Abstract
Dearomatization of phenols followed by oxidation affords cyclohexadienyloxyacetaldehydes, which produce hydrobenzofuranones via asymmetric intramolecular Stetter reaction in good to excellent yield. Quaternary as well as up to three contiguous stereocenters may be formed in good to excellent enantioselectivities and high diastereoselectivities.
Dearomatization of aromatic compounds provides a useful alicyclic synthetic building block due to its high economy and simple elegance.1 When coupled with a stereoselective process, it has the potential for affording enantioenriched material from commonly available precursors in rapid fashion.2 Reactivity umpolung3 affords an opportunity to take advantage of unobvious, complementary bond disconnections in the synthesis of complex molecules. One such reaction is the Stetter reaction, the nucleophile-catalyzed addition of an aldehyde to a Michael acceptor.4 If the Michael acceptor involves a prochiral alkene, this reaction generates new stereocenters.5 We have recently developed a family of catalysts capable of inducing highly enantioselective intramolecular Stetter reactions.6 As part of an effort to extend the power of this transformation, we considered that cyclohexadienones, readily available from dearomatization of phenols,7 could be suitable substrates for a desymmetrizing Stetter reaction. Herein we disclose that treatment of such substrates with chiral triazolium salts affords, in high yields and enantioselectivities, hydrobenzofurans, which are core skeletons found in many natural products.8
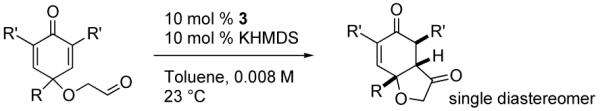
The requisite substrates are readily accessible from the corresponding phenols (Scheme 1). Hypervalent iodine reagents are used in conjunction with glycol to afford the dienone alcohols in good yield, whereupon Dess-Martin oxidation leads to the aldehydes and sets the stage for the asymmetric Stetter reaction. Upon cyclization, the desired product hydrobenzofuranones would contain at least two contiguous stereocenters.
Scheme 1.
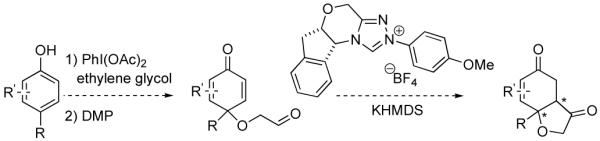
Asymmetric Synthesis of Hydrobenzofuranones
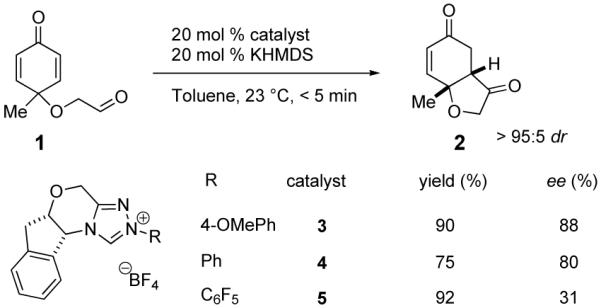
The reactivity of a series of catalysts6 was studied using substrate 1 (eq 1). The reactions were conducted in the presence of 20 mol% catalyst and 20 mol% KHMDS in toluene at 25 °C, and proceeded to completion in less than 5 min affording desired product 2. Aminoindanol-derived triazolium salt 3 bearing an anisyl substituent was found to be the best catalyst precursor for this reaction, again reflecting the subtle impact of electronics arising from aryl substitution.6
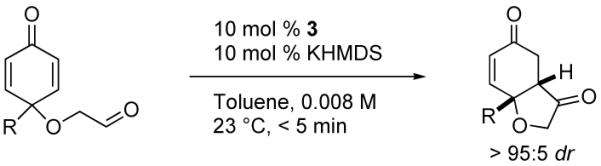
A range of substrates was synthesized following the general procedure (Scheme 1). Under optimized conditions,9 the asymmetric intramolecular Stetter reaction proceeds very well, Table 1. The enantioselectivities are excellent (92-94 % ee) when groups at the 4-position of the substrates are methyl, ethyl, isopropyl and tert-butyl (entries 1-4). Aromatic substituents result in slightly lower, but still synthetically useful enantioselectivities (Table 1, entries 5-6). Similar effects are observed with more functionalized sidechains at that position, entries 7-9. With all substrates, the reaction proceeds with very high diastereoselectivities (> 95:5 by 1H NMR).10
 |
(1) |
Table 1.
Asymmetric Intramolecular Stetter Reaction of Mono-Substituted Cyclohexadienones
 | |||||
|---|---|---|---|---|---|
| entrya | R | substrate | product | Yield (%) | ee (%)b |
| 1 | Me | 1 | 2 | 90 | 92 |
| 2 | Et | 6 | 7 | 86 | 94 |
| 3 | iPr | 8 | 9 | 87 | 94 |
| 4 | tBu | 10 | 11 | 86 | 94 |
| 5 | Ph | 12 | 13 | 87 | 88 |
| 6 | 4-BrPh | 14 | 15 | 78 | 85 |
| 7 | CH2OAc | 16 | 17 | 86 | 83 |
| 8 | CH2CH2OMe | 18 | 19 | 86 | 82 |
| 9 | CH2CH2CO2Me | 20 | 21 | 94 | 87 |
All reactions conducted in the presence of 10 mol% catalyst and 10 mol% KHMDS in toluene at 23 °C.
Determined by HPLC or GC using a chiral stationary phase.
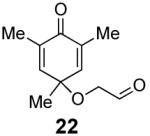
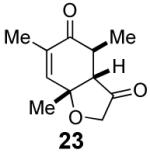
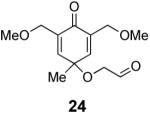
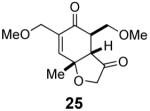
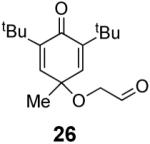
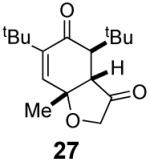
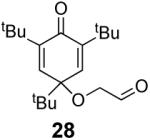
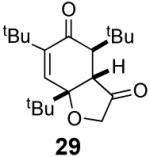
We have previously documented the ability of the Stetter reaction to form contiguous stereocenters with trisubstituted alkene acceptors.6d The use of 2,4,6-trisubstituted phenols as precursors in this chemistry allow a rapid entry into suitably functionalized substrates to test whether the desymmetrization would proceed. In the event, the dienone derived from 2,4,6-trimethylphenol proved remarkably efficient. This reaction provides hexahydrobenzofuranone 23 possessing three contiguous stereocenters in excellent yield and ee, entry 1 in Table 2. The reaction is tolerant of alkoxymethyl groups at the 2-position, providing 25 in excellent ee and good yield, entry 2. No elimination of the methoxy group is observed under these conditions. Remarkably, this reaction is also tolerant of as many as three tert-butyl groups in the vicinity of the reaction center and the substrates provide one (entry 3), or two (entry 4) neopentyl stereocenters in excellent selectivity. In each of the cases examined to date, a single diastereomer is formed.10
Table 2.
Reaction of Tri-Substituted Substrates
Commercially available 3,4,5-trimethylphenol provides an opportunity to test whether this approach is capable of forming quaternary stereocenters as per our previous report.6c In the event cyclization of substrate 30 affords one quaternary stereocenter adjacent to a tertiary ether in excellent enantioselectivity and good yield (eq 2).
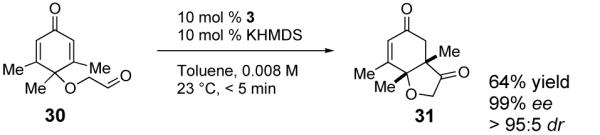 |
(2) |
Although the bulk of our work focused on oxygen-tethered substrates due to their accessibility, the reaction is also capable of forming carbocycles. To that end, when 32 is subjected to the reaction conditions, hydrindane 33 is isolated in good yield and 90% ee, eq 3.
In conclusion, a family of phenol-derived substrates have been
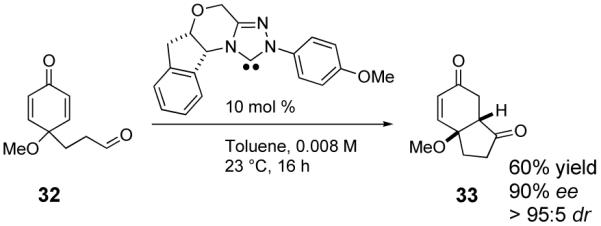 |
(3) |
successfully synthesized and found to undergo asymmetric intramolecular Stetter reactions in very good yields. Quaternary stereocenters and up to three contiguous stereocenters may be formed in excellent enantioselectivities and diastereoselectivities.
Supplementary Material
Acknowledgment
Dedicated to Professor David A. Evans on the occasion of his 65th birthday. We thank the National Institute of General Medical Sciences (GM72586) and Johnson and Johnson (Focused Giving) for partial support of this research. T.R. thanks Merck Research Laboratories, GlaxoSmithKline, Eli Lilly, Amgen, and Boehringer Ingelheim for unrestricted support, and the Monfort Family Foundation for a Monfort Professorship. T.R. is a fellow of the Alfred P. Sloan Foundation. We thank Jerry Murry and Michael C. Hillier (Merck Research Laboratories) for a generous gift of aminoindanol. We thank Mark S. Kerr, Oren P. Anderson and Susan M. Miller (CSU) as well as Charles Campana (Bruker AXS) for X-ray analysis.
References
- (1)(a).Woodward RB. J. Am. Chem. Soc. 1940;62:1208. [Google Scholar]; (b) Waring AJ. In: Advances in Alicyclic Chemistry. Hart H, Karabatsos GJ, editors. Academic Press; New York: 1966. pp. 129–256. [Google Scholar]; (c) Corey EJ, Girotra NN, Mathew CT. J. Am. Chem. Soc. 1969;91:1557–1559. [Google Scholar]; (d) Wiesner K, Tsai TYR, Nambiar KP. Can. J. Chem. 1978;56:1451–1454. [Google Scholar]; (e) Semmelhack MF, Harrison JJ, Thebtaranonth Y. J. Org. Chem. 1979;44:3275–3277. [Google Scholar]; (f) Corey EJ, Dittami JP. J. Am. Chem. Soc. 1985;107:256–257. [Google Scholar]; (g) Mander LN. Synlett. 1991;3:134–144. [Google Scholar]; (h) Kopach ME, Harman WD. J. Am. Chem. Soc. 1994;116:6581–6592. [Google Scholar]; (i) Maruoka K, Ito M, Yamamoto H. J. Am. Chem. Soc. 1995;117:9091–9092. [Google Scholar]; (j) Clive DLJ, Fletcher SP, Liu D. J. Org. Chem. 2004;69:3282–3293. doi: 10.1021/jo030364k. [DOI] [PubMed] [Google Scholar]; (k) Villar FV, Kolly-Kovac T, Equey O, Renaud P. Chem. Eur. J. 2003;9:1566–1577. doi: 10.1002/chem.200390180. [DOI] [PubMed] [Google Scholar]
- (2)(a).Pearson AJ, Zhu PY, Youngs WJ, Bradshaw JD, McConville DB. J. Am. Chem. Soc. 1993;115:10376–10377. [Google Scholar]; (b) Pearson AJ, Milletti MC, Zhu PY. Chem. Commun. 1995;8:853–854. [Google Scholar]; (c) Schultz AG. Chem. Commun. 1999:1263–1271. [Google Scholar]; (d) Imbos R, Minnaard AJ, Feringa BL. J. Am. Chem. Soc. 2002;124:184–185. doi: 10.1021/ja017200a. [DOI] [PubMed] [Google Scholar]; (e) Hayashi Y, Gotoh H, Tamura T, Yamaguchi H, Masui R, Shoji M. J. Am. Chem. Soc. 2005;127:16028–16029. doi: 10.1021/ja055740s. [DOI] [PubMed] [Google Scholar]; (f) Studer A, Schleth F. Synlett. 2005;20:3033–3041. [Google Scholar]
- (3).Seebach D. Angew. Chem., Int. Ed. Engl. 1979;18:239–258. [Google Scholar]
- (4)(a).Stetter H. Angew. Chem., Int. Ed. Engl. 1976;15:639–648. [Google Scholar]; (b) Stetter H, Kuhlmann H. Org. React. 1991;40:407–496. [Google Scholar]
- (5)(a).Enders D, Breuer K, Runsink J, Teles JH. Helv. Chim. Acta. 1996;79:1899–1902. [Google Scholar]; (b) Enders D, Balensiefer T. Acc. Chem. Res. 2004;37:534–541. doi: 10.1021/ar030050j. [DOI] [PubMed] [Google Scholar]; (c) Christmann M. Angew. Chem. Int. Ed. 2005;44:2632–2634. doi: 10.1002/anie.200500761. [DOI] [PubMed] [Google Scholar]; (d) Nair V, Bindu S, Sreekumar V. Angew. Chem. Int. Ed. 2004;43:5130–5135. doi: 10.1002/anie.200301714. [DOI] [PubMed] [Google Scholar]; (e) Zeitler K. Angew. Chem. Int. Ed. 2005;44:7506–7510. doi: 10.1002/anie.200502617. [DOI] [PubMed] [Google Scholar]; (f) Pesch J, Harms K, Bach T. Eur. J. Org. Chem. 2004;9:2025–2035. [Google Scholar]; (g) Mennen SM, Blank JT, Tran-Dube MB, Imbriglio JE, Miller S. J. Chem. Commun. 2005:195–197. doi: 10.1039/b414574g. [DOI] [PubMed] [Google Scholar]; (h) Nahm MR, Linghu X, Potnick JR, Yates CM, White PS, Johnson JS. Angew. Chem. Int. Ed. 2005;44:2377–2379. doi: 10.1002/anie.200462795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6)(a).Kerr MS, Read de Alaniz J, Rovis T. J. Am. Chem. Soc. 2002;124:10298–10299. doi: 10.1021/ja027411v. [DOI] [PubMed] [Google Scholar]; (b) Kerr MS, Rovis T. Synlett. 2003;12:1934–1936. [Google Scholar]; (c) Kerr MS, Rovis T. J. Am. Chem. Soc. 2004;126:8876–8877. doi: 10.1021/ja047644h. [DOI] [PubMed] [Google Scholar]; (d) Read de Alaniz J, Rovis T. J. Am. Chem. Soc. 2005;127:6284–6289. doi: 10.1021/ja0425132. [DOI] [PubMed] [Google Scholar]
- (7)(a).Pelter A, Elgendy SMA. J. Chem. Soc. Perkin Trans. 1. 1993:1891–1896. [Google Scholar]; (b) Moriarty RM, Prakash O. Org. React. 2001;57:327–415. [Google Scholar]; (c) Trân-Huu-Dâu M, Wartchow R, Winterfeldt E, Wong Y. Chem. Eur. J. 2001;7:2349–2369. doi: 10.1002/1521-3765(20010601)7:11<2349::aid-chem23490>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]; (d) Canesi S, Bouchu D, Ciufolini MA. Org. Lett. 2005;7:175–177. doi: 10.1021/ol048094v. [DOI] [PubMed] [Google Scholar]
- (8)(a).Chu-Moyer MY, Danishefsky SJ, Schulte GK. J. Am. Chem. Soc. 1994;116:11213–11228. [Google Scholar]; (b) Guth H. Helv. Chim. Acta. 1996;79:1559–1571. [Google Scholar]; (c) Yamashita M, Ohta N, Shimizu T, Matsumoto K, Matsuura Y, Kawasaki I, Tanaka T, Maezaki N, Ohta S. J. Org. Chem. 2003;68:1216–1224. doi: 10.1021/jo020619e. [DOI] [PubMed] [Google Scholar]; (d) Clive DLJ, Fletcher SP. Chem. Commun. 2003:2464–2465. doi: 10.1039/b307937f. [DOI] [PubMed] [Google Scholar]; (e) Booker-Milburn KI, Hirst P, Charmant JPH, Taylor LHJ. Angew. Chem. Int. Ed. 2003;42:1642–1644. doi: 10.1002/anie.200250507. [DOI] [PubMed] [Google Scholar]; (f) Taber DF, Neubert TD, Rheingold AL. J. Am. Chem. Soc. 2002;124:12416–12417. doi: 10.1021/ja027882h. [DOI] [PubMed] [Google Scholar]; (g) Takao K, Tsujita T, Hara M, Tadano K. J. Org. Chem. 2002;67:6690–6698. doi: 10.1021/jo0203140. [DOI] [PubMed] [Google Scholar]; (h) Burke SD, Cobb JE, Takeuchi K. J. Org. Chem. 1985;50:3420–3421. [Google Scholar]; (i) Yao S, Johannsen M, Hazell RG, Jørgensen KA. J. Org. Chem. 1998;63:118–121. doi: 10.1021/jo971528y. [DOI] [PubMed] [Google Scholar]; (j) Jonasson C, Rönn M, Bäckvall J. J. Org. Chem. 2000;65:2122–2126. doi: 10.1021/jo991787i. [DOI] [PubMed] [Google Scholar]; (k) Germain J, Deslongchamps P. J. Org. Chem. 2002;67:5269–5278. doi: 10.1021/jo025873l. [DOI] [PubMed] [Google Scholar]
- (9).Lower catalyst loading or higher concentration result in comparable yields of product but lower enantioselectivity (2 mol% catalyst: 84% yield, 83% ee; 0.04 M: 90% yield, 88% ee).
- (10).Relative and absolute configurations determined by nOe and X-ray for several compounds and the rest assigned by analogy (see SI for details). We have been unable to form the complementary diastereomers even by base-induced epimerization. A semi-empirical calculation of 23, 25, 27 and 29 suggests the minor diastereomers are as much as 9 kcal/mol less stable. Examination of GC traces suggests that this reaction is likely far more selective than 95% of a single diastereomer.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.