Abstract
Carbohydrate-restriction is a common weight-loss approach that modifies hepatic metabolism by increasing gluconeogenesis and ketosis. Because little is known regarding the effect of carbohydrate-restriction on the origin of gluconeogenic precursors (gluconeogenesis from glycerol (GNGglycerol) and lactate/amino acids (GNGPEP)) or its consequence to hepatic energy homeostasis, we studied these parameters in a group of overweight/obese subjects undergoing weight-loss via dietary restriction. We used 2H and 13C tracers and nuclear magnetic resonance spectroscopy to measure the sources of hepatic glucose and TCA cycle flux in weight-stable subjects(n=7) and subjects following carbohydrate-(n=7) or calorie-restriction(n=7). The majority of hepatic glucose production in carbohydrate-restricted subjects came from GNGPEP. The contribution of glycerol to gluconeogenesis was similar in all groups despite evidence of increased fat oxidation in carbohydrate-restricted subjects. A strong correlation between TCA cycle flux and GNGPEP was found, though the reliance on TCA cycle energy production for gluconeogenesis was attenuated in subjects undergoing carbohydrate restriction. Together, these data imply that the TCA cycle is the energetic patron of gluconeogenesis. However, the relationship between these two pathways is modified by carbohydrate restriction, suggesting an increased reliance of the hepatocyte on energy generated outside of the TCA cycle when GNGPEP is maximal. In conclusion, carbohydrate-restriction modifies hepatic gluconeogenesis by increasing reliance on substrates like lactate or amino acids but not glycerol. This modification is associated with a reorganization of hepatic energy metabolism suggestive of enhanced hepatic β-oxidation.
Since the seminal observation of Keys in 1980, the recommended diet in the United States has been the low-calorie, low-fat diet (1). This diet originated primarily as an inexpensive approach to prevent cardiovascular disease but has now become the recommended treatment for overweight and obesity in clinical practice (2). Despite the success of clinicians and the US Public Health Service in reducing the US population’s fat intake and increasing it’s carbohydrate intake over the past 30 years, the prevalence of obesity has continued to rise (3, 4). During this same period, metabolic liver disease has become increasingly prevalent, taking the form of excess triglyceride accumulation in liver that can result in inflammation, fibrosis, and cirrhosis (5, 6). The transition of this type of liver disease, known as non-alcoholic fatty liver disease (NAFLD), from relatively bland, inactive steatosis to a more morbid inflammatory condition, termed non-alcoholic steatohepatitis (NASH), occurs in a subset of individuals. The reason this transition takes place is unclear; however, some investigators have found a strong association between dietary carbohydrate intake and severity of both steatosis and steatohepatitis (7, 8). Current evidence suggests that a high carbohydrate diet leads to increased hepatic de novo lipogenesis (9), likely as the result of the molecular mediators carbohydrate and sterol response element binding protein (5). Such an increase in hepatic fat synthesis would be anticipated to be associated with hepatic steatosis; however, the connection between carbohydrate intake and inflammatory activity remains elusive. The changes in hepatic metabolism and energy production that occur as a consequence of changes in dietary carbohydrate intake may be important in the pathogenesis and progression of NAFLD.
Several studies have used stable isotopes to investigate the impact of carbohydrate intake on hepatic glucose metabolism (10-12). These studies clearly show that low-carbohydrate diets result in a reorganization of hepatic glucose production by changing the rate of glycogenolysis and, to a lesser degree, the rate of gluconeogenesis. However, little is known about the effect of carbohydrate restriction on the origin of gluconeogenic precursors (gluconeogenesis from lactate/amino acids or glycerol) or its consequence on hepatic energy homeostasis. There is an empirical relationship between gluconeogenesis and TCA cycle flux (13): suppressed rates of gluconeogenesis result in impaired hepatic TCA cycle flux (14, 15), while increased gluconeogenesis is accompanied by elevated TCA cycle flux (16). This relationship appears to be an “energetic rheostat,” allowing liver to match energy production with the requirements of gluconeogenesis. If the increased rates of gluconeogenesis observed during carbohydrate restriction are the result of increased conversion of lactate/amino acids to glucose, energy production at the level of the TCA cycle may be altered in a coordinated manner.
To gain a better understanding of hepatic energy production and its relationship to gluconeogenesis under conditions of varied macronutrient intake, we used 2H and 13C tracers combined with nuclear magnetic resonance (NMR) spectroscopy (17) to simultaneously assess endogenous glucose production, glycogenolysis, gluconeogenesis from lactate/amino acids (GNGPEP), gluconeogenesis from glycerol (GNGglycerol) pyruvate cycling, and TCA cycle flux in human subjects following a carbohydrate-restricted or calorie-restricted diet.
RESEARCH DESIGN AND METHODS
Materials
Seventy-percent deuterium oxide (2H2O) and 99% [U-13C]propionate was obtained from Cambridge Isotopes (Andover, MA). Sterility and pyrogen tested [3,4-13C]glucose was obtained from Omicron Biochemicals, Inc. (South Bend, IN). Other common reagents were purchased from Sigma (St. Louis, MO).
Participants
The study was comprised of two groups of 7 subjects aged 18- to 65-years that were matched for age, body mass index (BMI), gender, and ethnicity. To eliminate confounding variables, participants chosen were of stable health, using no medications known to alter hepatic glucose metabolism, participating in no weight loss diet or using no diet pills at enrollment or within 6 months prior to enrollment, and without baseline ketonuria. The study was restricted to subjects whose body mass index (BMI) was >25 kg/m2 and <35 kg/m2. All subjects had a normal nutritional status, no ethanol intake, and did not participate in regular exercise above the activity required for daily living.
An additional group of 7 subjects who were lean (BMI < 25 kg/m2) was also recruited to act as a weight-stable comparison group. These subjects were all of normal health, had a normal nutritional status, no ethanol intake, and did no participate in regular exercise above the activity required for daily living.
The protocol and consent form were approved by the Institutional Review Board of the University of Texas Southwestern Medical Center and all participants provided written informed consent prior to enrollment.
Design
At enrollment, subjects were assigned to either a low-carbohydrate diet or a calorie-restricted diet. Prior to initiating the diet, all subjects underwent a teaching session with the General Clinical Research Center (GCRC) dietician to explain appropriate use of the diet history questionnaire, determine food preferences, and provide dietary instruction. Two weeks after enrollment, subjects were admitted to the inpatient GCRC for overnight metabolic studies. On admission (16:00) subjects were provided a dinner consistent with their assigned diet, after which they were fasted until completion of the study. Between 22:00 and 09:00, subjects received two stable isotope tracers orally: [U-13C]propionate (∼1200 mg) at 08:30 and divided doses of 70% 2H2O (5 g/kg body water, calculated as 60% of body weight in men and 50% of body weight in women) at 22:00, 02:00, and 06:00. Subjects were allowed to drink 0.5% 2H2O ad libitum during the study. Between 08:30 and 09:00 subjects underwent measurement of their respiratory quotient (RQ) using a Delta Trak II indirect calorimeter (Sensormedics, Yorba Linda, CA). An intravenous catheter was placed and subjects were given a 2.25 mg/kg bolus of [3,4-13C]glucose followed by a two-hour infusion (0.0225 mg/kg/min). At the end of the infusion period, a 50 cc blood draw was performed.
Low-Carbohydrate Diet
Subjects assigned to the low-carbohydrate diet were instructed by the GCRC dietician to limit carbohydrate intake to less than 20 g/d (18) and were provided with instructional handouts detailing allowed and disallowed foods. Participants were encouraged to eat three regular-size meals and to consume eight 8-ounce glasses of water a day. Subjects initiated this diet on their own for the first 7 days of the study, keeping a detailed dietary record. Food for the final 7 days was provided to subjects as frozen meals pre-prepared by the GCRC kitchen in accordance with the caloric intake documented in the dietary record.
Calorie-Restricted Diet
This diet was designed to place individuals in negative energy balance relative to their typically daily caloric intake. Subjects were asked to continue their regular diet without calorie restriction for the first 7 days of the study and record their food intake in a diet record to determine average daily caloric intake as well as dietary macronutrient composition. Based upon this diet record, the GCRC dietician and kitchen staff prepared all meals for the final 7 days of the study in accordance with the dietary composition recorded by the individual, but reduced in caloric content by 800 kcal/d.
Weight-Stable Diet
Subjects were asked to continue their regular diet, but were placed on a diet which was 40% carbohydrate, 30% fat, and 30% protein for three days prior to study. The daily caloric value of the meals was 1700 kcal for women and 2000 kcal for men.
Blood Samples
Blood was collected in heparinized tubes and centrifuged immediately to isolate plasma from red blood cells. Plasma was extracted with perchloric acid and the glucose was purified as previously described (19, 20). Purified plasma glucose was converted to 1,2-isopropylidene glucofuranose (a.k.a. MAG) before NMR analysis as detailed previously (20-22). The synthesized MAG was then resuspended in 150 μL of HPLC-grade acetonitrile and 20 μL of deuterium-depleted H2O and loaded into a 3 mm NMR tube.
Magnetic Resonance Spectroscopy
Samples were analyzed on a 14.1 T Varian Inova spectrometer (Varian Instruments, Palo Alto CA). Deuterium NMR spectra of MAG were collected on a 3 mm broadband probe tuned to 92 MHz with a 90 degree pulse width, 1 sec acquisition time, and no additional delay. Deuterium spectra were performed without field frequency lock and were acquired in 250 scan blocks. These were combined at the end of the experiment by aligning the acetonitrile frequency. Proton-decoupled 13C NMR spectra were collected after the addition of 20 μL of deuterated acetonitrile (for frequency locking), using a 3 mm broadband probe tuned to 150 MHz. A 50 degree pulse width, 1.5 sec acquisition time, and no further delay were used to acquire the 13C spectra. Resonance areas were determined using the 1-D NMR processor in ACD/Labs 9.0 (Advanced Chemistry Development, Toronto, Ontario, Canada).
Isotopomer Analysis
The relative deuterium enrichments in glucose H2, H5 and H6s were assessed by 2H NMR and these values were used to determine the fractional contribution of gluconeogenesis and glycogenolysis to endogenous glucose production as previously detailed (17, 19, 20). Pathways intersecting the TCA cycle were evaluated by 13C isotopomer analysis of glucose C2. The 13C NMR multiplets in glucose generated by the tracer [U-13C]propionate were evaluated to determine flux through anaplerosis, pyruvate cycling and GNGPEP relative to TCA cycle (23, 24). Here, we use anaplerosis as a measure of PEPCK flux since this pathway is the predominant outlet of anaplerosis in the liver. This represents a maximal estimate of PEPCK flux since we are disregarding other minor pathways (e.g. malic enzyme) which may also contribute to the flux of intermediates out of the TCA cycle. This measurement is also an estimate of pyruvate carboxylase (PC) flux since PC is the main anaplerotic pathway in the liver. Endogenous glucose production was measured by 13C NMR analysis of the multiplets of glucose C3 and C4 as previously described (25). Fractional glycogenolysis and gluconeogenesis measured by 2H NMR was combined with PEPCK, pyruvate cycling and gluconeogenesis relative to TCA cycle flux measured by 13C NMR and the rate of endogenous glucose production (μmol/kgLBW/min) to yield the absolute fluxes through each of these pathways (14, 17, 25).
Statistical Analysis
Statistical analyses were performed using SigmaStat 3.0 (SPSS, Inc., Chicago, IL). Differences between two groups were evaluated using unpaired t-tests (means) or Mann-Whitney rank sums tests (medians). Differences between two repeated measures were analyzed using paired t-test. One-way ANOVA was used for the comparison of more than two groups. Correlations between variables were determined using the Pearson product moment test. Statistical significance was taken at P<0.05.
RESULTS
Subjects
Subjects in the low-carbohydrate group were matched to those in the low-calorie group (Table 1). The majority of participants (10/14) were obese (BMI > 30 kg/m2), with the remainder classified as overweight (25 kg/m2 < BMI < 30 kg/m2). All subjects in the low-carbohydrate arm of the study demonstrated urinary ketones at the end of the study except for one. Despite the absence of ketosis, this individual had a respiratory quotient of 0.84 at study-end and experienced a 3.5 kg weight loss over the period of study.
Table 1.
Treatment Group General Characteristics
| Low-Calorie (n=7) |
P-value | Low-Carbohydrate (n=7) |
|
|---|---|---|---|
| Age (years) | 43 + 9 | 0.982 | 44 + 13 |
| Race/Ethnicity (n) | |||
| Caucasian | 5 | 5 | |
| African-American | 1 | 1.000 | 1 |
| Hispanic | 1 | 1 | |
| Gender Ratio (F:M) | 5:2 | 1.000 | 5:2 |
| Body Weight (kg) | 90 + 11 | 0.327 | 96 + 12 |
| Body Mass Index (kg/m2) | 32 + 4 | 0.618 | 33 + 3 |
| Total Cholesterol (mg/dL) | 201 + 15 | 0.722 | 198 + 18 |
| Triglyceride (mg/dL) | 102 + 51 | 0.899 | 107 + 75 |
| HDL-c (mg/dL) | 51 + 17 | 0.599 | 47 + 11 |
| LDL-c (mg/dL) | 131 + 23 | 0.883 | 129 + 16 |
| AST (U/L) | 22 + 5 | 0.631 | 21 + 5 |
| ALT (U/L) | 21 + 10 | 0.839 | 20 + 8 |
| Fasting Glucose (mg/dL) | 94 + 10 | 0.964 | 94 + 13 |
| HgbA1c (%) | 5.0 + 0.4 | 0.132 | 5.4 + 0.5 |
Note: Values are presented as mean + SD.
Intervention
Subjects in the low-carbohydrate arm experienced greater total weight loss over the two-week period than those in the low-calorie arm (Table 2). There was no significant change in weight in the weight-stable groups (data not shown). As expected, respiratory quotients were significantly lower and total plasma ketone bodies were significantly higher among carbohydrate restricted subjects. Insulin, lactate, and triglyceride levels did not differ between the two dietary-restriction groups after two weeks. There was no significant difference in caloric intake between the two groups, although a trend toward lower caloric intake in the low-carbohydrate group was noted. The dietary macronutrient proportions were significantly different in the two arms of the study; however, absolute dietary macronutrient intake was only significantly different with regard to protein and carbohydrate - absolute daily fat intake in the dietary groups was similar. Concordant with this, the absolute daily intake of saturated, monounsaturated, and polyunsaturated fats also did not differ between the two groups.
Table 2.
Treatment Group Characteristics at Study-End
| Low-Calorie (n=7) |
P-value | Low-Carbohydrate (n=7) |
|
|---|---|---|---|
| Total Weight Loss (kg) | 2.3 + 1.7 | 0.024 | 4.4 + 1.4 |
| Respiratory Quotient | 0.90 + 0.08 | 0.006 | 0.79 + 0.04 |
| Insulin (ng/mL) | 3.4 + 1.3 | 0.727 | 3.8 + 1.9 |
| Lactate (mmol/L) | 1.3 + 1.2 | 0.366 | 0.9 + 0.2 |
| Glucose (mg/dL) | 90 + 9 | 0.295 | 95 + 8 |
| Triglyceride (mg/dL) | 99 + 16 | 0.700 | 91 + 36 |
| Total Ketones (μmol/L) | 436 + 146 | 0.002 | 1280 + 407 |
| Caloric Intake (kcal/d) | 1980 + 727 | 0.153 | 1520 + 328 |
| Diet Composition | |||
| Protein (%) | 17 + 3 | <0.001 | 34 + 4 |
| Fat (%) | 36 + 8 | <0.001 | 61 + 5 |
| Carbohydrate (%) | 47 + 6 | <0.001 | 5 + 1 |
| Protein (g/d) | 81 + 17 | 0.001 | 126 + 20 |
| Fat (g/d) | 83 + 50 | 0.347 | 104 + 30 |
| Carbohydrate (g/d) | 228 + 65 | <0.001 | 19 + 1 |
| Fat Intake (g/d) | |||
| Saturated | 28 + 15 | 0.184 | 39 + 14 |
| Monounsaturated | 35 + 24 | 0.822 | 37 + 10 |
| Polyunsaturated | 18 + 11 | 0.705 | 16 + 8 |
Note: Values are presented as mean + SD.
Fractional Contribution of Glycogen, Glycerol, and Phosphoenolpyruvate to Glucose Production: Analysis of plasma glucose 2H enrichment by 2H-NMR
The method for analyzing the 2H spectra of plasma glucose is presented in Figure 1. The fraction of glucose derived from gluconeogenesis in the low-carbohydrate group was significantly higher than the low-calorie group (79+9 vs. 61+8%; P=0.001). As anticipated, the contribution of glycogenolysis to glucose production was significantly lower in carbohydrate-restricted subjects than that measured in calorie-restricted subjects (21+9 vs. 39+8%; P=0.001) (Table 3). Surprisingly, the fraction of gluconeogenesis arising from glycerol (GNGglycerol) was similar between the low-carbohydrate and the low-calorie group. Thus, the observed increase in the fractional contribution of gluconeogenesis to glucose production in the carbohydrate-restricted group was solely the result of an increase in gluconeogenesis originating from substrates such as lactate or amino acids that must pass through the TCA cycle and PEPCK (GNGPEP) (64+5 vs. 47+11%; P=0.002) (Table 3).
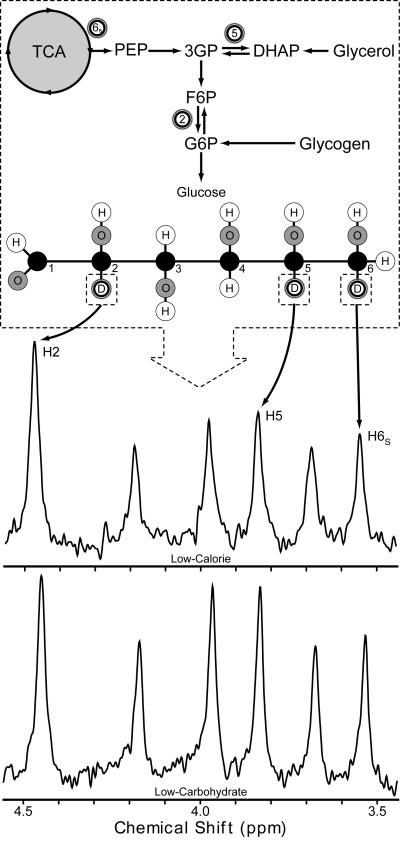
Figure 1.
2H-NMR Analysis of the Incorporation of Deuterium Oxide into Glucose. Protons derived from water are incorporated into glucose at three distinct steps of its production. After enrichment of total body water by oral ingestion of deuterium oxide (2H2O), deuterons are incorporated into glucose via the same mechanism. All glucose produced, whether from gluconeogenesis or from glycogenolysis, becomes enriched at carbon 2 of glucose (H2). All glucose derived from gluconeogenesis becomes enriched at carbon 5 of glucose (H5) while only gluconeogenesis occurring from precursors originating in the TCA cycle (PEP) becomes enriched at carbon 6s of glucose (H6s). The relative enrichment of deuterium at each of the carbon positions of glucose, after conversion to its monoacetone derivative, is easily discerned by computing the peak areas obtained by 2H-NMR spectroscopy. The ratio of H5/H2 gives the proportion of glucose that is derived from gluconeogenesis while (H5-H6s)/H2 gives the proportion of glucose derived from PEP. The H5/H2 ratio of the two depicted spectra are quite different: the low-calorie spectra indicates that gluconeogenesis accounts for ∼50% of glucose production while the low-carbohydrate spectra indicates that ∼80% of glucose is derived from gluconeogenesis.
Table 3.
Metabolic Flux Data
| Weight-Stable (n=7) |
Low-Calorie (n=7) |
Low-Carbohydrate (n=7) |
|
|---|---|---|---|
| Relative Data | |||
| Fractional Glucose Production | (%) | ||
| Glycogenolysis | 37 + 6 | 39 + 8† | 21 + 9* |
| Gluconeogenesis(PEP) | 50 + 9 | 47 + 11† | 64 + 5* |
| Gluconeogenesis(glycerol) | 13 + 6 | 14 + 9 | 16 + 8 |
| TCA Cycle | (Relative to Citrate Synthase) | ||
| PEPCK | 6.6 + 1.4 | 7.9 + 2.2 | 11.0 + 4.1* |
| Pyruvate Cycling | 4.7 + 1.0 | 5.3 + 1.9 | 7.0 + 2.2* |
| Gluconeogenesis(PEP) | 1.9 + 0.5 | 2.7 + 0.8 | 4.1 + 2.1* |
| Absolute Data | (μmol/kgLBW/min) | ||
| Endogenous Glucose Production | 19.2 + 3.3 | 17.1 + 2.7 | 18.9 + 4.2 |
| Glycogenolysis | 7.1 + 1.8 | 6.7 + 2.1† | 3.7 + 1.6* |
| Gluconeogenesis(PEP) triose units | 19.5 + 5.4 | 16.0 + 4.0† | 24.0 + 5.3 |
| Gluconeogenesis(glycerol) triose units | 4.9 + 1.9 | 4.8 + 3.0 | 6.4 + 4.9 |
| PEPCK | 69.2 + 20.3 | 51.7 + 25.9 | 70.0 + 20.9 |
| Pyruvate Cycling | 49.6 + 16.1 | 35.8 + 23.0 | 45.9 + 18.0 |
| TCA Cycle | 10.5 + 2.0 | 6.9 + 3.1* | 7.2 + 3.4* |
significantly different from weight-stable group
significantly different from low-carbohydrate group
These data were also compared to a group of lean, weight-stable individuals. In this comparison, no differences were noted between the low-calorie group and the weight stable group with respect to fractional glycogenolysis or GNGPEP (Table 3). Interestingly, regardless of dietary composition or weight status, the fractional contribution of glycerol to gluconeogenesis was similar for all groups studied (P=0.799).
TCA Cycle Flux: Isotopomer analysis of plasma glucose 13C enrichment by13C-NMR
To simultaneously measure hepatic TCA cycle turnover and fractional glucose production, subjects ingested [U-13C]propionate. Details regarding the analysis of the 13C-NMR spectra of the glucose isotopomers produced as a result of the incorporation of the 13C carbons from propionate into the TCA cycle are presented in Figure 2. This analysis allowed the determination of maximal flux through PEPCK, pyruvate cycling, and GNGPEP, all relative to TCA cycle flux (Table 3). There was a trend towards a higher flux through PEPCK in carbohydrate-restricted subjects when compared to calorie-restricted subjects (P=0.051). The combined flux through the malic enzyme (malate → pyruvate) and pyruvate kinase (PEP → pyruvate), with the ultimate return of pyruvate to the TCA cycle via pyruvate carboxylase (pyruvate → OAA) constitutes pyruvate cycling (Figure 2); the flux through this cycle was similar between the two groups (P=0.112). The relative rate of GNGPEP is simply the difference between the rate of production of PEP (PEPCK flux) and the rate of return of PEP to the TCA cycle (pyruvate cycling). Concordant with PEPCK flux, we found a trend towards an increase in GNGPEP relative to the rate of TCA cycle flux in carbohydrate-restricted individuals (P=0.063) (Table 3).
Figure 2.
13C-NMR Analysis of the Incorporation of [U-13C]propionate into Glucose. After ingestion, propionate is avidly taken up by liver and enters the TCA cycle as [1,2,3-13]succinyl-CoA. Within the TCA cycle, this labeled intermediate can have one of three fates: 1) exit the TCA cycle as phosphoenolpyruvate (PEP) immediately upon conversion to oxaloacetate (OAA) via the action of phosphoenolpyruvate carboxykinase (PEPCK), ultimately leading to the formation of [1,2,3-13C]glucose and [1,2-13C]glucose in a 1:1 proportion; 2) proceed through a complete turn of the TCA cycle, passing through citrate synthase, and exiting the cycle as PEP, ultimately yielding [2-13C]glucose and [2,3-13C]glucose; and 3) exit the TCA cycle as PEP with subsequent conversion from PEP to pyruvate to OAA and back to PEP (so-called pyruvate cycling), ultimately leading to the formation of [1,2,3-13C]glucose and [1,2-13C]glucose in a 1:3 proportion. By examining the C2 of glucose using 13C-NMR after conversion to its monoacetone derivative, each of these glucose isotopomers is readily apparent: [1,2,3-13C]glucose is denoted in the spectrum by the quartet (Q); [2,3-13C]glucose is represented by the doublet D23; [1,2-13C]glucose is represented by the doublet D12; and [2-13C]glucose is represented by the singlet peak in the center of the C2 spectrum (not labeled). The areas under each of these peaks provides the information necessary to calculate PEPCK flux, pyruvate cycling, and the rate of production GNGPEP relative to citrate synthase flux. These pathways and the isotopomers they produced can also be described by a full set of analytical equations (24).
A comparison of the 13C data from the low-carbohydrate group to the weight stable group revealed all fluxes relative to TCA cycle flux were significantly higher in the low-carbohydrate group (Table 3). When compared to the weight-stable group, the measures in the low-calorie weight loss group were not significantly different.
Integration of Relative Fluxes with Endogenous Glucose Production
By combining the relative fluxes determined from 2H and 13C data with the rate of endogenous glucose production (EGP) determined by [3,4-13C]glucose dilution, absolute flux rates of the metabolic pathways connecting gluconeogenesis and the TCA cycle were calculated (17). The absolute hepatic fluxes for the low-calorie and low-carbohydrate groups are expressed in terms of lean body weight (calculated) and presented in Table 3. There was no difference in EGP between any of the groups studied. The absolute rate of glycogenolysis in the low-carbohydrate group was reduced 39% compared to the low-calorie group (P<0.001). This decrease in glycogenolysis in the low-carbohydrate group was offset by an increase in the absolute rate of GNGPEP (P=0.010) while there was no significant difference with regard to gluconeogenesis from glycerol (P=0.421).
Rates of glycogenolysis, GNGPEP, and GNGGlycerol did not differ significantly between the weight-stable and low-calorie groups. Though the rate of glycogenolysis was lower in the low-carbohydrate group when compared to the weight stable group (P=0.001), the rate of GNGPEP and GNGglycerol were similar (Table 3).
Contrary to the trend observed in the relative data above, absolute PEPCK flux and rates of pyruvate cycling did not differ between any of the three groups studied (Table 3). Of interest is the fact that TCA cycle flux was not different between the two weight-loss groups (P=0.822), indicating similar rates of energy production within the TCA cycle. However, both weight-loss groups had significantly lower TCA cycle flux rates when compared to the weight-stable group (P<0.05).
Hepatic Energy Generation and Gluconeogenesis
Energy generation in the hepatic TCA cycle has a powerful influence on flux through PEPCK and ultimately gluconeogenesis (13, 14, 16, 26, 27). A similar relationship is apparent in this present study (Figure 3), with TCA cycle flux and PEPCK flux showing a strong correlation (r=0.660; P=0.001). To further examine this relationship, we compared the energy generated in the TCA cycle to that required to drive gluconeogenic flux in molar units of ATP (Table 4). In the fasted state, the major precursors for PEP formation are lactate (derived from the Cori Cycle) and alanine (derived from the nitrogen-exporting glucose-alanine cycle). Assuming GNGPEP was solely derived from lactate, significantly more energy was produced by the TCA cycle than could be consumed by gluconeogenesis in both the weight-stable and low-calorie group. However, within the low-carbohydrate group, the rate of energy production in the TCA cycle and energy consumption of the measured pathways was nearly identical. Conversely, assuming alanine was the sole precursor for GNGPEP, energy production and consumption of the measured pathways were identical in the weight-stable and low-calorie group while energy consumption significantly outpaced energy production in the low-carbohydrate group (P=0.028, data not shown). While in reality, lactate and alanine are both important gluconeogenic precursors, these calculations suggest that regardless of the gluconeogenic substrate, there is an increased dependence on energy derived upstream of the TCA cycle in individuals undergoing carbohydrate restriction (e.g. β-oxidation/ketogenesis).
Figure 3.
Relationship Between PEPCK Flux and TCA Cycle Flux.
Table 4.
Energetics of Measured Pathways
| Weight-Stable | Low-Calorie ATP μmole/kgLBW/min |
Low-Carbohydrate | |
|---|---|---|---|
| Energy Consuming | |||
|
| |||
| Glucose Production | |||
| PEP → 1,3-PGA (-ATP) | -19.5 + 5.4 | -16.0 + 4.0 | -24.0 + 5.3 |
| OAA → PEP (-GTP) | -69.2 + 20.3 | -51.8 + 25.9 | -70.0 + 20.9 |
| Anaplerosis | |||
| Pyruvate → OAA (-ATP) | -69.2 + 20.3 | -51.8 + 25.9 | -70.0 + 20.9 |
|
| |||
| Total ATP Consumption | -157.8 + 45.2 | -119.5 + 55.0 | -163.9 + 45.3 |
|
| |||
| Energy Producing | |||
|
| |||
| Pyruvate Cycling | |||
| PEP → pyruvate (+ATP) | +49.6 + 16.1 | +35.8 + 23.0 | +45.9 + 18.0 |
| β-Oxidation | |||
| Fatty Acyl-CoA → Acetyl-CoA (+NADH, +FADH2) | +52.5 + 10.2 | +34.2 + 15.7 | +30.4 + 20.7 |
| TCA Cycle | |||
| citrate → α-KG (+NADH) | +31.5 + 6.1 | +20.5 + 9.4 | +21.5 + 10.2 |
| a-KG → SUCC-CoA (+NADH) | +31.5 + 6.1 | +20.5 + 9.4 | +21.5 + 10.2 |
| SUCC-CoA → SUCC (+GTP) | +10.5 + 2.0 | +6.9 + 3.1 | +7.2 + 3.4 |
| SUCC → FUM (+FADH2) | +21.0 + 4.1 | +13.7 + 6.3 | +14.3 + 6.8 |
| FUM → OAA (+NADH) | +31.5 + 6.1 | +20.5 + 9.4 | +21.5 + 10.2 |
|
| |||
| Total ATP Production | +228.1 + 48.1 | +152.2 + 74.2 | +167.8 + 72.4 |
|
| |||
| Consumption vs. Production | P= 0.001 | P= 0.013 | P=0.851 |
Note: Values are presented as mean + SD.
Abbreviations: gluconeogenesis from phosphoenolpyruvate, GNGPEP; phosphoenolpyruvate, PEP; phosphoglycerate, PGA; oxaloacetate, OAA; α-ketoglutarate, α-KG; succinyl-CoA, SUCC-CoA; succinate, SUCC; fumarate, FUM; adenosine triphosphate, ATP; guanosine triphosphate, GTP; nicontinamide adenine dinucleotide, NADH; flavin adenine dinucleotide, FADH2.
These calculations assume that lactate contributes 100% of the carbons required for gluconeogenesis. Further, the energetic comparison is in terms of ATP consumption and production; therefore, the reducing equivalents NADH and FADH2 have been converted to their stoichiometric ATP values (3 and 2 ATP, respectively).
DISCUSSION
In this study, the effect of carbohydrate restriction on flux through the metabolic pathways of hepatic glucose production and the TCA cycle were simultaneously assessed by isotopomer analysis of glucose using 2H and 13C NMR spectroscopy. We found that carbohydrate restriction increased the rate of gluconeogenesis and decreased the rate of glycogenolysis. However, the observed increase in gluconeogenesis in the low-carbohydrate group was solely the result of increased GNGPEP rather than GNGglycerol. Despite the energetic investment required to increase GNGPEP, TCA cycle flux in the low-carbohydrate group was similar to the low-calorie group, indicating similar rates of energy generation. Interestingly, in the groups consuming carbohydrate as a significant proportion of their diet (weight-stable, low-calorie), the TCA cycle alone provided sufficient energy to drive gluconeogenesis regardless of whether the gluconeogenic substrate was assumed to be lactate or alanine. This was not the case in individuals undergoing carbohydrate restriction, indicating that a reorganization of hepatic energy metabolism occurred in tandem with the changes in hepatic carbohydrate metabolism.
Among previous studies of carbohydrate restriction, it remained unclear which gluconeogenic precursors were primarily responsible for increased gluconeogenesis. Evidence of a negative correlation between alanine conversion to glucose and dietary carbohydrate content suggests that anaplerosis and GNGPEP are increased with decreased dietary intake of carbohydrate (28, 29). However, increased fat oxidation during carbohydrate restriction might be expected to increase availability of the gluconeogenic precursor glycerol (10). In the present study, we showed that the increase in gluconeogenesis associated with carbohydrate restriction is due to the induction of GNGPEP. This suggests that in fasted human subjects undergoing weight loss, the elevated gluconeogenesis associated with carbohydrate restriction is driven by substrates such as lactate or amino acids. While it seems likely this increase is due to amplified protein turnover, we could not rule out enhanced cycling of lactate from the periphery back to liver (Cori Cycle) as a source of increased gluconeogenesis. Plasma lactate levels were similar between the two weight loss groups (Table 2); however, these were static measurements and gave no insight into the rate of production of lactate by muscle and uptake by liver. Likewise, protein turnover measurements were not performed so the contribution of amino acids also remains unknown. However, it is interesting to note that individuals on a low-carbohydrate diet increased their protein intake in favor of fat (Table 2), possibly as method to stave off non-dietary protein breakdown for the formation of glucose.
The contribution of glycerol as a substrate for gluconeogenesis was surprisingly unresponsive to dietary macronutrient composition. Though GNGglycerol appeared to be numerically higher in the low-carbohydrate group, this failed to reach statistical significance. It is possible that the small sample size of the study and/or the sensitivity of our technique limited our ability to detect modest changes in this measure. However, prior data in fasting man suggests that gluconeogenesis from glycerol occurs at a relatively fixed rate (30). Our findings would further support this observation. Indeed, insulin levels were similar between the groups undergoing dietary restriction, suggesting that rates of peripheral lipolysis were also similar (Table 2). This was somewhat surprising as prior data in lean individuals clearly demonstrates a reduction in insulin levels and increase in free fatty acid levels as a result of carbohydrate restriction (31). However, the present data is akin to that of Allick et al. in which overweight/obese individuals with diabetes maintained similar insulin and free fatty acid levels regardless of dietary macronutrient composition (32). Further studies are needed to verify this finding.
It should be noted that prior studies assessing the impact of carbohydrate restriction on hepatic glucose metabolism show that the main effect is a reduction in hepatic glucose output, predominantly via a reduction in glycogenolysis (31, 32). This is in contrast to the present study in which hepatic glucose production was similar between the dietary groups. The low-carbohydrate group was able to maintain hepatic glucose production at the levels observed for the weight-stable and low-calorie groups by increasing GNGPEP to match the reduction in glycogenolysis. This observation is reminiscent of “hepatic autoregulation” by which endogenous glucose production remains unchanged in the setting of altered gluconeogenesis or glycogenolysis because the two pathways tend to compensate for each other (28, 33). This finding may also be the result of the much larger intake of dietary protein in the low-carbohydrate group (∼34%) as compared to prior studies (11-15%) (31, 32), possibly yielding an enhanced supply of gluconeogenic substrate.
The multi-tracer approach used in the present study allowed for the simultaneous assessment of both hepatic glucose production as well as TCA cycle flux. Knowledge of metabolic flux through these pathways provided insight into the relationship between hepatic glucose and energy metabolism (Table 3). Energetic coupling of GNGPEP and the TCA cycle was observed as a correlation between TCA cycle flux and PEPCK flux (Figure 3), the metabolic pathway responsible for the delivery of substrate for gluconeogenesis. It is, however, intriguing that the increased GNGPEP in the low-carbohydrate group was not associated with increased TCA cycle flux (i.e., energy production). Indeed, assuming net glucose synthesis predominated (i.e., alanine or other amino acids acted as the gluconeogenic substrate as opposed to lactate), energy production in the TCA cycle would be unable to meet the energetic demands of gluconeogenesis in the low-carbohydate group. This would suggest a greater reliance on sources of energy upstream from the terminal oxidation of fat in the TCA cycle in this group, possibly β-oxidation/ketogenesis. Prior data has demonstrated a relationship between GNGPEP and ketogenesis: in general, ketogenesis parallels the rate of gluconeogenesis under most circumstances (30). Indeed, static measurements of ketone bodies were markedly higher in subjects undergoing carbohydrate restriction, indicating that the availability of acetyl-CoA to HMG-CoA synthase may have been greater. However, absolute rates of fatty acid delivery to liver as well as ketone body production were not measured, limiting our ability to further interpret the above findings.
Every attempt was made to equalize caloric intake between the two dietary restriction groups. However, a trend was noted towards decreased caloric intake in the group undergoing carbohydrate restriction (Table 2). It is possible that the differences observed between these two groups are solely the result of differences in caloric intake and weight loss. However, prior data obtained under weight-stable, isocaloric conditions showed similar changes in hepatic glucose metabolism in lean individuals (11). Likewise, fractional and absolute glucose production was similar between the weight-stable and low-calorie group despite the difference in energy balance between the two. It should also be noted that the present study was not designed to determine the effectiveness of these two weight-loss diets in weight reduction, but was simply designed to assess hepatic metabolism under the differing macronutrient compositions during negative energy balance. Additionally, it was our desire to examine changes in hepatic metabolism under conditions likely to be encountered in a clinical setting; hence, dietary choices of the subjects were more varied than what would be encountered in a strict physiologic study.
In conclusion, we have shown that the sources from which endogenous glucose is produced are dependent upon dietary macronutrient composition. Carbohydrate restriction yields a decreased rate of glycogenolysis and an increased rate of gluconeogenesis compared to calorie restriction. We have shown for the first time that this increased rate of hepatic gluconeogenesis is the result of an increased rate of utilization of substrates like lactate and amino acids, but not glycerol. Additionally, the TCA cycle appears to be the energetic patron of GNGPEP, as TCA cycle flux and PEPCK flux were highly correlated. Furthermore, it appears that the shift in glucose metabolism associated with a low carbohydrate diet leads to an increased contribution of energy generated outside of the TCA cycle to gluconeogenesis. This shift is consistent with enhanced β-oxidation/ketogenesis, which could be beneficial in individuals with NAFLD due to enhanced disposal of hepatic triglyceride. These findings may explain, in part, the correlation between carbohydrate intake and severity of liver disease in individuals with NAFLD (7). Understanding the alterations to cellular energetics that occur with simple macronutrient manipulation may be important for understanding and treating NAFLD and other metabolic disorders associated with obesity (34).
ACKNOWLEDGEMENTS
We would like to thank the staff of the GCRC at the University of Texas Southwestern Medical Center and Jay D. Horton, Dean Sherry, and Elizabeth Parks for helpful discussions.
Financial Support: This research was supported by NIH RL1DK081187 (JB, SB), 1K23DK074396-01 (JB), RR02584 (CM, SB), and DK078184 (SB).
List of Abbreviations
- GNG
gluconeogenesis
- PEP
phosphoenolpyruvate
- MAG
monoacetone glucose
- PEPCK
phosphoenolpyruvate carboxykinase
- OAA
oxaloacetate
- EGP
endogenous glucose production
- BMI
body mass index
- GCRC
General Clinical Research Center
- RQ
respiratory quotient
REFERENCES
- 1.Keys A. Seven Countries, A multivariate analysis of diet and coronary heart disease. Harvard University Press; Cambridge and London: 1980. [Google Scholar]
- 2.Expert Panel on the Identification E, and Treatment of Overweight and Obesity in Adults,. Executive summary of the clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults. Arch. Intern. Med. 1998;158:1855–1867. doi: 10.1001/archinte.158.17.1855. [DOI] [PubMed] [Google Scholar]
- 3.Heini AF, Weinsier RL. Divergent trends in obesity and fat intake patterns: the American paradox. Am. J. Med. 1997;102:259–264. doi: 10.1016/S0002-9343(96)00456-1. [DOI] [PubMed] [Google Scholar]
- 4.Flegal KM, Carroll MD, Kuczmarski RJ, Johnson CL. Overweight and obesity in the United States: prevalence and trends, 1960-1994. Int. J. Obes. Relat. Metab. Disord. 1998;22:39–47. doi: 10.1038/sj.ijo.0800541. [DOI] [PubMed] [Google Scholar]
- 5.Browning JD, Horton JD. Molecular mediators of hepatic steatosis and liver injury. J. Clin. Invest. 2004;114:1–4. doi: 10.1172/JCI22422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Browning JD, Szczepaniak LS, Dobbins R, Nuremberg P, Horton JD, Cohen JB, Grundy SM, et al. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology. 2004;40:1387–1395. doi: 10.1002/hep.20466. [DOI] [PubMed] [Google Scholar]
- 7.Solga S, Alkhuraishe AR, Clark JM, Torbenson M, Greenwald A, Diehl AM, Magnuson T. Dietary composition and nonalcoholic fatty liver disease. Dig. Dis. Sci. 2004;49:1578–1583. doi: 10.1023/b:ddas.0000043367.69470.b7. [DOI] [PubMed] [Google Scholar]
- 8.Kang H, Greenson JK, Omo JT, Chao C, Peterman D, Anderson L, Foess-Wood L, et al. Metabolic syndrome is associated with greater histologic severity, higher carbohydrate, and lower fat diet in patients with NAFLD. Am. J. Gastroenterol. 2006;101:2247–2253. doi: 10.1111/j.1572-0241.2006.00719.x. [DOI] [PubMed] [Google Scholar]
- 9.Schwarz JM, Linfoot P, Dare D, Aghajanian K. Hepatic de novo lipogenesis in normoinsulinemic and hyperinsulinemic subjects consuming high-fat, low-carbohydrate and low-fat, high-carbohydrate isoenergetic diets. Am. J. Clin. Nutr. 2003;77:43–50. doi: 10.1093/ajcn/77.1.43. [DOI] [PubMed] [Google Scholar]
- 10.Schwarz JM, Neese RA, Turner S, Dare D, Hellerstein MK. Short-term alterations in carbohydrate energy intake in humans. Striking effects on hepatic glucose production, de novo lipogenesis, lipolysis, and whole-body fuel selection. J. Clin. Invest. 1995;96:2735–2743. doi: 10.1172/JCI118342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bisschop PH, Arias AM Pereira, Ackermans MT, Endert E, Pijl H, Kuipers F, Meijer AJ, et al. The effects of carbohydrate variation in isocaloric diets on glycogenolysis and gluconeogenesis in healthy men. J. Clin. Endocrinol. Metab. 2000;85:1963–1967. doi: 10.1210/jcem.85.5.6573. [DOI] [PubMed] [Google Scholar]
- 12.Allick G, Bisschop PH, Ackermans MT, Endert E, Meijer AJ, Kuipers F, Sauerwein HP, et al. A low-carbohydrate/high-fat diet improves glucoregulation in type 2 diabetes mellitus by reducing postabsorptive glycogenolysis. J. Clin. Endocrinol. Metab. 2004;89:6193–6197. doi: 10.1210/jc.2004-1041. [DOI] [PubMed] [Google Scholar]
- 13.Burgess SC, He T, Yan Z, Lindner J, Sherry AD, Malloy CR, Browning JD, et al. Cytosolic phosphoenolpyruvate carboxykinase does not solely control the rate of hepatic gluconeogenesis in the intact mouse liver. Cell Metab. 2007;5:313–320. doi: 10.1016/j.cmet.2007.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burgess SC, Hausler N, Merritt M, Jeffrey FM, Storey C, Milde A, Koshy S, et al. Impaired tricarboxylic acid cycle activity in mouse livers lacking cytosolic phosphoenolpyruvate carboxykinase. J. Biol. Chem. 2004;279:48941–48949. doi: 10.1074/jbc.M407120200. Epub 42004 Sep 48943. [DOI] [PubMed] [Google Scholar]
- 15.Hakimi P, Johnson MT, Yang J, Lepage DF, Conlon RA, Kalhan SC, Reshef L, et al. Phosphoenolpyruvate carboxykinase and the critical role of cataplerosis in the control of hepatic metabolism. Nutr. Metab. (Lond) 2005;2:33. doi: 10.1186/1743-7075-2-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hausler N, Browning J, Merritt M, Storey C, Milde A, Jeffrey FM, Sherry AD, et al. Effects of insulin and cytosolic redox state on glucose production pathways in the isolated perfused mouse liver measured by integrated 2H and 13C NMR. Biochem. J. 2006;394:465–473. doi: 10.1042/BJ20051174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jones JG, Solomon MA, Cole SM, Sherry AD, Malloy CR. An integrated (2)H and (13)C NMR study of gluconeogenesis and TCA cycle flux in humans. Am. J. Physiol. Endocrinol. Metab. 2001;281:E848–856. doi: 10.1152/ajpendo.2001.281.4.E848. [DOI] [PubMed] [Google Scholar]
- 18.Atkins RC. Dr. Atkins’ New Diet Revolution. Avon Books; New York: 1998. [Google Scholar]
- 19.Landau BR, Wahren J, Chandramouli V, Schumann WC, Ekberg K, Kalhan SC. Use of 2H2O for estimating rates of gluconeogenesis. Application to the fasted state. J. Clin. Invest. 1995;95:172–178. doi: 10.1172/JCI117635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Burgess SC, Nuss M, Chandramouli V, Hardin DS, Rice M, Landau BR, Malloy CR, et al. Analysis of gluconeogenic pathways in vivo by distribution of 2H in plasma glucose: comparison of nuclear magnetic resonance and mass spectrometry. Anal. Biochem. 2003;318:321–324. doi: 10.1016/s0003-2697(03)00158-1. [DOI] [PubMed] [Google Scholar]
- 21.Burgess SC, Weis B, Jones JG, Smith E, Merritt ME, Margolis D, Sherry A Dean, et al. Noninvasive evaluation of liver metabolism by 2H and 13C NMR isotopomer analysis of human urine. Anal. Biochem. 2003;312:228–234. doi: 10.1016/s0003-2697(02)00465-7. [DOI] [PubMed] [Google Scholar]
- 22.Schleucher J, Vanderveer PJ, Sharkey TD. Export of carbon from chloroplasts at night. Plant Physiol. 1998;118:1439–1445. doi: 10.1104/pp.118.4.1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jones JG, Naidoo R, Sherry AD, Jeffrey FM, Cottam GL, Malloy CR. Measurement of gluconeogenesis and pyruvate recycling in the rat liver: a simple analysis of glucose and glutamate isotopomers during metabolism of [1,2,3-(13)C3]propionate. FEBS Lett. 1997;412:131–137. doi: 10.1016/s0014-5793(97)00764-3. [DOI] [PubMed] [Google Scholar]
- 24.Sherry AD, Jeffrey FM, Malloy CR. Analytical solutions for 13C isotopomer analysis of complex metabolic conditions: substrate oxidation, multiple pyruvate cycles, and gluconeogenesis. Metab. Eng. 2004;6:12–24. doi: 10.1016/j.ymben.2003.10.007. [DOI] [PubMed] [Google Scholar]
- 25.Jin ES, Jones JG, Burgess SC, Merritt ME, Sherry AD, Malloy CR. Comparison of [3,4-13C2]glucose to [6,6-2H2]glucose as a tracer for glucose turnover by nuclear magnetic resonance. Magn. Reson. Med. 2005;53:1479–1483. doi: 10.1002/mrm.20496. [DOI] [PubMed] [Google Scholar]
- 26.Burgess SC, Leone TC, Wende AR, Croce MA, Chen Z, Sherry AD, Malloy CR, et al. Diminished hepatic gluconeogenesis via defects in tricarboxylic acid cycle flux in peroxisome proliferator-activated receptor gamma coactivator-1alpha (PGC-1alpha)-deficient mice. J. Biol. Chem. 2006;281:19000–19008. doi: 10.1074/jbc.M600050200. Epub 12006 May 19002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Satapati S, He T, Inagaki T, Potthoff M, Merritt ME, Esser V, Mangelsdorf DJ, et al. Partial resistance to PPAR{alpha} agonists in Zucker diabetic fatty (ZDF) rats is associated with defective hepatic mitochondrial metabolism. Diabetes. 2008 doi: 10.2337/db08-0226. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Clore JN, Helm ST, Blackard WG. Loss of hepatic autoregulation after carbohydrate overfeeding in normal man. J. Clin. Invest. 1995;96:1967–1972. doi: 10.1172/JCI118243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eisenstein AB, Strack I, Steiner A. Increased hepatic gluconeogenesis without a rise of glucagon secretion in rats fed a high fat diet. Diabetes. 1974;23:869–875. doi: 10.2337/diab.23.11.869. [DOI] [PubMed] [Google Scholar]
- 30.Cahill GF., Jr. Starvation in man. N. Engl. J. Med. 1970;282:668–675. doi: 10.1056/NEJM197003192821209. [DOI] [PubMed] [Google Scholar]
- 31.Bisschop PH, de Sain-van der Velden MGM, Stellaard F, Kuipers F, Meijer AJ, Sauerwein HP, Romijn JA. Dietary Carbohydrate Deprivation Increases 24-Hour Nitrogen Excretion without Affecting Postabsorptive Hepatic or Whole Body Protein Metabolism in Healthy Men 10.1210/jc.2002-021087. J Clin Endocrinol Metab. 2003;88:3801–3805. doi: 10.1210/jc.2002-021087. [DOI] [PubMed] [Google Scholar]
- 32.Allick G, Bisschop PH, Ackermans MT, Endert E, Meijer AJ, Kuipers F, Sauerwein HP, et al. A Low-Carbohydrate/High-Fat Diet Improves Glucoregulation in Type 2 Diabetes Mellitus by Reducing Postabsorptive Glycogenolysis 10.1210/jc.2004-1041. J Clin Endocrinol Metab. 2004;89:6193–6197. doi: 10.1210/jc.2004-1041. [DOI] [PubMed] [Google Scholar]
- 33.Clore JN, Glickman PS, Nestler JE, Blackard WG. In vivo evidence for hepatic autoregulation during FFA-stimulated gluconeogenesis in normal humans. Am. J. Physiol. 1991;261:E425–E429. doi: 10.1152/ajpendo.1991.261.4.E425. [DOI] [PubMed] [Google Scholar]
- 34.Seidell JC. Obesity, insulin resistance and diabetes--a worldwide epidemic. Br. J. Nutr. 2000;83:S5–S8. doi: 10.1017/s000711450000088x. [DOI] [PubMed] [Google Scholar]