥9.1 μmol/L were older, had lower vitamin B12 levels and higher systolic blood pressure than subjects with tHcy<9.1 μmol/L (all p<0.05).
Table 2.
Carotid Artery Intima-Media Thickness (CIMT) Progression by Treatment Group
| B-Vitamins (n=248) | Placebo (n=242) | p-value* | |
|---|---|---|---|
| All subjects, adjusted for baseline CIMT strata (<≥0.75 mm) | 0.0022 (0.0005)† | 0.0029 (0.0007) | 0.31 |
| Baseline fasting plasma total homocysteine | |||
| <9.1 μmol/L (n=126/119)‡ | 0.0028 (0.0006) | 0.0021 (0.0007) | 0.41 |
| ≥9.1 μmol/L (n=122/123) | 0.0016 (0.0007) | 0.0038 (0.0007) | 0.02 |
p-values from mixed effects models
Mean (SE) carotid artery intima-media thickness (CIMT) progression rate (mm/year)
Sample sizes in sub-group analysis (B-vitamins/Placebo)
In mixed effects models in the entire sample of 490 subjects, baseline and on-trial levels of tHcy, folic acid, vitamin B12, and vitamin B6 were not significantly associated with CIMT progression rate. Both baseline (p=0.03) and on-trial PML (p=0.01) (2-hour post-methionine tHcy minus fasting tHcy) were positively associated with CIMT progression. On-trial PML was also significantly positively associated with CIMT progression within each treatment group.
CAC and AC Progression
Treatment groups did not differ on baseline CAC and AC (Table 3). Changes in calcium measures did not differ between treatment groups overall or by baseline median fasting tHcy. Among subjects who showed no CAC on baseline scan, incidence of new CAC at follow-up was 19% in B-vitamin-treated and 17% in placebo-treated subjects (p=0.61).
Table 3.
Coronary Artery and Aortic Calcium by Treatment Group
| B-Vitamins (n=224) | Placebo (n=219) | p-value* | |
|---|---|---|---|
| Coronary Artery Calcium | |||
| Baseline | 0 (0,52)† | 0 (0,90) | 0.23 |
| 2.5 years | 1.2 (0,115) | 3.9 (0,166) | 0.31 |
| Change | 0 (0,43) | 0 (0,52) | 0.82 |
| Aortic Calcium | |||
| Baseline | 202 (3,1539) | 253 (0,1561) | 0.52 |
| 2.5 years | 480 (55,2284) | 486 (12,2684) | 0.44 |
| Change | 224 (20,669) | 175 (3,737) | 0.75 |
| Incidence of coronary artery calcium | 24/126 (19%) | 19/115 (17%) | 0.61‡ |
p-values from Wilcoxon rank sum test
Median (interquartile range)
p-value from Fisher’s Exact test
Homocysteine and B-vitamins
Baseline fasting tHcy, PML and B-vitamin levels did not significantly differ between treatment groups (Table 4). Average fasting tHcy and PML levels significantly decreased in B-vitamin-treated subjects and increased significantly in placebo-treated subjects (p<0.0001). Within both B-vitamin-treated and placebo-treated subjects, average plasma B-vitamin levels significantly increased from baseline (p<0.004). On-trial levels and changes from baseline showed highly significant treatment group differences (all p<0.0001) with the B-vitamin-treated group demonstrating reduced fasting tHcy and PML levels and increased B-vitamin levels relative to the placebo-treated group. B-vitamin supplementation did not affect lipid or apolipoprotein levels (data not shown).
Table 4.
Plasma Homocysteine and B-Vitamin Levels
| B-Vitamins (n=248) | Placebo (n=242) | p-value between treatment groups | |
|---|---|---|---|
| Fasting Total | |||
| Homocysteine (μmol/L) | |||
| Baseline | 9.5 (2.7)* | 9.8 (4.4) | 0.40 |
| Average on-trial | 8.8 (1.8) | 11.2 (3.8) | <0.001 |
| Change | −0.7 (2.7) | 1.4 (3.3) | <0.001 |
| p-value within group | <0.001 | <0.001 | |
| Post Methionine | |||
| Loading Homocysteine – Fasting Homocysteine (μmol/L) | |||
| Baseline | 14.7 (6.7) | 14.8 (6.1) | 0.88 |
| Average on-trial | 12.8 (4.4) | 15.7 (6.5) | <0.001 |
| Change | −2.0 (4.7) | 0.9 (3.9) | <0.001 |
| p-value within group | <0.001 | <0.001 | |
| B-Vitamins | |||
| Folic acid (ng/ml) | |||
| Baseline | 9.7 (5.7) | 9.2 (5.0) | 0.26 |
| Average on-trial | 75.4 (72.7) | 10.3 (6.3) | <0.001 |
| Change | 65.6 (72.2) | 1.2 (6.2) | <0.001 |
| p-value within group | <0.001 | 0.004 | |
| B12 (pg/ml) | |||
| Baseline | 400 (199) | 394 (143) | 0.68 |
| Average on-trial | 748 (314) | 432 (159) | <0.001 |
| Change | 347 (213) | 38 (95) | <0.001 |
| p-value within group | <0.001 | <0.001 | |
| B6 (pmol/ml) | |||
| Baseline | 65 (33) | 73 (62) | 0.06 |
| Average on-trial | 350 (131) | 83 (57) | <0.001 |
| Change | 285 (120) | 9 (57) | <0.001 |
| p-value within group | <0.001 | 0.01 | |
Mean (SD)
P-value between treatment groups tests mean group differences by independent t-tests. P-value within treatment group tests significance of change (mean on-trial minus baseline) by paired t-test.
Compliance
Mean (SD) pill compliance was 90.4% (15.0%) among B-vitamin-treated subjects and 90.4% (16.0%) among placebo-treated subjects (p=0.97).
Tablet Stability
Folic acid and vitamin B6 remained stable at 100% of their initial pill content over 3 years. Vitamin B12 remained stable at 100% of its initial pill content until month 9; at months 12, 18, 24 and 36 vitamin B12 dropped respectively to 86%, 77%, 73% and 66% of its initial pill content. No single pill lot remained in circulation beyond 24 months.
Clinical Events
A total of 9 (3.5%) B-vitamin-treated and 11 (4.4%) placebo-treated subjects had at least 1 cardiovascular event during the trial (p=0.66). Two placebo-treated subjects died during the trial (p=0.25). A total of 31 subjects (16 (6.3%) B-vitamin, 15 (6.0%) placebo, p=1.00) were diagnosed with cancer during the trial. Of the subjects who had normal WBC at baseline, 35/232 (15.1%) B-vitamin-treated and 24/227 (10.6%) placebo-treated subjects demonstrated at least 1 instance of low WBC during the trial (p=0.16). Infectious illness did not differ between treatment groups (57 (22.2%) B-vitamin-treated, 60 (23.8%) placebo-treated subjects reported at least 1 infectious illness over the trial, p=0.72). Treatment groups did not differ in antibiotic use over the trial (28 (11.0%) B-vitamin-treated, 41 (16.3%) placebo-treated; p=0.09).
DISCUSSION
Supplementation with combination high-dose folic acid, vitamin B12 and vitamin B6 significantly raised the plasma concentrations of these vitamins and lowered fasting and PML tHcy relative to placebo. However, the high-dose combination of B-vitamins did not reduce the progression of CIMT, CAC, or AC over a 3.1-year period. Subgroup analysis however, revealed a statistically significant treatment effect of high-dose combination B-vitamins on CIMT progression in individuals with a baseline fasting tHcy≥9.1 μmol/L.
Although the subgroup analysis was done post-hoc and the results need confirmation, the findings are consistent with the literature indicating that CVD risk substantially increases when plasma fasting tHcy levels exceed 8–9 μmol/L.6,7 As such, the demonstration of a B-vitamin supplementation effect on CIMT in individuals with fasting tHcy≥9.1 μmol/L is highly relevant. Fasting tHcy levels have fallen in the general U.S. population since the FDA-mandated folate fortification of cereal-grains (estimated to increase folate intake 70–120 ug/d) was instituted in January 199826 and likely accounts for the lack of baseline folic acid difference between individuals with baseline fasting tHcy levels <and≥9.1 μmol/L. A folate dosage of 5 mg/day, 50 times the intake of folate from cereal-grains, is sufficient to overcome any confounding effects of the FDA fortification policy since there was a highly significant reduction in fasting tHcy relative to placebo. Subjects with baseline fasting tHcy≥9.1 μmol/L had a significantly lower vitamin B12 level than subjects with a baseline tHcy<9.1 μmol/L possibly contributing to the beneficial effect of B-vitamin supplementation on CIMT progression in the former group. Additionally, reduction of on-trial PML with B-vitamin supplementation (as a reflection of vitamin B6 supplementation) also possibly contributed to the reduction in the progression of CIMT relative to the placebo-treated group since on-trial PML was positively associated with CIMT progression. Together, these data suggest that FDA-mandated folate fortification likely has reduced folate as a substantial risk for the progression of atherosclerosis whereas vitamin B12 and vitamin B6 (perhaps through PML levels) remain additional targets for further reducing atherosclerosis progression.
BVAIT indicates that individuals of an average age of 61 years who are at low-risk for CVD with a fasting tHcy≥9.1 μmol/L benefit from 3-years of B-vitamin supplementation. A similar study indicated that lower B-vitamin dosages (daily folic acid 2.5 mg + vitamin B12 0.5 mg + vitamin B6 25 mg) over a 1-year treatment period slowed the progression of CIMT relative to placebo.27 The cohort was selected with a baseline CIMT≥1 mm and CIMT progression was significantly dependent on the baseline vitamin B12 concentration.
Randomized controlled trials of secondary prevention have failed to demonstrate a reduction of CVD with B-vitamin supplementation.10,11 The discordance between BVAIT and observational studies and secondary prevention randomized controlled trials may be the result of different timing of B-vitamin supplementation according to the stage (early versus advanced) of atherosclerosis.28,29 Consistent with this hypothesis was the fact that B-vitamin supplementation had no effect on CAC or AC, markers of late-stage atherosclerosis.
Pill compliance was high and the activity of the folic acid and vitamin B6 components of the combination pill was stable over the trial. Vitamin B12 was less stable. The significant rise in plasma B-vitamin levels and reduction of fasting tHcy and PML relative to placebo confirm the high level of compliance and pill activity.
Although nonsignificant, the implication of the WBC-lowering effect of high-dose B-vitamin supplementation relative to placebo in this trial is unclear. Previous reports indicate a relationship between high WBC levels and CVD and reduction of WBC levels could theoretically contribute to a reduction in atherosclerosis.30 Safety data failed to indicate any adverse effect of B-vitamin supplementation on infections or antibiotic use. An inverse association between plasma B-vitamin and WBC levels has been reported from previous observational studies;31 the mechanism is unknown.
Subjects randomized to BVAIT were at low-risk for CVD with baseline plasma B-vitamin levels defined as normal based on population distributions. There are no optimally-defined plasma B-vitamin levels in the context of vascular health. BVAIT indicates that currently defined “normal” plasma B-vitamin levels are insufficient for atheroprotection and perhaps a better way to define optimal B-vitamin levels for vascular health is reflected through fasting tHcy levels; that is, optimal levels of B-vitamins to maintain a fasting tHcy<9.1 μmol/L. Targeting tHcy levels is supported by BVAIT since there was a significant treatment interaction according to baseline tHcy.
The results from BVAIT are limited to individuals without pre-existing CVD. Although B-vitamin supplementation reduced the progression of athersoclerosis among subjects with tHcy≥9.1 μmol/L, BVAIT had insufficient power to statistically compare CVD outcomes.
In conclusion, BVAIT indicates that B-vitamin supplementation significantly reduces progression of early stage subclinical atherosclerosis in well-nourished healthy B-vitamin “replete” individuals at low-risk for CVD with a fasting tHcy≥9.1 μmol/L. Further studies to determine whether reducing tHcy levels prevents plaque rupture and clinical events in a population similar to BVAIT are warranted.
Acknowledgments
Funding/Support: This study was supported by grant R01AG-17160 from the National Institute on Aging, National Institutes of Health. Leiner Health Products provided the B-vitamin supplements and placebo.
Role of the Sponsors: The National Institutes of Health and Leiner Health Products had no role in the design and conduct of the study; collection, management, analysis and interpretation of the data; preparation, review or approval of the manuscript; and no role in reporting of the study or in the decision to submit the manuscript for publication.
Footnotes
Author Contributions: Dr. Hodis had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Hodis, Mack
Acquisition of data: Hodis, Mack, Dustin, Mahrer, Azen, Detrano, Selhub, Alaupovic, CR Liu, CH Liu, Hwang, Wilcox, Selzer
Analysis and interpretation of data: Hodis, Mack, Dustin
Drafting of the manuscript: Hodis, Mack
Critical revision of the manuscript for important intellectual content: Mahrer, Azen, Detrano, Selhub, Alaupovic, Hwang, Wilcox, Selzer
Statistical analysis: Mack, Dustin
Obtaining funding: Hodis, Mack
Administrative, technical, or material support: Hodis, Mack, Mahrer, Azen, Detrano, Selhub, Alaupovic, Hwang, Wilcox, Selzer
Study supervision: Hodis, Mack
Financial Disclosures: None
BVAIT Research Group: Study Chairman: Howard N. Hodis MD; Clinical Investigators: Peter R. Mahrer MD and Alex Sevanian PhD (deceased); Clinical Center Staff (USC): Martha Charlson RD, Sandra Engle MA, Christine Gesselman, Thelma LaBree MA, Sonia Moss MA, Charlene Moya, Jan St John, MaryAnn Spahn MS, Frank Watcher, Liny Zurbrugg RN; Clinical Center Staff (Kaiser Permanente): Robert Browning RN, Phyllis Scutella RN; Ultrasound Image Acquisition and Processing Laboratory: Robert H. Selzer MS (Director), Zenaida Lee, Chao-ran Liu MD, Ci-hua Liu MD; Vascular Calcium Image Acquisition: Alison G. Wilcox MD (Director), Clifford Martizorena, Tom Pham, Donna Proby, John Vicario; Vascular Calcium Image Processing Laboratory: Robert Detrano MD (Director), Christopher Dailing, Agnes Papa; Data Coordinating Center: Wendy J. Mack PhD (Director), Nicole Aguirre, Stanley P. Azen PhD, Laurie Dustin MS, Molly Hubbard, Michael Hutchinson, George Martinez, Olga Morales, Christina Trujillo; Core Lipid/Lipoprotein Laboratory: Juliana Hwang, PharmD (Director), Orlando Bolusan, Gail Izumi, Arletta Ramirez; Vitamin Metabolism Laboratory: Jacob Selhub PhD (Director); Apolipoprotein Laboratory: Petar Alaupovic PhD (Director); Data Safety Monitoring Board: Meir Stampfer MD (Chairman), B. Greg Brown MD, Joan Hilton PhD, Joanna Badinelli (NIA ex-officio), Andre J. Premen PhD (NIA ex-officio).
Trial Registration: BVAIT is registered with http://www.ClinicalTrials.gov, NCT00114400.
References
- 1.Boushey CJ, Beresford SAA, Omenn GS, Motulsky AG. A quantitative assessment of plasma homocysteine as a risk factor for vascular disease. JAMA. 1995;274:1049–1057. doi: 10.1001/jama.1995.03530130055028. [DOI] [PubMed] [Google Scholar]
- 2.Refsum H, Ueland PM, Nygard O, Vollset SE. Homocysteine and cardiovascular disease. Annu Rev Med. 1998;49:31–62. doi: 10.1146/annurev.med.49.1.31. [DOI] [PubMed] [Google Scholar]
- 3.Graham IM, Daly LE, Refsum HM, Robinson K, Brattstrom LE, Ueland PM, Palma-Reis RJ, Boers GH, Sheahan RG, Israelsson B, Uiterwaal CS, Meleady R, McMaster D, Verhoef P, Witteman J, Rubba P, Bellet H, Wautrecht JC, de Valk HW, Sales Luis AC, Parrot-Rouland FM, Tan KS, Higgins I, Garcon D, Andria G. Plasma homocysteine as a risk factor for vascular disease: the European Concerted Action Project. JAMA. 1997;277:1775–1781. doi: 10.1001/jama.1997.03540460039030. [DOI] [PubMed] [Google Scholar]
- 4.Bostom AG, Jacques PF, Nadeau MR, Williams RR, Ellison RC, Selhub J. Post-methionine load hyperhomocysteinemia in persons with normal fasting total plasma homocysteine: initial results from the NHLBI Family Heart Study. Atherosclerosis. 1995;116:147–151. doi: 10.1016/0021-9150(95)05529-6. [DOI] [PubMed] [Google Scholar]
- 5.Verhoef P, Kok FJ, Kruyssen DACM, Schouten EG, Witteman JCM, Grobbee DE, Ueland PM, Refsum H. Plasma total homocysteine, B vitamins, and risk of coronary atherosclerosis. Arterioscler Thromb Vasc Biol. 1997;17:989–995. doi: 10.1161/01.atv.17.5.989. [DOI] [PubMed] [Google Scholar]
- 6.Hopkins PN, Wu LL, Wu J, Hunt SC, James BC, Vincent GM, Williams RR. Higher plasma homocysteine and increased susceptibility to adverse effects of low folate in early familial coronary artery disease. Arterioscler Thromb Vasc Biol. 1995;15:1314–1320. doi: 10.1161/01.atv.15.9.1314. [DOI] [PubMed] [Google Scholar]
- 7.Nygard O, Nordrehaug JE, Refsum H, Ueland PM, Farstad M, Vollset SE. Plasma homocysteine levels and mortality in patients with coronary artery disease. N Engl J Med. 1997;337:230–236. doi: 10.1056/NEJM199707243370403. [DOI] [PubMed] [Google Scholar]
- 8.Rimm EB, Willett WC, Hu FB, Sampson L, Colditz GA, Manson JE, Hennekens C, Stampfer MJ. Folate and vitamin B6 from diet and supplements in relation to risk of coronary heart disease among women. JAMA. 1998;279:359–364. doi: 10.1001/jama.279.5.359. [DOI] [PubMed] [Google Scholar]
- 9.Verhoef P, Stampfer MJ, Buring JE, Gaziano JM, Allen RH, Stabler SP, Reynolds RD, Kok FJ, Hennekens CH, Willett WC. Homocysteine metabolism and risk of myocardial infarction: relation with vitamins B6, B12, and folate. Am J Epidemiol. 1996;143:845–859. doi: 10.1093/oxfordjournals.aje.a008828. [DOI] [PubMed] [Google Scholar]
- 10.Bazzano LA, Reynolds K, Holder KN, He J. Effect of folic acid supplementation on risk of cardiovascular diseases: a meta-analysis of randomized controlled trials. JAMA. 2006;296:2720–2726. doi: 10.1001/jama.296.22.2720. [DOI] [PubMed] [Google Scholar]
- 11.Jamisom RL, Hartigan P, Kaufman JS, Goldfarb DS, Warren SR, Guarino PD, Gaziano JM. Effect of homocysteine lowering on mortality and vascular disease in advanced chronic kidney disease and end-stage renal disease: a randomized controlled trial. JAMA. 2007;298:1163–1170. doi: 10.1001/jama.298.10.1163. [DOI] [PubMed] [Google Scholar]
- 12.Hodis HN, Mack WJ, Lobo RA, Shoupe D, Sevanian A, Mahrer PR, Selzer RH, Liu CR, Liu CH, Azen SP. Estrogen in the prevention of atherosclerosis: a randomized, double-blind, placebo-controlled trial. Ann Intern Med. 2001;135:939–953. doi: 10.7326/0003-4819-135-11-200112040-00005. [DOI] [PubMed] [Google Scholar]
- 13.Hodis HN, Mack WJ, LaBree L, Mahrer PR, Sevanian A, Liu CR, Liu CH, Hwang J, Selzer RH, Azen SP. Alpha tocopherol supplementation in healthy individuals reduces low-density lipoprotein oxidation but not atherosclerosis: the Vitamin E Atherosclerosis Prevention Study (VEAPS) Circulation. 2002;106:1453–1459. doi: 10.1161/01.cir.0000029092.99946.08. [DOI] [PubMed] [Google Scholar]
- 14.Selzer RH, Hodis HN, Kwong-Fu H, Mack WJ, Lee PL, Liu CR, Liu CH. Evaluation of computerized edge tracking for quantifying intima-media thickness of the common carotid artery from B-mode ultrasound images. Atherosclerosis. 1994;111:1–11. doi: 10.1016/0021-9150(94)90186-4. [DOI] [PubMed] [Google Scholar]
- 15.Selzer RH, Mack WJ, Lee PL, Kwong-Fu H, Hodis HN. Improved common carotid elasticity and intima-media thickness measurement from computer analysis of sequential ultrasound frames. Atherosclerosis. 2001;154:185–193. doi: 10.1016/s0021-9150(00)00461-5. [DOI] [PubMed] [Google Scholar]
- 16.Detrano R, Kang X, Mahaisavariya P, Tang W, Colombo A, Sabee M, Garner D, Nickerson S. Accuracy of quantifying coronary hydroxyapatite with electron beam tomography. Invest Radiol. 1994;29:733–738. doi: 10.1097/00004424-199408000-00001. [DOI] [PubMed] [Google Scholar]
- 17.Mahaisavariya P, Detrano R, Kang X, Garner D, Vo A, Georgiou D, Molloi S, Brundage BH. Quantitation of in vitro coronary artery calcium using ultrafast computed tomography. Cathet Cardiovasc Diagn. 1994;32:387–393. doi: 10.1002/ccd.1810320421. [DOI] [PubMed] [Google Scholar]
- 18.Reed JE, Rumberger JA, Davitt PJ. System for quantitative analysis of coronary calcification via electron beam computed tomography. Proc SPIE. 1994;2168:36–43. [Google Scholar]
- 19.Yaghoubi S, Tang W, Wang S, Reed J, Hsiai J, Detrano R, Brundage B. Offline assessment of atherosclerotic coronary calcium from electron beam tomograms. Am J Card Imaging. 1995;9:231–236. [PubMed] [Google Scholar]
- 20.Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M, Detrano R. Qunatification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15:827–832. doi: 10.1016/0735-1097(90)90282-t. [DOI] [PubMed] [Google Scholar]
- 21.Lipid Research Clinics Program. The Manual of Laboratory Operations: Lipid and Lipoprotein Analysis. Bethesda, MD: National Institutes of Health; 1974. DHEW Publication No., N.I.H., 75–628. [Google Scholar]
- 22.Curry MD, Alaupovic P, Suenram CA. Determination of apolipoprotein A and its constitutive A-I and A-II polypeptides by separate electroimmunoassays. Clin Chem. 1976;22:315–322. [PubMed] [Google Scholar]
- 23.Curry MD, Gustafson A, Alaupovic P, McConathy WJ. Electroimmunoassay, radioimmunoassay and radial immunodiffusion assay evaluation for quantification of human apolipoprotein B. Clin Chem. 1978;24:280–286. [PubMed] [Google Scholar]
- 24.Araki A, Sako Y. Determination of free and total homocysteine in human plasma by high-performance liquid chromatography with fluorescence detection. J Chromatography. 1987;422:43–52. doi: 10.1016/0378-4347(87)80438-3. [DOI] [PubMed] [Google Scholar]
- 25.Shin-Buehring Y, Rasshofer R, Endres W. A new enzymatic method for pyridoxal-5′-phosphate determination. J Inherit Metab Disorders. 1981;4:123–124. [Google Scholar]
- 26.Malinow MR, Duell PB, Hess DL, Anderson PH, Kruger WD, Phillipson BE, Gluckman RA, Block PC, Upson BM. Reduction of plasma homocyst(e)ine levels by breakfast cereal fortified with folic acid in patients with coronary heart disease. N Engl J Med. 1998;338:1009–1015. doi: 10.1056/NEJM199804093381501. [DOI] [PubMed] [Google Scholar]
- 27.Till U, Rohl P, Jentsch A, Till H, Muller A, Bellstedt K, Plonne D, Fink HS, Ruiger V, Sliwka U, Herrmann FH, Petermann H, Riezler R. Decrease of carotid intima-media thickness in patients at risk to cerebral ischemia after supplementation with folic acid, vitamins B6 and B12. Atherosclerosis. 2005;181:131–135. doi: 10.1016/j.atherosclerosis.2004.12.043. [DOI] [PubMed] [Google Scholar]
- 28.Steinberg D, Witztum JL. Is the oxidative modification hypothesis relevant to human atherosclerosis? Do the antioxidant trials conducted to date refute the hypothesis? Circulation. 2002;105:2107–2111. doi: 10.1161/01.cir.0000014762.06201.06. [DOI] [PubMed] [Google Scholar]
- 29.Hodis HN, Mack WJ, Sevanian A. Antioxidant vitamin supplementation and cardiovascular disease. In: Bendich A, Deckelbaum RJ, editors. Preventive Nutrition: The Comprehensive Guide for Health Professionals. 3. Totowa, NJ: Humana Press Inc; 2005. pp. 245–277. [Google Scholar]
- 30.Danesh J, Collins R, Appleby P, Peto R. Association of fibrinogen, C-reactive protein, albumin, or leukocyte count with coronary heart disease: meta-analysis of prospective studies. JAMA. 1998;279:1477–1482. doi: 10.1001/jama.279.18.1477. [DOI] [PubMed] [Google Scholar]
- 31.Folsom AR, Desvarieux M, Nieto FJ, Boland LL, Ballantyne CM, Chambless LE. B-vitamin status and inflammatory markers. Atherosclerosis. 2003;169:169–174. doi: 10.1016/s0021-9150(03)00161-8. [DOI] [PubMed] [Google Scholar]